Abstract
The early discovery of the human Cu+-ATPases and their link to Menkes and Wilson's diseases brought attention to the unique role of these transporters in copper homeostasis. The characterization of bacterial Cu+-ATPases has significantly furthered our understanding on the structure, selectivity and transport mechanism of these enzymes, as well as their interplay with other elements of Cu+ distribution networks. This review focuses on the structural-functional insights that have emerged from studies of bacterial Cu+-ATPase at the molecular level and how these observations have contributed to draw up a comprehensive picture of cellular copper homeostasis.
Distribution of Cu+-ATPases in biological systems and their relevance for copper homeostasis
Rhizobium meliloti fixI/copA2, identified as a P-type ATPase associated with N2 fixation, was probably the first gene encoding for a Cu+-ATPase mentioned in the literature1. It was later established that FixI/CopA2 translocates cytoplasmic Cu+ for metallation of the cbb3-type cytochrome complex1–4. The early observation of the role of Enterococcus hirae CopA and CopB in copper tolerance linked the large family of P-type ATPases with the transmembrane copper transport5, 6. The contemporary discovery of the human Cu+-ATPases associated with Menkes and Wilson's diseases, ATP7A and ATP7B respectively, generated significant interest and further drove the study of this subfamily of proteins7–9.
From a functional-phylogenetic standpoint, Cu+-ATPases are classified within the P1B subfamily of P-type ATPases. Members of this subfamily transport various heavy metals including Cu+, Cu2+, Zn2+, Mn2+, Co2+ and Fe2+10–14. Cu+-ATPases are widely distributed in all kingdoms of life12, 15, 16. Their key role in copper tolerance is well established in bacteria and archea17–19. They contribute to maintain the cellular Cu quota (i.e. total Cu in the cell) by driving the cytoplasmic Cu+ efflux. Several Cu+-sensing transcriptional regulators (CueR, CopY, CsoR) control Cu+-ATPase levels17–19. In recent years, the involvement of bacterial Cu+-ATPases in the metallation of secreted, periplasmic, and plasma membrane cuproproteins has been uncovered20–24. Again, this is directly linked to the molecular function of all Cu+-ATPases, that is, the energy dependent efflux of cytoplasmic Cu+.
These essential biological functions; i.e., Cu+ tolerance and enzyme metallation, are also common to the eukaryote Cu+-ATPases. For instance, studies of Saccharomyces cerevisiae CCC2 provided the initial evidence for the ATP-mediated Cu+ transport into the secretory pathway for the metallation of iron oxidase FET3 that is required for iron uptake25, 26. The genomes of yeasts, insects and nematodes encode a single Cu+-ATPase; however, gene duplication occurred in chordates (birds, fish and mammals)27. Interestingly, the genomes of photosynthetic organisms have an array of genes coding for Cu+-ATPases28, 29. The model plant Arabidopsis thaliana, for example, contains four Cu+-ATPase isoforms (AtHMA5–AtHMA8). AtHMA5 is involved in copper compartmentalization and detoxification within root cells30. Localized in the post-Golgi network, AtHMA7 is responsible for metallation of ethylene receptors31. AtHMA6 transport Cu+ across the chloroplast inner envelope membrane into the stroma and is required for Cu+ loading into Cu/Zn-SOD. AtHMA6 also works in tandem with AtHMA8, located in the thylakoid membrane, providing Cu+ to plastocyanin32, 33.
The human genome encodes only two Cu+-ATPases, ATP7A and ATP7B, and mutations in the corresponding genes lead to Menkes and Wilson's diseases, respectively7–9. ATP7A and ATP7B display tissue and cell type-specific expression patterns generating distinct physiological functions. ATP7A is present in most extrahepatic tissues, whereas ATP7B is preferentially found in liver34–36. Cu+ efflux by ATP7A located in the basolateral membrane of the intestinal epithelia is responsible for copper absorption in humans34. Consequently, dysfunction of ATP7A leads to a severe systemic copper deficiency associated with Menkes disease, occipital horn syndrome and ATP7A-related distal motor neuropathy34, 35. In particular, Menkes disease is characterized by severe developmental abnormalities, dramatic neurologic impairment, and death during early childhood37, 38. ATP7B-deficiency results in Wilson disease. This is associated with copper overload in the liver and brain, manifested as progressive neurological and hepatic abnormalities as well as neuropsychiatric disorders35, 39–41. ATP7A and ATP7B are located in the trans-Golgi network and transport Cu+ into the secretory pathway for incorporation into cuproenzymes such as dopamine-β-hydroxylase, tyrosinase, lysyl oxidase, peptidylglycine-α-amidating monooxygenase and extracellular SOD3 (ATP7A) or ceruloplasmin (ATP7B)34, 42, 43. Importantly, in response to changes in the intracellular copper quota, ATP7A and ATP7B traffics to the plasma membrane to extrude ion excess42, 44. Upon restoring physiological levels, these enzymes are relocated to the trans-Golgi network45,46. Cu+-ATPases might also play an important role at the host-pathogen interface. It has been proposed that macrophages recruit ATP7A to phagosomes containing encapsulated bacteria inducing toxic Cu+ stress as part of the immune response47. The importance of bacterial Cu+-ATPases in virulence, further supports this attractive hypothesis48.
The molecular structure responsible for copper transmembrane translocation
The hallmark of P-type ATPases is the catalytic mechanism driven by phosphorylation of the Asp residue in the invariant DKTGT sequence. Cu+-ATPases, as all P-type ATPases, have three large cytoplasmic domains (Fig. 1)49–53. The nucleotide (N) domain binds and positions ATP for phosphorylation of the DKTGT region located in the phosphorylation (P) domain. This drives the movement of the actuator (A) domain, leading to the rearrangement of transmembrane segments (TMs) necessary for substrate translocation across the permeability barrier54, 55. Most P-type enzymes have 6–12 TMs with the substrate-binding TMs flanking the P and N domains12, 54, 56. Cu+-ATPases have eight TMs (Fig. 1). Metal binding assays performed in Archaeoglobus fulgidus CopA57, Escherichia coli CopA23 and A. thaliana HMA6 and HMA822 have shown the presence of two metal binding sites in the transmembrane region of Cu+-ATPases. Transmembrane fragments 6th, 7th and 8th contain conserved residues for the two transmembrane metal binding sites (TM-MBS) that coordinate Cu+ during transport (Fig. 1)12, 57, 58. Site I is constituted by two Cys in TM6 and a Tyr in TM7. An Asn in TM7 and Met and Ser in TM8 form Site II. Except for the Asn amino group, the absence of side chains capable of acid/base protonation/deprotonation is conspicuous in these sites. On the other hand, coordination by intermediate (N) or soft (S) Lewis base ligands is congruent with Cu+ soft Lewis acid attributes59. Interestingly, a single transmembrane Cu+ binding site has been observed in Legionella pneumophila CopA60. While this enzyme has the six invariant residues in the 6th, 7th and 8th transmembrane segments, only the two Cys in the 4th and the Met in the 8th appear to coordinate the ion.
