Abstract
Background/Purpose
Many factors appear to be related to physical activity after stroke, yet it is unclear how these factors interact and which ones might be the best predictors. Therefore, the purpose of this study was twofold: 1) to examine the relationship between walking capacity and walking activity, and 2) to investigate how biopsychosocial factors and self-efficacy relate to walking activity, above and beyond walking capacity impairment post-stroke.
Methods
Individuals greater than 3 months post-stroke (n=55) completed the Yesavage Geriatric Depression Scale (GDS), Fatigue Severity Scale (FSS), Modified Cumulative Illness Rating (MCIR) Scale, Walk 12, Activities Specific Balance Confidence (ABC) Scale, Functional Gait Assessment (FGA), and oxygen consumption testing. Walking activity data was collected via a StepWatch Activity Monitor (SAM). Predictors were grouped into 3 constructs: (1) Walking Capacity: oxygen consumption and FGA; (2) Biopsychosocial: GDS, FSS, and MCIR; (3) Self-Efficacy: Walk 12 and ABC. Moderated sequential regression models were used to examine what factors best predicted walking activity.
Results
Walking capacity explained 35.9% (p<0.001) of the variance in walking activity. Self-efficacy (ΔR2 = 0.15, p<0.001) and the interaction between the FGA*ABC (ΔR2 = 0.047, p<0.001) significantly increased the variability explained. FGA (β=0.37, p=0.01), MCIR (β=−0.26, p=0.01), and Walk 12 (β=−0.45, p=0.00) were each individually significantly associated with walking activity.
Discussion/Conclusion
While measures of walking capacity and self-efficacy significantly contributed to "real-world" walking activity, balance self-efficacy moderated the relationship between walking capacity and walking activity. Improving low balance self-efficacy may augment walking capacity and translate to improved walking activity post-stroke.
Keywords: stroke, physical activity, walking
INTRODUCTION
In the United States, stroke is the leading cause of disability,1 affecting approximately 795,000 people each year.2 Daily walking activity in persons with chronic stroke (>6 months) is well below the activity level of even the most sedentary adults (<5,000 steps/day).3, 4 A recent 3 year longitudinal study suggested that at the 3rd year of recovery, stroke survivors spend only 9% of the time walking.5 This is concerning since declines in activity can lead to an increased risk of co-morbidities, including subsequent stroke6 and mortality.1 Therefore it is important to understand factors that affect walking activity after stroke.
Performance measures of physical capacity, including short distance walking speed, balance, walking endurance and metabolic cost, are related to walking activity after stroke.7–9 Regardless of the physical performance measure examined, however, the variability in walking activity accounted for by these factors was relatively small10, 11 suggesting that there are other factors impacting post-stroke walking activity. In particular, several studies have found that self-efficacy is a strong predictor of “real-world” walking activity after stroke.11–14 Using a self-reported measure of walking activity, Schmid and colleagues11 found that while physical performance measures were correlated with activity, only balance self-efficacy significantly predicted walking activity in a step-wise regression model comprising both measures of physical performance and self-efficacy.
There may also be influential biopsychosocial factors related to walking activity after stroke including fatigue, depression and co-morbidity burden.15–17 Studies of fatigue among stroke survivors are limited; however self-reported fatigue has been shown to be predictive of functional dependency after stroke.18 Fatigue research in other neurological populations (Parkinson’s Disease, Multiple Sclerosis) supports the relationship between fatigue and activity.19–21 Depression may affect upwards of 63% of those living with stroke22 and has been shown to be correlated with reduced participation in community walking.12 Moreover, depression severity and social inactivity appear to be associated with each other12, 23–26 and both have been linked to functional27 limitations in performing tasks like walking12 and stair climbing post-stroke.28 Fatigue and depression together have been associated with reduced participation in instrumental Activities of Daily Living (ADLs)29 suggesting decreased participation in community activities.12 Lastly, it is not clear how the presence of comorbidities, in addition to stroke, relates to “real-world” walking activity. The number of comorbidities has been related to the difficulty of walking and ADLs in individuals post-stroke.12, 27 A study in other populations with chronic conditions suggest that co-morbidity burden may be related to physical activity.16
In summary, many factors appear to be related to physical activity after stroke.30 However the aforementioned studies did not address factors that account for the variability of walking activity above and beyond physical performance measures and did not holistically capture constructs and account for interactions between potential predictors in various constructs (i.e. physical vs personal factors). In addition, the variability in walking activity accounted for by the models in these studies was small, suggesting that they were incomplete. In order to design interventions aimed at improving walking activity after stroke it is important to understand not only the factors that predict walking activity, but also how these factors interact with each other to impact “real-world” walking in individuals post-stroke. Therefore, the purpose of this study was twofold: 1) to examine the relationship between walking capacity (captured through performance measures of dynamic walking and energy cost) and daily walking activity in individuals post-stroke, and 2) to investigate how biopsychosocial factors (depression, fatigue, comorbidities) and self-efficacy (for balance and walking) relate to walking activity above and beyond walking capacity impairment. We hypothesized that biopsychosocial factors and self-efficacy would be significant predictors of daily walking activity after stroke, above and beyond walking capacity. In addition, since there appears to be many factors that play a role in walking activity post-stroke we hypothesized that understanding the interactions between constructs may also be critical to obtaining a complete picture of post-stroke walking activity.
