Abstract
The lytic phage ST79 of Burkholderia pseudomallei can lyse a broad range of its host including antibiotic resistant isolates from within using a set of proteins, holin, lysB, lysC and endolysin, a peptidoglycan (PG) hydrolase enzyme. The phage ST79 endolysin gene identified as peptidase M15A was cloned, expressed and purified to evaluate its potential to lyse pathogenic bacteria. The molecular size of the purified enzyme is approximately 18 kDa and the in silico study cited here indicated the presence of a zinc-binding domain predicted to be a member of the subfamily A of a metallopeptidase. Its activity, however, was reduced by the presence of Zn2+. When Escherichia coli PG was used as a substrate and subjected to digestion for 5 min with 3 μg/ml of enzyme, the peptidase M15A showed 2 times higher in lysis efficiency when compared to the commercial lysozyme. The enzyme works in a broad alkaligenic pH range of 7.5–9.0 and temperatures from 25 to 42 °C. The enzyme was able to lyse 18 Gram-negative bacteria in which the outer membrane was permeabilized by chloroform treatment. Interestingly, it also lysed Enterococcus sp., but not other Gram-positive bacteria. In general, endolysin cannot lyse Gram-negative bacteria from outside, however, the cationic amphipathic C-terminal in some endolysins showed permeability to Gram-negative outer membranes. Genetically engineered ST79 peptidase M15A that showed a broad spectrum against Gram-negative bacterial PG or, in combination with an antibiotic the same way as combined drug methodology, could facilitate an effective treatment of severe or antibiotic resistant cases.
Keywords: Endolysins, Enzyme, Gene expression, Peptidoglycan
Introduction
A bacteriophage or phage is the virus of bacteria that is very specific to its host. It can replicate, multiply after transfection and either lyse the host or become integrated into its genome (Golkar et al. 2014). Eastern Europe such as Poland and Georgia reported the phage as an alternative therapy for infectious diseases (Abedon et al. 2011). Phage therapy has also been evaluated in the United States (Ho 2001). The discovery of antibiotics changed the paradigm of curing infectious diseases and helped protect enormous numbers of people who suffered from bacterial infections in the twentieth century, however, the numbers of antibiotic-resistant bacteria have also increased (Golkar et al. 2014). Even though the post-antibiotic era is not at hand, the use of broad-spectrum antibiotics has proven to disturb the beneficial bacterial community in humans and animals, especially gut microbiota, that affect immunity (O’Hara and Shanahan 2006; Round and Mazmanian 2009). Therefore, the use of phages as an alternative treatment came under the spotlight again because the phage is highly specific, can overcome antibiotic resistant bacteria and are available in large quantities in nature.
The enzymes related to the lysis mechanism of the phages that are used to lyse the bacterial host during the release of their progeny have been studied and revealed the classical holin-endolysin lysis system found in phages of both Gram-positive and Gram-negative bacteria (Young et al. 2000). Holin is used to make pores in the cytoplasmic membrane that assists endolysin to access and cleave peptidoglycan to lyse the bacteria from the inside out. In addition, some phages of Gram-negative bacteria also have Rz/Rz1 or lysB/lysC accessory proteins that help in the lysis of the process (Berry et al. 2008). Endolysins have been considered as a new class of antibiotics as they can destroy the peptidoglycan (PG) of Gram-positive bacterial cell walls. Endolysin has five specific activities on PG which are muramidase (lysozyme), transglycosylase, glucosaminidase that digests N-acetylmuramic acids (NAM) and N-acetylglucosamine (NAG), amidase that digests NAM and peptides and endopeptidases digested within the peptide chain of PG (Borysowski et al. 2006). The enzymes or the phages themselves are extensively applied in several fields, for example, the food industry and biological control of unwanted bacteria (Ruyter et al. 1997) including pathogenic bacteria in medicine as it shows neither toxicity nor stimulates hyperimmune sera in the mouse model (Jado et al. 2003).
Burkholderia pseudomallei is a Gram-negative soil bacterium that causes severe septic infectious disease called melioidosis. The disease can be found in both humans and animals in endemic areas (Leelarasamee and Bovornkitti 1989). This pathogenic bacterium is intrinsically resistant to several antibiotics and it can produce high levels of biofilms that protect the bacterium from the killing by either antibiotics or the host immune response (Sawasdidoln et al. 2010; Pibalpakdee et al. 2012; Mongkolrob et al. 2015). The drug of choice is a third generation cephalosporin such as ceftazidime that needs long-term treatment to prevent relapse. Currently, there is no commercial vaccine available (Limmathurotsakul et al. 2015). Phages that have shown some specificity in lysing B. pseudomallei have been reported (Sariya et al. 2006; Yordpratum et al. 2011; Gatedee et al. 2011; Kvitko et al. 2012; Guang-Han et al. 2016). The genome of ST79, a novel lytic phage that lyses B. pseudomallei was sequenced and submitted to GenBank (GI:509141608) (manuscript in preparation). The lysis cassette of ST79 was also characterized (Khakhum et al. 2016) and its modified phages were shown to lyse a wide range of B. pseudomallei isolates and could significantly reduce biofilm formation of the bacteria especially at the early stage of attachment (Kulsuwan et al. 2015).
In this study, the peptidase M15A, known as endolysin or peptidoglycan hydrolase from the ST79 lytic phage that could lyse a broad spectrum of B. pseudomallei and other Gram-negative bacteria from within was cloned, expressed and characterized. More information on the enzymes and phages themselves could facilitate the application of them as adjunct standard antibiotic therapy for B. pseudomallei.
