Abstract
BACKGROUND AND PURPOSE
The objectives of this pilot study were to 1) evaluate the feasibility and investigate the efficacy of a 3-week, high volume (450 minutes/week) Adapted Tango intervention for community dwelling individuals with mild-moderate PD, and to 2) investigate the potential efficacy of Adapted Tango in modifying electromyographic (EMG) activity and center of body mass (CoM) displacement during automatic postural responses to support surface perturbations.
METHODS
Individuals with PD (n=26) were recruited for high volume Adapted Tango (15 lessons, 1.5 hour each over 3 weeks). Twenty participants were assessed with clinical balance and gait measures before and after the intervention. Nine participants were also assessed with support-surface translation perturbations.
RESULTS
Overall adherence to the intervention was 77%. At posttest, peak forward CoM displacement was reduced (4.0±0.9 cm, pretest, vs. 3.7±1.1 cm, posttest; P=0.03; Cohen’s d=0.30) and correlated to improvements on Berg Balance Scale (BBS; rho=−0.68; P=0.04) and Dynamic Gait Index (rho=−0.75; P=0.03). Overall antagonist onset time was delayed (27 ms; P=0.02; d=0.90) and duration was reduced (56 ms, ≈39%, P=0.02; d=0.45). Reductions in EMG magnitude were also observed (P<0.05).
DISCUSSION AND CONCLUSIONS
Adherence was acceptable and improvements on clinical measures of balance and gait were comparable to that obtained with lower volume, 12-week programs. Following participation in Adapted Tango, changes in kinematic and some EMG measures of perturbation responses were observed in addition to improvements in clinical measures. We conclude that 3-week, high volume Adapted Tango is feasible and represents a viable alternative to longer duration adapted dance programs. Video Abstract available for more insights from the authors (see Supplemental Digital Content 1)
Keywords: Parkinson’s Disease, Postural Balance, Dance Therapy, Electromyography, Exercise Therapy
INTRODUCTION
Balance problems are common in Parkinson disease (PD) and are challenging to treat via pharmacotherapy or surgical interventions.1,2 Improvements on clinical measures of balance and gait have been demonstrated after several rehabilitative exercise programs for individuals with PD,3–6 including Adapted Tango dance.7,8 Adapted Tango elicits clinically-measured balance improvements that are superior to exercise,9 non-partnered dance,7 other dance-based and martial arts-based interventions,10,11 no intervention,8,11 and health education.12 Recently, the importance of rehabilitation volume has received increased attention.13,14 In PD, high volume rehabilitation may be particularly effective, as individuals exhibit superior increases in gait speed after higher volume/low intensity exercise therapy (12 weeks, 150 minutes/week, 40%–50% of heart rate reserve) compared to lower volume/high intensity exercise therapy (12 weeks, 90 minutes/week, 70%–80% of heart rate reserve) with comparable overall work.14 Further, exercise therapy of at least 180 minutes/week is required to improve gait speed in older adults.15 Recently, 450 minutes/week has been demonstrated to be the upper threshold of exercise volume (i.e., the sweet spot) required for lowered mortality risk (by 39%), compared to sedentary older adults.16 Previously, individuals with PD demonstrated functional improvements after two weeks of high volume (450 minutes/week) Adapted Tango training.17 Although these improvements are promising, it is unknown if longer-term therapy (i.e., 3 weeks) with similar volume is feasible and possibly more effective. Also, it is unknown whether clinical changes after Adapted Tango are associated with alterations in responses to postural perturbations assessed in a laboratory setting, a common paradigm in human neurophysiology research.18–20
Previous studies in populations other than PD suggest that improvements in clinical measures of balance after rehabilitation may be associated with improved kinematic and electromyographic measures during balance and gait.21–24 For example, three weeks of high volume (450 minutes/week) Tai Chi training advanced agonist muscle activation onset times and reduced co-contraction in response to support-surface perturbations during walking in mildly balance-impaired older adults whose balance had only slightly improved, as measured by a 2 point increase on the Berg Balance Scale (BBS).22 In individuals with post-stroke hemiparesis, agility exercise therapy (10 weeks, 180 minutes/week) improved gait speed, and reduced muscle activation onset times in response to support-surface perturbations during standing.23 Locomotor rehabilitation also improved timing of ankle plantar flexors during gait in hemiparetic individuals (12 weeks, 120 minutes/week).24 However, it is unknown whether changes in clinical measures after Adapted Tango, a dance-based rehabilitation, which may address PD impairments through different mechanisms than Tai Chi and agility exercise, 25 would be associated with changes in muscle activity or kinematic measures during postural responses.
Electromyographic (EMG) and kinematic abnormalities during responses to support surface translation perturbations in PD18,20 differ from those of older adults22 and stroke survivors.23,24 During translation perturbations of the support surface, the center of mass is displaced and medium- (≥ 80 ms) and long-latency (≥ 100 ms) corrective responses are generated in leg and trunk muscles, referred to as the automatic postural response.19 Unlike the delayed responses in balance-impaired older adults22 and in individuals with post-stroke hemiparesis,23,24 in individuals with PD, automatic postural response onset latency is typically normal or earlier than normal in agonist muscles18 and typically earlier than normal in antagonist muscles, leading to inappropriate co-contraction.18,20 PD is also associated with increased total center of mass displacement during perturbation responses.26 We initiated this investigation because, to the best of our knowledge, no studies have examined changes in postural responses in PD before and after Adapted Tango,.
The primary objectives of this pilot study were to determine the feasibility and investigate the efficacy of 3 weeks of high volume (450 minutes/week) Adapted Tango in improving clinical measures of balance and gait in community-dwelling individuals with PD. We performed a repeated measures observational study of high volume Adapted Tango with duration increased to 3 weeks to estimate adherence and investigate efficacy. We predicted that 1) three-week high volume Adapted Tango would be feasible for individuals with mild-moderate PD, as demonstrated by adherence with a 95% confidence interval lower bound of ≥60%, 2) clinical measures of balance and disease severity would improve from pretest to posttest, and be retained for at least one month, based on results from previous studies demonstrating retention for three months,8,12 and that 3) clinical measures would be stable over the 1 month before pretest, when tested in a subset of participants.
The secondary, exploratory, objectives of this pilot study were to evaluate postural responses before and after Adapted Tango to examine the feasibility of and utility of using kinematic and electromyographic outcome measures in this type of intervention. We allocated a convenience sample of intervention participants to receive additional perturbation response testing at pretest and posttest. Muscle onset time measurements have been demonstrated to be stable across multiple days in healthy young individuals 27 and across multiple months in individuals with PD.28 Thus, we determined that we would consider a randomized trial to be feasible and justified if we obtained preliminary efficacy evidence, as determined by reductions in CoM displacement or in antagonist muscle onset time, duration, or magnitude. In order to further investigate preliminary evidence of efficacy, we also examined associations between changes in clinical measures and changes in CoM displacement or in muscle activity measures after Adapted Tango.
METHODS
Study design
This study was a repeated measures, observational study without a control group. A double baseline procedure was employed to improve internal validity of clinical outcomes. Multiple post-test periods were used to assess stability of observed changes in clinical outcome measures. A convenience sample of study participants was allocated to additional perturbation testing before and after the intervention.
Participants and setting
Participants were recruited at PD outreach events, senior centers, and the Emory Movement Disorders clinic.. Participants met the following inclusion criteria: Hoehn & Yahr stage I-IV, diagnosis of “definite” idiopathic PD,29 age ≥ 35 years. Exclusion criteria were: deep brain stimulation, other significant comorbidities, or significant musculoskeletal impairment as determined by the investigators. Participants were observed for outcome measures on three separate occasions. All participants were assessed within one week before (pretest) and within one week after (posttest) the intervention. Participants recruited early in the trial (n=7) were assessed 1 month before the beginning of the intervention (one-month pretest) to establish a double baseline for these participants and examine stability of clinical measures. Participants recruited later in the trial (n=13) were observed in a follow up appointment, 1 month after the intervention’s cessation (one-month post). The double baseline was conducted to examine the stability of measures between one-month pretest and pretest: a time period (~1 month) that was similar to the intervention time period of 3 weeks. The one-month posttest (follow-up) was used to detect retention (or loss) of changes between posttest and one-month posttest also over a period of time that was similar to the interventional time period. Adapted Tango classes and clinical assessments were performed in a large multipurpose room on a university campus. Perturbation response assessments were performed in a dedicated balance laboratory elsewhere on campus. Participants provided written informed consent according to protocols approved by institutional review boards at Emory University and the Georgia Institute of Technology.