METHODS
Participants
Participants were recruited from local physical therapy clinics, stroke support groups, and newspaper advertisements. Individuals aged 21–85 years were included in the study if they had sustained a stroke more than 3 months prior, were able to walk without assistance (the use of orthotics or assistive devices were allowed), were able to walk 5 minutes at a self-selected pace on the treadmill, were able to walk outside the home prior to stroke, walked less than 10,000 steps per day (SPD), and were able to communicate with the investigators. Individuals post-stroke were not included in the study if they had experienced more than one stroke, had evidence of a cerebellar stroke, additional neurologic diseases, cardiac event less than 3 months prior, had received Botox in lower extremities less than 4 months prior, pain that limited walking, unexplained dizziness in the past 6 months, and if they were participating in skilled physical therapy services. All participants post-stroke received medical clearance prior to beginning the study and signed an informed consent approved by the Human Subjects Review Board at University of Delaware prior to participation.
Outcome Measures
Questionnaires and the Functional Gait Assessment (FGA) were completed at a clinical evaluation session followed by oxygen consumption testing at least 1 week later. During the interim week, activity data was collected while subjects wore a calibrated activity monitor (StepWatch Activity Monitor [SAM], Orthocare Innovations, Seattle Washington). Previous studies have shown excellent reliability and accuracy of the SAM in persons post-stroke.31 The SAM was placed above the ankle on the non-paretic lower extremity and calibrated to the participants' height and walking characteristics per manufacturer's instructions. To calibrate the SAM, participants walked 30 strides at their self-selected pace and 10 strides at a slightly faster pace. If the number of steps differed from manual counting by > 2 strides, the sensitivity of the SAM was adjusted until accuracy was obtained. The numbers of strides were counted in each consecutive 10 second interval (changed from the SAM default interval of 60 seconds). During the initial session subjects were verbally educated and then demonstrated understanding in donning/doffing the SAM unit. They were given verbal and written instructions on the wear and care of the SAM unit, along with contact information for researchers in case questions arose at home. Participants wore the SAM for all waking hours, except during bathing and swimming activities for one week. Following recommendations from a previous study using the SAM in persons post-stroke, at least 3 days of data32 were required to calculate the mean SPD. Days with less than 10 hours of recorded data were examined to determine whether the number of hours recorded were consistent with previous days. To be conservative, if the number of hours was substantially less than other days, the day was not included in the analysis. All testing was completed by 3 research physical therapists who had established reliability between themselves. Sampling bias was reduced by recruiting subjects from a variety of community sources, including newspaper advertisements.
To holistically capture walking capacity as a construct, the energy cost of transport (CT), or the oxygen consumption per unit distance walked (mL O2/kg/m) was determined to assess cardiovascular capacity and dynamic walking balance was assessed via the FGA. Subjects were advised to employ the assistive device most commonly used in their daily life, during dynamic walking balance testing. A Parvo Medics metabolic cart was used to measure oxygen consumption (VO2) as subjects walked at an over ground self-selected pace while on a treadmill for 5 minutes. VO2 over the last 1 minute of walking was normalized to body mass and speed, resulting in the energy cost per meter walked, otherwise known as CT. The FGA is a 10-item assessment of postural stability during various walking tasks such as ambulating backwards, gait with a narrow base of support, and gait with eyes closed.33 Each item is scored on a 4-level (0–3 points) ordinal scale with a maximum possible score of 30 points.33 The FGA has demonstrated excellent test- retest, inter-rater and intra-rater reliability, and criterion and construct validity with other balance measures when used to assess individuals post-stroke.34, 35
To represent the biopsychosocial construct as it relates to function after stroke, measures included the Yesavage Geriatric Depression Scale (GDS), Fatigue Severity Scale (FSS), and the Modified Cumulative Illness Rating (MCIR) Scale. The GDS is a 15-item self-rating tool that assesses depression.36 A yes or no answer is provided by the participant and a point is given for each answer indicative of depression; the total score is summed. The GDS has demonstrated excellent test-retest, inter-rater and intra-rater reliability, internal consistency, and concurrent validity for other measures of depression in individuals post-stroke.37–39 The FSS is a 9-item self-report scale that measures the severity of fatigue and its effect on a person's activities and lifestyle.21 The items are scored on a 7 point scale with 1 = strongly disagree and 7= strongly agree; the higher the score the greater the fatigue severity. In neurologic populations, FSS has demonstrated excellent test-retest reliability, internal consistency, and correlates well with other fatigue related scales.37, 40, 41 The MCIR is a 14-item rating scale used to indicate medical burden by rating impairment across 13 different organ systems as well as psychiatric/behavioral disturbances (excluding dementia).42 Estimates of impairment severity encompass aspects of current disability, treatment and prognosis, and ratings are made on a 0 (no impairment) to 4 (extremely severe impairment) scale. Studies have confirmed the validity and reliability of this scale as an indicator of health status in multiple patient populations.42–46 In those post-stroke, it has been correlated (comorbidity index: −0.24 P≤0.02; severity index: −0.32 P≤0.002) to a tool (Functional Independence Measure (FIM)) that is known to measure dependence in individuals post-stroke.47
