Abstract
IMPORTANCE
The appropriate use of surgery or radiotherapy-based approaches for organ preservation has been the subject of much debate. Unfortunately, there has been a lack of improvement in overall survival for patients with laryngeal carcinoma in the last 30 years.
OBJECTIVE
To assess the rates of laryngeal preservation and laryngectomy-free survival in patients receiving cetuximab and radiotherapy (CRT) and patients receiving radiotherapy alone.
DESIGN, SETTING, AND PARTICIPANTS
Patients were enrolled in a multicenter, open-label, stratified, randomized, phase 3 study from April 1, 1999, through March 31, 2002, from 73 centers in the United States and 14 other countries. A secondary subgroup analysis of patients with hypopharyngeal and laryngeal carcinoma was undertaken. Rates of laryngeal preservation and laryngectomy-free survival were estimated by the Kaplan-Meier method. The hazard ratios (HRs) were calculated using a Cox proportional hazards regression model. Quality of life was evaluated using the European Organization for Research and Treatment of Cancer core questionnaire and head and neck module.
MAIN OUTCOMES AND MEASURES
Laryngeal preservation and laryngectomy-free survival.
RESULTS
Of the 424 patients included in the trial, 168 treated patients with cancer of the larynx or hypopharynx were included in this analysis (90 in the CRT group and 78 in the radiotherapy alone group). The median (range) age of the patients was 59 (40-80) years in the CRT group and 61 (35-81) years in the radiotherapy alone group. In the CRT group, 72 patients (80.0%) were male and 18 (20.0%) were female. In the radiotherapy alone group, 62 (79.5%) were male and 16 (20.5%) were female. The rates of laryngeal preservation at 2 years were 87.9% for CRT vs 85.7% for radiotherapy alone, with an HR of 0.57 (95% CI, 0.23-1.42; P = .22). Similarly, the HR for laryngectomy-free survival comparing CRT vs radiotherapy alone was 0.78 (95% CI, 0.54-1.11; P = .17). This study was not powered to assess organ preservation. Median overall survival was 27 (95% CI, 20-45) vs 21 (95% CI, 17-35) months for the CRT and radiotherapy alone groups, respectively, with an HR of 0.87 (95% CI, 0.60-1.27). No differences between treatments were reported regarding overall quality of life, need for a feeding tube, or speech.
CONCLUSIONS AND RELEVANCE
The results of a possible cetuximab-related laryngeal preservation benefit for patients with hypopharyngeal or laryngeal cancer are intriguing; these results need to be interpreted in the context of a retrospective subset analysis with limited sample size.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00004227
Historically, locoregionally advanced squamous cell cancers of the larynx or hypopharynx have been treated with surgical resection, usually involving laryngectomy with or without postoperative radiotherapy.1 Although laryngectomy is an effective treatment, investigators have sought therapeutic strategies that result in voice preservation. Various modifications of surgical techniques have been explored.2-5 Alternatively, in the early 1980s, investigators evaluated curative primary radiotherapy with salvage surgery as an option for these patients.6,7 Retrospective studies8,9 that compared primary radiotherapy with salvage surgery to initial surgery with postoperative radiotherapy found similar rates of survival in patients with advanced laryngeal or hypopharyngeal cancers.
After the realization that many patients could avoid total laryngectomy with the use of primary radiotherapy, several combination chemoradiotherapy strategies were introduced for patients with laryngeal or hypopharyngeal cancers. In large phase 3 randomized clinical trials, induction chemotherapy followed by radiotherapy demonstrated equivalent overall survival compared with surgical resection and postoperative radiotherapy in patients with laryngeal10,11 and hypopharyngeal cancers.12 More recently, the Radiation Therapy Oncology Group 91-11 trial revealed that radiotherapy with concomitant cisplatin resulted in a superior laryngeal preservation rate compared with induction with cisplatin and fluorouracil followed by radiotherapy or radiotherapy alone for these advanced laryngeal cancers.13,14 However, this treatment did not produce a survival benefit.
The appropriate use of surgery or radiotherapy-based approaches for organ preservation has been the subject of much debate.15-18 Unfortunately, there has been a lack of improvement in overall survival for patients with laryngeal carcinoma in large database studies19-21 examining the last 30 years of care. Investigators have reported that poor follow-up may contribute to the potential for larger and less curable recurrences, and surgeons experienced with radiotherapy with salvage surgery need to be involved as early as possible.20,21
The analysis described in this report was performed to assess the laryngeal preservation rates of patients with locoregionally advanced laryngeal and hypopharyngeal cancers who were entered into a randomized clinical trial of cetuximab and radiotherapy (CRT) compared with radiotherapy alone.22,23 This group of patients represents a subgroup (168 patients) of a larger population (424 patients) that also included patients with oropharyngeal cancers. It is noteworthy that this randomized clinical trial was not designed to enroll a sufficient number of patients to definitively and prospectively address the subject of the analysis reported herein. Previous reports22,23 of the overall randomized clinical trial found locoregional control and survival advantages associated with the addition of cetuximab to radiotherapy.