3-week high volume Adapted Tango intervention
Participants received high volume, moderate intensity Adapted Tango, taught by a professional dance instructor,30 and were to complete fifteen 90-minute Adapted Tango sessions in three weeks. Classes were designed to induce expenditure of ≥3 Metabolic Equivalents of Task (METs) per minute, as per estimates for typical ballroom dance, which is considered light-moderate intensity exercise by the United States Center for Disease Control.31 Classes began with standing warm-ups to upbeat music, and continued with dancing to commercial music selections. Participants spent equal time leading and following dance steps, performed in an adapted ballroom frame, holding forearms, and classes were progressive. (See Video Abstract, Supplemental Digital Content 1, for an example of the adapted ballroom frame.) Individuals with PD were coupled with individuals without PD. Participants spent 1/3 of class working on rhythmic entrainment to the beat during the warm-ups, such as tapping of toes or heels, or sequentially opening and closing the hands. Further, the participants spent ample time (i.e., 20–30 minutes) simply walking to various tango rhythms intended to enhance their musicality, i.e., the ability to control the gait cycle in a more complex rhythm than typical gait. As in previous studies,7,9,10,12 participants were allowed to take breaks as needed throughout the classes in order to decrease fatigue.
Outcome measures
Clinical balance and gait measures
Assessments were administered in the same order at each evaluation in order to minimize the effects of fatigue on measurements. Participants were assessed for general health, and were observed at each visit with clinical measures including: Parkinson’s disease severity (Unified Parkinson Disease Rating Scale [UPDRS] motor subscale III32), dyskinesia (total of scores 0–4 for each limb and face), the Berg Balance Scale (BBS33), Dynamic Gait Index (DGI34), Fullerton Advanced Balance Scale (FAB35,36), the two-footed Jump test, a test of neuromuscular synergies and musculoskeletal health,37 6 minute walk test (6MWT38), functional reach (FR39), Single/Dual Timed Up and Go (TUG34), fast and preferred gait speed and cadence were measured using a stopwatch over a 20′ path.40 The Activities-Specific Balance Confidence questionnaire (ABC41), and the Freezing of Gait questionnaire (FOG42) were also administered. FAB was recently validated in community-dwelling individuals with PD,36 and was used to avoid BBS ceiling effects. For each participant, all assessments occurred at a standardized time of day coinciding with a self-determined optimal ON period to minimize pharmacologically-related motor fluctuations. Clinical balance and gait measures were performed by an experienced rehabilitation scientist or by trained research assistants. An experienced rehabilitation scientist certified by the Movement Disorders Society administered the UPDRS-III. To minimize the variability of individual UPDRS items, including the retropulsion test,43 the same rehabilitation scientist administered the exam at each observation. Clinical data were entered and cross-verified by research assistants.
Response to perturbation
A convenience sample of the study participants was allocated to receive additional perturbation response assessments within two weeks before (pretest) and within two weeks after (posttest) the intervention. These participants were assessed at a standardized time of day (either 9 AM or 1 PM) coinciding as closely as possibly to the participants’ self-determined ON time. While wearing a safety harness, participants stood with each foot on a 6-axis (3D ground-reaction forces and moments) force plate (OR6-6, AMTI, Watertown, MA) embedded in a custom perturbation platform that translated in the horizontal plane. They were instructed to cross their arms over their chest, to focus on a landscape scene 3 m ahead, and to maintain balance with their feet in place but to take protective steps if necessary. Three perturbations were induced in each of the forward (displacing the center of mass anterior towards the toes) and backward (displacing the center of mass posterior towards the heels) directions of body sway.19 These perturbations were induced within a set of 36 perturbations spanning all directions in the horizontal plane and delivered in random order. At pretest, three to six test perturbations were delivered to select the highest perturbation level each participant could maintain balance without stepping. These perturbations were excluded from analysis to control for startle effects.44 Participants PR7 and PR9 (“PR” designates “Perturbation Response”) used level 4 (peak displacement 10 cm; peak velocity 20 cm/s; peak acceleration 0.2 g; 700 ms total duration); all others used level 3 (7.5 cm; 15 cm/s; 0.1 g; 700 ms). Self-selected stance width was measured at pretest and subsequently enforced at posttest. (See Video Abstract, Supplemental Digital Content 1, for video of the perturbation apparatus.)
Platform kinematics, surface EMG, and ground-reaction forces were sampled at 1080 Hz and processed in Matlab (The MathWorks, Natick, MA). Trials with stepping responses or arm movement were excluded from analysis. Ground reaction forces were low-pass filtered (100 Hz, third-order zero-lag Butterworth filter), and CoM acceleration in the medial-lateral and anterior-posterior directions was calculated by adding horizontal-plane ground reaction force components at each foot and dividing by participant mass (acceleration = force/mass).19,45,46 Because estimates of CoM position from kinematic marker data were unreliable, we then integrated the acceleration twice, assuming zero initial velocity and zero initial displacement at the onset of the perturbation, to arrive at the displacement of the center of mass.47–51 Linear trends were removed from acceleration signals before integration to avoid introducing integration constants in velocity signals, and computed velocity and displacement signals were set to zero at perturbation onset to enforce the assumed initial conditions. Surface EMG (Konigsberg Instruments, Pasadena, CA) was collected from leg and trunk muscles, high-pass filtered (35 Hz, third-order zero-lag Butterworth filter), demeaned, rectified, and low-pass filtered (40 Hz).48,52,53 EMG was analyzed from ankle muscles tibialis anterior (TA) and medial gastrocnemius (MG), recorded bilaterally.22,54 During backward sway, agonist TA is lengthened and antagonist MG is shortened. During forward sway, agonist MG is lengthened and atagonist TA is shortened. To minimize variability in electrode placement between pretest and posttest silver/silver chloride disc electrodes were placed at 2-cm interelectrode distance according to standard EMG electrode placement guidelines19,55 by the same experimenter at each assessment. EMG records from each trial were normalized to the maximum value observed during each assessment after averaging across similar trials and across 50 ms bins.
Before statistical analysis, computed CoM displacement signals in the anterior-posterior direction and normalized EMG signals from each recorded muscle were averaged across similar trials for each participant at each assessment. The peak of each average CoM displacement signal was calculated. Onset and offset times of each average EMG signal were calculated with a computer program and corrected as necessary (14 records, ≈11%). For each average EMG signal, the first sample within a window between 80 ms and 300 ms after perturbation onset to cross a threshold of M + 6 × SD was first identified. Onset time was then determined as the last sample prior to the threshold-crossing sample for which the preceding 10 samples were all below M + 2 × SD. Offset time was determined as the first sample subsequent to the threshold-crossing sample for which the following 10 samples were all below M + 2 × SD. To avoid including responses to platform deceleration,54 offset times were truncated to the earlier of 280 ms after EMG onset or 450 ms after perturbation onset. The duration of each average EMG signal was calculated as offset time - onset time. The magnitude of each average EMG signal was calculated by averaging over a window 80–450 ms after perturbation onset after removing background level.18,20 After all processing, kinematic and electromyographic data of each participant were summarized as a dataset containing 13 variables (CoM displacement, one variable, and 3 variables [Onset, Duration, and Magnitude] for each of the 4 muscles analyzed [TA from the left and right leg and MG from the left and right leg], for a total of 13 variables) for each level of the independent variables Time [pretest, posttest], Perturbation Direction [forward, backward], and Perturbation Level [3, 4]).
Sample size
Sample size for the intervention (n=26) was selected to achieve effect sizes in clinical balance and gait measures comparable to a previous 2-week intervention (conducted with n=14)17 after allowing for ~40% attrition given the longer term of the intervention. Sample size for the group of participants allocated to postural response testing (n=10) was selected based on previous literature demonstrating the feasibility of identifying effects of interest in electromyographic and kinematic measurements of individuals with PD in cross-sectional56 and longitudinal57 studies.
Statistical Analyses
Descriptive analyses and effect sizes
Descriptive statistics were calculated for all outcomes at each timepoint. Magnitude effect sizes representing changes from pretest to posttest was calculated with Cohen’s d,58 which describes the difference in means scaled to units of standard deviation, in this case taken from pretest.
Sampling and stability of clinical measures at pretest
To test that participants allocated to perturbation response testing represented an unbiased sample of the study population, baseline demographic characteristics were compared between perturbation response participants and the rest of the study participants with 1-way Analyses of Variance (ANOVAs) (Group [allocation to perturbation response testing vs. non-allocation to perturbation response testing]) or Kruskal-Wallis 1-way ANOVAs on ranks for nonparametric data. To establish test-retest stability of clinical balance and gait measures in this cohort, intra-class correlation coefficients were calculated between one-month pretest (screening) and pretest. Intra-class correlation coefficient values > 0.75 and > 0.40 were characterized as “excellent” and “fair to good,” respectively.