The construct of self-efficacy as it relates to function after stroke was represented by the Walk 12 and Activities Specific Balance Confidence (ABC) Scale. The Walk 12 is a self-report scale that was developed to measure the impact of stroke on walking from the perspective of the person with stroke.48 The scale consists of 12-items and asks about limitations due to the stroke during the previous 2 weeks, in tasks like walking and climbing stairs; the need for support indoors and outdoors; and effort and concentration when walking. The response to each question is a 5 point ordinal scale from 1 (not at all) to 5 (extremely). The total score of the Walk 12 is reported on a 0–100 scale. A score of 0 indicates no self-perceived limitation in walking and 100 indicates maximum limitation.48 There is a moderate correlation between the Walk 12 and gait performance tests (ρ=−0.70 velocity and ρ=−0.59 cadence) in people post-stroke and the alpha coefficient for those post stroke was 0.95 suggesting relatively high internal consistency.48 The ABC is a measure of balance self-efficacy, or the confidence in performing position changes and walking activities ranging from sweeping the flooring to walking on icy sidewalks..49 The ABC is a 16-item questionnaire with self-reported confidence rated on an 11-point ordinal scale ranging from 0% (no confidence) to 100% (complete confidence). Item scores are averaged to determine an overall balance confidence score ranging from 0% to 100%.50 The ABC has demonstrated high test-retest reliability, excellent internal consistency, and correlation with the Berg Balance Scale and gait speed in individuals more than 1 year post-stroke.51
Statistical Analysis/Analysis Plan
Moderated sequential regression models were used to examine the relationship between walking capacity, biopsychosocial measures, self-efficacy and walking activity post-stroke. Sequential regression allows for the testing of specific subsets or blocks of predictors as they are added to see if they significantly improve the model.52 This approach enables researchers to subsequently add constructs of interest after adjusting for the constructs already in the model. Moderation was tested using interaction effects in the final block. Variables were centered prior to calculating interactions and being entered into the model to remove any multicollinearity caused by the inclusion of the interaction effects. All assumptions for regression models were tested. Initially, the model violated the assumption of normality, after performing a Box-Cox test and applying the suggested transformation along with removing two outliers all assumptions were satisfied.
This study grouped predictors into 3 constructs that were added in the following order: walking capacity (block 1: FGA and CT), biopsychosocial factors (block 2: GDS, FSS, MCIR), and self-efficacy measures (block 3: Walk 12, ABC). A fourth block included the interactions between selected measures in each construct. This study grouped potential predictors into 3 constructs to best capture the diversity within the construct. For example, block 1 captures two aspects of walking capacity: dynamic walking balance (FGA) and cardiovascular capacity (CT). Doing so reduced the number of specific predictors tested, instead looking at the construct as a whole, i.e. block significance was assessed. Limited by sample size and the number of regression parameters already included in the model, only five interaction terms between significant predictors were tested (FGA*Walk 12, FGA*ABC, FGA*MCIR, MCIR*Walk 12, and MCIR*ABC). Because we did not have a priori hypotheses about specific interactions, we opted to include only the significant interactions. This allowed for the investigation of the interactions of the predictors of walking activity above and beyond the individual constructs alone.
The change in R2 was tested to evaluate if each construct was significantly related to SPD after adjusting for the previous blocks. Block one included measures of walking capacity (FGA and CT) so that our first hypothesis was solely investigated. Additional blocks were added in ascending order based on our hypotheses; block 2 included biopsychosocial measures (GDS, FSS, and MCIR), block 3 comprised measures of self-efficacy (Walk 12 and ABC), and block 4 contained the interactions between significant predictors. Significant interactions were probed using the simple slope method, at three values for the moderator (−1SD, Mean, and +1SD).53 To keep the model as simple as possible, only significant interactions were kept for the final model. All analyses were performed using SPSS (Version 21.0; Chicago, IL, USA); α=0.05. Given our sample size of n=55 with α=0.05, the ability to detect an effect with an R2 = 0.11 with power = 0.8 was calculated.
RESULTS
Data was analyzed from a clinical research database for stroke studies at the University of Delaware. All data available at the time with complete data sets were used; therefore, analyses were conducted on 55 participants. The basic subject characteristics are given in Table 1, descriptive statistics by subject characteristics are given in Table 2, summary results of the sequential regression are given Table 3. The initial block of walking capacity (hypothesis 1), FGA and CT, was significant, (R2 = 0.36, p < 0.001).
Table 1.
Summary Subject Characteristics
| Gender | Age (years) |
Side of Stroke |
Time Since Stroke (months) |
Self-Selected Gait Speed (m/s) |
Assistive Device |
Orthotic |
|---|---|---|---|---|---|---|
| M: n=33 F: n=22 |
Mean: 54 +/− 11 |
R CVA: n=37 L CVA: n=18 |
Mean: 47 Range: 4–366 |
Mean: 0.72 Range: 0.14–1.8 |
SPC: 7 RW: 2 SW: 1 QC: 6 |
AFO: n=18 Bioness: n=1 |
M: Male; F: Female; R CVA: Right Cerebral Vascular Accident; L CVA: Left Cerebral Vascular Accident; SPC: Single Point Cane; RW: Rolling Walker; SW: Standard Walker; QC: Quad Cane; AFO: Ankle Foot Orthosis.