Figure 1. Molecular organization of Cu+ transport across bacterial plasma membrane.
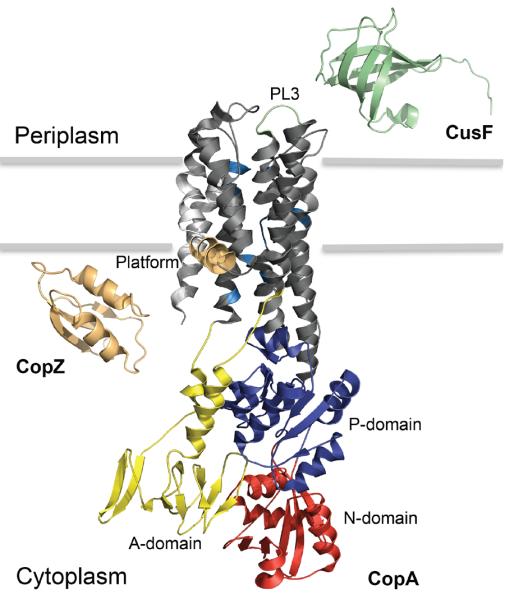
Crystal structures and subcellular localization of Enterococcus hirae CopZ (PDB entry 1CPZ; light orange), Legionella pneumophila CopA (PDB entry 3RFU) and Escherichia coli CusF (PDB entry 1ZEQ; light green) are presented. Specific domains and features within the CopA structure are described. The actuator (yellow), nucleotide binding (red) and phosphorylation (dark blue) domains are shown. The transmembrane segments (TM1-TM2, light grey) leading to the platform helix (orange; involved in CopZ docking) and the periplasmic loop 3 (light green; involved in CusF docking) are emphasized. Within the TM3-TM8 membrane region (dark grey), residues implicated in Cu+ entry site (M148/E205/D337), high-affinity binding sites: site-1 (C382/C384/Y688) and site-2 (N689/M717/S721) and exit site (E189) are highlighted in sky blue.
X-ray spectroscopic analysis showed the trigonal planar coordination of Cu+ at both TM-MBS57. Cu+ is relatively unique among transition metals in that it capable of adopting 2, 3 and 4-coordinate complexes61. Nevertheless, it could be considered that despite binding Cu+ with very high affinity, TM-MBSs only transiently interact with the metal as in the presence of ATP they are transported across the membrane. Moreover, the vectorial ion release (i.e. across the permeability barrier) would occur upon minimal coordination shifts. Then, observation of novel ligands (various Lewis bases) and the trigonal coordination architecture in these proteins might be a functional requirement. Similar trigonal coordination is apparent in the Ctr and Cus Cu+ transport systems59, 62, 63.
Description of high-resolution structures of L. pneumophila CopA had a significant impact in our understanding of Cu+ transport52, 53 (Fig. 1). These confirmed the architecture of the A, N, and P domains and gave a detailed first view of the transmembrane region when the enzyme is an E2 conformation (Fig. 2). Although the obtained structures do not reveal the metal bound architecture, they show the proximity of residues proposed to form the two TM-MBSs. Importantly, the structures revealed a platform formed by the bending of the 2nd TM and the proximity of invariant residues forming a putative entry site into the permeation path (Fig.1). Cu+ ions access the ATPase bound to cytoplasmic Cu+-chaperones (see below). Then, it was proposed that the platform might serve as a landing pad for the chaperone-ATPase interaction, as the invariant residues might be involved in ligand exchange and Cu+ transfer to the TM-MBSs52. Subsequent studies yielded experimental evidence consistent with this hypothesis64. On the other hand, Cu+ extrusion through a specific permeation path with participation of invariant Met and Glu amino acids has been proposed53. Different studies have also shown that similar to the cytoplasmic platform, periplasmic (luminal) loops provide a docking site for metal accepting apo-chaperones or alternative cuproproteins (Fig. 1)23, 65.
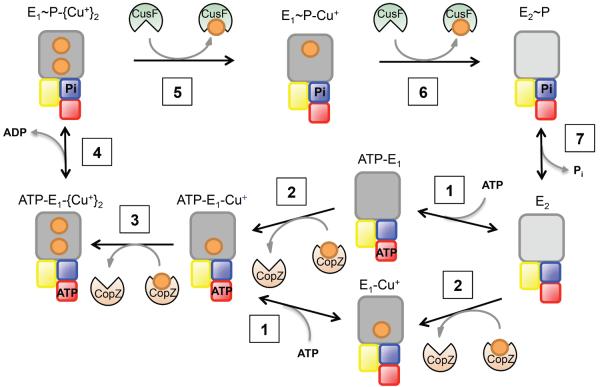
Figure 2. The catalytic mechanism of Cu+-ATPases.
The scheme emphasizes the two enzyme conformations (E1 dark grey) and (E2 light), The Cu+ transfer from cytoplasmic chaperones (CopZ, orange), binding of ATP and the subsequent enzyme phosphorylation. This is followed by Cu+ release to the periplasmic chaperone (CusF, green) and the return of the enzyme to the E2 form. The scheme includes the Cu+-ATPase transmembrane region (grey), P (blue), N (red) and A (yellow) domains, Cu+ ions (orange), CopZ (orange) and CusF (green).
Cu+-ATPases also contain Cu+ sensing regulatory domains (N-MBDs) in their cytoplasmic N-terminus. The presence of N- or C-terminal regulatory domains is a common feature of various P-type ATPases66–68. Bacterial Cu+-ATPases contain one or two N-MBD while eukaryote enzymes contain up to six69. These 70 amino acids long domains present a classic βαββαβ ferredoxin-like folding with an invariant GXXCXXC Cu+-binding motif69–71. Less common N-MBDs with cuprodoxin folding have been recently identified72. Mechanistic studies have uncovered the regulatory role of N-MBDs in bacteria73. These are not required for enzyme activity or transport; however, Cu+ binding to N-MBDs increases enzyme velocity. The interaction of N-MBD and N domains in the absence of Cu+ has been observed51, 74, 75. Then, if this interaction slows the nucleotide hydrolysis, it provides a structural mechanism by which N-MBD can affect the enzyme turnover rate. Studies in eukaryote systems have shown the importance of these domains in the trafficking and cellular roles of ATP7A and ATP7B34, 76.