Methods
Patients
Patients were enrolled in the multicenter, open-label, stratified, randomized, phase 3 study from April 1, 1999, through March 31, 2002, from 73 centers in the United States and 14 other countries. Patients who entered the trial had stage III or IV non-metastatic squamous cell carcinoma of the oropharynx, hypopharynx, or larynx (Figure 1 and Table 1). Patients were eligible if they had pathologically confirmed stage III or IV squamous cell carcinoma, had measurable disease, had no distant metastases, had no prior therapy for the tumor under study, and were medically suitable to undergo definitive radiotherapy. This subgroup includes the patients with hypopharyngeal and laryngeal cancers. The protocol was approved by the ethics review boards at the participating institutions, and all the patients provided written informed consent.
Figure 1. Trial Profile for the Subpopulation of Patients With Hypopharyngeal or Laryngeal Carcinoma.
a Data are from Bonner et al.22
b Data are from Bonner et al.23
Table 1.
Patient Characteristics and Radiotherapy Fractionationa
| Characteristic | Cetuximab Plus Radiotherapy (n = 90) |
Radiotherapy Alone (n = 78) |
|---|---|---|
| Age, median (range), y | 59 (40-80) | 61 (35-81) |
|
| ||
| Sex | ||
|
| ||
| Male | 72 (80.0) | 62 (79.5) |
|
| ||
| Female | 18 (20.0) | 16 (20.5) |
|
| ||
| Karnofsky performance status | ||
|
| ||
| 90-100 | 56 (62.2) | 47 (60.3) |
|
| ||
| 60-80 | 34 (37.8) | 30 (38.5) |
|
| ||
| Missing | 0 | 1 (1.3) |
|
| ||
| Site of primary tumor | ||
|
| ||
| Hypopharynx | 35 (38.9) | 27 (34.6) |
|
| ||
| Larynx | 55 (61.1) | 51 (65.4) |
|
| ||
| AJCC stage | ||
|
| ||
| III | 34 (37.8) | 22 (28.2) |
|
| ||
| IV | 56 (62.2) | 56 (71.8) |
|
| ||
| Tumor stage | ||
|
| ||
| T1-2 | 18 (20.0) | 12 (15.4) |
|
| ||
| T3 | 47 (52.2) | 38 (48.7) |
|
| ||
| T4 | 25 (27.8) | 28 (35.9) |
|
| ||
| Node stage | ||
|
| ||
| N0 | 29 (32.2) | 23 (29.5) |
|
| ||
| N1 | 20 (22.2) | 15 (19.2) |
|
| ||
| N2-3 | 41 (45.6) | 40 (51.3) |
|
| ||
| Radiotherapy fractionation | ||
|
| ||
| Once daily | 22 (24.4) | 25 (32.1) |
|
| ||
| Twice daily | 20 (22.2) | 11 (14.1) |
|
| ||
| Concomitant boost | 48 (53.3) | 42 (53.8) |
Abbreviation: AJCC, American Joint Commission on Cancer.
Data are presented as number (percentage) of patients unless otherwise indicated.
Patients were initially evaluated with a comprehensive head and neck examination, which included panendoscopy. The primary tumors and lymph nodes were staged by the American Joint Committee on Cancer staging classification of 1998. Initial evaluation included computed tomography or magnetic resonance imaging of the head and neck region and chest radiography. Patients began treatment within 2 weeks after this initial evaluation, as determined by the randomization procedure.22 Patients who underwent no surgery or less extensive operations were censored at death or date of last contact.
Treatment
The primary radiotherapy was delivered with curative intent. Investigators selected 1 of 3 radiation fractionation regimens: once daily, twice daily, or concomitant boost regimen (Figure 1). Cervical lymph node drainage regions, considered to be at high risk for subclinical disease, were treated with a dose of 50 to 54 Gy. The primary tumor and gross nodal disease received full-dose radiotherapy (70-76.8 Gy, depending on fractionation). However, if a postoperative neck dissection was planned, gross nodal disease could be treated with 60 Gy.
For patients randomized to CRT, cetuximab treatment consisted of an initial dose of 400 mg/m2 and was delivered as a 120-minute intravenous infusion. This initial dose was delivered 1 week before the initiation of CRT. Patients received weekly cetuximab infusions during the radiotherapy treatment. Seven weekly infusions were delivered at a dose of 250 mg/m2 for a period of 60 minutes each.
For patients who were scheduled to have a planned neck dissection, surgery was scheduled to take place 4 to 8 weeks after completion of CRT or radiotherapy alone.