Table 2.
Descriptive Statistics by Subject Characteristic
| Range | Mean (SD) | |
|---|---|---|
| SPD (steps) | 377–14,433 | 5,816 (3,293) |
| CT (mLO2/kg/m) | 0.12–1.26 | 0.34 (0.20) |
| FGA (points) | 4–25 | 13 (5) |
| GDS (points) | 0–11 | 3 (3) |
| FSS (points) | 9–60 | 34 (14) |
| MCIR (points) | 14–26 | 19 (2) |
| Walk 12 (%) | 18–93 | 67 (19) |
| ABC (%) | 20–99 | 73 (18) |
SPD: steps per day; CT: energy cost of transport; FGA: Functional Gait Assessment; GDS: Yesavage Geriatric Depression Scale; FSS: Fatigue Severity Scale; MCIR: Modified Cumulative Illness Rating Scale; ABC: Activities Specific Balance Confidence Scale; SD: Standard Deviation.
Table 3.
Summary of Regression Analyses Predicting Walking Activity Post Stroke
| Block | Predictors | R2 | Model p | ΔR2 | ΔR2 p |
|---|---|---|---|---|---|
| 1 | Walking Capacity | 0.359* | <0.001 | ||
| 2 | Biopsychosocial Factors | 0.414 | <0.001 | 0.055 | 0.216 |
| 3 | Self-Efficacy | 0.566* | <0.001 | 0.151 | 0.001 |
| 4 | Interactions | 0.612* | <0.001 | 0.047 | 0.023 |
represents significance
To address our second hypothesis, after adjusting for walking capacity, including the biopsychosocial factors, GDS, FSS, and MCIR did not significantly improve the model. After adjusting for both walking capacity and biopsychosocial factors, self-efficacy, Walk 12 and ABC, did significantly increase the variance accounted for (ΔR2 = 0.15, p < 0.001). In the final model, FGA (β=0.37, p=0.01), MCIR (β=−0.26, p=0.01), Walk 12 (β=−0.45, p=0.00) and the FGA*ABC interaction (β=−0.29, p=0.02) were significant (Table 4).
Table 4.
Regression Coefficients of the Predictors of Walking Activity Post-Stroke
| Block | Predictor | β | p |
|---|---|---|---|
| 1 | FGA | 0.37* | 0.01 |
| CT | 0.18 | 0.24 | |
| 2 | FSS | 0.00 | 1.00 |
| GDS | 0.08 | 0.53 | |
| MCIR | −0.26* | 0.01 | |
| 3 | Walk 12 | −0.45* | 0.00 |
| ABC | 0.14 | 0.28 | |
| 4 | ABC*FGA | −0.29 | 0.02 |
FGA: Functional Gait Assessment; CT: energy cost of transport; FSS: Fatigue Severity Scale; GDS: Yesavage Geriatric Depression Scale; MCIR: Modified Cumulative Illness Rating (MCIR); ABC: Activities Specific Balance Confidence Scale;
represents significance; p<0.05.
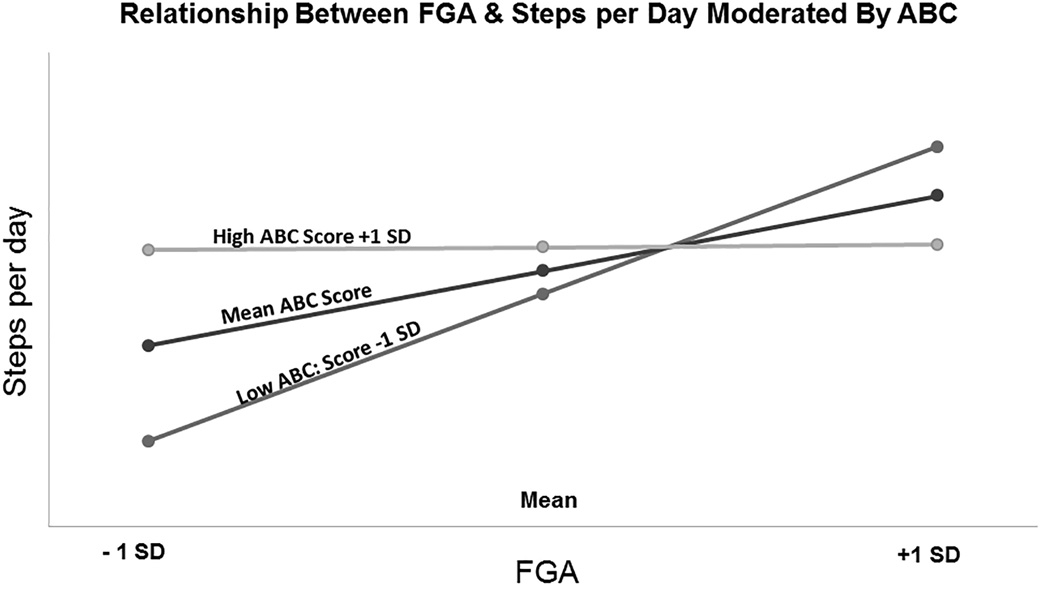
Examining the simple slopes for the interaction between FGA*ABC show that as ABC goes down, the relationship between FGA and SPD becomes stronger (Figure 1). Participants with an ABC score +1 standard deviation above the mean have a weak relationship between FGA and SPD. In contrast, participants with a low ABC score, −1 standard deviation below the mean, FGA is strongly positively related to SPD.