The transport mechanism of Cu+-ATPases
Cu+-ATPases drive the efflux of cytoplasmic Cu+ using the energy provided by ATP hydrolysis. They perform this task following the classical Albers-Post cycle described for P-ATPases (Fig. 2)77. During transport, the enzyme adopts two basic conformations (E1↔E2). In the case of Cu+-ATPases, these conformational changes were verified using partial proteolytic digestions while placing the enzyme in alternating conformations78, 79. The cornerstone of the catalysis is however the enzyme phosphorylation upon binding of ATP and the outward transported cytoplasmic substrate (Fig. 2, steps 1–4). This is, metal binding sites face the cytoplasm when ATP occupies the N domain. As a consequence of this structural/mechanistic constrain, all Cu+ ATPases drive the efflux of cytoplasmic Cu+. In vivo studies have repeatedly shown this phenomenon17–19. In addition, detailed transport studies using membrane vesicles and taking advantage of the sidedness of ATP hydrolysis have experimentally shown a consistent outward direction of transport21, 80. Alternatively, the necessary coupling of ATP hydrolysis and ion transport has enabled the study of the catalytic cycle by measuring ATPase activity. Cu+-ATPases transport Cu+ at a relatively slow rate (200 min−1) when compared to the faster proton or alkali transporting P-type ATPases (10,000 min−1). This appears to be a general characteristic of transition metal transporters59.
In vitro studies have shown that Cu+-ATPases transport and are activated by free Cu+ in solution81. These enzymes are also activated by non-cognate ions such as Ag+ and Au+ 81, 82. These ions can apparently fit into the TM-MBSs and enable the required catalytic conformational changes. There is no evidence of the relative affinities of Cu+ ATPases for Ag+ or Au+. However, kinetics characterization suggests that Ag+ drives a higher enzyme turnover with a larger K1/281. It can be then hypothesized that the non-cognate ions have a poorer interaction (lower binding affinity) with a faster efflux associated with poor metal coordination. Since the metal release appears as the rate-limiting step in the transport cycle81, a higher Vmax for non-cognate ions could be expected. CopA binds two Cu+ at the TM-MBSs with affinities in the 0.1-1 fM range57. Access of Cu+ to these sites is not sequential but rather independent to either site57. The high affinity of these sites prevents the backward release of Cu+ from inward facing sites, since this would lead to free Cu+ in the cytoplasm with the ensuing toxic effects. Cu+ occupancy of both sites is required to trigger enzyme phosphorylation by ATP (Fig. 2, step 4)57, 58. Protein phosphorylation is followed by E1P→E2P conformational change concomitant with the opening of TM-MBS to the periplasm (gram− bacteria) or extracellular milieu (Fig. 2, steps 5 & 6). In this conformation the TM-MBS have an undetermined but lower affinity for Cu+ that is released from the TM-MBS to bind a soluble target protein23.
Chaperone mediated ion uptake and release of Cu+-ATPases
As aforementioned, Cu+ ATPases can operate obtaining Cu+ from the assay solution and releasing the ion into a vesicular compartment21, 80, 83. The high affinity of metal sensing transcriptional regulators for Cu+ is congruent with the virtual absence of free Cu+ in cells84, 85. Consequently, in vivo Cu+ gains access and leaves the ion-transporting ATPases bound to soluble chaperones23, 86. Thus, metallochaperones are a major component of Cu+ distribution networks, enabling accurate metal targeting to transmembrane transporters and metalloproteins. The cytoplasmic Cu+ chaperone CopZ shares high sequence-structure homology with the N-MBD of Cu+-ATPases69, 71 (Fig. 1). As N-MBDs, the two Cys in the CXXC sequence linearly coordinate one Cu+ ion. Nevertheless, it has been hypothesized that in vivo the participation of glutathione leading to a trigonal Cu+ coordination might prevent the chaperone homodimer formation observed in vitro69, 87. The cytosolic concentration of CopZ appears in the 0.1 - 1 μM range with pM KD for Cu+ 88, 89. Extensive studies have demonstrated that CopZ interact with the regulatory N-MBD of Cu+-ATPases, exchanging Cu+ with Keq≈169–71, 90. This equilibrium of the Cu+-N-MBD pool with Cu+-CopZ is in line with the regulation of ATPase activity by N-MBDs upon “sensing” the Cu+-CopZ pool. The N-MBD/CopZ recognition appears mediated by electrostatic interactions69, 71. Upon specific docking, Cu+ is transferred via ligand exchange. This Cu+ exchange between CopZ and N-MBDs is independent of the delivery of Cu+ to the ATPase permeation path for transmembrane transport64, 86 (see below).
CopZ loads the Cu+ substrate into TM-MBS of Cu+-ATPases48, 64, 75, 86 (Fig. 2, steps 2 & 3). Experiments using truncated A. fulgidus CopA lacking the cytoplasmic MBD, support the idea that the chaperone mediated Cu+ loading of TM-MBS is independent of the presence of Cu+ transfer to the N-MBD86. However, the sequence of events in the full length ATPase, this is, what site is loaded first N-NMD or TM-MBS, has not been established. Similar experiments performed with archaeal and eukaryote proteins have shown that MBD could not transfer Cu+ to TM-MBS21, 63. Homology modeling to available chaperone structures and mutagenesis experiment suggest that the docking of the negatively charged surface of CopZ with the electropositive platform region of CopA appears to direct the chaperone Cu+-binding residues toward the ion permeation path52, 64 (Fig. 3). The electrostatic interaction seems quite specific, as no activation by homologous N-MBD/chaperone domains has been observed22, 52, 64, 86. Mutagenesis experiments have also shown the requirement for the Cu+ transfer of three invariant Met, Glu and Asp residues located close to the platform52, 64. It has been proposed that these form a ligand exchange, “Cu+ entrance site”, which bridges the Cu+ access to the TM-MBSs. This model for Cu+ delivery by the chaperone and access the permeation path in the ATPase is however distinct in the case of Streptococcus pneumoniae72, 91. In this organism, the membrane associated chaperone CupA, as the N-MBDs in the ATPase CopA, have singular cupredoxin-like folds. While the specific protein-protein interaction is still required for conferring Cu+ tolerance, mutations of the platform structure or a His residue in the possible “Cu+ entrance site” of the ATPase do not impair the copper tolerance. This is the chaperone docking and the Cu+ transfer still occurs. These observations suggest an alternative Cu+ delivery model for the chaperone/ATPase system of S. pneumoniae.
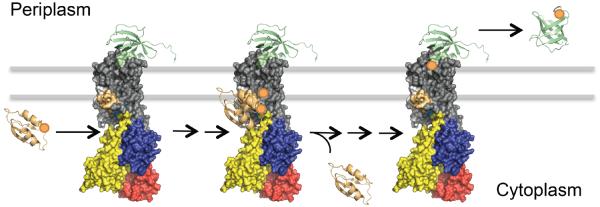
Figure 3. Chaperone mediated Cu+ uptake and release of Cu+-ATPase.