Materials and methods
Bacterial strains and ST79 phage
Burkholderia pseudomallei strain P37 was isolated from a blood sample from a patient admitted to Srinagarind Hospital, Faculty of Medicine, Khon Kaen University, Thailand. The B. pseudomallei lytic phage ST79, isolated from soil in the northeast of Thailand, was used as a source of the peptidase M15A for cloning (Yordpratum et al. 2011). Escherichia coli BL21 (DE3) was used as the host for cloning and protein expression processes (Thermo Fisher Scientific, Waltham, MA, USA). Eighteen Gram-negative bacteria, five of which were E. coli host strains; Top10, LMG194 (Invitrogen, CA, USA), DH5α, BL21 (DE3) and XL1-Blue (Thermo Fisher Scientific, Waltham, MA, USA), two B. pseudomallei isolates, P37 and G1; two Burkholderia mallei isolates, EY2233 and EY2237; Burkholderia thailandensis UE5 (kindly provided by MORU, Mahidol University, Thailand), Klebsiella pneumoniae, Vibrio parahaemolyticus, Pseudomonas vasculitis, P. aeruginosa, Acinetobacter baumannii, Salmonella gr. D, Shigella gr. D and Citrobacter freundii and seven Gram-positive bacteria included Enterococcus sp., Streptococcus epidermidis, Staphylococcus aureus, Bacillus sp., Micrococcus sp., β-streptococcus gr. B and Corynebacterium diphtheria were obtained from the Department of Microbiology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand and used in this study. All of bacterial strains and ST79 phage were deposited in culture collection belonging to World Data Centre For Microorganism (WDCM) as MRCKKU (registration number 1130). ST79 phage is available for research collaborators.
Bioinformatic analysis
The peptidase M15A amino acid sequence (YP_008060500.1) from the ST79 phage genome (NC_02134.1) was submitted to BLASTP homology search (Altschul et al. 1997) in the NCBI database (http://www.ncbi.nlm.nih.gov/). The peptidase information resource and sequence analysis were performed by the MEROPS batch Blast tool (Rawlings and Morton 2008). The Interproscan 4 software v.4.8 (Zdobnov and Apweiler 2001) was used to analyze protein functional domains. The protein conserved domain and structure was predicted using Pfam (Finn et al. 2016) and SWISS-MODEL (Biasini et al. 2014).
ST79 phage propagation and DNA extraction
The ST79 lytic phage was propagated in liquid culture using B. pseudomallei strain P37 as the propagating strain (Yordpratum et al. 2011). The phage DNA was extracted with a phenol–chloroform extraction method as described elsewhere (Sambrook and Russell 2001).
Cloning and expression of peptidase M15A and Western blot analysis
The peptidase M15A gene was amplified from the ST79 genomic DNA by the polymerase chain reaction (PCR) using in house designed peptidase M15A forward primer (5′-TATAAAGAGCTCTATGCAGTTGACGGACCATTTC-3′) with SacI restriction site (underlined sequence) and a peptidase M15A reverse primer (5′-ATAATAGGTACCTCATGCGCCCACCGTGTA-3′) with the KpnI restriction site (underlined sequence). The PCR product was cloned into the pQE31 vector (Qiagen, Hilden, Germany) containing the N-terminal 6xhistidine tag and transformed into E. coli BL21 (DE3). E. coli cells containing the recombinant plasmid with peptidase M15A gene were propagated in Luria and Bertani (LB) medium containing 100 µg/ml ampicillin until mid-exponential phase (OD600 nm = 0.6) and induced with 1 mM final concentration of isopropyl-β-d-thiogalactopyranoside (IPTG) (Sigma, St. Louis, Missouri, USA). Cells were further cultured for 4 h and collected as a pellet followed by sonication (10 cycles of 30 s pulses and 30 s rest at 200 Watts in an ice bath, MSE Soniprep 150, MSE, London, UK) in 5 ml lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole pH 8.0). The protein supernatant and pellet fractions were separated by 15 % SDS-PAGE (Sambrook and Russell 2001) using the Novex Sharp Pre-Stained Protein Standard (Thermo Fisher Scientific, Waltham, MA, USA) as the protein molecular weight marker, then blotted onto nitrocellulose by a semi-dry blotting system (BioRad, CA, USA). The proteins on the membrane were detected by anti-6X His tag® antibody (HRP) (Abcam, Cambridge, UK) and chemiluminescent SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA, USA).
Protein purification
The recombinant E. coli BL21 (DE3) containing the peptidase M15A gene-plasmid was induced by IPTG and the His-tagged proteins inside E. coli cells were purified using Ni–NTA agarose (Qiagen, Hilden, Germany) by a gravity-flow chromatography method. SDS-PAGE was used to analyze the eluted fractions for the 18 kDa of the peptidase M15A protein. The fraction containing the protein was refolded by buffer exchanging with 25 mM sodium acetate buffer, pH 6.5 and filtered through the Amicon® Ultra 10 K centrifugal filter device (Millipore, Darmstadt, Germany) according to the manufacturer’s instructions. The purified protein was quantified by the bicinchoninic acid assay (BCA assay) (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions using a Spectronic 20D+ spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Zymogram analysis
The peptidase M15A enzyme activity was observed using zymogram or renaturing SDS-PAGE (Piuri and Hatfull 2006) with some modifications as follows: An overnight culture of E. coli XL1-Blue (0.2 % w/v) was autoclaved and added to the SDS-PAGE solution (15 % w/v) before polymerization. The purified peptidase M15A enzyme was mixed with 2× sample-refolding buffer (0.5 mM Tris–HCl pH 6.8, 20 % glycerol, 0.2 % bromophenol blue), then loaded into the SDS-PAGE containing E. coli. After electrophoresis, the gel was incubated at 37 °C for 16 h in 1 % triton X-100, 25 mM Tris–HCl pH 8.5 and washed once with water and stained for 3 h with 0.5 % methylene blue in 0.01 % KOH. The peptidoglycan hydrolase activity of peptidase M15A on E. coli lysate was observed as a clear zone.