Evaluations
Evaluations, including physical examination, hematologic testing, and chemical profiles, were performed weekly for the duration of the study. A pharmacologic profile was also performed weekly on patients receiving cetuximab infusion (CRT group). Patients provided a history and underwent a physical examination, which included a fiberoptic examination of the tumor, as well as an assessment of all study end points at required time points after the completion of treatment.22 Post-treatment assessments were performed 4 and 8 weeks after completion of radiotherapy. Patients who were scheduled for a planned postoperative neck dissection could have their 8-week assessment at the 6-week time point, along with post-treatment computed tomography. Subsequently, patients were evaluated every 4 months during the first and second years. They were evaluated semiannually during years 3 to 5. These follow-up assessments included imaging studies, consisting of computed tomography or magnetic resonance imaging of the head and neck region.
Quality of Life
The instruments used in this study were the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 version 3.0 and the Quality of Life Head and Neck Module 35. The self-administered core questionnaire consisted of 30 questions and incorporated 5 functional scales, 3 symptom scales, and a global health and quality-of-life scale. The remaining single items assessed additional symptoms commonly reported by patients with cancer, as well as the perceived financial effect of disease and treatment. All scales and single items met the standards for reliability. The 35-item module comprised 1 multi-item scale and a series of single items assessing head and neck–associated symptoms and adverse effects from conventional therapy.
Statistical Analysis
The randomization was not stratified by primary site of cancer; hence, the results presented below are a retrospective analysis of the selected subset of patients. A CONSORT flow diagram was included with the original publication of the trial.23 A trial profile for the analysis presented here is shown in Figure 1. The primary end point of the study was to examine differences in the rate of locoregional disease control maintained for 1 year. The duration of locoregional control was defined as the absence of locoregional disease progression at the scheduled follow-up visits and was determined through a masked review of the investigator-generated data by an independent committee of experts. The duration of locoregional control was from randomization to the first documented progression or recurrence of locoregional disease or death from any cause. Overall survival was calculated from randomization to death from any cause. Tumor present in a neck dissection, which was performed for any reason after 15 weeks after radiotherapy, constituted locoregional progression. Distribution of time-to-event parameters was estimated by the Kaplan-Meier method, and treatment effects were compared using a log-rank test. The Cox proportional hazards regression model was used to calculate the nonstratified hazard ratio (HR). The Kaplan-Meier method was also used to estimate the rate of preservation of larynx.24 Patients who underwent no surgery or lesser operations were censored at death or date of last contact.
Results
Characteristics of the Patients
A total of 424 patients participated in the study and 168 were included in the subanalysis (90 in the CRT group or 78 in the radiotherapy alone group). Most patients from both treatment groups were treated with the concomitant boost fractionation regimen. The 2 treatment groups were well balanced with respect to sex, tumor stage, node stage (Table 1), and the rate of neck surgery (16% in the CRT group and 14% in the radiotherapy alone group).
Treatment Adherence
The median durations of treatment were 52, 46, and 43 days for once daily, twice daily, or concomitant boost, respectively, for patients treated with CRT and 52, 44, and 43 days, respectively, for patients treated with radiotherapy alone. For patients who received cetuximab, the median number of cetuximab infusions was 8 (range, 1–11). The median cumulative cetuximab dose was 2154 mg/m2 (range, 14-2887 mg/m2). The number of patients who received 7 or more cetuximab infusions was 80 (88.9%). The radiotherapy quality assurance review found that the mean and median doses for the once daily, twice daily, and concomitant boost regimens were 68.7 and 70.0 Gy, 74.3 and 74.4 Gy, and 70.8 and 72.0 Gy, respectively; these doses were similar in both treatment groups. Radiotherapy adherence for patients who received CRT or radiotherapy alone was balanced; 65 (72.2%) received CRT and 56 (71.8%) received radiotherapy alone as planned or with minor deviation. A total of 121 patients (72%) were treated as planned or with minor deviation. Major acceptable deviations in the CRT and radiotherapy alone groups were found in 13 (14.4%) and 13 (16.7%) patients, and major unacceptable deviations were observed in 3 (3.3%) and 5 (6.4%) patients, respectively. A total of 9 (10.0%) and 4 (5.1%) patients in these respective groups were not evaluable for a radiation quality assurance review. The differences in radiotherapy adherence between the 2 treatment groups were not statistically significant. Neck dissections were performed in 14 patients (15.6%) treated with CRT and 11 patients (14.1%) treated with radiotherapy alone.
Efficacy
Locoregional control was the primary end point of the phase 3 study. For this subgroup of patients with laryngeal and hypopharyngeal cancers, locoregional control was slightly improved for patients who received CRT compared with radiotherapy alone (HR, 0.80; 95% CI, 0.56-1.13) (Table 2). The 2-year rates of locoregional control were 36.9% in the CRT group and 25.7% in the radiotherapy alone group. Survival was also slightly improved for patients who received CRT compared with radiotherapy alone (HR, 0.87; 95% CI, 0.60-1.27) (Table 2). The 3-year survival rates were 41.9% in the CRT group and 39.0% in the radiotherapy alone group.