The model represents the docking of cytoplasmic Cu+-CopZ and periplasmic apo-CusF with CopA and the release of apo-CopZ and Cu+-CusF during the transmembrane translocation of Cu+. The scheme was built using the available 3D structures of Enterococcus hirae CopZ (PDB entry 1CPZ; light orange), Legionella pneumophila CopA (PDB entry 3RFU; colour pattern explained in Figure 1), Escherichia coli apo-CusF (PDB entry 1ZEQ; light green, bound to CopA) and Escherichia coli holo-CusF (PDB entry 2VB2; light green, unbound) and reported docking calculations23, 48.
The proposed mechanism for the delivery of Cu+ by CopZ would imply that Cu+ access the transport path dehydrated. Interestingly, in vitro, the Cu+-CopZ drives a higher Vmax than free Cu+ 86. A possible explanation is that the ligand exchange site can strip off Cu+ from the chaperone faster than from the hydration shell surrounding a free Cu+. An alternative hypothesis is that the presence of the chaperone rebinding the released ion prevents the backward binding of Cu+ to outward facing binding sites. The inhibition of transport at high Cu+ concentration is well documented92. Cu+ transfer from Cu+-CopZ to CopA appears essentially stoichiometric75. This could be explained by a model where CopA has high affinity for Cu+-CopZ such as total transfer will occur even when the ratio of Cu+-CopZ to CopA is low. The underlying implication is the preponderance of apo-CopZ under physiological conditions. Alternatively, CopA appears to have very low affinity for apo-CopZ. This is a logic mechanistic requirement as CopZ carry a single Cu+ and the enzyme requires the binding of two Cu+ to undergo catalysis and transport.
A similar Cu+ transfer is observed at the exit site of Cu+-ATPase. Using E. coli CopA and CusF, direct Cu+ transfer from the ATPase to the periplasmic chaperone was observed23 (Fig. 3). CusF is the periplasmic soluble chaperone (Fig. 1), part of CusCBA system responsible for periplasmic Cu+ efflux to the extracellular milieu. CusF binds Cu+ via conserved His36, Trp44, Met47, and Met49 with binding affinities in the nM/pM range93, 94. In silico analysis and biochemical assays have shown the specific interaction of the Cu+-bound form of CopA with apo-CusF for subsequent metal transfer upon ATP hydrolysis (Fig. 2, step 5 & 6). That is, the Cu+ bound CopA has a higher affinity for the apo-CusF than for the holo-CusF (Fig. 3). It is apparent that the conserved electropositive surface of CusF interact with the 3rd extracellular loop of the Cu+-ATPase (Fig. 1), as replacement of polar amino acids in these surfaces leads to a decrease in Cu+ transfer23, 52, 53. The presence of the chaperone does not seem to affect the transport stoichiometry because both Cu+ leave the ATPase to load two CusF equivalents, provided that the chaperone is in excess23.
Cu+ transfer to and from chaperones is an integral part of the ATPase catalytic cycle. The conformations that the enzyme adopts during the catalytic cycle interact differentially with alternative chaperones. The Cu+-CopZ interacts with the enzyme in the E1 conformation (cytoplasmic facing TM-MBSs) (Fig. 2, steps 1 & 3)75. Upon binding of two Cu+, the ATPase is phosphorylated and transitions to the E1·P state, where Cu+ ions are in an occluded state (Fig. 2, step 4). Subsequently, the enzyme moves into the E2 form, where Cu+ is released (Fig. 2, step 5 and 6). Accordingly, the metal bound E1 and E1P forms of CopA interact with apo-CusF23. In line with these observations, there is no evident interaction of apo-CopZ or Cu+-CusF with CopA. A gradient in the relative affinities of chaperones and transporters has been considered as a mechanism to direct the outward flux of cytoplasmic Cu+. A central implication of the structural ligand exchange between chaperone and Cu+-ATPase, the high affinity of CopZ-Cu+/CopA or CopA-Cu+/CusF and the stoichiometrical transfer is that the affinities of each protein for Cu+ is not likely relevant for the transfer. Protein affinities for Cu+ describe the preference of Cu+ to be in water vs being bound and are likely determined by koff rather than diffusion limited kon. On the other hand, in vivo Cu+ transfer occurs in an apparently dehydrate environment and it is dependent on the preference of Cu+ for the alternating ligands. Extrapolation of the energetics provided by KD to the ion partition between two different non-polar environments (compared to water) might not be straightforward.
Functional diversity of homologous Cu+-ATPases
Bacterial genomes, with very few exceptions, encode at least one Cu+-ATPase that maintains the cytoplasmic metal quota12, 19. Furthermore, the presence of multiple Cu+-ATPase genes in bacterial genomes has long been recognized12, 48. Why do simple bacterial systems require multiple Cu+-ATPases? The described specificity in the chaperone-transporter-chaperone relay provides a sound hypothesis where alternative ATPases function delivering Cu+ to different targets. For instance, consider periplasmic chaperones that deliver Cu+ either to efflux systems or target cuproenzymes. It seems then reasonable that distinct proteins might interact with specific partners. Moreover, while the “Cu+-efflux network” might be under Cu+ sensing regulators, alternative Cu+ distribution networks might be under different control.
In support of these ideas, the metallation of membrane and periplasmic cuproproteins has been linked to the ATPase mediated Cu+ transport23, 24, 95. For instance, Pseudomonas aeruginosa contains two homologous Cu+-ATPases. CopA1 mediates copper detoxification and CopA2 is involved in cytochrome c oxidase metallation80. A second, perhaps more complex example is Sinorhizobium meliloti, a symbiotic organism that contains five homologous Cu+-ATPases. CopA1a and CopA1b seem to maintain the cytoplasmic Cu+ levels at different stages of symbiotic life-cycle. CopA2a and CopA2b are necessary for the assembly of two different cytochrome c oxidases which are synthesized at different life-stages. Finally, CopA3 is expressed in response to redox stress and shows low enzymatic activity. This suggest a role in metallation of periplasmic or membrane-bound cuproenzymes4. The pathogen Salmonella typhimurium contains two Cu+-ATPases, CopA and GolT96. These are required to maintain cytosolic quota or deliver Cu+ to the periplasmic chaperone CueP for metallation of periplasmic Cu/Zn- superoxide dismutase SodCII24. Strikingly, both ATPases are functionally redundant, which arises new questions about functional paralogs among Cu+-ATPases.
