Abstract
Background
HIV infection is associated with high rates of acute rejection following kidney transplantation. The underlying mechanisms for such predisposition are incompletely understood. Pathological immune activation is a hallmark of chronic HIV infection that persists despite effective antiretroviral therapy. We hypothesized that the baseline levels of T cell activation in HIV+ candidates would correlate with their risk of acute rejection following kidney transplantation.
Methods
Single-center retrospective cohort analysis of HIV+ adult kidney transplants performed between October 2006 and September 2013. The frequency of CD3+HLA-DR+ cells measured by flow cytometry served as a surrogate marker of immune activation. Patients were categorized into tertiles of activation, and the rates of biopsy-proven acute rejection were compared across groups.
Results
(1) Compared to matched HIV− controls, the baseline number of CD3+HLA-DR+ cells was higher in HIV+ kidney transplant candidates. (2) Abnormally high levels of activation did not decrease with transplant-associated immunosuppression. (3) Patients categorized within the lower and middle CD3+HLA-DR+ tertiles had higher probability of rejection during the first 3 years post-transplant compared to those in the higher activation tertile (36.9% vs. 0%; log-rank P= .04).
Conclusions
Pathological immune activation in HIV+ transplant candidates does not explain their increased susceptibility to allograft rejection. Paradoxically, those with the highest levels of immune activation seem to be less prone to rejection.
Keywords: HIV, immune activation, HLA-DR, acute rejection, kidney transplant
1. Introduction
Human immunodeficiency virus (HIV) infection was once considered a contraindication for kidney transplantation. With the advent of effective antiretroviral therapy (ART), a growing number of people living with HIV in the United States are aged 50 and older. The aging of the HIV population and the high prevalence of end-stage renal disease in HIV-infected (HIV+) individuals [1-2], has led to a rise in the number of kidney transplants performed in HIV+ recipients over the last fifteen years [3]. Large cohort studies and registry data indicate that patient and graft survival rates following kidney transplantation in HIV+ recipients are comparable to that of HIV-uninfected (HIV−) controls [3-6]. Concerns remain, however, regarding the high incidence of acute rejection in HIV+ recipients [3, 5, 7]. A two to three-fold higher-than-expected rejection rate was observed in the largest prospective trial of kidney transplantation in HIV+ recipients [5]. The underlying mechanisms for the increased susceptibility to rejection in HIV+ recipients remain poorly understood.
Chronic HIV infection is characterized by pathological immune activation [8-12]. Even among HIV+ individuals with evidence of viral load suppression and normalization of the CD4 count (>900 cells/μL) in response to ART, the frequency of activated (HLA-DR+) effector memory CD4+ T cells remains elevated compared to HIV− persons [10]. Thus, it could be expected that HIV+ kidney transplant candidates with well controlled HIV infection can have abnormal levels of immune activation at the time of transplantation. It is increasingly recognized that the levels of immune activation in HIV+ individuals correlate with the incidence of non-AIDS morbidity and mortality [11]; we hypothesized that the baseline levels of T cell activation in HIV+ candidates would correlate with their risk of acute rejection following kidney transplantation.
2. Objective
In the present study, we investigated the influence of pre-transplant immune activation levels on the cumulative incidence of acute rejection in a group of 36 HIV− to HIV+ kidney allograft recipients transplanted at our institution over a 7-year period.
3. Patients and methods
3.1 Study subjects
Single-center retrospective cohort analysis; HIV− to HIV+ adult, first-time kidney transplants performed between October 2006 and September 2013, with available information on baseline (within 3 months prior to transplant) immune activation levels were identified. All HIV+ recipients had an undetectable viral load at the time of transplant. All patients but one (a kidney-liver recipient) had CD4 count >200 cells/mm3 at the time of transplant. A group of 118 age-, transplant year- and type of donor (living vs. deceased)-matched HIV− kidney transplant recipients served as a control group in selected analyses. The study was approved by the institutional review board (#20150614).