Enzyme activity
The outer membrane of E. coli XL1-Blue was permeabilized by chloroform to expose the peptidoglycan for digestion as described by Lavigne et al. (2004) with some modifications and used as the substrate for peptidase M15A. In brief, bacteria were grown until the mid-exponential phase (OD600 nm = 0.6), then centrifuged at 4000g at 4 °C, for 15 min to collect the cell pellet. The outer membranes were permeabilized by chloroform-saturated 0.05 M Tris–HCl buffer, pH 7.5 and gently shaken at room temperature for 45 min. The permeabilized E. coli were then washed, resuspended in 10 mM phosphate buffer, pH 8.0 and adjusted to OD600 nm = 0.6–1.0. The E. coli suspension of 270 µl was added into 96-well BD Falcon microplates (BD Bioscience, San Jose, CA, USA), then the purified peptidase M15A enzyme was added at the amount of 0.1 (0.3 μg/ml), 0.5 (1.6 μg/ml), 1 (3 μg/ml) and 5 μg (16 μg/ml) (30 µl) and the turbidity reduction at OD600 nm was measured by a Gen5 microplate reader (Biotek, Vermont, USA) as the kinetic assay for 15 min (1 min time intervals). The chicken egg white lysozyme (Sigma, St. Louis, Missouri, USA) (3 μg/ml) was used as a positive control and 10 mM phosphate buffer, pH 8.0, was used as a negative control (Briers et al. 2007a).
The effect of pH, temperature and divalent metal ions
To evaluate the effect of pH on enzymatic activity, permeabilized E. coli XL1-Blue cells were resuspended in 900 μl of 10 mM citrate buffer for pH 3.0–5.5, 10 mM phosphate buffer for pH 6.0–7.0 or 10 mM Tris–HCl buffer for pH 7.5–9.0 and then 100 μl of enzyme (5 μg) was added. The percentage of OD600 nm reduction was determined after incubation at 30 °C for 15 min. The effect of temperatures at 25, 30, 37 and 42 °C on enzyme activity were tested in the same manner at pH = 8.0.
The effect of metal ion, particularly Zinc, on enzyme activity was observed using 100 μl (5 μg) of purified peptidase M15A mixed with 900 μl of permeabilized E. coli XL1-Blue cells (OD600 nm ~ 1.0) in various concentrations of ZnCl2 (5, 10, 50 and 100 μM), MgCl2, MnCl2 or CaCl2 (100 and 1000 μM). The relative lytic activity was calculated as follows: {ΔOD600 nm sample (endolysin added) − ΔOD600 nm (buffer only)}/initial OD600 nm (Plotka et al. 2015) and compared with the control without metal ions for 100 % of relative activity (Son et al. 2012).
Spectrum of antibacterial lytic activity
A total of 18 Gram-negative and seven Gram-positive bacteria were tested for the peptidoglycan hydrolase spectrums. Gram-negative bacteria were permeabilized by chloroform and their peptidoglycans were used as substrates for enzyme digestion as previously described (Briers et al. 2007a). For Gram-positives, each strain was grown to mid-exponential phase then centrifuged at 3000g to collect cell pellets. The pellets were washed and resuspended in 10 mM phosphate buffer pH 8.0, the OD600 nm to 0.6–1.0 was adjusted. To test the spectrum of antimicrobial lytic activity, 5 and 20 μg in 100 μl of purified peptidase M15A was added into 900 μl permeabilized cell suspensions of Gram-negative or cell suspension of Gram-positive. The score was estimated from % relative lytic activity after incubation at 30 °C for 15 min. The % relative activity was defined as—(no lytic activity), + (1–30 %), ++ (31–60 %) and +++ (61–100 %) (Son et al. 2012).
Statistical analysis
The data of OD600 nm in the turbidity reduction test at different pHs and temperatures were analyzed by a Student’s t test and with a p value of <0.05 considered as significant.
Results
Peptidase M15A sequence analysis
The protein sequence of peptidase M15A from the lytic phage ST79 showed a conserved domain as Peptidase_M15_3 (PF08291) when analyzed by BLASTP and Pfam (Fig. 1a). The Interproscan 4 (version 4.8) protein functional domain indicated a Hedgehog signaling/DD-peptidase zinc-binding domain (SSF55166). The SWISS-MODEL protein structure prediction was matched with the 1lbu.1.A template, which was muramoyl-pentapeptide carboxypeptidase (MEROPS data). When the MEROPS batch Blast tool was used to detect peptidases and their non-peptidase homologues sequences in ST79 genome, it showed common amino acids among peptidases found in other bacterial genome, such as: B. glumae, B. thailandensis, B. cenocepacia, Burkholderia sp. CCGE1002, Asticcacaulis excentricus and Pseudomonas putida but not B. pseudomallei and B. mallei (Fig. 1b). This study’s analysis also identified a homologue catalytic domain from amino acid positions 3 to 134 (total 132 residues) with peptidase from P. putida (MER087996). The active site residues were located at the tryptophan (W117H) position and metal ligands at histidine (H77), aspartic acid (D84) and histidine (H119) (Fig. 1c). The analysis also indicated the presence of motifs HXXXXXXD and WXH, which were typical for peptidase M15 subfamily A.