Statistical analyses of changes in clinical measures across pretest, posttest, and follow-up
To investigate the efficacy of the intervention in improving clinical measures of balance and gait, repeated measures ANOVAs (Time [pretest, posttest, follow-up]), with Holms-Sidak post hoc tests determined significance of changes in clinical measures between pretest, posttest, and follow-up. Greenhouse-Geisser corrections to degrees of freedom were applied when sphericity was violated as per Mauchly’s Test. The last observation was carried forward in cases of missing data. Additional paired t-tests on individual UPDRS-III items and on average tremor score (the average of the scores of items III.20 and III.21; cf.59) were performed post-hoc to identify items that changed from pretest to posttest.
Statistical analyses of changes in postural responses from pretest to posttest
To investigate the potential efficacy of the intervention in altering CoM displacement and muscle activity during perturbation responses, separate repeated measures ANOVAs (Time [pretest, posttest], with Perturbation Level [3, 4] included as a covariate) were initially run. Perturbation level was entered as a covariate in these analyses to control for the potential effects of perturbation level on CoM displacement and muscle activity demonstrated in previous studies.19,27 No statistical testing of differences between perturbation level 3 and 4 was performed. These ANOVAs determined the significance of changes in peak CoM displacement and in onset time, duration, and magnitude of each recorded muscle (TA-L, TA-R, MG-L, MG-R) in each perturbation direction.
Changes in postural responses based on pooled EMG data
Secondary univariate ANOVAs were also conducted on EMG variables onset time, duration, and magnitude after recoding data from individual muscles as either TA or MG, or as agonist or antagonist. In these analyses: 1) Data from TA-L and TA-R were pooled for analysis as “TA,” and data from MG-L and MG-R were pooled for analysis as “MG.” Secondary ANOVAs (Time [pretest, posttest] × Perturbation Level [3, 4] × Participant [1–9]; with Participant as a nested factor within Perturbation Level, and a Time × Participant interaction term) were then conducted to determine significance of changes in onset time, duration, and magnitude of TA and MG in each perturbation direction. 2) Data from MG during forward CoM perturbations and from TA during backward CoM perturbations were pooled for analysis as “agonists,” and data from TA during forward CoM perturbations and from MG during backward CoM perturbations were pooled for analysis as “antagonists.” Secondary ANOVAs (Time [pretest, posttest] × Perturbation Level [3, 4] × Participant [1–9]; with Participant as a nested factor within Perturbation Level, and a Time × Participant interaction term) were then conducted to determine significance of changes in onset time, duration, and magnitude of agonists and antagonists.
Associations between changes in clinical measures and changes in postural responses
To test whether improvements in clinical measures of balance function after Adapted Tango were associated with alterations in perturbation responses, and to detect possible evidence of efficacy of the intervention in altering CoM displacement and muscle activity during perturbation responses, associations between changes on BBS, FAB, and DGI and changes in perturbation response measures were determined with Spearman’s correlation coefficients in a complete-case analysis. Statistical analyses were performed using IBM SPSS 20 software and SAS University Edition. All tests were performed with 2 tails and considered significant at P < 0.05. Summary statistics are reported as M ± SD unless otherwise noted.
RESULTS
Participant flow and recruitment
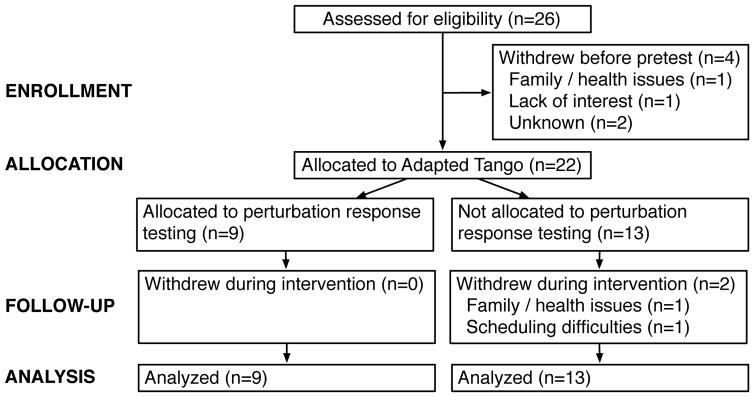
A flow-chart of participants through the study is presented in Figure 1. Twenty-six participants were recruited for the trial. Of these, 4 withdrew before pretest assessment (family/health issues, n=1; lack of interest, n=1; loss of contact/unknown, n=1). These individuals were excluded from analyses of outcome measures as no data were available, but they were included in estimates of adherence to the intervention. Of the remaining 22 participants, 2 participants withdrew before posttest (family/health issues, n=1; scheduling difficulties, n=1); all others completed all planned assessments. Adherence to the intervention exceeded previously expected targets (20/26 observed vs. 15/26 expected), providing evidence that 3-week high volume Adapted Tango is feasible. Overall adherence to the intervention was 77%, with 95% confidence interval (61%, 93%). Adherence was higher among those who attended at least 1 class (91% [95% CI 78%–100%]). Posttest and one-month post data were unavailable for participants who withdrew before posttest (n=2). One-month post data were not collected for those (n=7) allocated to one-month pretest (screening) assessments. Participants who completed at least one clinical assessment were invited to participate in postural response assessments until the Adapted Tango intervention began and enrollment for additional testing was closed. Nine participants were enrolled in postural response testing. Demographic characteristics of the 22 participants included in the final analysis (68% female, 65.4 ± 12.8 years) are summarized in Table 1. Detailed characteristics of the 9 participants allocated to additional postural response testing are summarized in Table 2.
Figure 1.
Diagram depicting flow of participants through the study.
Table 1.
Characteristics of participants in the 3-week high volume Adapted Tango rehabilitative intervention.
| Variable | All participants (n=22) | Participants allocated to receive perturbation response testing (n=9) | Participants not allocated to receive perturbation response testing (n=13) | P-valuesa |
|---|---|---|---|---|
| Age, y (M±SD) | 65.4 ± 12.8 | 68.0 ± 14.6 | 63.5 ± 11.7 | 0.46 |
| Sex (n, %) | 0.65 | |||
| Male | 7, 32% | 2, 22% | 5, 38% | |
| Female | 15, 68% | 7, 78% | 8, 62% | |
| Height, m (M±SD) | 1.72 ± 0.11 | 1.76 ± 0.07 | 1.68 ± 0.12 | 0.06 |
| Weight, kg (M±SD) | 74.3 ± 13.7 | 73.2 ± 11.4 | 75.0 ± 15.5 | 0.76 |
| PD duration, y (M±SD) | 6.1 ± 3.8 | 6.0 ± 3.9 | 6.2 ± 3.8 | 0.89 |
| UPDRS III (M±SD) | 30.4 ± 6.1 | 30.0 ± 4.7 | 30.6 ± 7.0 | 0.81 |
| H & Y (n, %) | 0.83 | |||
| Stage 1.5 | 1, 4% | 0, 0% | 1, 8% | |
| Stage 2 | 12, 55% | 5, 56% | 7, 54% | |
| Stage 2.5 | 4, 18% | 1, 11% | 3, 23% | |
| Stage 3 | 5, 23% | 3, 33% | 2, 15% | |
| Dyskinesia score (M±SD) | 1.8 ± 2.5b | 2.1 ± 2.5c | 1.6 ± 2.5d | 0.65 |
| Tremor score (M±SD) | 0.4 ± 0.4 | 0.5 ± 0.7 | 0.3 ± 0.2 | 0.32 |
Abbreviations: PD, Parkinson’s disease; UPDRS III, Unified Parkinson’s Disease Rating Scale Motor Subscale III; H & Y, modified Hoehn and Yahr stage.
P-values are from independent-samples t-tests for continuous variables or Fisher’s exact tests for categorical variables comparing participants allocated to receive perturbation response testing to those not allocated to receive perturbation response testing.
n=19.
n=7.
n=12.
Table 2.
Detailed characteristics of participants in the 3-week high volume Adapted Tango rehabilitative intervention allocated to receive perturbation response testing.
| Participant | Age, y | Sex | Height, m | Weight, kg | PD duration, y | UPDRS III (/108) | H & Y | Dysk (/20) | Medications |
|---|---|---|---|---|---|---|---|---|---|
| PR1 | 68 | M | 1.80 | 80.6 | 5 | 26 | 2 | 1 | C/L, Ent., Rop. |
| PR2 | 79 | M | 1.68 | 68.0 | 3 | 40 | 2 | 0 | C/L, Ama. |
| PR3 | 64 | M | 1.75 | 79.3 | 11 | 25 | 2.5 | 6 | C/L, Ent. |
| PR4 | 81 | M | 1.78 | 83.8 | 3 | 35 | 3 | 0 | C/L, Ent., Ras. |
| PR5 | 74 | M | 1.73 | 76.1 | 5 | 28 | 2 | 0 | C/L |
| PR6 | 73 | F | 1.80 | 62.5 | 4 | 28 | 3 | 0 | C/L, Ras. |
| PR7 | 36 | M | 1.83 | 74.7 | 6 | 29 | 2 | 4 | C/L |
| PR8 | 81 | F | 1.65 | 48.9 | 14 | 31 | 3 | 4 | C/L, Rop. |
| PR9 | 56 | M | 1.85 | 82.9 | 3 | 28 | 2 | 1 | C/L |
Abbreviations: PD, Parkinson’s disease; UPDRS III, Unified Parkinson’s Disease Rating Scale Motor Subscale III; H & Y, modified Hoehn and Yahr stage; Dysk, dyskinesia score, C/L, carbidopa/levodopa; Ent., entacapone; Rop., ropinirole; Ama., amantadine; Ras., Rasagiline.