3.2 Immunosuppression protocol
All patients received induction immunosuppression with anti-thymocyte globulin (ATG, 1mg/kg IV × 3 doses; two additional doses were given to kidney-pancreas and kidney recipients with slow or delayed graft function) plus anti-CD25 mAb × 2 doses and methylprednisolone (500 mg IV daily × 3 doses). Maintenance immunosuppression regimen consisted of tacrolimus, mycophenolate and prednisone. At our center, tacrolimus is started soon after transplant, typically on post-operative day 1 or 2. Target level: 6-8 ng/mL during the first three months and 5-7 ng/mL after three months post-transplant. Higher levels are targeted for highly sensitized patients.
3.3 Flow cytometry
The frequency of activated (CD3+HLA-DR+) T cells was routinely measured in peripheral blood samples at baseline, 4, 12, 26 and 52 weeks post-transplant. Surface staining was performed on whole blood using the Lyse/No-Wash protocol. At least 5,000 events were collected on the lymphocyte gate for each sample. Cells were acquired on a BD FACSCalibur™ flow cytometer (BD Systems) and analyzed using the BD Multiset software.
3.4 Statistics
We stratified the cohort by tertiles of T cell activation as measured by HLA-DR expression. The Kaplan-Meier plots with a log-rank test, Fisher exact test, Kruskal-Wallis test, Wilcoxon signed-rank test were used where appropriate. Statistical analyses were performed using SAS 9.2 (Cary, NC).
4. Results
4.1 Patient characteristics
A total of 36 HIV+ kidney recipients had T cell activation data available within 3 months prior to transplantation (Table 1). The median post-transplant follow-up was 2.3 years (IQR, 0.9-4.3). The median age at the time of transplant was 47 years (range, 30-68). Most patients were males (75%) and African-American (72%). The median duration of HIV diagnosis prior to transplant was 10 years. Median CD4 count at time of transplant was 586 (IQR, 345-704) cells/mm3. All patients attained ART-induced viral load suppression (<400 copies/ml) post-transplant.
Table 1.
Baseline Characteristics of 36 HIV+ Kidney Transplant Recipients by immune activation tertiles
| Characteristic | CD3+HLA-DR+ tertiles | Overall N=36 | P Valuea | ||
|---|---|---|---|---|---|
| Lower n=11 | Middle n=13 | Upper n=12 | |||
| Age at time of transplant, No. (%), y | |||||
| 18-34 | 1 (9.1) | 4 (11.1) | 0 | 5 (13.9) | |
| 35-49 | 7 (63.6) | 6 (46.1) | 7 (58.3) | 20 (55.6) | |
| 50-65 | 2 (18.2) | 3 (23.1) | 5 (41.7) | 10 (27.8) | .24 |
| >65 | 1 (9.1) | 0 | 0 | 1 (2.8) | |
| Male, No. (%) | 9 (81.8) | 8 (61.5) | 10 (83.3) | 27 (75) | .44 |
| African-American, No. (%) | 8 (72.7) | 9 (69.2) | 9 (75) | 26 (72.2) | >.99 |
| Co-morbidities, No. (%) | |||||
| Diabetes mellitus | 2 (18.2) | 3 (23.1) | 1 (8.3) | 6 (16.7) | .75 |
| Hypertension | 7 (63.6) | 6 (46.1) | 8 (66.7) | 21 (58.3) | .59 |
| HIVAN b | 8 (80) | 7 (53.8) | 9 (75) | 24 (66.7) | .43 |
| Hepatitis C | 3 (27.3) | 1 (7.7) | 1 (8.3) | 5 (13.9) | .40 |
| Immunosuppressive agents, No. (%) | |||||