Fig. 1.

The bioinformatics analysis of ST79 peptidase M15A protein sequence. a The Peptidase_M15_3 conserved domain analyzed using Pfam; #HMM: consensus of the Hidden Markov Models (HMMs), Capital letters indicated the most conserved positions, #MATCH: the match between the query sequence and the HMM, ‘ + ’ indicates a positive score which can be interpreted as a conservative substitution, #PP: posterior probability, which is the degree of confidence in each individual aligned residue, 0 means 0–5 %, 1 means 5–15 % and so on and 9 means 85–95 %, ‘*’ means 95–100 % posterior probability, #SEQ: query sequence, ‘-’ indicated deletions in the query sequence with respect to the HMM, b Multiple alignment of peptidase protein sequences from the MEROPS database showed identity of peptidase M15A sequence from ST79 phage with peptidase enzymes from B. glumae, B. thailandensis, B. cenocepacia, Burkholderia spp. CCGE1002, A. excentricus and P. putida using the CLUSTAL X program. Conserved amino acids of all sequences were marked with an asterisk. c The full amino acids sequence analysis of ST79 peptidase M15A using MEROPS batch Blast. The peptidase unit was labeled in green, active site in red, metal ion ligand in orange and the conserved domain in underlined letters
Expression, purification and zymogram analysis of the Peptidase M15A
The peptidase M15A gene was successfully cloned and expressed in E. coli BL21 (DE3). The estimated molecular weight of purified peptidase M15A on SDS-PAGE was approximately 18 kDa (with 6xhis tag) (Fig. 2). For zymogram analysis, the peptidase M15A enzyme in the gel lysed the peptidoglycan substrate from E. coli in which it appeared as a transparent band (Fig. 2).
Fig. 2.

The SDS-PAGE analysis of proteins and zymogram analysis of the lytic activity of Peptidase M15A against E. coli peptidoglycan. The Coomassie Brilliant blue stained SDS-PAGE gel shows protein molecular weight marker (M), induced E. coli cells containing pQE31 vector (lane 1), induced cells containing peptidase M15A/pQE31 plasmid (lane 2), 5 μg of purified peptidase M15A protein (lane 3) and the zymogram that is stained with 0.1 % methylene blue in 0.01 % KOH of SDS-PAGE refolding gel (lane 4). The clear lysis of PG substrate by the enzyme on zymogram located at the same position of the over-expressed protein band in lane 2 and purified enzyme in lane 3. The arrow indicates the 18 kDa lytic band of peptidase M15A
Lytic activity test
At the concentration of 1.6 μg/ml onward at 5 min of digestion, the enzyme reduced the turbidity of permeabilized E. coli XL1-Blue more than the lysozyme. When longer times of 10 and 15 min were observed, the turbidities from each concentration including lysozyme were similar. When the same concentrations of lysozyme and purified peptidase M15A (3 μg/ml) were compared at 5 min of digestion, the lysozyme gave a 22 % relative lytic activity while the peptidase M15A resulted in 52 %. The purified peptidase M15A digested the substrate approximate 2 times more than the lysozyme at this point (Fig. 3).
Fig. 3.
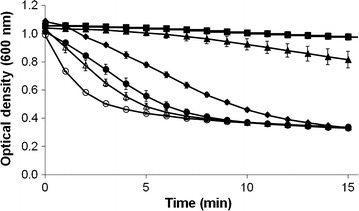
The turbidity reduction curve of peptidase activity against chloroform-treated E. coli. The peptidase M15A activity of various amounts was used to lyse chloroform E. coli XL1-Blue cell suspensions. The graph of turbidity reduction using peptidase M15A of 0.3 μg/ml (filled triangle), 1.6 μg/ml (filled circle), 3 μg/ml (open triangle) and 16 μg/ml (open circle) were evaluated. Phosphate buffer (filled square) was used as a negative control and 3 μg/ml of lysozyme (filled diamond) was used as positive control. Each point represents the mean of triplicate experiments and error bars indicate the standard deviation (SD)
Effect of pH, temperature and divalent metal ions on the enzyme activity
The peptidase M15A showed highest the activity at pH 7.5–9.0 with relative activity above 60 % (Fig. 4a). The enzyme could work in broad temperature ranges of 25, 30, 37 and 42 °C (Fig. 4b). The enzyme activity was decreased when Zn2+ concentrations were increased (Fig. 5). On the other hand, the 100 μM of Mg2+ and Mn2+ could only increase approximately 10 % of relative activity. When the 1000 μM concentration of Mg2+ and Mn2+ was used, the enzyme activity was reduced to 50.3 and 65.6 %. The Ca2+ ion showed little effect on the enzyme activity as seen by an approximately 6 % increase with 100 μM of the ion and a 5 % decrease when 1000 μM was used (Table 1).
Fig. 4.
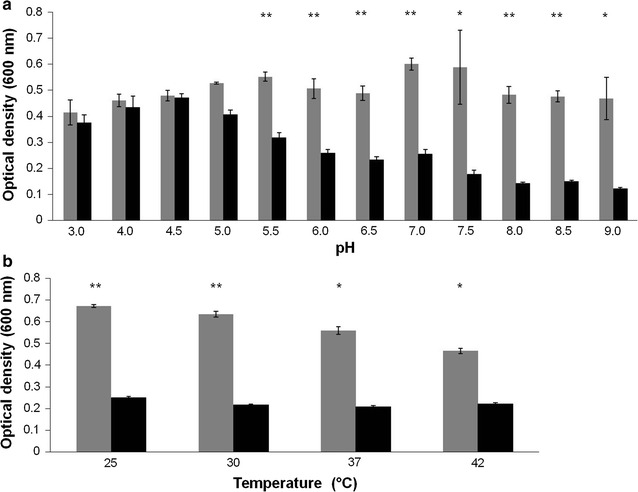
The effect of pH and temperature on the peptidase M15A lytic activity against E. coli XL1-Blue peptidoglycan. The optical density at 600 nm of cell suspensions was used to observe the effects of pH a and temperature b on the peptidase M15A lytic activity against E. coli XL1-Blue peptidoglycan. The grey bars show negative controls without enzyme and black bars indicate the reaction with enzyme. p value <0.05 (*) and 0.01 (**) indicate the significance. Each column represents the mean of triplicate experiments and error bars indicate SD
Fig. 5.