Figure 1.
Results of the moderated regression analysis. FGA: Functional Gait Assessment; ABC: Activities Specific Balance Confidence Scale. Light grey lines represent data from subjects who scored ABC scores 1 standard deviation above the mean (high), black lines represent mean ABC scores and dark grey lines represent ABC scores 1 standard deviation below the mean (low). Each symbol represents the mean (middle symbol) ± 1 standard deviation (first and third symbol) of the group data for the FGA. The ABC score appears to moderate the relationship between the FGA and ABC. Subjects with a high ABC score demonstrate a weak relationship between the FGA and SPD. In contrast, participants with a low ABC score exhibit a strong positive relationship between the FGA and SPD.
Discussion
The purpose of this study was to examine the relationship between walking capacity, biopsychosocial factors, self-efficacy and daily walking activity in those post-stroke. We hypothesized that biopsychosocial factors and self-efficacy would be significant predictors of daily walking activity after stroke, above and beyond walking capacity, and that interactions between predictors may moderate walking activity.
The results support our hypothesis for self-efficacy. Specifically, above and beyond walking capacity and biopsychosocial factors, self-efficacy significantly predicted number of steps per day. This result extends previous findings by demonstrating that not only is self-efficacy related to walking activity after stroke, it is a significant predictor even after physical or other personal factors have been considered. This suggests that when physical and biopsychosocial capacity are reasonably intact after stroke, walking activity may be significantly limited due to poor self-efficacy.
Conversely, as a group, biopsychosocial factors did not contribute to daily walking activity above and beyond walking capacity. However, when considered individually, the MCIR is a predictor of walking activity after stroke. This finding demonstrates that individuals post-stroke with a greater number and severity of comorbidities walk less. This is consistent with the more general results of a previous study that found that the number of comorbidities was related to self-reported difficulty of walking and activities of daily living in individuals post-stroke.54 The present study adds to those findings by demonstrating that having a greater number and severity of comorbidities predicts the amount of walking activity in individuals post-stroke. Thus, comorbidity burden influences not only the person’s perception of their difficulty with walking and daily activities,54 but also the actual, observed amount of walking. Given that previous studies demonstrate significant discrepancies between self-reported and actual physical activity post-stroke,12, 55 establishing the relationship between actual steps per day and comorbidity burden is important.
The results of the present study demonstrate, for the first time, that the interaction between individual factors also plays an important role in daily walking activity post-stroke. Particularly the interaction between the ABC (balance confidence) and FGA (dynamic walking balance) accounted for a significant amount of the variability in walking activity. This interaction predicted walking activity above and beyond physical capacity, biospsychosocial factors and self-efficacy constructs. These results indicate that balance confidence moderates the relationship between walking capacity and the amount of daily walking activity in individuals post-stroke. Those that scored higher on the ABC (more confident) had a weak relationship between walking capacity and walking activity. For these individuals it appears that balance confidence is more important for walking activity than walking capacity. Individuals post-stroke that scored lower on the ABC (less confident) had a stronger relationship between walking capacity and walking activity. Thus, it seems that walking capacity plays a more important role in walking activity after stroke when balance self-efficacy is low. It should be noted that having low walking capacity and high self-efficacy may be inappropriate in certain situations (e.g.-individuals with decreased cognition, impulsive behavior, lack of insight). To maintain safety in this cohort it would be important to educate individuals post-stroke and caregivers on environmental safety, appropriate device usage and assistance levels while still promoting walking activity.
Overall, balance self-efficacy has been correlated with physical functioning, perceived health status, and avoidance of walking and participation in the community post-stroke.11, 12, 56, 57 Schmid et al.11 identified that balance self-efficacy, not physical aspects of gait, were independently associated with self-reported measures of activity and participation in stroke survivors. The results of this study provide additional insight into the role of self-efficacy and suggest that when attempting to promote increased daily “real-world” walking activity after stroke, self-efficacy is particularly important in those with greater impairments in walking capacity. Instead of solely focusing on improving walking capacity, rehabilitation clinicians may need to take a patient centered approach to also improve balance self-efficacy by designing interventions where the patient consistently and successfully completes dynamic tasks and is educated about their balance capacity.
In community dwelling older adults the ability to consistently and successfully completing dynamic tasks, combined with falls-risk knowledge and assertiveness to ask for assistance when needed, builds confidence with upright mobility.50, 58 Some studies suggest that building confidence is just as important as physical training for decreasing the fear of falling49, 50, 59 and improving activity.59 The present study may suggest the improving low balance self-efficacy that in individuals post-stroke can augment walking capacity and translate to improved walking activity. To our knowledge there are no studies that focus on improving balance self-efficacy to promote “real-world” walking (such as measured in this study using the SAM) as a component of an intervention in the post-stroke population. As such, this will be an important direction for future research.