| ATG | 11 (100.0) | 13 (100.0) | 12 (100.0) | 36 (100.0) | >.99 |
| Basiliximab | 11 (100.0) | 13 (100.0) | 12 (100.0) | 36 (100.0) | >.99 |
| Methylprednisolone | 11 (100.0) | 13 (100.0) | 12 (100.0) | 36 (100.0) | >.99 |
| IVIG | 0 | 4 (30.8) | 1 (8.3) | 5 (13.9) | .10 |
| Rituximab | 1 (9.1) | 2 (15.4) | 1 (8.3) | 4 (11.1) | >.99 |
| Tacrolimus | 11 (100.0) | 13 (100.0) | 11 (91.7) | 35 (97.2) | .64 |
| MMF | 11 (100.0) | 13 (100.0) | 11 (91.7) | 35 (97.2) | .64 |
| Prednisone | 10 (90.9) | 10 (76.9) | 10 (78.3) | 30(83.3) | .85 |
| Sirolimus | 2 (18.2) | 0 | 1 (8.3) | 3 (8.3) | .19 |
| Cyclosporine | 0 | 1 (7.7) | 1 (8.3) | 2 (5.6) | >.99 |
| Donor age, median (IQR), y | 30 (21-48) | 36 (21-45) | 46 (35-50) | 37 (28-48) | .13 |
| Living donor, No. (%) | 0 | 5 (38.5) | 4 (33.3) | 9 (25) | .06 |
| Cold ischemia time ≥36 hours, No. (%)c | 3 (42.9) | 2 (22.2) | 2 (16.7) | 7 (25) | .47 |
| Delayed-graft function, No. (%)d | 2 (18.2) | 1 (7.7) | 3 (25) | 6 (16.7) | .55 |
| ABC-PRA <5%, No. (%)e | 9 (90) | 12 (100) | 11 (91.7) | 32 (94.1) | .74 |
| DR-PRA <5%, No. (%)e | 9 (90) | 11 (91.7) | 11 (91.7) | 31 (91.2) | >.99 |
| HLA-ABDR mismatches >5, No. (%) | 4 (36.4) | 5 (38.5) | 5 (41.7) | 14 (38.9) | >.99 |
| CMV viremia >500 copies/mL, No. (%)f | 0 | 2(18.2) | 0 | 2(5.9) | .20 |
| BK viremia >10,000 copies/mL, No. (%)f | 2(25) | 0 | 1(8.3) | 3(10.3) | .34 |
| Duration of HIV diagnosis, median (IQR), y | 7 (5-13) | 9.5 (5-12) | 14.5 (8-17) | 10 (5-15) | .16 |
| Study follow-up, median (IQR), y | 1.2 (0.7-2.8) | 3.2 (1.9-4.9) | 2.8 (1-4.3) | 2.3 (0.9-4.3) | .27 |
| Pre-transplant CD4+, median (IQR), cells/μl | 483 (315-666) | 628 (382-686) | 580 (291-723) | 586 (345-704) | .80 |
| Pre-transplant CD4/CD8 ratio, median (IQR) | 0.7 (0.6-1.3) | 0.7 (0.7-1) | 0.6 (0.35-0.8) | 0.7 (0.6-1) | .26 |
| Pre-transplant % CD4+ CD25high, median (IQR) | 2.87 (2.47-4.11) | 2.09 (1.54-2.61) | 1.84 (1.54-2.34) | 2.32 (1.68-2.82) | .04 |
| ART class, No. (%)g | |||||
| NRTI | 11 (100.0) | 12 (92.3) | 10 (83.3) | 33 (91.7) | .63 |
| NNRTI | 1 (12.5) | 2 (15.4) | 5 (41.7) | 8 (22.2) | .16 |
| INSTI | 2 (18.2) | 4 (30.8) | 4 (33.3) | 10 (27.8) | .72 |
| PI | 8 (72.7) | 11 (84.6) | 7 (58.3) | 26 (72.2) | .37 |
| Tacrolimus level at 4 weeks, median (IQR), ng/mLj | 5.9 (4.4-10.9) | 6.1 (5.7-10.2) | 7.6 (3.9-9.2) | 6.6 (4.1-10.2) | .95 |
| Tacrolimus level at 12 weeks, median (IQR), ng/mLj | 6.3 (5-8.3) | 6 (5-7.9) | 5.6 (4.8-6.7) | 5.9 (4.8-7.7) | .63 |
| Tacrolimus level at 26 weeks, median (IQR), ng/mLj | 6 (4-7.2) | 7.2 (4.9-10) | 6.3 (6.1-6.7) | 6.2 (4.9-8.7) | .54 |
| Tacrolimus level at 52 weeks, median (IQR), ng/mLj | 5.3 (4.4-7) | 6.6 (3.9-8.4) | 7.2 (6.3-10.9) | 6.4 (4.7-8.3) | .32 |
Abbreviations: ART, antiretroviral therapy; ATG, anti-thymocyte globulin; CMV, cytomegalovirus; HIV, human immunodeficiency virus; HIVAN, HIV-associated nephropathy; INSTI, integrase strand transfer inhibitors; IQR, interquartile range; IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; PI, protease inhibitors. PRA, panel reactive antibody.