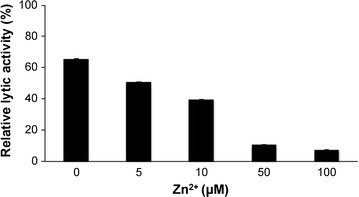
The percentage of E. coli XL1-Blue peptidoglycan reduction when treated with peptidase M15A in the presence of Zn2+. The effect of Zn2+ on peptidase M15A activity was calculated from the difference between optical densities at 600 nm of enzyme treated cells and untreated cells. Each column represents the mean of triplicate experiments and error bars indicate SD
Table 1.
The effect of divalent metal ions on lytic activity of peptidase M15A enzyme
| Relative lytic activity (%)a | ||
|---|---|---|
| Without metal ions (control) | 100.0 ± 1.98 | |
| Metal ions | 100 μM | 1000 μM |
| Mg2+ | 110.0 ± 2.6 | 50.3 ± 6.8 |
| Ca2+ | 106.1 ± 5.4 | 95.3 ± 4.2 |
| Mn2+ | 111.9 ± 0.4 | 65.6 ± 21.5 |
aThe activities are shown as percentages in relation to the non-treated peptidase M15A control. Values represent the mean ± standard deviation of triplicate experiments
Spectrum of antibacterial lytic activity
Eighteen chloroform permeabilized Gram-negative and seven Gram-positive bacteria were used for susceptibility tests against 5 and 20 μg of the peptidase M15A (Table 2). The enzyme lysed the peptidoglycan from all Gram-negative bacteria investigated. The enzyme effectively lysed E. coli, K. pneumoniae, Shigella gr. D and C. freundii and moderately lysed Burkholderia spp., V. parahaemolyticus, P. vasculitis, P. aeruginosa, A. baumannii and Salmonella gr. D (36–82 %). For Gram-positive, it only lyzed Enterococcus sp. (49 %).
Table 2.
Spectrum of antibacterial lytic activity against Gram-negative and Gram-positive bacteria
| Bacterial strains | % Relative lytic activitya |
|---|---|
| Gram–negative bacteria | |
| E. coli Top10 | +++ |
| E. DH5α | +++ |
| E. coli BL21 (DE3) | +++ |
| E. coli LMG194 | +++ |
| E. coli XL1-Blue | +++ |
| B. pseudomallei P37 | ++ |
| B. pseudomallei G1 | ++ |
| B. mallei EY2233 | ++ |
| B. mallei EY2237 | ++ |
| B. thailandensis UE5 | ++ |
| K. pneumoniae | +++ |
| V. parahaemolyticus | ++ |
| P. vasculitis | ++ |
| P. aeruginosa | ++ |
| A. baumannii | ++ |
| Salmonella gr. D | ++ |
| Shigella gr. D | +++ |
| C. freundii | +++ |
| Gram–positive bacteria | |
| Enterococcus sp. | ++ |
| S. epidermidis | – |
| S. aureus | – |
| Bacillus sp. | – |
| Micrococcus sp. | – |
| β-streptococcus gr. B | – |
| C. diphtheriae | – |
a% Relative lytic activity was calculated from different optical densities at 600 nm between the samples treated with buffer and enzyme. The score is indicated as: –no activity and +, ++, +++ indicating the different ranges including 1–30 %, 31–60 % and 61–100 %
Discussion
The increase in antibiotic-resistant bacteria makes the use of possible phage therapy as an alternative treatment for bacterial infections as one of multiple options for treatment. Similar to the concept of a mixed viral vaccine, phages also could be used as a portion of a cocktail for broad host range lysis and more phage could be added into suit the resistance situation (Chan et al. 2013). PG is the major component of Gram-positive bacteria and also the lining under the outer membrane of Gram-negative bacteria. Endolysins are a group of PG hydrolyzing enzymes well characterized in phages for their function on the release of the progeny out of the bacterial host during the lytic cycle. The information on the specificity of the phage and its enzymes against bacteria could facilitate their use safely.
A novel lytic phage ST79 and its modified phage that lyses B. pseudomallei has been reported to effectively lyse a broad range of the bacterium (Yordpratum et al. 2011; Kulsuwan et al. 2015). ST79 endolysin-like protein, peptidase M15A, was identified from the phage genome sequence and predicted to contain 149 amino acids (approximately 16 kDa) with catalytic but not the binding domain (Khakhum et al. 2016). This was similar to other lysins from phages that infect Gram-negative bacterial hosts, which contain single catalytic domains with a molecular mass of 15–20 kDa (Nelson et al. 2012). Endolysins KZ144 and EL188 from a Pseudomonas phage, however, were shown to contain both lytic and N-terminal binding domains (Briers et al. 2007b).