Potential Limitations
This study was limited by sample size. A larger sample would have allowed the investigation of a greater number of potential predictors of walking activity, as well as the simultaneous testing of all the interaction effects of interest. Furthermore, the findings of this study are only generalizable to persons >3 months post-stroke who are able to walk without the assistance of another person. The importance of walking capacity may be greater in those who cannot walk independently. The effects of walking on a treadmill versus over-ground on oxygen consumption testing in individuals post-stroke is not known. However, this study attempted to accommodate for this by having subjects walk at an over-ground self-selected pace while walking on the treadmill. Lastly, we decided to use the GDS as a measure of depression. This tool is mainly used to screen subjects for signs of depression. Using a more detailed tool to measure depression may have provided more insight into the effects of depression on walking activity in individuals post-stroke.
Conclusions
Measures of physical walking performance and self-efficacy, in respective order, significantly contributed to “real-world” walking activity post-stroke. Most notably balance self-efficacy moderated the relationship between walking capacity and walking activity. This interaction accounts for the variability in walking activity in individuals post-stroke above and beyond walking capacity, biopsychosocial factors, and self-efficacy measures. This suggests that rehabilitation clinicians should address balance self- efficacy in addition to walking capacity to facilitate “real-world” walking activity improvements in patients post-stroke.
Supplementary Material
Acknowledgments
Funding from NIH grant R21HD07142 and NIH NR010786
Footnotes
This work was presented as a platform, at the American Physical Therapy Association-Combined Sections Meeting 2014.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Roos MA, Rudolph KS, Reisman DS. The structure of walking activity in people after stroke compared with older adults without disability: a cross-sectional study. Phys Ther. 2012;92(9):1141–1147. doi: 10.2522/ptj.20120034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunkel D, Fitton C, Burnett M, Ashburn A. Physical inactivity post-stroke: a 3-year longitudinal study. Disabil Rehabil. 2015;37(4):304–310. doi: 10.3109/09638288.2014.918190. [DOI] [PubMed] [Google Scholar]
- 6.Hornnes N, Larsen K, Boysen G. Little change of modifiable risk factors 1 year after stroke: a pilot study. Int J Stroke. 2010;5(3):157–162. doi: 10.1111/j.1747-4949.2010.00424.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmid A, Duncan PW, Studenski S, Lai SM, Richards L, Perera S, Wu SS. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38(7):2096–2100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 8.Macko RF, Ivey FM, Forrester FM, Hanley D, Sorkin JD, Katzel LI, Silver KH, Goldberg AP. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. 2005;36(10):2206–2211. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 9.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005;86(8):1552–1556. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Andrews AW, Chinworth SA, Bourassa M, Garvin M, Benton D, Tanner S. Update on distance and velocity requirements for community ambulation. J Geriatr Phys Ther. 2010;33(3):128–134. [PubMed] [Google Scholar]
- 11.Schmid AA, Van Puymbroeck M, Altenburger PA, Dierks TA, Miller KK, Damush TM, Williams LS. Balance and balance self-efficacy are associated with activity and participation after stroke: a cross-sectional study in people with chronic stroke. Arch Phys Med Rehabil. 2012;93(6):1101–1107. doi: 10.1016/j.apmr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Robinson CA, Shumway-Cook A, Ciol MA, Kartin D. Participation in community walking following stroke: subjective versus objective measures and the impact of personal factors. Phys Ther. 2011;91(12):1865–1876. doi: 10.2522/ptj.20100216. [DOI] [PubMed] [Google Scholar]
- 13.Hellström K, Lindmark B, Wahlberg B, Fugl-Meyer AR. Self-efficacy in relation to impairments and activities of daily living disability in elderly patients with stroke: a prospective investigation. J Rehabil Med. 2003;35(5):202–207. doi: 10.1080/16501970310000836. [DOI] [PubMed] [Google Scholar]
- 14.Salbach NM, Mayo NE, Robichaud-Ekstrand S, Hanley JA, Richards CL, Wood-Dauphinee S. Balance self-efficacy and its relevance to physical function and perceived health status after stroke. Arch Phys Med Rehabil. 2006;87(3):364–370. doi: 10.1016/j.apmr.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Rimmer JH, Wang E, Smith D. Barriers associated with exercise and community access for individuals with stroke. J Rehabil Res Dev. 2008;45(2):315–322. doi: 10.1682/jrrd.2007.02.0042. [DOI] [PubMed] [Google Scholar]