P values were calculated with the use of Fisher exact test for categorical data and Kruskal-Wallis for continuous data.
Includes presumptive diagnosis (i.e., no kidney biopsy available).
Data available for 28 patients.
Defined as need for hemodialysis during the first week post-transplant.
Data available for 34 patients.
Refers to CMV and BK viremia during first year post-transplant.
Refers to ART regimen post-transplant.
Patients were categorized into tertiles based on the pre-transplant percentage of CD3+HLA-DR+ cells as follows: ≤4.72 (lower); 4.73-9.36 (middle) and ≥9.37 (upper). The mean (SD) percentage of CD3+HLA-DR+ was 8.8 (6.9) for the entire cohort, and 3 (1.2), 6.2 (1) and 17 (6) for the lower, middle, and upper tertiles, respectively. There were no differences in the baseline characteristics, immunosuppressive or ART regimens between groups (Table 1). Notably, despite the fact that a significant proportion of patients were on protease inhibitor- and/or nonnucleoside reverse transcriptase inhibitor-containing ART regimens (which pose challenging drug-drug interactions with calcineurin inhibitors), the median levels of tacrolimus at 4, 12, 26 and 52 weeks post-transplant were within the therapeutic range, and were comparable across groups by HLA-DR strata (Table 1).
4.2 Levels of immune activation by HIV status
Compared to a group of 118 age-, transplant year- and type of donor-matched HIV− controls, the baseline percentage of CD3+HLA-DR+ cells was higher in HIV+ candidates (median [IQR], 6.13 [5.96] vs. 3.8 [3.48]; p<0.0003). These abnormally high levels of T cell activation in HIV+ recipients were not reduced by the induction/maintenance immunosuppressive therapy; on the contrary, the frequency of CD3+HLA-DR+ cells appeared to increase from baseline at six months and one year post-transplant (6.08 [7.35], 4.74 [6.25], 9.72 [12.9] and 11.8 [11.4] at 4, 12, 26 and 52 weeks post-transplant, respectively). A similar trajectory of the number of HLA-DR-expressing T cells was observed in HIV− recipients (data not shown).