Future directions
Significant progress has been made to understand the similarities, and differences, of Cu+-ATPases with the very well characterized alkali metal transporting ATPases. Nevertheless, Cu+ permeation along the protein is poorly understood. Upon enzyme phosphorylation, Cu+ is released from the TM-MBS. However, it must travel a 7–12 Å short permeation path before exiting the protein. The L. pneumophila CopA structure suggest that the ions exit through a narrow, dehydrated channel. It has been proposed that conserved Met and Glu residues at the periplasmic exit suggest a mechanism of extrusion driven by hydrophilic interactions53. While mutation of the invariant residues impairs the function, this interesting hypothesis invites further experimental corroboration. Similarly, only the fundamental aspects of Cu+ transfer between chaperones and ATPases has been explored. Further studies are required to understand the role of putative ligand exchange residues and specificity determinants.
While alternative periplasmic Cu+-chaperones targeting the ion to different proteins have been described24, 94, 97, this is not the case for the cytoplasmic equivalents. How is the Cu+ distribution network configured? Clearly, Cu+-ATPases are central to Cu+ distribution and perhaps the best characterized elements of the proposed networks. The identification of nodes, links, forks, etc. is perhaps the challenge ahead.
Acknowledgements
We thank Dr. Robert Dempski (Worcester Polytechnic Institute) for helpful discussions and suggestion during the preparation of this manuscript. JMA acknowledges the NIH (R01 GM114949) for research funding.
Notes and references
‡ Bacterial Cu+-ATPases have received various names (CopA, PacS, CueA, SilP, ActP, CtpV, etc.). For simplicity, we will refer to them as CopA.
- 1.Kahn D, David M, Domergue O, Daveran ML, Ghai J, Hirsch PR, Batut J. Rhizobium meliloti fixGHI sequence predicts involvement of a specific cation pump in symbiotic nitrogen fixation. J Bacteriol. 1989;171:929–939. doi: 10.1128/jb.171.2.929-939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preisig O, Anthamatten D, Hennecke H. Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for a nitrogen-fixing endosymbiosis. Proc Natl Acad Sci U S A. 1993;90:3309–3313. doi: 10.1073/pnas.90.8.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preisig O, Zufferey R, Hennecke H. The Bradyrhizobium japonicum fixGHIS genes are required for the formation of the high-affinity cbb(3)-type cytochrome oxidase. Arch Microbiol. 1996;165:297–305. doi: 10.1007/s002030050330. [DOI] [PubMed] [Google Scholar]
- 4.Patel SJ, Padilla-Benavides T, Collins JM, Argüello J. Functional diversity of five homologous Cu+-ATPases present in Sinorhizobium meliloti. Microbiology. 2014 doi: 10.1099/mic.0.079137-0. DOI: 10.1099/mic.0.079137-0. [DOI] [PubMed] [Google Scholar]
- 5.Odermatt A, Suter H, Krapf R, Solioz M. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J. Biol. Chem. 1993;268:12775–12779. [PubMed] [Google Scholar]
- 6.Solioz M, Vulpe C. CPx-type ATPases: a class of P-type ATPases that pump heavy metals. Hum. Mol. Genet. 1994;3:1647–1656. [PubMed] [Google Scholar]
- 7.Mercer JF, Livingston J, Hall B, Paynter JA, Begy C, Chandrasekharappa S, Lockhart P, Grimes A, Bhave M, Siemieniak D, et al. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat Genet. 1993;3:20–25. doi: 10.1038/ng0193-20. [DOI] [PubMed] [Google Scholar]
- 8.Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat. Genet. 1993;3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- 9.Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM, et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- 10.Guan G, Pinochet-Barros A, Gaballa A, Patel SJ, Arguello JM, Helmann JD. PfeT, a P -type ATPase, effluxes ferrous iron and protects Bacillus subtilis against iron intoxication. Mol Microbiol. 2015 doi: 10.1111/mmi.13158. DOI: 10.1111/mmi.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmgren MG, Axelsen KB. Evolution of P-type ATPases. Biochim. Biophys. Acta. 1998;1365:37–45. doi: 10.1016/s0005-2728(98)00041-3. [DOI] [PubMed] [Google Scholar]
- 12.Argüello JM. Identification of ion selectivity determinants in heavy metal transport P1B-type ATPases. J. Membr. Biol. 2003;195:93–108. doi: 10.1007/s00232-003-2048-2. [DOI] [PubMed] [Google Scholar]
- 13.Rosenzweig AC, Argüello JM. Toward a molecular understanding of metal transport by P(1B)-type ATPases. Current topics in membranes. 2012;69:113–136. doi: 10.1016/B978-0-12-394390-3.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padilla-Benavides T, Long JE, Raimunda D, Sassetti CM, Argüello JM. A novel P(1B)-type Mn2+-transporting ATPase is required for secreted protein metallation in mycobacteria. J Biol Chem. 2013;288:11334–11347. doi: 10.1074/jbc.M112.448175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernández-Montes G, Argüello JM, Valderrama B. Evolution and diversity of periplasmic proteins involved in copper homeostasis in gamma proteobacteria. BMC microbiology. 2012;12:249–263. doi: 10.1186/1471-2180-12-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith AT, Smith KP, Rosenzweig AC. Diversity of the metal-transporting P1B-type ATPases. J Biol Inorg Chem. 2014;19:947–960. doi: 10.1007/s00775-014-1129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupont CL, Grass G, Rensing C. Copper toxicity and the origin of bacterial resistance-new insights and applications. Metallomics. 2011;3:1109–1118. doi: 10.1039/c1mt00107h. [DOI] [PubMed] [Google Scholar]
- 18.Argüello JM, Raimunda D, Padilla-Benavides T. Mechanisms of Copper Homeostasis in Bacteria. Front. Cell. Infect. Microbiol. 2013;3 doi: 10.3389/fcimb.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osman D, Cavet JS. Copper homeostasis in bacteria. Adv Appl Microbiol. 2008;65:217–247. doi: 10.1016/S0065-2164(08)00608-4. [DOI] [PubMed] [Google Scholar]
- 20.Tottey S, Rich PR, Rondet SAM, Robinson NJ. Two Menkes-type ATPases supply copper for photosynthesis in Synechocystis PCC 6803. J Biol Chem. 2001;276:19999–20004. doi: 10.1074/jbc.M011243200. [DOI] [PubMed] [Google Scholar]