- 16.Shumway-Cook A, Ciol MA, Yorkston KM, Hoffman JM, Chan L. Mobility limitations in the Medicare population: prevalence and sociodemographic and clinical correlates. J Am Geriatr Soc. 2005;53(7):1217–1221. doi: 10.1111/j.1532-5415.2005.53372.x. [DOI] [PubMed] [Google Scholar]
- 17.Melzer D, Gardener E, Guralnik JM. Mobility disability in the middle-aged: cross-sectional associations in the English Longitudinal Study of Ageing. Age Ageing. 2005;34(6):594–602. doi: 10.1093/ageing/afi188. [DOI] [PubMed] [Google Scholar]
- 18.Glader EL, Stegmayr B, Asplund K. Poststroke fatigue: A 2-year follow-up study of stroke patients in Sweden. Stroke. 2002;33(5):1327–1333. doi: 10.1161/01.str.0000014248.28711.d6. [DOI] [PubMed] [Google Scholar]
- 19.Garber CE, Friedman JH. Effects of fatigue on physical activity and function in patients with Parkinson's disease. Neurology. 2003;60(7):1119–1124. doi: 10.1212/01.wnl.0000055868.06222.ab. [DOI] [PubMed] [Google Scholar]
- 20.Herlofson K, Larson JP. Measuring fatigue in patients with Parkinson's disease - the Fatigue Severity Scale. Eur J Neurol. 2002;9(6):595–600. doi: 10.1046/j.1468-1331.2002.00444.x. [DOI] [PubMed] [Google Scholar]
- 21.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JL, Minarik PA, Nyström KV, Bautista C, Gorman MJ. Poststroke depression incidence and risk factors: an integrative literature review. J Neurosci Nurs. 2006;38(4):316–327. doi: 10.1097/01376517-200609000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Ayabe M, Brubaker PH, Mori Y, Kumahara H, Kiyonaga A, Tanaka H, Aoki J. Self-monitoring moderate-vigorous physical activity versus steps/day is more effective in chronic disease exercise programs. J Cardiopulm Rehabil Prev. 2010;30(2):111–115. doi: 10.1097/HCR.0b013e3181be7c80. [DOI] [PubMed] [Google Scholar]
- 24.Mutai H, Furukawa T, Araki K, Misawa K, Hanihara T. Long-term outcome in stroke survivors after discharge from a convalescent rehabilitation ward. Psychiatry Clin Neurosci. 2013;67(6):434–440. doi: 10.1111/pcn.12075. [DOI] [PubMed] [Google Scholar]
- 25.Carod-Artal J, Egido JA, González JL, Varela de Seijas E. Quality of life among stroke survivors evaluated 1 year after stroke: experience of a stroke unit. Stroke. 2000;31(12):2995–3000. doi: 10.1161/01.str.31.12.2995. [DOI] [PubMed] [Google Scholar]
- 26.Whyte EM, Mulsant BH, Vanderbilt J, Dodge HH, Ganguli M. Depression after stroke: a prospective epidemiological study. J Am Geriatr Soc. 2004;52(5):774–778. doi: 10.1111/j.1532-5415.2004.52217.x. [DOI] [PubMed] [Google Scholar]
- 27.Carod-Artal FJ, Ferreira Coral L, Trizotto DS, Menezes Moreira C. Poststroke depression: prevalence and determinants in Brazilian stroke patients. Cerebrovasc Dis. 2009;28(2):157–165. doi: 10.1159/000226114. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin RD, Devanand DP. Stroke, depression, and functional health outcomes among adults in the community. J Geriatr Psychiatry Neurol. 2008;21(1):41–46. doi: 10.1177/0891988707311041. [DOI] [PubMed] [Google Scholar]
- 29.van de Port IG, Kwakkel G, Schepers VP, Heinemans CT, Lindeman E. Is fatigue an independent factor associated with activities of daily living, instrumental activities of daily living and health-related quality of life in chronic stroke? Cerebrovasc. Dis. 2007;23(1):40–45. doi: 10.1159/000095757. [DOI] [PubMed] [Google Scholar]
- 30.Robinson CA, Shumway-Cook A, Matsuda PN, Ciol MA. Understanding physical factors associated with participation in community ambulation following stroke. Disabil Rehabil. 2011;33(12):1033–1042. doi: 10.3109/09638288.2010.520803. [DOI] [PubMed] [Google Scholar]
- 31.Mudge S, Stott NS, Walt SE. Criterion validity of the StepWatch Activity Monitor as a measure of walking activity in patients after stroke. Arch Phys Med Rehabil. 2007;88(12):1710–1715. doi: 10.1016/j.apmr.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 32.Orendurff MS, Schoen JA, Bernatz GC, Segal AD, Klute GK. How humans walk: bout duration, steps per bout, and rest duration. J Rehabil Res Dev. 2008;45(7):1077–1089. doi: 10.1682/jrrd.2007.11.0197. [DOI] [PubMed] [Google Scholar]
- 33.Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther. 2004;84(10):906–918. [PubMed] [Google Scholar]
- 34.Thieme H, Ritschel C, Zange C. Reliability and validity of the functional gait assessment (German version) in subacute stroke patients. Arch Phys Med Rehabil. 2009;90(9):1565–1570. doi: 10.1016/j.apmr.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Lin JH, Hsu MJ, Hsu HW, Wu HC, Hsieh CL. Psychometric comparisons of 3 functional ambulation measures for patients with stroke. Stroke. 2010;41(9):2021–2025. doi: 10.1161/STROKEAHA.110.589739. [DOI] [PubMed] [Google Scholar]
- 36.Cheng ST, Yu EC, Lee SY, Wong JY, Lau KH, Chan LK, Chan H, Wong MW. The geriatric depression scale as a screening tool for depression and suicide ideation: a replication and extention. Am J Geriatr Psychiatry. 2010;18(3):256–265. doi: 10.1097/JGP.0b013e3181bf9edd. [DOI] [PubMed] [Google Scholar]