During the study no adverse events or deviations from the intervention were observed. Two small deviations from the perturbation response assessment protocol occurred. In one participant (PR1) self-selected stance width was not correctly enforced. This participant used a 9.5 cm wider stance width at posttest. These data were retained in analyses as stance width minimally affects forward and backward perturbations.18 Due to equipment failure, only MG recorded from the right leg (MG-R) was available at posttest for participants PR4, PR5, and PR6. Adapted Tango classes and all assessments were conducted from August through October 2011.
Baseline data
At pretest, no significant effects of Group (allocated to perturbation response testing vs. not allocated to perturbation response testing) were identified in age, sex, height, weight, disease duration, UPDRS-III, Hoehn and Yahr stage, or dyskinesia score. Correlational analyses showed very strong correlations between FAB and BBS (r=0.81; P <0.001) and between FAB and DGI (r=0.87; P<0.001). Test-retest analyses demonstrated that clinical measures were stable over the month before treatment, with “excellent” intra-class correlation coefficient values (>0.75) obtained for BBS (0.93), DGI (0.90), FR (0.79), ABC (0.94), and FOG (0.88) and intra-class correlation coefficients characterized as “fair to good” (>0.4) obtained for 6MWT (0.60).
Clinical measures
Descriptive statistics, change scores, and effect sizes for all clinical measures are tabulated in Table 3. At posttest, scores increased on BBS, (P<0.01), FAB (P<0.001), and DGI (P=0.01) (Figure 2). All significant increases at posttest remained significant at one-month post testing in post-hoc tests (BBS, P<0.001, FAB, P<0.001, DGI, P=0.04). Participants also increased preferred and fast cadence (preferred, P<0.01; fast, P=0.03) and exhibited decreased UPDRS-III (motor subscale) total scores (P<0.01) from pretest to follow-up. Paired t-tests performed post-hoc on individual UPDRS-III items identified significant improvements on postural stability (item III.30; 0.95±0.58, pretest, vs. 0.60±0.68, posttest, M±SD, P=0.03), and speech (item III.1; 1.18±0.73, pretest vs. 1.00±0.79, posttest; P=0.02). No changes were observed on 6MWT (P=0.11), FR (P=0.48), ABC (P=0.22), FOG (P=0.38), gait speed (preferred, P=0.69; fast, P=0.18), Jump (P=0.06) or TUG (P=0.30).
Table 3.
Mean values (±SD) of clinical measures of balance and gait before and after the 3-week, high volume Adapted Tango rehabilitative intervention.
| Pretest (n=20) | Posttest (n=20) | Follow-up (n=13) | Change Scores (n=20) | Effect Size | |
|---|---|---|---|---|---|
| PD severity | |||||
| UPDRS-III (/108)* | 30.4±6.1 | 27.5±6.3 | 23.6±5.6† | −2.9±5.4i | −0.47 |
| Dyskinesia | 1.7±2.1a | 1.3±2.1 | 1.8±2.1 | −0.3±1.8f | −0.14 |
| FOGB | 5.2±5.0 | 5.3±5.2 | 4.9±4.9d | −0.3±1.9 | −0.05 |
| Static and dynamic balance | |||||
| BBS (/56)* | 49.3±6.4 | 53.1±3.5† | 54.4±1.7† | 3.8±4.2ii | 0.59 |
| FAB (/40)* | 27.1±6.6 | 31.0±5.3† | 31.6±7.4e, † | 3.7±3.5 | 0.56 |
| DGI (/24)* | 19.1±3.5 | 21.2±2.1c,† | 20.9±2.6e,† | 1.8±2.4c,iii | 0.53 |
| FR (m) | 0.30±0.07 | 0.32±0.07 | 0.30±0.07 | 0.01±0.06 | 0.14 |
| ABC (/100) | 77.0±23.0b | 79.6±23.3 | 85.1±16.4d | 2.9±12.6c | 0.12 |
| TUG (sec) | 9.3±3.1 | 8.2±2.1 | 8.9±3.6e | −1.0±2.1 | −0.31 |
| TUG cognitive (sec) | 12.6±4.3 | 11.2±3.7 | 11.0±6.0e | −1.4±3.0 | −0.32 |
| TUG manual (sec) | 11.3±3.9 | 10.5±3.7 | 10.8±3.8e | −0.6±2.0 | −0.16 |
| Two-Footed Jump (m) | 0.50±0.39 | 0.60±0.37c | 0.66±0.47e | 0.09±0.19c | 0.23 |
| Gait | |||||
| 6MWT (m) | 396.3±87.7 | 437.1±86.0 | 450.3±93.2e | 32.4±83.0iv | 0.37 |
| Preferred gait speed (m/sec) | 1.19±0.21 | 1.23±0.26 | 1.26±0.19e | 0.02±0.19v | 0.10 |
| Preferred cadence (steps/min)* | 109.6±11.1 | 115.3±9.7 | 120.2±8.1e,† | 6.1±10.7 | 0.55 |
| Fast gait speed (m/sec) | 1.67±0.26 | 1.78±0.34 | 1.77±0.31e | 0.09±0.18 | 0.35 |
| Fast cadence (steps/min)* | 136.3±16.4b | 141.9±12.6 | 144.1±15.4e,† | 7.6±11.8c | 0.47 |
Abbreviations: UPDRS, Unified Parkinson’s Disease Rating Scale; BBS, Berg Balance Scale; FAB, Fullerton Advanced Balance Scale; DGI, Dynamic Gait Index; 6MWT, Six Minute Walk Test; FR, Functional Reach; ABC, Activities-specific Balance Confidence Scale; FOGB, Freezing Of Gait questionnaire B; TUG, Timed Up and Go test. Values are shown as M±SD at each timepoint. Effect sizes (Cohen’s d) calculated as difference in means between posttest and pretest divided by standard deviation at pretest.
Main effect of time (P < 0.05), repeated measures ANOVA with Holms-Sidak post hoc tests;
Significantly different from pretest. The last observation was carried forward in cases of missing data.
n=18.
n=21.
n=19.
n=14.
n=12.
n=16.
Where available, Minimal Clinically-Important Differences (MCID) are noted.
MCID = 2.5 points.60
MCID (estimated from balance-impaired older adults) = 7 points,62 Minimal Detectable Change (estimated from PD) = 5 points.69
MCID (estimated from community-dwelling older adults) = 1.9 points.61
MCID (estimated from geriatric individuals and stroke survivors) = 47 m.70
MCID (estimated from geriatric individuals and stroke survivors) = 0.10 m/s.70
Figure 2.
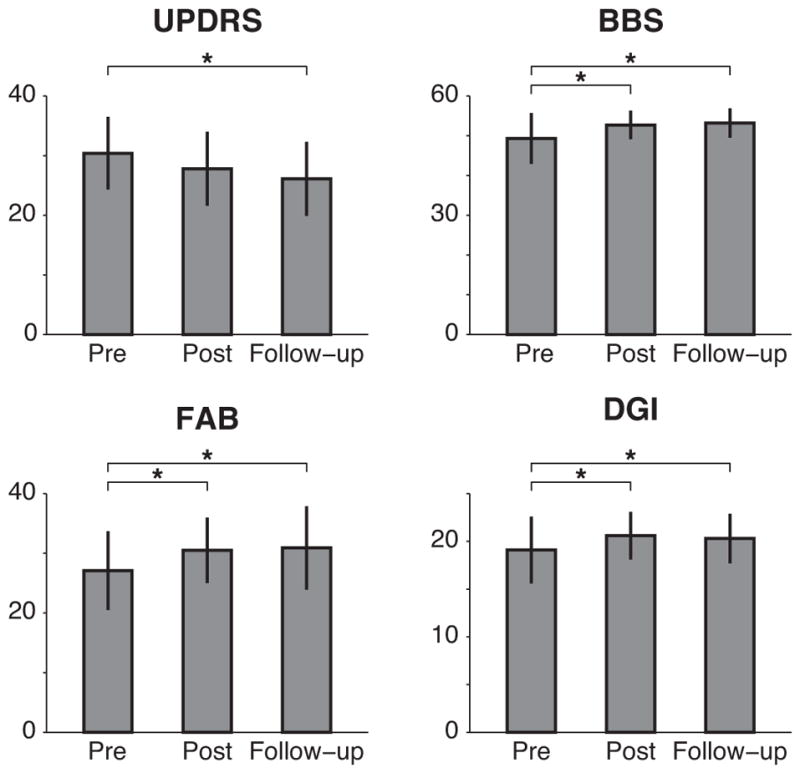
Clinical measures of balance and gait before and after the intervention.