The peptidase M15A amino acid sequence analyzed by MEROPS showed a high identity with conserved amino acid sequences of the peptidase M15 subfamily A that is typical for metallopeptidases with a metal binding part (Rawlings and Morton 2008). A similar prediction was observed with BLASTP and Pfam results, detecting a conserved domain of peptidase M15_3_superfamily. The peptidase M15A from ST79 phage has 132 amino acid residues with active sites containing a Zn2+ ion-binding site at the following amino acids: His77, Asp84 and His199. The peptidase enzyme of a Streptomyces phage also contains His154, Asp161 and His197 of the D-Ala-D-Ala carboxypeptidase zinc specific cleavage site. When the Zn2+ ion was present, the activity of peptidase M15A from ST79 phage was inhibited while that of Streptomyces works more effectively (Courvalin 2006). Likewise, the Zn2+ inhibition effect is also found in the phage T5 endolysin, in which a 10 mM concentration completely inactivated the enzyme immediately after addition. It was also observed that this specific endolysin requires Ca2+ instead of Zn2+ or Mn2+ at the stage of the phage developmental cycle (Mikoulinskaia et al. 2009). Activity of the peptidase M15A was inhibited with addition of 100 μM Zn2+ (10 % of relative activity remain) while 100 μM of Mg2+, Ca2+ and Mn2+ caused a slightly increased enzyme activity. When 1000 μM of Mg2+, Mn2+ and Ca2+ were used, the activity was decreased. On the contrary, Zn2+ and Mn2+ are required for full enzymatic activity of LysB4 endolysin from the B. cereus-infecting phage B4 (Son et al. 2012) and this requirement is also seen in Ply500 endolysin from the Listeria monocytogenes phages (Loessner et al. 1995).
Characterization of some biochemical properties of the peptidase M15A showed a broad range of optimal pHs varying from 7.5 to 9.0 (% relative lytic activity >60 %), which is in alkalophilic range similar to other previously reported peptidases as observed for phage T5 endolysin (optimum pH 8.5) (Mikoulinskaia et al. 2009), lysB4 (pH 8.0–10.0) (Son et al. 2012), transglycosylase endolysin of the phage SPN1S (pH 7.0–10.5), and the highly thermostable Ts2631 amidase endolysin from the Thermus scotoductus phage vB_tsc2631 (7.0–11.0) (Plotka et al. 2015). The Peptidase M15A worked at 25–37 °C and also 42 °C which is the optimum temperature for cultivation of B. pseudomallei in the laboratory (Chen et al. 2003; Palasatien et al. 2008). The enzyme from phages mostly works in an alkalophilic pH.
In this study, all the chloroform permeabilized Gram-negative bacteria were prepared and used as a substrate to test the specificity of endolysin against PG (Briers et al. 2007a). The PG from 18 strains of Gram-negative bacteria including drug resistant strains B. pseudomallei G1, B. mallei EY2233 can be lysed by ST79 peptidase M15A. The enzyme may be more specific to peptidoglycans of Gram-negatives as the enzyme can lyse only Enterococcus sp. among eight Gram-positive bacteria tested. The amino acid composition and sequence of PG in Gram-negative bacteria is known to have a low variation and the PG type belong to A1γ. Even though PG from C. diphtheriae contains A1γ as in Gram-negative bacteria (Schleifer and Kandler 1972), its cell wall is distinct from others with a predominance of meso-diaminopimelic acid in the murein wall and multiple repetitions of arabinogalactan (Besserer et al. 2006). The PG is A1γ type, similar to Bacillus sp., but has modification like deacetylation and resists lysozyme digestion (Davis and Weiser 2011). Both of them were resistant to the ST79 peptidase M15A. For other Gram-positive bacteria, S. epidermidis, S. aureus, Micrococcus spp. and β- streptococcus group B, their PG belongs to the A3α type (Schleifer and Kandler 1972), preventing lysis by the peptidase M15A. Interestingly, Enterococcus sp., which is a Healthcare–Associated Infections (HAI) bacterium, was effectively lysed by ST79 peptidase M15A. The enzyme could act on the D-Ala-D-Ala termini of Enterococcus cell wall peptidoglycan (Arthur et al. 1996). Therefore, the action of the ST79 peptidase M15A may be specific to the peptidoglycan type A1γ of Gram-negative bacteria and to the Gram-positive Enterococcus sp.
Even though, in general, endolysin cannot attack the PG which is located under the outer membrane in Gram-negative bacteria, but permeabilized the outer membrane for example with 10 mM EDTA in combination with 50 mg/ml of the Pseudomonas endolysin EL188 can decrease the viable P. aeruginosa cells by 3 or 4 orders of magnitude in 30 min (Briers et al. 2011). Interestingly, LysAB2, the endolysin from A. baumannii phage ϕAB2 was reported to have an antibacterial effect against both Gram-negative (A. baumannii, E. coli, Salmonella enterica) and Gram-positive (Streptococcus sanguis, S. aureus, B. subtilis) strains (Lai et al. 2011). LysAB2 contains a C-terminal amphipathic region that is necessary for the antibacterial activity as also reported in the Lys1521 endolysin from a B. amyloliquefaciens phage that contains two cationic C-terminal regions. The cationic region was demonstrated to permeabilize the outer membrane of P. aeruginosa (Muyombwe et al. 1999). It is therefore possible to genetically engineer endolysin to have the cationic C-terminal regions to lyse Gram-negative bacteria from outside the same way as suggested by Nelson et al. (2012).