- 21.Raimunda D, González-Guerrero M, Leeber BW, 3rd, Argüello JM. The transport mechanism of bacterial Cu(+)-ATPases: distinct efflux rates adapted to different function. Biometals. 2011;24:467–475. doi: 10.1007/s10534-010-9404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaby-Haas CE, Padilla-Benavides T, Stube R, Arguello JM, Merchant SS. Evolution of a plant-specific copper chaperone family for chloroplast copper homeostasis. Proc Natl Acad Sci U S A. 2014;111:5480–5487. doi: 10.1073/pnas.1421545111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padilla-Benavides T, George Thompson AM, McEvoy MM, Argüello JM. Mechanism of ATPase-Mediated Cu+ Export and Delivery to Periplasmic Chaperones: The Interaction of Escherichia coli CopA and CusF. J Biol Chem. 2014;289:20492–20501. doi: 10.1074/jbc.M114.577668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osman D, Patterson CJ, Bailey K, Fisher K, Robinson NJ, Rigby SE, Cavet JS. The copper supply pathway to a Salmonella Cu,Zn-superoxide dismutase (SodCII) involves P(1) (B) -type ATPase copper efflux and periplasmic CueP. Mol Microbiol. 2012;87:466–477. doi: 10.1111/mmi.12107. [DOI] [PubMed] [Google Scholar]
- 25.Yuan DS, Stearman R, Dancis A, Dunn T, Beeler T, Klausner RD. The Menkes/Wilson disease gene homologue in yeast provides copper to a ceruloplasmin-like oxidase required for iron uptake. Proc Natl Acad Sci. 1995;92:2632–2636. doi: 10.1073/pnas.92.7.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu D, Beeler TJ, Dunn TM. Sequence, mapping and disruption of CCC2, a gene that cross-complements the Ca(2+)-sensitive phenotype of csg1 mutants and encodes a P-type ATPase belonging to the Cu(2+)-ATPase subfamily. Yeast. 1995;11:283–292. doi: 10.1002/yea.320110310. [DOI] [PubMed] [Google Scholar]
- 27.Veldhuis NA, Gaeth AP, Pearson RB, Gabriel K, Camakaris J. The multi-layered regulation of copper translocating P-type ATPases. Biometals. 2009;22:177–190. doi: 10.1007/s10534-008-9183-2. [DOI] [PubMed] [Google Scholar]
- 28.Yruela I. Copper in plants: acquisition, transport and interactions. Funct. Plant Biol. 2009;36:409–430. doi: 10.1071/FP08288. [DOI] [PubMed] [Google Scholar]
- 29.Williams LE, Mills RF. P1B-ATPases - an ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci. 2005;10:491–502. doi: 10.1016/j.tplants.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Andrés-Colas N, Sancenon V, Rodríguez-Navarro S, Mayo S, Thiele DJ, Ecker JR, Puig S, Penarrubia L. The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 2006;45:225–236. doi: 10.1111/j.1365-313X.2005.02601.x. [DOI] [PubMed] [Google Scholar]
- 31.Hirayama T, Kieber JJ, Hirayama N, Kogan M, Guzman P, Nourizadeh S, Alonso JM, Dailey WP, Dancis A, Ecker JR. RESPONSIVE-TO-ANTAGONIST1, a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell. 1999;97:383–393. doi: 10.1016/s0092-8674(00)80747-3. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Ghany SE, Muller-Moule P, Niyogi KK, Pilon M, Shikanai T. Two P-type ATPases are required for copper delivery in Arabidopsis thaliana chloroplasts. Plant Cell. 2005;17:1233–1251. doi: 10.1105/tpc.104.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shikanai T, Muller-Moule P, Munekage Y, Niyogi KK, Pilon M. PAA1, a P-type ATPase of Arabidopsis, functions in copper transport in chloroplasts. Plant Cell. 2003;15:1333–1346. doi: 10.1105/tpc.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 35.Kaler SG. ATP7A-related copper transport diseases—emerging concepts and future trends. Nature reviews Neurology. 2011 doi: 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Telianidis J, Hung Y, Materia S, Fontaine S. Role of the P-Type ATPases, ATP7A and ATP7B in brain copper homeostasis. Frontiers in Aging Neuroscience. 2013;5 doi: 10.3389/fnagi.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petris MJ, Mercer JFB. The Menkes protein (ATP7A; MNK) cycles via the plasma membrane both in basal and elevated extracellular copper using a C- terminal di-leucine endocytic signal. Hum. Mol. Genet. 1999;8:2107–2115. doi: 10.1093/hmg/8.11.2107. [DOI] [PubMed] [Google Scholar]
- 38.Lutsenko S, Petris MJ. Function and Regulation of the Mammalian Copper-transporting ATPases: Insights from Biochemical and Cell Biological Approaches. J. Membr. Biol. 2003;191:1–12. doi: 10.1007/s00232-002-1040-6. [DOI] [PubMed] [Google Scholar]
- 39.Lutsenko S, LeShane ES, Shinde U. Biochemical basis of regulation of human copper-transporting ATPases. Arch Biochem Biophys. 2007;463:134–148. doi: 10.1016/j.abb.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forbes JR, Cox DW. Copper-dependent trafficking of Wilson disease mutant ATP7B proteins. Hum. Mol. Genet. 2000;9:1927–1935. doi: 10.1093/hmg/9.13.1927. [DOI] [PubMed] [Google Scholar]
- 41.Gitlin JD. Wilson disease. Gastroenterol. 2003;125:1868–1877. doi: 10.1053/j.gastro.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. Embo J. 1996;15:6084–6095. [PMC free article] [PubMed] [Google Scholar]
- 43.Mercer JF, Barnes N, Stevenson J, Strausak D, Llanos RM. Copper-induced trafficking of the Cu-ATPase: a key mechanism for copper homeostasis. Biometals. 2003;16:175–184. doi: 10.1023/a:1020719016675. [DOI] [PubMed] [Google Scholar]
- 44.La Fontaine S, Mercer JF. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch Biochem Biophys. 2007;463:149–167. doi: 10.1016/j.abb.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 45.Nyasae L, Bustos R, Braiterman L, Eipper B, Hubbard A. Dynamics of endogenous ATP7A (Menkes protein) in intestinal epithelial cells: copper-dependent redistribution between two intracellular sites. American journal of physiology. Gastrointestinal and liver physiology. 2007;292:94. doi: 10.1152/ajpgi.00472.2006. [DOI] [PubMed] [Google Scholar]