Abbreviations: UPDRS, Unified Parkinson’s Disease Rating Scale, motor subscale III; BBS, Berg Balance Scale; FAB, Fullerton Advanced Balance Scale; DGI, Dynamic Gait Index. Bars and error bars indicate M±SD. The last observation was carried forward in cases of missing data. All measures shown exhibited a main effect of time (P < 0.05) in repeated measures ANOVA. Asterisks (*) indicate significant differences from pretest determined with Holms-Sidak post hoc tests, P < 0.05.
Postural responses
Changes in postural responses from pretest to posttest
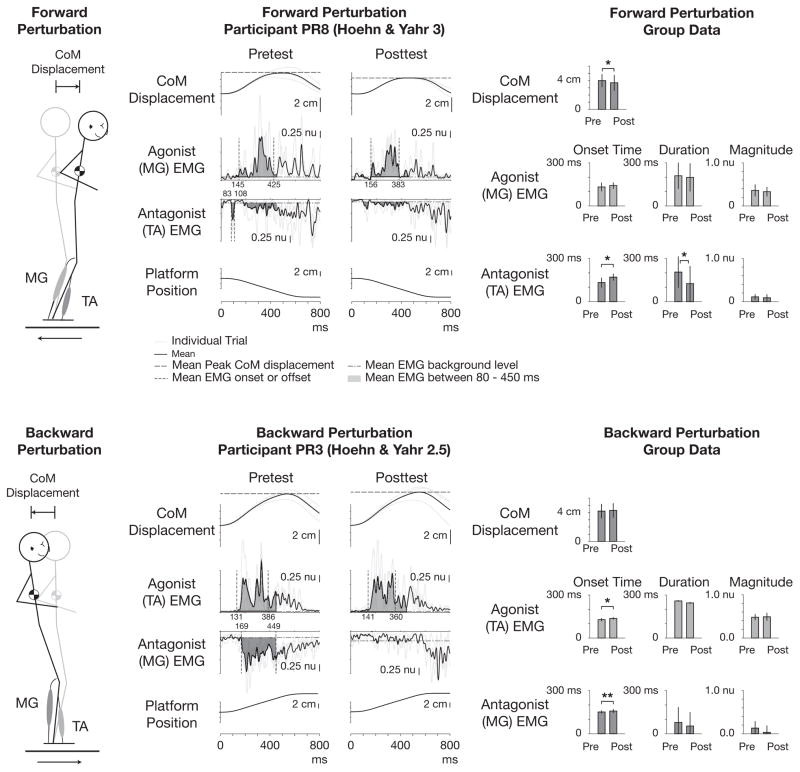
Descriptive statistics and effect sizes for kinematic and individual EMG measures are tabulated in Table 4. At posttest, CoM displacement was reduced during forward CoM perturbations (P=0.03) and unchanged during backward CoM perturbations (P=0.39) (Figure 3). Initial analyses of individual muscles revealed significant reductions in TA-L magnitude (P=0.02) and MG-R magnitude (P=0.01) during forward CoM perturbations and no statistically significant changes in onset, duration, or magnitude of any individual muscles during backward CoM perturbations.
Table 4.
Mean values (±SD) of primary kinematic and electromyographic measures of perturbation responses before and after the 3-week, high volume Adapted Tango rehabilitative intervention.
| Pretest
|
Posttest
|
Effect Size | |||
|---|---|---|---|---|---|
| n | M±SD | n | M±SD | ||
| Kinematic | |||||
| CoM displacement, backward (cm) | 9 | 4.17±0.97 | 9 | 4.26±1.04 | 0.09 |
| CoM displacement, forward (cm)* | 9 | 3.97±0.93 | 9 | 3.69±1.13 | −0.30 |
| Individual muscle analyses | |||||
| Backward perturbation | |||||
| TA-L onset (ms) | 9 | 124±10 | 6 | 138±10 | 1.40 |
| TA-L duration (ms) | 9 | 243±63 | 6 | 231±77 | −0.19 |
| TA-L magnitude (nu) | 9 | 0.48±0.08 | 6 | 0.50±0.08 | 0.30 |
| TA-R onset (ms) | 9 | 133±14 | 6 | 136±7 | 0.21 |
| TA-R duration (ms) | 9 | 274±19 | 6 | 259±33 | −0.79 |
| TA-R magnitude (nu) | 9 | 0.50±0.07 | 6 | 0.48±0.11 | −0.22 |
| MG-L onset (ms) | 4 | 152±14 | 2 | 153±22 | 0.07 |
| MG-L duration (ms) | 9 | 100±121 | 6 | 85±131 | −0.12 |
| MG-L magnitude (nu) | 9 | 0.14±0.16 | 6 | 0.06±0.16 | −0.52 |
| MG-R onset (ms) | 4 | 149±10 | 3 | 161±12 | 1.20 |
| MG-R duration (ms) | 9 | 58±91 | 9 | 34±71 | −0.26 |
| MG-R magnitude (nu) | 9 | 0.12±0.17 | 9 | 0.02±0.17 | −0.64 |
| Forward perturbation | |||||
| TA-L onset (ms) | 8 | 137±29 | 3 | 175±28 | 1.31 |
| TA-L duration (ms) | 9 | 203±118 | 6 | 96±122 | −0.91 |
| TA-L magnitude (nu)* | 9 | 0.11±0.07 | 6 | 0.09±0.09 | −0.30 |
| TA-R onset (ms) | 9 | 129±40 | 5 | 167±23 | 0.95 |
| TA-R duration (ms) | 9 | 206±108 | 6 | 154±128 | −0.48 |
| TA-R magnitude (nu) | 9 | 0.11±0.06 | 6 | 0.09±0.08 | −0.30 |
| MG-L onset (ms) | 9 | 141±36 | 6 | 141±27 | 0.00 |
| MG-L duration (ms) | 9 | 183±90 | 6 | 174±96 | −0.10 |
| MG-L magnitude (nu) | 9 | 0.34±0.09 | 6 | 0.32±0.16 | −0.23 |
| MG-R onset (ms) | 9 | 124±20 | 8 | 143±20 | 0.95 |
| MG-R duration (ms) | 9 | 236±89 | 9 | 216±101 | −0.22 |
| MG-R magnitude (nu)** | 9 | 0.39±0.18 | 9 | 0.34±0.09 | −0.28 |
Abbreviations: MG, medial gastrocnemius; TA, tibialis anterior; nu, normalized units.
P ≤ 0.05;
P ≤ 0.01, ANOVA.
Figure 3.
Examples of center of mass (CoM) displacement and muscle activity during automatic postural responses to forward (above) and backward (below) perturbations before and after the intervention. From left to right in each row, a cartoon describing perturbation direction, exemplar data of one participant at pretest and posttest, and group data across participants are shown. Shaded regions in exemplar data plots designate area under average EMG curves 80 ms – 450 ms after perturbation onset. Note scale is reversed for antagonist muscles, and absolute CoM displacement is shown as positive for both perturbation directions. Bars and error bars in group data designate M ± SD. *P < 0.05, **P < 0.01; ANOVA.
Secondary analyses using pooled EMG data
Descriptive statistics and effect sizes for pooled EMG measures are tabulated in Table 5. Secondary analyses of EMG data pooled across legs revealed significant delays in TA onset time (forward CoM perturbations, P=0.04; backward, P=0.03), TA duration (forward, P=0.02), and MG onset time (backward, P<0.01). Secondary analyses of EMG data pooled across legs and across perturbation directions revealed significant delays in antagonist onset time (27 ms; P=0.02), agonist onset time (10 ms, P<0.05), and a significant reduction in antagonist duration (56 ms, ≈39%, P=0.02).
Table 5.