Table 2.
Antitumor Efficacy
| Variable | Cetuximab Plus Radiotherapy (n = 90) |
Radiotherapy Alone (n = 78) |
|---|---|---|
| Locoregional control | ||
|
| ||
| Duration, median (95% CI), mo | 14 (11-17) | 12 (8-14) |
|
| ||
| Rate at 1 year, % (95% CI) | 55.8 (44.9-65.5) | 46.1 (34.6-56.8) |
|
| ||
| Rate at 2 years, % (95% CI) | 36.9 (26.8-46.9) | 25.7 (16.5-36.0) |
|
| ||
| Overall survival | ||
|
| ||
| Duration, median (95% CI), mo | 27 (20-45) | 21 (17-35) |
|
| ||
| Rate at 2 years, % (95% CI) | 51.3 (40.5-61.2) | 48.3 (36.8-58.9) |
|
| ||
| Rate at 3 years, % (95% CI) | 41.9 (31.4-51.9) | 39.0 (28.1-49.7) |
|
| ||
| Laryngeal preservation | ||
|
| ||
| Patients with laryngectomy, No. (%) |
8 (8.9) | 11 (14.1) |
|
| ||
| Rate at 2 years, % (95% CI) | 87.9 (77.0-93.8) | 85.7 (73.0-92.7) |
|
| ||
| Rate at 3 years, % (95% CI) | 87.9 (77.0-93.8) | 76.8 (61.0-86.9) |
|
| ||
| Laryngectomy-free survival | ||
|
| ||
| Duration, median (95% CI), mo | 21 (14-30) | 18 (13-24) |
|
| ||
| Rate at 2 years, % (95% CI) | 44.5 (34.0-54.5) | 40.5 (29.6-51.2) |
|
| ||
| Rate at 3 years, % (95% CI) | 37.4 (27.3-47.4) | 28.5 (18.9-38.9) |
The rates of laryngeal preservation (no need for salvage laryngectomy) were 87.9% at 2 and 3 years in the CRT group compared with 85.7% at 2 years and 76.8% at 3 years in the radiotherapy alone group. The 2.2% and 11.1% absolute improvements in the rates of laryngeal preservation, at 2 and 3 years, respectively, favored CRT compared with radiotherapy alone (Figure 2). However, this improvement was not statistically significant (HR, 0.57; 95% CI, 0.23-1.42; P = .22). In addition, there was a 4.0% and 8.9% absolute improvement in laryngectomy-free survival at 2 and 3 years, respectively. Again, these differences did not reach statistical significance (HR, 0.78; 95% CI, 0.54-1.11; P = .17) (Figure 2).
Figure 2. Kaplan-Meier Plots.
CRT indicates cetuximab plus radiotherapy; NE, not estimable.
Safety
The most frequently reported adverse events are given in Table 3. The frequency of grades 3 and 4 mucositis/stomatitis and odynophagia was not significantly different among patients who received CRT compared with radiotherapy alone. Patients who received CRT had a greater rate of acneiform rash compared with patients who received radiotherapy alone (Table 3). Chills, fever, and headache were the only toxic events reported to occur at any grade with significantly higher frequency (P = .002, P = .01, and P = .004, respectively) in patients in the CRT group than in the radiotherapy alone group; however, their incidences were low (chills, 16 [17.8%] vs 2 [2.6%]; fever, 20 [22.2%] vs 6 [7.7%]; headache, 20 [22.2%] vs 5 [6.4%]).
Table 3.
Number (Percentage) of Adverse Eventsa
| Cetuximab Plus Radiotherapy (n = 90) |
Radiotherapy Alone (n = 78) |
|||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Adverse Event | All Grades | Grade 3 | Grade 4 | All Grades | Grade 3 | Grade 4 |
| Mucositis/stomatitis | 75 (83.3) | 36 (40.0) | 0 | 62 (79.5) | 24 (30.8) | 1 (1.3) |
|
| ||||||
| Acneiform rash | 79 (87.8) | 13 (14.4) | 1 (1.1) | 7 (9.0) | 0 | 0 |
|
| ||||||
| Radiation dermatitis | 75 (83.3) | 21 (23.3) | 2 (2.2) | 71 (91.0) | 14 (17.9) | 0 |
|
| ||||||
| Weight loss | 70 (77.8) | 12 (13.3) | 0 | 49 (62.8) | 4 (5.1) | 0 |
|
| ||||||
| Dry mouth | 61 (67.8) | 6 (6.7) | 0 | 52 (66.7) | 2 (2.6) | 0 |
|
| ||||||
| Dysphagia | 57 (63.3) | 18 (20.0) | 0 | 48 (61.5) | 18 (23.1) | 2 (2.6) |
|
| ||||||
| Asthenia | 48 (53.3) | 5 (5.6) | 1 (1.1) | 39 (50.0) | 3 (3.8) | 2 (2.6) |
Adverse events of special interest occurring at grade 3 or higher in at least 5% of patients and at any grade in at least 50% of all patients in any arm. Calculated P values demonstrated no statistically significant differences between the 2 arms. No P value could be calculated for acneiform rash. There were no grade 5 adverse events.