Lysozyme, also known as muramidase, is a well-known endolysin enzyme that is generally used as a standard for comparisons of endolysin activity. For example, A. baumannii phage phiAB2 endolysin activity was only 30 % activity of chicken egg white lysozyme (Lai et al. 2011). When the ST79 peptidase M15A activity was compared with chicken egg white lysozyme, it could reduce PG substrate approximately 2 times higher in lysis efficiency than lysozyme. Nevertheless, the transglycosylase SPN1S endolysin from Salmonella typhimurium-infecting phage can reduce 50 % OD of a 1 ml EDTA pretreated cell suspension when only 50 nanograms was used. This phage enzyme, therefore, has approximately 30 times higher activity than lysozyme (Lim et al. 2012) and also more than ST79 peptidase M15A.
In conclusion, ST79 peptidase M15A is specific to A1γ PG and cleaves PG at the peptide chains. The enzyme can work in a broad alkaligenic range and temperature, has higher activity when compared to lysozyme and is also active against a broad range of Gram-negative bacterial PG and also Enterococcus sp. that make the enzyme an outstanding one for further development. The cationic amphipathic C-terminal in some endolysins that showed permeability to Gram-negative bacteria may be genetically engineered into ST79 peptidase M15A and used as an adjunct to standard antibiotic therapy for B. pseudomallei infection. The combination of a compound that permeabilizes B. pseudomallei’s outer membrane with the enzyme that attacks PG may provide a more effective treatment in severe or drug resistant cases. Intensive investigation is, however, definitely required.
Authors’ contributions
NK, UY, AB, UT, JLR and RWS designed the experiments. NK performed experiments. NK and RWS drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Prof. James A. Will for editing the manuscript via Publication Clinic, Khon Kaen University, Thailand.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable since this article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This work was supported by The Royal Golden Jubilee Ph.D. program (Grant No. 4.O.KK/52/I.1.N.XX) for NK and RWS and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the heath cluster, project “Specific Health Problem in Greater Mekong Sub-region (SHeP-GMS)” of Khon Kaen University.
Abbreviations
- PG
peptidoglycan
- NAM
N-acetylmuramic acids
- NAG
N-acetylglucosamine
- NCBI
National Center for Biotechnology Information
- PCR
polymerase chain reaction
- LB
Luria and Bertani medium
- IPTG
isopropyl-β-d-thiogalactopyranoside
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- HAI
Healthcare–Associated Infections
- EDTA
Ethylenediaminetetraacetic acid
- OD
optical density
Contributor Information
Nittaya Khakhum, Email: nittaya_kha@hotmail.com.
Umaporn Yordpratum, Email: umapornyo@kku.ac.th.
Atcha Boonmee, Email: atcha@kku.ac.th.
Unchalee Tattawasart, Email: unchalee@kku.ac.th.
Jorge L. M. Rodrigues, Email: jmrodrigues@ucdavis.edu
Rasana W. Sermswan, Phone: 66-43-363265, Phone: 66-43-348386, Email: rasana@kku.ac.th
References
- Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. Phage treatment of human infections. Bacteriophage. 2011;1:66–85. doi: 10.4161/bact.1.2.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M, Reynolds P, Courvalin P. Glycopeptide resistance in enterococci. Trends Microbiol. 1996;4:401–407. doi: 10.1016/0966-842X(96)10063-9. [DOI] [PubMed] [Google Scholar]
- Berry J, Summer EJ, Struck DK, Young R. The final step in the phage infection cycle: the Rz and Rz1 lysis proteins link the inner and outer membranes. Mol Microbiol. 2008;70:341–351. doi: 10.1111/j.1365-2958.2008.06408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besserer A, Puech-Pagès V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, Portais J-C, Roux C, Bécard G, Séjalon-Delmas N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006;4:e226. doi: 10.1371/journal.pbio.0040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Cassarino TG, Bertoni M, Bordoli L, Schwede T. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014 doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysowski J, Weber-Dabrowska B, Górski A. Bacteriophage endolysins as a novel class of antibacterial agents. Exp Biol Med (Maywood) 2006;231:366–377. doi: 10.1177/153537020623100402. [DOI] [PubMed] [Google Scholar]
- Briers Y, Lavigne R, Volckaert G, Hertveldt K. A standardized approach for accurate quantification of murein hydrolase activity in high-throughput assays. J Biochem Biophys Meth. 2007;70:531–533. doi: 10.1016/j.jbbm.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Briers Y, Volckaert G, Cornelissen A, Lagaert S, Michiels CW, Hertveldt K, Lavigne R. Muralytic activity and modular structure of the endolysins of Pseudomonas aeruginosa bacteriophages phiKZ and EL. Mol Microbiol. 2007;65:1334–1344. doi: 10.1111/j.1365-2958.2007.05870.x. [DOI] [PubMed] [Google Scholar]
- Briers Y, Walmagh M, Lavigne R. Use of bacteriophage endolysin EL188 and outer membrane permeabilizers against Pseudomonas aeruginosa. J Appl Microbiol. 2011;110:778–785. doi: 10.1111/j.1365-2672.2010.04931.x. [DOI] [PubMed] [Google Scholar]