- 46.Polishchuk R, Lutsenko S. Golgi in copper homeostasis: a view from the membrane trafficking field. Histochemistry and cell biology. 2013;140:285–295. doi: 10.1007/s00418-013-1123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Argüello JM, González-Guerrero and M, Raimunda D. Bacterial transition metal P1B-ATPases: transport mechanism and roles in virulence. Biochemistry. 2011;50:9940–9949. doi: 10.1021/bi201418k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sazinsky MH, Agarwal S, Argüello JM, Rosenzweig AC. Structure of the Actuator Domain from the Archaeoglobus fulgidus Cu+-ATPase. Biochemistry. 2006;45:9949–9955. doi: 10.1021/bi0610045. [DOI] [PubMed] [Google Scholar]
- 50.Sazinsky MH, Mandal AK, Argüello JM, Rosenzweig AC. Structure of the ATP binding domain from the Archaeoglobus fulgidus Cu+-ATPase. J. Biol. Chem. 2006;281:11161–11166. doi: 10.1074/jbc.M510708200. [DOI] [PubMed] [Google Scholar]
- 51.Wu CC, Rice WJ, Stokes DL. Structure of a copper pump suggests a regulatory role for its metal-binding domain. Structure. 2008;16:976–985. doi: 10.1016/j.str.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gourdon P, Liu XY, Skjorringe T, Morth JP, Moller LB, Pedersen BP, Nissen P. Crystal structure of a copper-transporting PIB-type ATPase. Nature. 2011;475:59–64. doi: 10.1038/nature10191. [DOI] [PubMed] [Google Scholar]
- 53.Andersson M, Mattle D, Sitsel O, Klymchuk T, Nielsen AM, Moller LB, White SH, Nissen P, Gourdon P. Copper-transporting P-type ATPases use a unique ion-release pathway. Nat Struct Mol Biol. 2014;21:43–48. doi: 10.1038/nsmb.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmgren MG, Nissen P. P-type ATPases. Annu Rev Biophys. 2011;40:243–266. doi: 10.1146/annurev.biophys.093008.131331. [DOI] [PubMed] [Google Scholar]
- 55.Olesen C, Picard M, Winther AM, Gyrup C, Morth JP, Oxvig C, Moller JV, Nissen P. The structural basis of calcium transport by the calcium pump. Nature. 2007;450:1036–1042. doi: 10.1038/nature06418. [DOI] [PubMed] [Google Scholar]
- 56.Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-type ATPase superfamily. J. Mol. Evol. 1998;46:84–101. doi: 10.1007/pl00006286. [DOI] [PubMed] [Google Scholar]
- 57.González-Guerrero M, Eren E, Rawal S, Stemmler TL, Argüello J. Cu+ transporting ATPases: Structure of the Two Transmembrane Cu+ Transport Sites. J. Biol. Chem. 2008;283:29753–29759. doi: 10.1074/jbc.M803248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandal AK, Yang Y, Kertesz TM, Argüello JM. Identification of the transmembrane metal binding site in Cu+-transporting PIB-type ATPases. J. Biol. Chem. 2004;279:54802–54807. doi: 10.1074/jbc.M410854200. [DOI] [PubMed] [Google Scholar]
- 59.Argüello JM, Raimunda D, González-Guerrero M. Metal transport across biomembranes: Emerging models for a distinct chemistry. J Biol Chem. 2012;287:13510–13517. doi: 10.1074/jbc.R111.319343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mattle D, Zhang L, Sitsel O, Pedersen LT, Moncelli MR, Tadini-Buoninsegni F, Gourdon P, Rees DC, Nissen P, Meloni G. A sulfur-based transport pathway in Cu+-ATPases. EMBO Rep. 2015;16:728–740. doi: 10.15252/embr.201439927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubino JT, Franz KJ. Coordination chemistry of copper proteins: how nature handles a toxic cargo for essential function. J Inorg Biochem. 2012;107:129–143. doi: 10.1016/j.jinorgbio.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 62.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci U S A. 2009;106:4237–4242. doi: 10.1073/pnas.0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Long F, Su CC, Lei HT, Bolla JR, Do SV, Yu EW. Structure and mechanism of the tripartite CusCBA heavy-metal efflux complex. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2012;367:1047–1058. doi: 10.1098/rstb.2011.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Padilla-Benavides T, McCann CJ, Argüello JM. The mechanism of Cu+ transport ATPases: Interaction with Cu+ chaperones and the role of transient metal-binding sites. J Biol Chem. 2013;288:69–78. doi: 10.1074/jbc.M112.420810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barry AN, Otoikhian A, Bhatt S, Shinde U, Tsivkovskii R, Blackburn NJ, Lutsenko S. The Lumenal Loop Met(672)-Pro(707) of Copper-transporting ATPase ATP7A Binds Metals and Facilitates Copper Release from the Intramembrane Sites. Journal of Biological Chemistry. 2011;286:26585–26594. doi: 10.1074/jbc.M111.229039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harper JF, Hong B, Hwang I, Guo HQ, Stoddard R, Huang JF, Palmgren MG, Sze H. A novel calmodulin-regulated Ca2+-ATPase (ACA2) from Arabidopsis with an N-terminal autoinhibitory domain. J. Biol. Chem. 1998;273:1099–1106. doi: 10.1074/jbc.273.2.1099. [DOI] [PubMed] [Google Scholar]
- 67.Penniston JT, Enyedi A. Modulation of the plasma membrane Ca2+ pump. J Membr Biol. 1998;165:101–109. doi: 10.1007/s002329900424. [DOI] [PubMed] [Google Scholar]
- 68.Palmgren MG, Sommarin M, Serrano R, Larsson C. Identification of an autoinhibitory domain in the C-terminal region of the plant plasma membrane H(+)-ATPase. J. Biol. Chem. 1991;266:20470–20475. [PubMed] [Google Scholar]
- 69.Boal AK, Rosenzweig AC. Structural biology of copper trafficking. Chem Rev. 2009;109:4760–4779. doi: 10.1021/cr900104z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banci L, Bertini I, Cantini F, Ciofi-Baffoni S. Cellular copper distribution: a mechanistic systems biology approach. Cell. Mol. Life Sci. 2010;67:2563–2589. doi: 10.1007/s00018-010-0330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Banci L, Bertini I, McGreevy KS, Rosato A. Molecular recognition in copper trafficking. Nat Prod Rep. 2010;27:695–710. doi: 10.1039/b906678k. [DOI] [PubMed] [Google Scholar]
- 72.Fu Y, Tsui HC, Bruce KE, Sham LT, Higgins KA, Lisher JP, Kazmierczak KM, Maroney MJ, Dann CE, 3rd, Winkler ME, Giedroc DP. A new structural paradigm in copper resistance in Streptococcus pneumoniae. Nat Chem Biol. 2013;9:177–183. doi: 10.1038/nchembio.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mandal AK, Argüello JM. Functional roles of metal binding domains of the Archaeoglobus fulgidus Cu+-ATPase CopA. Biochemistry. 2003;42:11040–11047. doi: 10.1021/bi034806y. [DOI] [PubMed] [Google Scholar]