Mean values (±SD) of secondary pooled electromyographic measures of perturbation responses before and after the 3-week, high volume Adapted Tango rehabilitative intervention.
| Pretest
|
Posttest
|
Effect Size | |||
|---|---|---|---|---|---|
| n | M±SD | n | M±SD | ||
| TA, pooled | |||||
| Backward perturbation | |||||
| TA onset* | 18 | 128±12 | 12 | 137±8 | 0.75 |
| TA duration | 18 | 259±48 | 12 | 245±58 | −0.29 |
| TA magnitude | 18 | 0.49±0.07 | 12 | 0.49±0.09 | 0.06 |
| Forward perturbation | |||||
| TA onset* | 17 | 132±34 | 8 | 170±24 | 1.12 |
| TA duration* | 18 | 205±109 | 12 | 125±123 | −0.73 |
| TA magnitude | 18 | 0.11±0.06 | 12 | 0.09±0.08 | −0.31 |
| MG, pooled | |||||
| Backward perturbation | |||||
| MG onset** | 8 | 151±11 | 5 | 158±14 | 0.64 |
| MG duration | 18 | 79±106 | 15 | 54±99 | −0.24 |
| MG magnitude | 18 | 0.13±0.16 | 15 | 0.03±0.16 | −0.62 |
| Forward perturbation | |||||
| MG onset | 18 | 132±30 | 14 | 142±22 | 0.33 |
| MG duration | 18 | 210±91 | 15 | 199±98 | −0.12 |
| MG magnitude | 18 | 0.36±0.14 | 15 | 0.33±0.11 | −0.23 |
| Agonist, pooled | |||||
| TA, backward; MG, forward | |||||
| Agonist onset* | 36 | 130±23 | 26 | 140±17 | 0.43 |
| Agonist duration | 36 | 234±76 | 27 | 220±84 | −0.18 |
| Agonist magnitude | 36 | 0.43±0.13 | 27 | 0.40±0.13 | −0.19 |
| Antagonist, pooled | |||||
| MG, backward; TA, forward | |||||
| Antagonist onset* | 25 | 138±30 | 13 | 165±21 | 0.90 |
| Antagonist duration* | 36 | 142±124 | 27 | 86±114 | −0.45 |
| Antagonist magnitude | 36 | 0.12±0.12 | 27 | 0.06±0.13 | −0.52 |
Abbreviations: MG, medial gastrocnemius; TA, tibialis anterior; nu, normalized units.
P ≤ 0.05;
P ≤ 0.01, ANOVA.
Associations between clinical changes and changes in postural perturbations
Significant correlations were identified between reductions in forward CoM displacement and increased BBS scores (rho =−0.68; P=0.04) and DGI (rho=−0.75; P=0.03). No significant correlations were identified between increased BBS scores and delayed antagonist onset times (rho=0.78; P=0.07), between reductions in forward CoM displacement and increased FAB scores (rho=−0.49; P=0.19), nor between changes in backward CoM displacement and improvements in BBS (rho=0.37; P=0.33), FAB (rho=0.52; P=0.15), or DGI (rho=0.21; P=0.62).
DISCUSSION
The low attrition observed here (2/22 participants who began the intervention) and improvements observed in these individuals with mild-moderate PD on clinical measures of balance, gait, and disease severity after 3-week, high volume Adapted Tango demonstrate the program volume is feasible and may have efficacy comparable to longer programs with similar total doses. This pilot study is the first to measure automatic postural responses before and after Adapted Tango. In convenience sample of the study participants, we observed reductions in forward CoM displacement and changes in some measures of EMG magnitude and timing after Adapted Tango. Based on this, we consider a subsequent randomized Adapted Tango trial with kinematic and electromyographic outcome measures to be feasible and justified.
Benefits and feasibility of high volume exercise in persons with PD
High volume exercise (> 180 minutes/week) is necessary to improve older adults’ gait speed.15 We observed overall adherence to the high volume intervention of 77%, with 95% confidence interval 61%–93%, achieving the stated primary feasibility criterion of 60% and demonstrating that 3-week, high volume Adapted Tango is feasible in this population. After the intervention, we observed improvements in measures of disease severity and balance (changes: UPDRS, 2.9; BBS, 3.8) comparable to a previous 2-week high volume Adapted Tango trial (UPDRS, 4.6; BBS 2.8)17 and to two previous longer duration trials (i.e., 20 hours over 13 weeks: UPDRS, 1.6; BBS, 3.9; 20 hours over 10 weeks: BBS, 3.6).7,10 Minimal clinically important differences (MCID) have not been established for many of the outcome measures used in this population. However, clinically significant changes were observed in UPDRS-III (2.9 points vs. MCID 2.560) and marginally-significant changes were observed in DGI (1.8 points vs. MCID 1.9 in community-dwelling older adults61). While these results ostensibly support the benefits of the program, it is important to be extremely cautious in any interpretation. The results of this pilot study will require replication with a more appropriately powered sample size. Although the participants exhibited a 3.4 point improvement on BBS, this change is below the MCID of 7 points established in older adults with balance impairments62 and also below the Minimal Detectable Change (MDC) of 5 points established in individuals with PD. This cohort was relatively higher functioning than the reference population for the MDCs determined for the BBS in PD (49.3 ± 6.4 vs. 42 ± 11.2); therefore, there may have been some ceiling effects on this measure. Improvements were observed on the more challenging but lesser used FAB, and small improvements were observed in the postural stability UPDRS-III item (0.35 points, comparable to the difference observed in this item between the OFF and ON medication states63). However, clearly a similar cohort would need to be recruited and examined in comparison to a control group to make definitive conclusions about the efficacy of this high volume but short term dose of Adapted Tango. Vigorous ongoing exercise that increases heart rate and oxygen uptake could be neuroprotective for individuals with PD;64 however, high volume/low intensity exercise therapy may be superior to low volume/high intensity exercise therapy for changes in gait speed.14 We noted sustained gains 1 month after the high volume Adapted Tango treatment ended, consistent with prior work demonstrating gains maintained over one month,7 and three months,12 after intervention cessation. The 3-week Adapted Tango protocol may be useful in crossover designs that can be completed in a short overall time frame, which is beneficial for academic research studies that rely on student volunteer personnel over the course of an academic semester.
Electromyographic and kinematic measures from support-surface perturbation as rehabilitation outcome measures
Based on the observed reductions in forward CoM displacement and changes in antagonist onset and duration, we consider a subsequent randomized trial of Adapted Tango with kinematic and electromyographic outcome measures to be feasible and justified. Given the limited sample size, the observed changes in muscle activity could be attributed to chance in many cases. However, average effect sizes observed in individual muscle analyses were moderate (average effect size 0.50), and generally comparable to those observed in UPDRS-III (0.47), BBS (0.59), FAB (0.56), and DGI (0.53). Particularly because effect sizes are less susceptible to the influence of small sample sizes than P-values, we interpret these results as evidence that electromyographic and kinematic measures would be feasible and potentially useful when applied in a larger sample in this type of intervention. Associations between changes in clinical measures observed after the intervention and changes in kinematic measures provided additional evidence that laboratory-assessed balance measures are feasible as objective rehabilitative outcomes for Adapted Tango.
Generalizability
The feasibility results obtained here appear generalizable to subsequent controlled trials without substantial modifications to the basic protocol. We anticipate that a subsequent randomized trial would test the hypothesis that CoM displacement would be reduced and automatic postural response onset latency would be delayed from pretest to posttest after Adapted Tango, compared to standard care. The following modifications could improve the precision of subsequent studies. A reduced number of clinical outcomes, all collected at the same visit as postural response testing, would improve the precision of correlational analyses and reduce the potential for fatigue effects. The postural stability UPDRS item has known limitations in discriminating fallers from non-fallers63 and lower inter-rater reliability than tests including the Push and Release test.43 Balance outcome measures should be evaluated carefully to improve external validity and to reduce participant burden. At posttest, we did not observe reductions in backward CoM displacement, despite reductions in forward CoM displacement, altered antagonist activity, and improved postural stability as measured by the UPDRS. This may reflect the increased difficulty and fewer biomechanical strategies available to recover balance when falling backward in individuals with PD, who are particularly unstable during backward sway.20,65 Overall, the number of trials delivered in which a foot, heel, or toe lift or arm flailing occurred was reduced from 31% at pretest to 23% at posttest, suggesting that perturbations in both directions were less challenging at posttest, possibly due to altered postural strategies that were not captured in our analyses of CoM displacement. A more complete kinematic and kinetic dataset including variables such as center of pressure and stability margin65 should be collected in order to better characterize postural strategies during perturbation responses and investigate this asymmetric response. As posterior perturbation responses and the UPDRS postural stability item are correlated in the practically-defined 12-hour OFF,65 but not in the ON,66 medication state, testing should be performed in the practically-defined 12-hour OFF state to improve the precision of correlational analyses66 and the discriminatory ability of clinical measures.63,67
Limitations
This pilot study has several limitations that should be addressed in subsequent controlled trials. Caution should be used in interpreting these results, given the reported small effect sizes of most measures, the potential for Type II error and the lack of a control group. Further, the small sample size left the study underpowered. Although we provide test-retest reliability findings that demonstrate stability of clinical mobility measures within these individuals with PD, the absence of a parallel control group for electromyographic and kinematic measures prevents us from attributing changes in these measures to the effects of the intervention. The study used a large number of outcomes, which increases the likelihood of chance findings. In particular, a plausible mechanism for Adapted Tango in improving speech (UPDRS-III item 1) is unknown. PD is most often associated with hypokinetic dysarthria attributed to decreased range of motion in the speech mechanism.68 We observed improved preferred and fast cadence after the intervention, as well as improved speech – these changes may reflect a common underlying mechanism. However, it is also possible that this unexpected finding is spurious, and should be interpreted with caution. The study also used a convenience sample of participants for postural response outcome measures. Although these participants did not differ in demographic measures from the other participants in the study, unknown selection biases limit the generalizability of these findings. Since the Adapted Tango classes represent a form of group exercise, in future studies it would be valuable to assess changes in measures of social participation.71
CONCLUSIONS
These results demonstrate that a 3-week, high volume Adapted Tango rehabilitative intervention is feasible for individuals with mild-moderate PD and that randomized Adapted Tango trials using laboratory-assessed measures of postural responses are feasible and justified.