- 37.Valderramas S, Feres AC, Melo A. Reliability and validity study of a Brazilian-Portuguese version of the fatigue severity scale in Parkinson's disease patients. Arq Neuropsiquiatr. 2012;70(7):497–500. doi: 10.1590/s0004-282x2012000700005. [DOI] [PubMed] [Google Scholar]
- 38.Sivrioglu EY, Sivrioglu K, Ertan T, Ertan FS, Cankurtaran E, Aki O, Uluduz D, Ince B, Kirli S. Reliability and validity of the Geriatric Depression Scale in detection of poststroke minor depression. J Clin Exp Neuropsychol. 2009;31(8):999–1006. doi: 10.1080/13803390902776878. [DOI] [PubMed] [Google Scholar]
- 39.Agrell B, Dehlin O. Comparison of six depression rating scales in geriatric stroke patients. Stroke. 1989;20(9):1190–1194. doi: 10.1161/01.str.20.9.1190. [DOI] [PubMed] [Google Scholar]
- 40.Grace J, Mendelsohn A, Friedman JH. A comparison of fatigue measures in Parkinson's disease. Parkinsonism Relat Disord. 2007;13(7):443–445. doi: 10.1016/j.parkreldis.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Hagell P, Höglund A, Reimer J, Eriksson B, Knutsson I, Widner H, Cella D. Measuring fatigue in Parkinson's disease: a psychometric study of two brief generic fatigue questionnaires. J Pain Symptom Manage. 2006;32(5):420–432. doi: 10.1016/j.jpainsymman.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 42.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr. Sco. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 43.deGroot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity: a critical review of available methods. Journal of Epidemiology. 2003;56(3):221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 44.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness in burden in geropsychiatric practice and research: Application Cumulative Illness Rating Scale. Psychiat. Res. 1992;41(3):237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 45.Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc. 1995;43:130–137. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 46.Mistry R, Gokhman I, Bastani R, et al. Measuring medical burden using CIRS in older veterans enrolled in UPBEAT, a psychogeriatric treatment program: A pilot study. J Gerontol A Biol Sci. Med Sci. 2004;59A:1068–1075. doi: 10.1093/gerona/59.10.m1068. [DOI] [PubMed] [Google Scholar]
- 47.Giaquinto S. Comorbidity in post-sroke rehabilitation. European Journal of Neurology. 2003;10:235–238. doi: 10.1046/j.1468-1331.2003.00563.x. [DOI] [PubMed] [Google Scholar]
- 48.Holland A, O'Connor RJ, Thompson AJ, Playford ED, Hobart JC. Talking the talk on walking the walk: a 12-item generic walking scale suitable for neurological conditions? J Neurol. 2006;253(12):1594–1602. doi: 10.1007/s00415-006-0272-2. [DOI] [PubMed] [Google Scholar]
- 49.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28–M34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 50.Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci. Med. Sci. 1998;53(4):M287–M294. doi: 10.1093/gerona/53a.4.m287. [DOI] [PubMed] [Google Scholar]
- 51.Botner EM, Miller WC, Eng JJ. Measurement properties of the Activities-specific Balance Confidence Scale among individuals with stroke. Disabil Rehabil. 2005;27(4):156–163. doi: 10.1080/09638280400008982. [DOI] [PubMed] [Google Scholar]
- 52.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 6th. Pearson; 2012. p. 1024. [Google Scholar]
- 53.West SG, Aiken LS. Multiple Regression: Testing and Interpreting Interactions. Sage Publications, Inc; 1991. [Google Scholar]
- 54.Robinson CA, Matsuda PN, Ciol MA, Shumway-Cook A. Participation in community walking following stroke: the influence of self-perceived environmental barriers. Phys Ther. 2013;93(5):620–627. doi: 10.2522/ptj.20110217. [DOI] [PubMed] [Google Scholar]
- 55.Pang MY, Eng JJ. Fall-related self-efficacy, not balance and mobility performance, is related to accidental falls in chronic stroke survivors with low bone mineral density. Osteoporos. Int. 2008;19(7):919–927. doi: 10.1007/s00198-007-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pound P, Gompertz P, Ebrahim S. A patient-centred study of the consequences of stroke. Clin Rehabil. 1998;12(4):338–347. doi: 10.1191/026921598677661555. [DOI] [PubMed] [Google Scholar]
- 57.Letgers K. Fear of falling. Phys Ther. 2002;82(3):264–272. [PubMed] [Google Scholar]
- 58.Myers AM, Powell LE, Maki BE, Holliday PJ, Brawley LR, Sherk W. Psychological indicators of balance confidence: relationship to actual and perceived abilities. J Gerontol. Med Sci. 1996;51(A):M37–M43. doi: 10.1093/gerona/51a.1.m37. [DOI] [PubMed] [Google Scholar]
- 59.Tennstedt S, Howland J, Lachman M, Peterson E, Kasten L, Jette A. A randomized, controlled trial of a group intervention to reduce fear of falling and associated activity restriction in older adults. J Gerontol B Psychol Sci Soc Sci. 1998;53(6):384–392. doi: 10.1093/geronb/53b.6.p384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.