- 74.Tsivkovskii R, MacArthur BC, Lutsenko S. The Lys1010–Lys1325 fragment of the Wilson's disease protein binds nucleotides and interacts with the N-terminal domain of this protein in a copper-dependent manner. J Biol Chem. 2001;276:2234–2242. doi: 10.1074/jbc.M003238200. [DOI] [PubMed] [Google Scholar]
- 75.González-Guerrero M, Hong D, Argüello JM. Chaperone-mediated Cu+ delivery to Cu+ transport ATPases. Requirement of nucleotide binding. J Biol Chem. 2009;284:20804–20811. doi: 10.1074/jbc.M109.016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lutsenko S, Petrukhin K, Cooper MJ, Gilliam CT, Kaplan JH. N-terminal domains of human copper-transporting adenosine triphosphatases (the Wilson's and Menkes disease proteins) bind copper selectively in vivo and in vitro with stoichiometry of one copper per metal-binding repeat. J. Biol. Chem. 1997;272:18939–18944. doi: 10.1074/jbc.272.30.18939. [DOI] [PubMed] [Google Scholar]
- 77.Argüello J, Eren E, González-Guerrero M. The structure and function of heavy metal transport P1B-ATPases. Biometals. 2007;20:233–248. doi: 10.1007/s10534-006-9055-6. [DOI] [PubMed] [Google Scholar]
- 78.Hatori Y, Majima E, Tsuda T, Toyoshima C. Domain organization and movements in heavy metal ion pumps - papain digestion of CopA, a Cu+-transporting ATPase. J. Biol. Chem. 2007;282:25213–25221. doi: 10.1074/jbc.M703520200. [DOI] [PubMed] [Google Scholar]
- 79.Hatori Y, Lewis D, Toyoshima C, Inesi G. Reaction cycle of Thermotoga maritima copper ATPase and conformational characterization of catalytically deficient mutants. Biochemistry. 2009;48:4871–4880. doi: 10.1021/bi900338n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.González-Guerrero M, Raimunda D, Cheng X, Argüello JM. Distinct functional roles of homologous Cu(+) efflux ATPases in Pseudomonas aeruginosa. Mol. Mirobiol. 2010;78:1246–1258. doi: 10.1111/j.1365-2958.2010.07402.x. [DOI] [PubMed] [Google Scholar]
- 81.Mandal AK, Cheung WD, Argüello JM. Characterization of a thermophilic P-type Ag+/Cu+-ATPase from the extremophile Archaeglobus fulgidus. J. Biol. Chem. 2002;277:7201–7208. doi: 10.1074/jbc.M109964200. [DOI] [PubMed] [Google Scholar]
- 82.Espariz M, Checa SK, Audero ME, Pontel LB, Soncini FC. Dissecting the Salmonella response to copper. Microbiology. 2007;153:2989–2997. doi: 10.1099/mic.0.2007/006536-0. [DOI] [PubMed] [Google Scholar]
- 83.Yang Y, Mandal AK, Bredeston LM, González-Flecha FL, Argüello JM. Activation of Archaeoglobus fulgidus Cu+-ATPase CopA by cysteine. Bba-Biomembranes. 2007;1768:495–501. doi: 10.1016/j.bbamem.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 84.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O'Halloran TV. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 85.Changela A, Chen K, Xue Y, Holschen J, Outten CE, O'Halloran TV, Mondragon A. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science. 2003;301:1383–1387. doi: 10.1126/science.1085950. [DOI] [PubMed] [Google Scholar]
- 86.González-Guerrero M, Argüello JM. Mechanism of Cu+-transporting ATPases: Soluble Cu+ chaperones directly transfer Cu+ to transmembrane transport sites. P Natl Acad Sci USA. 2008;105:5992–5997. doi: 10.1073/pnas.0711446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Banci L, Bertini I, Del Conte R, Mangani S, Meyer-Klaucke W. X-ray absorption and NMR spectroscopic studies of CopZ, a copper chaperone in Bacillus subtilis: the coordination properties of the copper ion. Biochemistry. 2003;42:2467–2474. doi: 10.1021/bi0205810. [DOI] [PubMed] [Google Scholar]
- 88.Lippard SJ. Free copper ions in the cell? Science. 1999;284:748–749. doi: 10.1126/science.284.5415.748. [DOI] [PubMed] [Google Scholar]
- 89.Urvoas A, Moutiez M, Estienne C, Couprie J, Mintz E, Le Clainche L. Metal-binding stoichiometry and selectivity of the copper chaperone CopZ from Enterococcus hirae. Eur J Biochem. 2004;271:993–1003. doi: 10.1111/j.1432-1033.2004.04001.x. [DOI] [PubMed] [Google Scholar]
- 90.Sazinsky MH, LeMoine B, Orofino M, Davydov R, Bencze KZ, Stemmler TL, Hoffman BM, Argüello JM, Rosenzweig AC. Characterization and structure of a Zn2+ and [2Fe-2S]-containing copper chaperone from Archaeoglobus fulgidus. J. Biol. Chem. 2007;282:25950–25959. doi: 10.1074/jbc.M703311200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fu Y, Bruce KE, Wu H, Giedroc DP. The S2 Cu(i) site in CupA from Streptococcus pneumoniae is required for cellular copper resistance. Metallomics. 2016;8:61–70. doi: 10.1039/c5mt00221d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rice WJ, Kovalishin A, Stokes DL. Role of metal-binding domains of the copper pump from Archaeoglobus fulgidus. Biochem. Bioph. Res. Co. 2006;348:124–131. doi: 10.1016/j.bbrc.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 93.Xue Y, Davis AV, Balakrishnan G, Stasser JP, Staehlin BM, Focia P, Spiro TG, Penner-Hahn JE, O'Halloran TV. Cu(I) recognition via cation-pi and methionine interactions in CusF. Nat Chem Biol. 2008;4:107–109. doi: 10.1038/nchembio.2007.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim EH, Rensing C, McEvoy MM. Chaperone-mediated copper handling in the periplasm. Nat Prod Rep. 2010;27:711–719. doi: 10.1039/b906681k. [DOI] [PubMed] [Google Scholar]
- 95.Raimunda D, Padilla-Benavides T, Vogt S, Boutigny S, Tomkinson KN, Finney LA, Argüello JM. Periplasmic response upon disruption of transmembrane Cu(+) transport in Pseudomonas aeruginosa. Metallomics. 2013;5:144–151. doi: 10.1039/c2mt20191g. [DOI] [PubMed] [Google Scholar]
- 96.Pontel LB, Audero MEP, Espariz M, Checa SK, Soncini FC. GoIS controls the response to gold by the hierarchical induction of Salmonella-specific genes that include a CBA efflux-coding operon. Mol Microbiol. 2007;66:814–825. doi: 10.1111/j.1365-2958.2007.05963.x. [DOI] [PubMed] [Google Scholar]
- 97.Lohmeyer E, Schroder S, Pawlik G, Trasnea PI, Peters A, Daldal F, Koch HG. The ScoI homologue SenC is a copper binding protein that interacts directly with the cbb(3)-type cytochrome oxidase in Rhodobacter capsulatus. Biochim Biophys Acta. 2012;1817:2005–2015. doi: 10.1016/j.bbabio.2012.06.621. [DOI] [PMC free article] [PubMed] [Google Scholar]