Supplementary Material
Supplemental Digital Content 1. Video abstract.
Acknowledgments
Source of Funding: This work was supported in part by NIH R21 HD075612-01, NSF EFRI 1137229, Tango Under the Tent, Inc. and by the Emory Udall Center. JLM was supported by the Atlanta Clinical and Translational Science Institute KL2-Mentored Clinical and Translational Research Program (NIH RR025008, UL1TR000454 and KL2TR000455). MEH was supported by the Department of Veterans Affairs R&D service Career Development Awards E7108M and N0870W.
The authors thank H. Bartlett, J. Bingham, S. Chvatal, J. Jilk, K. Kramer, M. McCall, C. Pope, A. Ruedrich, S. Safavynia, H. Sohn, A. Adams, P. Dillard, E. Renz, A. Robinson, and A. Daftarian for assistance with experiments and data processing, K. Pirog Revill for assistance with recruitment, assessment, and study conduct, K. Lang for researching clinical instruments, and thank the Georgia Tech/Georgia State Center for Advanced Brain Imaging for providing space for the intervention.
Footnotes
Previous Publication: This work has been previously published in abstract form at the International Society for Posture and Gait Research.
Conflicts of Interest: The authors declare that they have no conflicts of interest.
References
- 1.Bloem BR, Grimbergen YAM, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson’s disease. J Neurol. 2001;248(11):950–958. doi: 10.1007/s004150170047. [DOI] [PubMed] [Google Scholar]
- 2.Melton LJ, 3rd, Leibson CL, Achenbach SJ, et al. Fracture risk after the diagnosis of Parkinson’s disease: Influence of concomitant dementia. Mov Disord. 2006 Sep;21(9):1361–1367. doi: 10.1002/mds.20946. [DOI] [PubMed] [Google Scholar]
- 3.Fisher BE, Wu AD, Salem GJ, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Archives of physical medicine and rehabilitation. 2008 Jul;89(7):1221–1229. doi: 10.1016/j.apmr.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smania N, Corato E, Tinazzi M, et al. Effect of balance training on postural instability in patients with idiopathic Parkinson’s disease. Neurorehabil Neural Repair. 2010 Nov-Dec;24(9):826–834. doi: 10.1177/1545968310376057. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Archives of physical medicine and rehabilitation. 2003;84(8):1109–1117. doi: 10.1016/s0003-9993(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 6.Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012 Feb 9;366(6):511–519. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackney ME, Earhart GM. Effects of dance on gait and balance in Parkinson’s disease: a comparison of partnered and nonpartnered dance movement. Neurorehabil Neural Repair. 2010 May;24(4):384–392. doi: 10.1177/1545968309353329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012 Feb;26(2):132–143. doi: 10.1177/1545968311421614. [DOI] [PubMed] [Google Scholar]
- 9.Hackney ME, Kantorovich S, Levin R, Earhart GM. Effects of tango on functional mobility in Parkinson’s disease: a preliminary study. J Neurol Phys Ther. 2007 Dec;31(4):173–179. doi: 10.1097/NPT.0b013e31815ce78b. [DOI] [PubMed] [Google Scholar]
- 10.Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson’s disease: a comparison of Argentine tango and American ballroom. J Rehabil Med. 2009 May;41(6):475–481. doi: 10.2340/16501977-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackney ME, Earhart GM. Health-related quality of life and alternative forms of exercise in Parkinson disease. Parkinsonism Relat Disord. 2009 Nov;15(9):644–648. doi: 10.1016/j.parkreldis.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKee KE, Hackney ME. The effects of adapted tango on spatial cognition and disease severity in Parkinson’s disease. J Mot Behav. 2013;45(6):519–529. doi: 10.1080/00222895.2013.834288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frazzitta G, Maestri R, Bertotti G, et al. Intensive rehabilitation treatment in early Parkinson’s disease: a randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair. 2015 Feb;29(2):123–131. doi: 10.1177/1545968314542981. [DOI] [PubMed] [Google Scholar]
- 14.Shulman LM, Katzel LI, Ivey FM, et al. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurology. 2013 Feb;70(2):183–190. doi: 10.1001/jamaneurol.2013.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopopolo RB, Greco M, Sullivan D, Craik RL, Mangione KK. Effect of therapeutic exercise on gait speed in community-dwelling elderly people: a meta-analysis. Physical Therapy. 2006 Apr;86(4):520–540. [PubMed] [Google Scholar]
- 16.Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015 Jun;175(6):959–967. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackney ME, Earhart GM. Short duration, intensive tango dancing for Parkinson disease: an uncontrolled pilot study. Complement Ther Med. 2009 Aug;17(4):203–207. doi: 10.1016/j.ctim.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimitrova D, Horak FB, Nutt JG. Postural Muscle Responses to Multidirectional Translations in Patients With Parkinson’s Disease. J Neurophysiol. 2004 Jan 1 2004;91(1):489–501. doi: 10.1152/jn.00094.2003. [DOI] [PubMed] [Google Scholar]
- 19.Welch TDJ, Ting LH. A Feedback Model Explains the Differential Scaling of Human Postural Responses to Perturbation Acceleration and Velocity. J Neurophysiol. 2009;101(6):3294–3309. doi: 10.1152/jn.90775.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter MG, Allum JH, Honegger F, Adkin AL, Bloem BR. Postural abnormalities to multidirectional stance perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004 Sep;75(9):1245–1254. doi: 10.1136/jnnp.2003.021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansfield A, Peters AL, Liu BA, Maki BE. Effect of a perturbation-based balance training program on compensatory stepping and grasping reactions in older adults: a randomized controlled trial. Physical Therapy. 2010;90(4):476–491. doi: 10.2522/ptj.20090070. [DOI] [PubMed] [Google Scholar]
- 22.Gatts SK, Woollacott M. Neural mechanisms underlying balance improvement with short term Tai Chi training. Aging-Clinical and Experimental Research. 2006;18(1):7–19. doi: 10.1007/BF03324635. [DOI] [PubMed] [Google Scholar]
- 23.Marigold DS, Eng JJ, Dawson AS, Inglis JT, Harris JE, Gylfadottir S. Exercise leads to faster postural reflexes, improved balance and mobility, and fewer falls in older persons with chronic stroke. J Am Geriatr Soc. 2005 Mar;53(3):416–423. doi: 10.1111/j.1532-5415.2005.53158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Routson RL, Clark DJ, Bowden MG, Kautz SA, Neptune RR. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait and Posture. 2013 Jul;38(3):511–517. doi: 10.1016/j.gaitpost.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ting Lena H, Chiel Hillel J, Trumbower Randy D, et al. Neuromechanical Principles Underlying Movement Modularity and Their Implications for Rehabilitation. Neuron. 2015;86(1):38–54. doi: 10.1016/j.neuron.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimitrova D, Nutt J, Horak FB. Abnormal force patterns for multidirectional postural responses in patients with Parkinson’s disease. Experimental Brain Research. 2004 May;156(2):183–195. doi: 10.1007/s00221-003-1770-4. [DOI] [PubMed] [Google Scholar]
- 27.Nonnekes J, Scotti A, Oude Nijhuis LB, et al. Are postural responses to backward and forward perturbations processed by different neural circuits? Neuroscience. 2013 Aug 15;245:109–120. doi: 10.1016/j.neuroscience.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 28.St George RJ, Carlson-Kuhta P, Burchiel KJ, Hogarth P, Frank N, Horak FB. The effects of subthalamic and pallidal deep brain stimulation on postural responses in patients with Parkinson disease. J Neurosurg. 2012 Jun;116(6):1347–1356. doi: 10.3171/2012.2.JNS11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson’s disease. Am J Med Genet. 1999 Oct 15;88(5):539–543. [PubMed] [Google Scholar]
- 30.Hackney ME, Earhart GM. Recommendations for implementing partnered dance classes for persons with Parkinson Disease. Am J Dance Ther. 2010;31(1):41–45. doi: 10.1007/s10465-010-9086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heyward VH. Advanced Fitness Assessment and Exercise Prescription. 6. Human Kinetics; 2010. [Google Scholar]