4.3 Immune activation and rejection rates
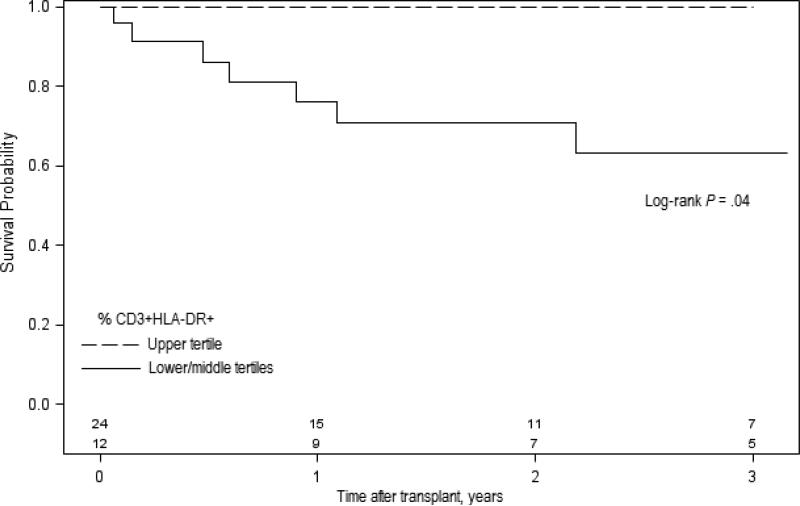
Eight (22%) HIV+ recipients had a first episode of biopsy-proven acute rejection during the study follow-up; six (75%) of them due to cellular or mixed rejection. There was a trend towards higher frequency of pre-transplant CD3+HLA-DR+ cells among patients without episodes of acute rejection at one year (median [IQR]: 7.05 [10.5] vs. 4.67 [1.38], P= .07) and three years (7.17 [10.5] vs. 4.73 [2.68], P= .10) compared to those who experienced rejection. The cumulative incidence of acute rejection at 1 and 3 years for patients in the lower, middle and upper tertiles of activation were as follows: 31.8%, 17.5%, and 0%; and 31.8%, 35.8%, and 0%, respectively. Patients categorized within the lower and middle CD3+HLA-DR+ tertiles had higher probability of rejection during the first 3 years post-transplant compared to those in the higher tertile (36.9% vs. 0%; log-rank P= .04; Figure 1). The rate of serious non-opportunistic infections (i.e., those requiring hospital admission) at six months was not different by CD3+HLA-DR+ tertiles (41.6 vs. 29.1 for the upper vs. lower/middle tertiles; P= .48). Pre-transplant levels of immune activation did not influence patient survival or death-censored graft survival (data not shown).
Figure 1.
Rejection-free survival at three years by baseline T cell activation status. Solid line represents the group of patients categorized within the lower and middle CD3+HLA-DR+ tertiles (n=24). Dashed line represents the group of patients within the upper CD3+HLA-DR+ tertile (n=12). Number of patients in each group is shown in the bottom.
5. Discussion
Supporting the notion that HIV infection is associated with increased risk of allograft rejection [3, 5, 7], the incidence of acute rejection among HIV+ individuals was higher than that previously reported by our group [13] in HIV− kidney recipients (22% versus 14% at 36 months, respectively). Similarly, consistent with previous reports [8, 10, 12] we observed abnormally high levels of immune activation in HIV+ individuals compared to HIV− controls.
To date no study has examined the impact of dysregulated immune activation, a hallmark of chronic HIV infection [8-12], on the increased risk of allograft rejection in HIV+ kidney transplant recipients. Contrary to our expectations, we observed a paradoxical protective effect of high levels of T cell activation in HIV+ transplant candidates.
Since the expression of HLA-DR on T cells mirrors that of programmed cell death-1 (PD-1) [10, 12], an inhibitory receptor and marker of immune exhaustion [14], we speculate that HIV+ transplant recipients with aberrant immune activation have an excess of exhausted T cells which favors immunological tolerance of the allograft. Although not statistically significant, the fact that there was a stepwise increase in the median duration of HIV diagnosis prior to transplant by activation tertiles (namely seven, ten, and fifteen years for those in the lower, middle and upper tertile, respectively [Table 1]) suggests that individuals with more protracted HIV infection might exhibit a more advanced stage of immune exhaustion that is protective against allograft rejection. Although this hypothesis requires further investigation, our data serve as a prelude to prospective studies aimed to examine the link between T cell activation and immune exhaustion on the risk of allograft rejection in HIV+ transplant recipients.
Reduced exposure to immunosuppressive agents, previous allosensitization and HIV infection of the allograft are also thought to contribute to the increased rates of rejection in HIV+ recipients [5, 15]. In addition, CMV replication is considered a significant cause of immune activation in chronic HIV infection [16] and a potential risk factor for acute rejection in kidney transplant recipients [17]. As shown in Table 1, the serum levels of tacrolimus and the number of sensitized patients in this cohort were similar across groups. All the patients studied here were CMV seropositive at the time of the transplant, and all received a minimum of three months of antiviral prophylaxis per protocol. Only two cases of transient CMV viremia >500 copies/mL were identified. Thus, the association between immune activation and acute rejection in HIV+ recipients observed here seems to be independent of CMV reactivation, previous allosensitization and drug-drug interactions between ART and immunosuppressive agents.