Quality of Life
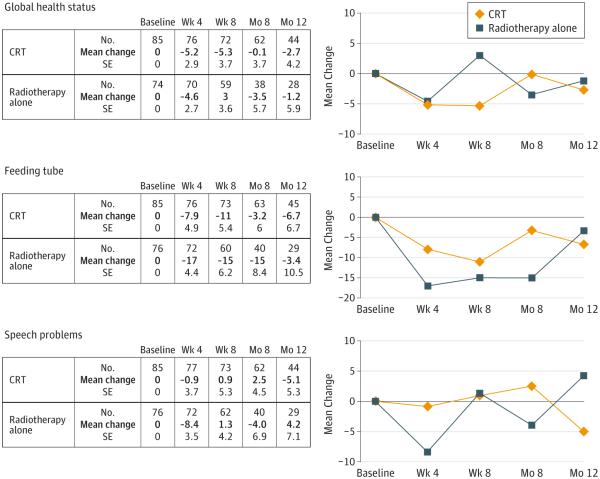
The quality-of-life responses were evaluated in a longitudinal design in all patients. Baseline assessment was performed at or just after randomization. Subsequent assessments took place before the beginning of the fourth week of radiotherapy, at the 8-week posttreatment evaluation, and at the 2 every-4-month follow-up evaluations of year 1. Plots of mean changes in global health status, use of a feeding tube, or speech problems reveal no differences between the treatment groups (Figure 3). A positive value in change indicates an improvement for the patient.
Figure 3. Mean Changes in Quality of Life.
Categorical quality-of-life scores were linearly transformed to 0- to 100-point scales according to the European Organization for Research and Treatment of Cancer scoring manual. Change was calculated as the postbaseline score minus the baseline score. A positive value indicates an improvement.
Differences in mean changes in swallowing at radiotherapy week 4 (−15 in the CRT group and −25 in the radiotherapy alone group, P = .02), loss of appetite at radiotherapy week 4 (−11 in the CRT group and −18 in the radiotherapy alone group, P = .03), nausea at week 8 after radiotherapy (−7.3 in the CRT group and 0.3 in the radiotherapy alone group, P = .04), coughing at radiotherapy week 4 (7.4 in the CRT group and −5.6 in the radiotherapy alone group, P = .02) and at week 8 after radiotherapy (−3.2 in the CRT group and 1.1 in the radiotherapy alone group, P = .04), constipation at month 8 (2.6 in the CRT group and −19 in the radiotherapy alone group, P < .001), and cognitive functioning at month 12 (−7.0 in the CRT group and 4.6 in the radiotherapy alone group, P = .04) were not seen at the other time points.
Discussion
When laryngectomy is the primary treatment for locoregionally advanced laryngeal and hypopharyngeal cancers, patients gradually adjust to the loss of natural phonation, but the condition substantially interferes with normal communication and social interactions.25 In addition, although the effect of the procedure on voice frequently receives the greatest consideration, the presence of the stoma may also adversely affect patients’ quality of life.26
In the analysis reported herein, laryngeal preservation rates were studied in a subgroup of patients who were part of a large phase 3 trial that compared primary radiotherapy with or without the antiepidermal growth factor receptor monoclonal antibody cetuximab. The use of CRT produced a higher rate of laryngeal preservation compared with radiotherapy alone for patients with locoregionally advanced laryngeal or hypopharyngeal cancers. Although the difference in the rates of laryngeal preservation between the 2 groups did not reach statistical significance, the initial trial was not powered to assess this subgroup question. The HR of 0.57 is a strong indicator that cetuximab, when added to radiotherapy, may improve laryngeal preservation. The 87.9% rate of laryngeal preservation at 2 years for CRT found in this trial is similar to the rate reported for the use of radiotherapy and concomitant cisplatin in Radiation Therapy Oncology Group 91-11 trial (n = 547).13
Patients who undergo a laryngectomy often experience a difficult adjustment process. Even when the patient achieves a certain level of comfort with various techniques of artificial speech,27 the ability to communicate with others is hampered.26,28,29 Investigations have explored both the patient’s and the interviewer’s perceptions of the patient’s difficulty with communication after laryngectomy.26 Of interest, the interviewers perceived the patients’ impairment with communication as a much greater infringement on quality of life compared with the patients’ perceptions. Patients who underwent laryngectomy believed that interference with social activities resulted in a greater detrimental effect regarding their quality of life compared with the interviewers’ perception of this factor. Therefore, patients have their own perceptions, as well as the perceptions of others, as potential hurdles to normal activities after laryngectomy.