- 32.Fahn S, Elton RL. Members of the UPDRS Development Committee. The Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Vol. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- 33.Berg K, Wood-Dauphinee S, Williams J. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995;27(1):27–36. [PubMed] [Google Scholar]
- 34.Shumway-Cook A, Woollacott MH. Motor Control: Theory and Practical Applications. Baltimore, Md: Williams & Wilkins; 1995. [Google Scholar]
- 35.Klein PJ, Fiedler RC, Rose DJ. Rasch Analysis of the Fullerton Advanced Balance (FAB) Scale. Physiother Can. 2011 Winter;63(1):115–125. doi: 10.3138/ptc.2009-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlenstedt C, Brombacher S, Hartwigsen G, Weisser B, Moller B, Deuschl G. Comparing the Fullerton Advanced Balance scale with the Mini-BESTest and Berg Balance Scale to assess postural control in patients with Parkinson’s Disease. Archives of physical medicine and rehabilitation. 2014 Sep 24; doi: 10.1016/j.apmr.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Rose DJ, Lucchese N, Wiersma LD. Development of a multidimensional balance scale for use with functionally independent older adults. Archives of physical medicine and rehabilitation. 2006 Nov;87(11):1478–1485. doi: 10.1016/j.apmr.2006.07.263. [DOI] [PubMed] [Google Scholar]
- 38.Enright PL. The six-minute walk test. Respir Care. 2003 Aug;48(8):783–785. [PubMed] [Google Scholar]
- 39.Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol. 1990 Nov;45(6):M192–197. doi: 10.1093/geronj/45.6.m192. [DOI] [PubMed] [Google Scholar]
- 40.Hackney ME, Byers C, Butler G, Sweeney M, Rossbach L, Bozzorg A. Adapted Tango Improves Mobility, Motor-Cognitive Function, and Gait but Not Cognition in Older Adults in Independent Living. J Am Geriatr Soc. 2015 Oct 12;63(10):2105–2113. doi: 10.1111/jgs.13650. [DOI] [PubMed] [Google Scholar]
- 41.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995 Jan;50A(1):M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 42.Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Related Disord. 2000;6(3):165–170. doi: 10.1016/s1353-8020(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs JV, Horak FB, Van Tran K, Nutt JG. An alternative clinical postural stability test for patients with Parkinson’s disease. J Neurol. 2006 Nov;253(11):1404–1413. doi: 10.1007/s00415-006-0224-x. [DOI] [PubMed] [Google Scholar]
- 44.St George RJ, Nutt JG, Burchiel KJ, Horak FB. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology. 2010 Oct 5;75(14):1292–1299. doi: 10.1212/WNL.0b013e3181f61329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Safavynia SA, Ting LH. Sensorimotor feedback based on task-relevant error robustly predicts temporal recruitment and multidirectional tuning of muscle synergies. J Neurophysiol. 2013 Jan;109(1):31–45. doi: 10.1152/jn.00684.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welch TDJ, Ting LH. A Feedback Model Reproduces Muscle Activity During Human Postural Responses to Support-Surface Translations. Journal of Neurophysiology. 2008;99(2):1032–1038. doi: 10.1152/jn.01110.2007. [DOI] [PubMed] [Google Scholar]
- 47.Lockhart DB, Ting LH. Optimal sensorimotor transformations for balance. Nat Neurosci. 2007;10(10):1329–1336. doi: 10.1038/nn1986. [DOI] [PubMed] [Google Scholar]
- 48.Safavynia SA, Ting LH. Long-latency muscle activity reflects continuous, delayed sensorimotor feedback of task-level and not joint-level error. Journal of Neurophysiology. 2013 Sep;110(6):1278–1290. doi: 10.1152/jn.00609.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKay JL, Ting LH. Optimization of muscle activity for task-level goals predicts complex changes in limb forces across biomechanical contexts. PLoS Comput Biol. 2012;8(4):e1002465. doi: 10.1371/journal.pcbi.1002465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macpherson JM. Changes in a postural strategy with inter-paw distance. Journal of Neurophysiology. 1994 Mar;71(3):931–940. doi: 10.1152/jn.1994.71.3.931. [DOI] [PubMed] [Google Scholar]
- 51.Ting LH, Macpherson JM. Ratio of shear to load ground-reaction force may underlie the directional tuning of the automatic postural response to rotation and translation. Journal of Neurophysiology. 2004 Aug;92(2):808–823. doi: 10.1152/jn.00773.2003. [DOI] [PubMed] [Google Scholar]
- 52.Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. Journal of Neurophysiology. 2006 Sep;96(3):1530–1546. doi: 10.1152/jn.00810.2005. [DOI] [PubMed] [Google Scholar]
- 53.Welch TD, Ting LH. Mechanisms of motor adaptation in reactive balance control. PLoS One. 2014;9(5):e96440. doi: 10.1371/journal.pone.0096440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McIlroy WE, Maki BE. The ‘deceleration response’ to transient perturbation of upright stance. Neurosci Lett. 1994 Jul 4;175(1–2):13–16. doi: 10.1016/0304-3940(94)91066-9. [DOI] [PubMed] [Google Scholar]
- 55.Basmajian J, Blumenstein R. Electrode placement in EMG biofeedback. Baltimore, MD: Williams & Wilkins; 1980. [Google Scholar]
- 56.Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci. 1992 Aug;111(1):46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 57.Kelly VE, Israel SM, Samii A, Slimp JC, Goodkin R, Shumway-Cook A. Assessing the effects of subthalamic nucleus stimulation on gait and mobility in people with Parkinson disease. Disabil Rehabil. 2010;32(11):929–936. doi: 10.3109/09638280903374139. [DOI] [PubMed] [Google Scholar]
- 58.Cohen J. A power primer. Psychol Bull. 1992 Jul;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 59.Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord. 2013 May;28(5):668–670. doi: 10.1002/mds.25383. [DOI] [PubMed] [Google Scholar]
- 60.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson’s disease rating scale. Arch Neurol. 2010 Jan;67(1):64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 61.Pardasaney PK, Latham NK, Jette AM, et al. Sensitivity to change and responsiveness of four balance measures for community-dwelling older adults. Phys Ther. 2012 Mar;92(3):388–397. doi: 10.2522/ptj.20100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Godi M, Franchignoni F, Caligari M, Giordano A, Turcato AM, Nardone A. Comparison of reliability, validity, and responsiveness of the mini-BESTest and Berg Balance Scale in patients with balance disorders. Phys Ther. 2013 Feb;93(2):158–167. doi: 10.2522/ptj.20120171. [DOI] [PubMed] [Google Scholar]
- 63.Foreman KB, Addison O, Kim HS, Dibble LE. Testing balance and fall risk in persons with Parkinson disease, an argument for ecologically valid testing. Parkinsonism Relat Disord. 2011 Mar;17(3):166–171. doi: 10.1016/j.parkreldis.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011 Jul 19;77(3):288–294. doi: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson’s disease. Exp Neurol. 2005 Jun;193(2):504–521. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 66.Bloem BR, Beckley DJ, van Hilten BJ, Roos RA. Clinimetrics of postural instability in Parkinson’s disease. J Neurol. 1998 Oct;245(10):669–673. doi: 10.1007/s004150050265. [DOI] [PubMed] [Google Scholar]
- 67.Prodoehl J, Rafferty MR, David FJ, et al. Two-year exercise program improves physical function in Parkinson’s disease: the PRET-PD randomized clinical trial. Neurorehabil Neural Repair. 2015 Feb;29(2):112–122. doi: 10.1177/1545968314539732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tjaden K. Speech and Swallowing in Parkinson’s Disease. Top Geriatr Rehabil. 2008;24(2):115–126. doi: 10.1097/01.TGR.0000318899.87690.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008 Jun;88(6):733–746. doi: 10.2522/ptj.20070214. [DOI] [PubMed] [Google Scholar]
- 70.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 71.Dibble LE, Foreman KB, Addison O, Marcus RL, LaStayo PC. Exercise and medication effects on persons with Parkinson disease across the domains of disability: a randomized clinical trial. J Neurol Phys Ther. 2015 Apr;39(2):85–92. doi: 10.1097/NPT.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Video abstract.