Activated/effector T regulatory cells are depleted during HIV infection [18] and are thought to play an important role in controlling alloreactivity after kidney transplant [19]. In our cohort, the number of CD4+CD25high T cells was lowest amongst patients categorized to the upper activation tertile (Table 1); this finding suggests that the frequency of T cells with potential regulatory function do not explain the lower risk of allograft rejection we observed among patients with the highest levels of immune activation.
We observed an increased frequency of HLA-DR-expressing T cells at 26 and 52 weeks post-transplant in both HIV− and HIV+ cohorts. The reasons for this phenomenon require further investigation. We speculate that this increase in CD3+HLA-DR+ cells might reflect a relative paucity of naïve T cells with homeostatic expansion of the effector memory T cell repertoire, an immune reconstitution pattern that has been previously described in ATG-treated kidney transplant recipients [20].
We recently showed that ATG-treated HIV+ kidney transplant recipients with a baseline CD4<350 cells/mm3 have higher risk of severe lymphopenia (CD4<200 cells/mm3) and associated infectious complications [21]. Given the narrow therapeutic window of ATG in this population, it has been proposed that administration of this agent should be restricted to patients at very high immunologic risk for rejection [5, 21]. Considering the null rates of acute rejection at 3 years we observed among HIV+ recipients categorized to the upper CD3+HLA-DR+ tertile, it is conceivable that individuals with high pre-transplant immune activation levels, particularly those with CD4<350 cells/mm3, might benefit of less intense lymphodepletion during induction therapy.
One potential limitation of the present study is that we relied on a single marker of T cell activation. However, the utility of HLA-DR expression as a single surrogate marker of T cell activation has been validated previously in several HIV cohorts [10, 12, 22, 23]. Given the retrospective nature of the study we were unable to estimate the activation status of CD4 and CD8 naïve/memory T cell subsets; small sample size is another limitation and our findings need to be validated in larger prospective studies. Despite these limitations, our findings provide evidence for a previously unrecognized link between HIV-associated immune activation and the risk of allograft rejection in HIV+ kidney recipients.
To date no study has examined the impact of dysregulated immune activation, a hallmark of chronic HIV infection, on the increased risk of allograft rejection in HIV+ kidney transplant recipients.
Abnormally high levels of activation in HIV+ kidney allograft recipients did not decrease with transplant-associated immunosuppression.
Paradoxically, those individuals with the highest levels of immune activation seemed to be less prone to rejection.
Pathological immune activation in HIV+ transplant candidates does not explain their increased susceptibility to allograft rejection.