The perception of life without a larynx plays an important role in a patient’s treatment decision. In a recent trial from France, 269 patients were queried about the treatment choices they might make if they faced the diagnosis of advanced laryngeal cancer.30 Only 29% stated that they would not consider options with a lower chance of cure if told that the best option, with respect to survival, was total laryngectomy. However, their enthusiasm for seeking laryngeal-preserving options waned when told about potential adverse effects, such as tracheostomy or permanent gastrostomy. An older report31 of interviews with firefighters and executives who were asked to envision that they had advanced laryngeal cancer determined that they were willing to accept a 15% to 30% reduction in life expectancy for a laryngeal-preserving treatment compared with total laryngectomy. These studies30,31 demonstrate the importance of reviewing options and potential adverse effects with patients before making a decision regarding the best treatment.
Considering the importance of laryngeal function, many head and neck cancer treatments have been directed toward the goal of laryngeal preservation. One of the largest randomized clinical trials was designed to evaluate laryngeal preservation compared with radiotherapy alone, concomitant chemoradiotherapy, and induction chemotherapy followed by radiotherapy.13,14 Each treatment group included at least 180 patients, thus providing much greater statistical power than the current communication. The use of concomitant cisplatin and radiotherapy resulted in a statistically significant improvement in laryngeal preservation compared with radiotherapy alone, with a hazard ratio of 0.46. However, the induction regimen of cisplatin and fluorouracil did not improve laryngeal preservation over radiotherapy alone. Neither concomitant chemoradiotherapy nor induction chemotherapy followed by radiotherapy resulted in improved overall survival compared with radiotherapy alone. Furthermore, there were greater severe acute toxic effects from the chemoradiotherapy treatments compared with radiotherapy alone,13 but no significant differences in severe late toxic effects were noted in the comparison of the 3 treatment arms.14 Unfortunately, the concomitant chemoradiotherapy arm had an increase in deaths not attributable to cancer compared with the other 2 arms; this finding needs to be explored further to determine whether undetected fatal late treatment toxic effects are greater with concomitant regimens.
Although induction chemotherapy was not used in this trial, it has been the subject of many past and recent assessments involving laryngeal preservation, as well as other efficacy and safety outcomes.10-14 A retrospective review32 of the phase 3 TAX 324 trial (Induction Chemotherapy Comparing Taxotere Cisplatin and 5-Fluorouracil With Standard Cisplatin and 5-Fluorouracil Followed by Chemoradiation in Locally Advanced Head and Neck Cancer) in patients with laryngeal or hypopharyngeal cancers revealed that induction chemotherapy of docetaxel, cisplatin, and fluorouracil (n = 90) compared with cisplatin plus fluorouracil (n = 76) followed by chemoradiotherapy with weekly carboplatin resulted in significantly improved overall survival (P = .02) and progression-free survival (P = .03) for the docetaxel, cisplatin, and fluorouracil group. Among patients undergoing surgery, laryngectomy-free survival was also significantly greater with docetaxel, cisplatin, and fluorouracil (P = .03).
The TREMPLIN (Radiotherapy With Cisplatin Versus Radiotherapy With Cetuximab After Induction Chemotherapy for Larynx Preservation) trial compared the efficacy and safety of induction chemotherapy using docetaxel, cisplatin, and fluorouracil, followed by chemoradiotherapy with cisplatin (n = 60) or cetuximab (n = 56), for laryngeal preservation.33 No differences were found in laryngeal preservation, laryngeal function preservation, or overall survival between the 2 regimens. Newer studies, such as DeCIDE (Docetaxel-Based Chemotherapy Plus or Minus IC to Decrease Events in Head and Neck Cancer)34 and PARADIGM (Combination Chemotherapy and Radiation in Treating Patients With Stage III or IV Head and Neck Cancer),35 suggest no overall benefit from the addition of induction chemotherapy vs concomitant chemoradiotherapy alone.
Conclusions
The higher rate of laryngeal preservation that was achieved with the use of CRT compared with radiotherapy alone was encouraging. Furthermore, no significant increases in the most debilitating radiotherapy-induced toxic effects, such as mucositis/stomatitis and dysphagia, were observed. This treatment approach warrants further evaluation in larger populations to fully assess the potential value of cetuximab or other molecular targeting agents to augment laryngeal preservation rates.
Key Points.
Question
Does treatment with cetuximab and radiotherapy (CRT) increase the rate of laryngeal preservation and laryngectomy-free survival compared with radiotherapy alone in patients with hypopharyngeal and laryngeal carcinoma?
Findings
In this secondary analysis of a randomized clinical trial comparing CRT vs radiotherapy alone in 168 treated patients, there was no difference in the rates of laryngeal preservation at 2 years. There was a 4.0% and 8.9% absolute improvement in laryngectomy-free survival at 2 and 3 years, respectively, for CRT vs radiotherapy alone.
Meaning
The results of this secondary subset analysis reveal a possible cetuximab-related benefit in laryngeal preservation.
Acknowledgments
Funding/Support: Research funding was provided by Eli Lilly and Company.
Footnotes
Author Contributions: Dr Chin and Mr Hossain had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bonner, Harari, Hossain, Schulten, Chin, Baselga.
Acquisition, analysis, or interpretation of data: Bonner, Giralt, Spencer, Schulten, Hossain, Chang, Chin.
Drafting of the manuscript: Bonner, Hossain, Chin.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Bonner, Hossain.
Obtained funding: Chang.
Administrative, technical, or material support: Harari.
Study supervision: Giralt, Hossain, Chang, Chin, Baselga.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Bonner reported working as a consultant for Eli Lilly and Company, Merck Serono, and Bristol-Myers Squibb. Dr Schulten reported being an employee of Merck KGaA. Dr Chin and Mr Hossain reported being employees of Eli Lilly and Company. Dr Chang reported being a former employee of Eli Lilly and Company and currently owning Lilly stock. No other disclosures were reported.
Role of the Funder/Sponsor: The funding source had a role in the design and conduct of the study.
Additional Contributions: Nathalie Godinot, MS, of Eli Lilly and Company, provided medical writing support. Anastasia Perkowski, MBA, of Eli Lilly and Company, provided medical editing assistance. Neither was compensated outside their usual salary.
REFERENCES
- 1.Shah JP, Tollefsen HR. Epidermoid carcinoma of the supraglottic larynx: role of neck dissection in initial surgical treatment. Am J Surg. 1974;128(4):494–499. doi: 10.1016/0002-9610(74)90262-1. [DOI] [PubMed] [Google Scholar]
- 2.Pearson BW, Woods RD, II, Hartman DE. Extended hemilaryngectomy for T3 glottic carcinoma with preservation of speech and swallowing. Laryngoscope. 1980;90(12):1950–1961. doi: 10.1288/00005537-198012000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Pearson BW, DeSanto LW, Olsen KD, Salassa JR. Results of near-total laryngectomy. Ann Otol Rhinol Laryngol. 1998;107(10):820–825. doi: 10.1177/000348949810701002. pt 1. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal A, Moon J, Davis RK, et al. Southwest Oncology Group Transoral carbon dioxide laser supraglottic laryngectomy and irradiation in stage I, II, and III squamous cell carcinoma of the supraglottic larynx: report of Southwest Oncology Group Phase 2 Trial S9709. Arch Otolaryngol Head Neck Surg. 2007;133(10):1044–1050. doi: 10.1001/archotol.133.10.1044. [DOI] [PubMed] [Google Scholar]
- 5.Bussu F, Paludetti G, Almadori G, et al. Comparison of total laryngectomy with surgical (cricohyoidopexy) and nonsurgical organ-preservation modalities in advanced laryngeal squamous cell carcinomas: a multicenter retrospective analysis. Head Neck. 2013;35(4):554–561. doi: 10.1002/hed.22994. [DOI] [PubMed] [Google Scholar]
- 6.Harwood AR, Beale FA, Cummings BJ, Keane TJ, Payne D, Rider WD. T4NOMO glottic cancer: an analysis of dose-time volume factors. Int J Radiat Oncol Biol Phys. 1981;7(11):1507–1512. doi: 10.1016/0360-3016(81)90079-1. [DOI] [PubMed] [Google Scholar]
- 7.Harwood AR, Beale FA, Cummings BJ, et al. Supraglottic laryngeal carcinoma: an analysis of dose-time-volume factors in 410 patients. Int J Radiat Oncol Biol Phys. 1983;9(3):311–319. doi: 10.1016/0360-3016(83)90289-4. [DOI] [PubMed] [Google Scholar]
- 8.Bryce DP, Rider WD. Pre-operative irradiation in the treatment of advanced laryngeal carcinoma. Laryngoscope. 1971;81(9):1481–1490. doi: 10.1288/00005537-197109000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Keane TJ, Hawkins NV, Beale FA, et al. Carcinoma of the hypopharynx results of primary radical radiation therapy. Int J Radiat Oncol Biol Phys. 1983;9(5):659–664. doi: 10.1016/0360-3016(83)90231-6. [DOI] [PubMed] [Google Scholar]