ACKNOWLEDGEMENTS
Funding/support: This work was supported in part by a Miami Center for AIDS research (CFAR) pilot award to J.F.C., funded by a grant (P30AI073961) from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- ART
antiretroviral therapy
- ATG
anti-thymocyte globulin
- CMV
cytomegalovirus
- HIV
human immunodeficiency virus
- HIVAN
HIV-associated nephropathy
- INSTI
integrase strand transfer inhibitor
- IQR
inter-quartile range
- IVIG
intravenous immunoglobulin
- PRA
panel reactive antibody
- PI
protease inhibitor
- mAb
monoclonal antibody
- MMF
mycophenolate mofetil
- NRTI
nucleoside reverse transcriptase inhibitor
- NNRTI
non-nucleoside reverse transcriptase inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest and financial disclosures: none reported
REFERENCES
- 1.Abraham AG, Althoff KN, Jing Y, et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis. 2015;60:941–949. doi: 10.1093/cid/ciu919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucas GM, Ross MJ, Stock PG, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014. 59:e96–138. doi: 10.1093/cid/ciu617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locke JE, Mehta S, Reed RD, et al. A National Study of Outcomes among HIV-Infected Kidney Transplant Recipients. J Am Soc Nephrol. 2015;26(9):2222–2229. doi: 10.1681/ASN.2014070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawinski D, Forde KA, Eddinger K, et al. Superior outcomes in HIV-positive kidney transplant patients compared with HCV-infected or HIV/HCV-coinfected recipients. Kidney Int. 2015;88:341–349. doi: 10.1038/ki.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stock PG, Barin B, Murphy B, et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010;363(21):2004–2014. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar MS, Sierka DR, Damask AM, et al. Safety and success of kidney transplantation and concomitant immunosuppression in HIV-positive patients. Kidney Int. 2005;67:1622–1629. doi: 10.1111/j.1523-1755.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 7.Locke JE, James NT, Mannon RB, et al. Immunosuppression regimen and the risk of acute rejection in HIV-infected kidney transplant recipients. Transplantation. 2014;97:446–450. doi: 10.1097/01.TP.0000436905.54640.8c. [DOI] [PubMed] [Google Scholar]
- 8.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 9.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;15104(4):942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 10.Okulicz JF, Le TD, Agan BK, et al. Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals. JAMA Intern Med. 2015;175(1):88–99. doi: 10.1001/jamainternmed.2014.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camargo JF, Kulkarni H, Agan BK, et al. Responsiveness of T cells to interleukin-7 is associated with higher CD4+ T cell counts in HIV-1-positive individuals with highly active antiretroviral therapy-induced viral load suppression. J Infect Dis. 2009;199:1872–1882. doi: 10.1086/598858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciancio G, Gaynor JJ, Guerra G, et al. Randomized trial of three induction antibodies in kidney transplantation: long-term results. Transplantation. 2014;97:1128–1138. doi: 10.1097/01.TP.0000441089.39840.66. [DOI] [PubMed] [Google Scholar]
- 14.Thorp EB, Stehlik C, Ansari MJ. T-cell exhaustion in allograft rejection and tolerance. Curr Opin Organ Transplant. 2015;20(1):37–42. doi: 10.1097/MOT.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stock PG. Kidney infection with HIV-1 following kidney transplantation. J Am Soc Nephrol. 2014;25(2):212–215. doi: 10.1681/ASN.2013101112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203(10):1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96(4):333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 18.Simonetta F, Lecuroux C, Girault I, et al. Early and long-lasting alteration of effector CD45RA(-)Foxp3(high) regulatory T-cell homeostasis during HIV infection. J Infect Dis. 2012;205(10):1510–1519. doi: 10.1093/infdis/jis235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braza F, Dugast E, Panov I, et al. Central Role of CD45RA- Foxp3hi Memory Regulatory T Cells in Clinical Kidney Transplantation Tolerance. J Am Soc Nephrol. 2015;26(8):1795–1805. doi: 10.1681/ASN.2014050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearl JP, Parris J, Hale DA, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5(3):465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 21.Suarez JF, Rosa R, Lorio MA, et al. Pre-transplant CD4 count influences immune reconstitution and risk of infectious complications in HIV+ kidney allograft recipients. Am J Transplant. 2016 Mar 8; doi: 10.1111/ajt.13782. doi: 10.1111/ajt.13782 (PMID: 26953224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoufaly A, Kiepe JG, Hertling S, et al. Immune activation despite suppressive highly active antiretroviral therapy is associated with higher risk of viral blips in HIV-1-infected individuals. HIV Med. 2014;15(8):449–457. doi: 10.1111/hiv.12134. [DOI] [PubMed] [Google Scholar]
- 23.Leng Q, Borkow G, Weisman Z, et al. Immune activation correlates better than HIV plasma viral load with CD4 T-cell decline during HIV infection. J Acquir Immune Defic Syndr. 2001;27(4):389–397. doi: 10.1097/00126334-200108010-00010. [DOI] [PubMed] [Google Scholar]