- 10.Wolf GT, The Department of Veterans Affairs Laryngeal Cancer Study Group Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324(24):1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 11.Wolf GT, Fisher SG. Effectiveness of salvage neck dissection for advanced regional metastases when induction chemotherapy and radiation are used for organ preservation. Laryngoscope. 1992;102(8):934–939. doi: 10.1288/00005537-199208000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T, EORTC Head and Neck Cancer Cooperative Group Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. J Natl Cancer Inst. 1996;88(13):890–899. doi: 10.1093/jnci/88.13.890. [DOI] [PubMed] [Google Scholar]
- 13.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 14.Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31(7):845–852. doi: 10.1200/JCO.2012.43.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(suppl 111):1–13. doi: 10.1097/01.mlg.0000236095.97947.26. 9, pt 2. [DOI] [PubMed] [Google Scholar]
- 16.Chen AY, Halpern M. Factors predictive of survival in advanced laryngeal cancer. Arch Otolaryngol Head Neck Surg. 2007;133(12):1270–1276. doi: 10.1001/archotol.133.12.1270. [DOI] [PubMed] [Google Scholar]
- 17.Megwalu UC, Sikora AG. Survival outcomes in advanced laryngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(9):855–860. doi: 10.1001/jamaoto.2014.1671. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Travis LB, Chen R, et al. Impact of radiotherapy on laryngeal cancer survival: a population-based study of 13,808 US patients. Cancer. 2012;118(5):1276–1287. doi: 10.1002/cncr.26357. [DOI] [PubMed] [Google Scholar]
- 19.Olsen KD. Reexamining the treatment of advanced laryngeal cancer. Head Neck. 2010;32(1):1–7. doi: 10.1002/hed.21294. [DOI] [PubMed] [Google Scholar]
- 20.Wolf GT. Reexamining the treatment of advanced laryngeal cancer: the VA laryngeal cancer study revisited. Head Neck. 2010;32(1):7–14. doi: 10.1002/hed.21296. [DOI] [PubMed] [Google Scholar]
- 21.Forastiere AA. Larynx preservation and survival trends: should there be concern? Head Neck. 2010;32(1):14–17. doi: 10.1002/hed.21295. [DOI] [PubMed] [Google Scholar]
- 22.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 23.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 25.Mohide EA, Archibald SD, Tew M, Young JE, Haines T. Postlaryngectomy quality-of-life dimensions identified by patients and health care professionals. Am J Surg. 1992;164(6):619–622. doi: 10.1016/s0002-9610(05)80720-2. [DOI] [PubMed] [Google Scholar]
- 26.DeSanto LW, Olsen KD, Perry WC, Rohe DE, Keith RL. Quality of life after surgical treatment of cancer of the larynx. Ann Otol Rhinol Laryngol. 1995;104(10):763–769. doi: 10.1177/000348949510401003. pt 1. [DOI] [PubMed] [Google Scholar]
- 27.Hanna E, Sherman A, Cash D, et al. Quality of life for patients following total laryngectomy vs chemoradiation for laryngeal preservation. Arch Otolaryngol Head Neck Surg. 2004;130(7):875–879. doi: 10.1001/archotol.130.7.875. [DOI] [PubMed] [Google Scholar]
- 28.Natvig K. Study No. 1: Social, personal, and behavioral factors related to present mastery of the laryngectomy event. J Otolaryngol. 1983;12(3):155–162. [PubMed] [Google Scholar]
- 29.O’Sullivan B, Mackillop W, Gilbert R, et al. Controversies in the management of laryngeal cancer: results of an international survey of patterns of care. Radiother Oncol. 1994;31(1):23–32. doi: 10.1016/0167-8140(94)90410-3. [DOI] [PubMed] [Google Scholar]
- 30.Laccourreye O, Malinvaud D, Ménard M, Consoli S, Giraud P, Bonfils P. Total laryngectomy or laryngeal preservation for advanced laryngeal cancer: impact of the functional risk upon the patient’s preferences. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(2):93–97. doi: 10.1016/j.anorl.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 31.McNeil BJ, Weichselbaum R, Pauker SG. Speech and survival: tradeoffs between quality and quantity of life in laryngeal cancer. N Engl J Med. 1981;305(17):982–987. doi: 10.1056/NEJM198110223051704. [DOI] [PubMed] [Google Scholar]
- 32.Posner MR, Norris CM, Wirth LJ, et al. TAX 324 Study Group Sequential therapy for the locally advanced larynx and hypopharynx cancer subgroup in TAX 324: survival, surgery, and organ preservation. Ann Oncol. 2009;20(5):921–927. doi: 10.1093/annonc/mdn752. [DOI] [PubMed] [Google Scholar]
- 33.Lefebvre JL, Pointreau Y, Rolland F, et al. Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: the TREMPLIN randomized phase II study. J Clin Oncol. 2013;31(7):853–859. doi: 10.1200/JCO.2012.42.3988. [DOI] [PubMed] [Google Scholar]
- 34.Cohen EEW, Karrison TG, Kocherginsky M, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(25):2735–2743. doi: 10.1200/JCO.2013.54.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14(3):257–264. doi: 10.1016/S1470-2045(13)70011-1. [DOI] [PubMed] [Google Scholar]