- Chan BK, Abedon ST, Loc-Carrillo C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013;8:769–783. doi: 10.2217/fmb.13.47. [DOI] [PubMed] [Google Scholar]
- Chen YS, Chen SC, Kao CM, Chen YL. Effects of soil pH, temperature and water content on the growth of Burkholderia pseudomallei. Folia Microbiol. 2003;48:253–256. doi: 10.1007/BF02930965. [DOI] [PubMed] [Google Scholar]
- Courvalin P. Vancomycin resistance in gram-positive cocci. Clinic Infect Dis. 2006;42(Suppl 1):S25–S34. doi: 10.1086/491711. [DOI] [PubMed] [Google Scholar]
- Davis KM, Weiser JN. Modifications to the peptidoglycan backbone help bacteria to establish infection. Infect Immun. 2011;79:562–570. doi: 10.1128/IAI.00651-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A, Salazar GA, Tate J, Bateman A. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruyter PGGA, Kuipers OP, Meijer WC, de Vos WM. Food-grade controlled lysis of Lactococcus lactis for accelerated cheese ripening. Nat Biotech. 1997;15:976–979. doi: 10.1038/nbt1097-976. [DOI] [PubMed] [Google Scholar]
- Gatedee J, Kritsiriwuthinan K, Galyov EE, Shan J, Dubinina E, Intarak N, Clokie MRJ, Korbsrisate S. Isolation and characterization of a novel podovirus which infects Burkholderia pseudomallei. Virol J. 2011;8:366. doi: 10.1186/1743-422X-8-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar Z, Bagasra O, Pace DG. Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J Infect Dev Ctries. 2014;8:129–136. doi: 10.3855/jidc.3573. [DOI] [PubMed] [Google Scholar]
- Guang-Han O, Leang-Chung C, Vellasamy KM, Mariappan V, Li-Yen C, Vadivelu J. Experimental Phage Therapy for Burkholderia pseudomallei Infection. PLoS One. 2016;11(7):e0158213. doi: 10.1371/journal.pone.0158213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K. Bacteriophage therapy for bacterial infections: rekindling a memory from the pre-antibiotics era. Perspect Biol Med. 2001;44(1):1–16. doi: 10.1353/pbm.2001.0006. [DOI] [PubMed] [Google Scholar]
- Jado I, López R, García E, Fenoll A, Casal J, García P, Network on behalf of the SPIS Phagelytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J Antimicrob Chemother. 2003;52:967–973. doi: 10.1093/jac/dkg485. [DOI] [PubMed] [Google Scholar]
- Khakhum N, Yordpratum U, Boonmee A, Tattawasart U, Rodrigues JL, Sermswan RW. Identification of the Burkholderia pseudomallei bacteriophage ST79 lysis gene cassette. J Appl Microbiol. 2016 doi: 10.1111/jam.13151. [DOI] [PubMed] [Google Scholar]
- Kulsuwan R, Wongratanacheewin S, Wongratanacheewin RS, Yordpratum U, Tattawasart U. Lytic capability of bacteriophages (Family Myoviridae) on Burkholderia pseudomallei. Southeast Asian J Trop Med Public Health. 2015;45:1344–1353. [PubMed] [Google Scholar]
- Kvitko BH, Cox CR, DeShazer D, Johnson SL, Voorhees KJ, Schweizer HP. φX216, a P2-like bacteriophage with broad Burkholderia pseudomallei and B. mallei strain infectivity. BMC Microbiol. 2012;12:289. doi: 10.1186/1471-2180-12-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M-J, Lin N-T, Hu A, Soo P-C, Chen L-K, Chen L-H, Chang K-C. Antibacterial activity of Acinetobacter baumannii phage ϕAB2 endolysin (LysAB2) against both gram-positive and gram-negative bacteria. App Microbiol biotechnol. 2011;90:529–539. doi: 10.1007/s00253-011-3104-y. [DOI] [PubMed] [Google Scholar]
- Leelarasamee A, Bovornkitti S. Melioidosis: review and update. Rev Infect Dis. 1989;11:413–425. doi: 10.1093/clinids/11.3.413. [DOI] [PubMed] [Google Scholar]
- Lavigne R, Briers Y, Hertveldt K, Robben J, Volckaert G. Identification and characterization of a highly thermostable bacteriophage lysozyme. Cell Mol Life Sci. 2004;61:2753–2759. doi: 10.1007/s00018-004-4301-y. [DOI] [PubMed] [Google Scholar]
- Lim J-A, Shin H, Kang D-H, Ryu S. Characterization of endolysin from a Salmonella Typhimurium-infecting bacteriophage SPN1S. Res Microbiol. 2012;163:233–241. doi: 10.1016/j.resmic.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Limmathurotsakul D, Funnell SGP, Torres AG, Morici LA, Brett PJ, Dunachie S, Atkins T, Altmann DM, Bancroft G, Peacock SJ. Consensus on the development of vaccines against naturally acquired melioidosis. Emerg Infect Dis. 2015 doi: 10.3201/eid2106.141480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loessner MJ, Wendlinger G, Scherer S. Heterogeneous endolysins in Listeria monocytogenes bacteriophages: a new class of enzymes and evidence for conserved holin genes within the siphoviral lysis cassettes. Mol Microbiol. 1995;16:1231–1241. doi: 10.1111/j.1365-2958.1995.tb02345.x. [DOI] [PubMed] [Google Scholar]
- Muyombwe A, Tanji Y, Unno H. Cloning and expression of a gene encoding the lytic functions of Bacillus amyloliquefaciens phage: evidence of an auxiliary lysis system. J Biosci Bioeng. 1999;88:221–225. doi: 10.1016/S1389-1723(99)80206-0. [DOI] [PubMed] [Google Scholar]
- Mikoulinskaia GV, Odinokova IV, Zimin AA, Lysanskaya VY, Feofanov SA, Stepnaya OA. Identification and characterization of the metal ion-dependent l-alanoyl-d-glutamate peptidase encoded by bacteriophage T5. FEBS J. 2009;276:7329–7342. doi: 10.1111/j.1742-4658.2009.07443.x. [DOI] [PubMed] [Google Scholar]
- Mongkolrob R, Taweechaisupapong S, Tungpradabkul S. Correlation between biofilm production, antibiotic susceptibility and exopolysaccharide composition in Burkholderia pseudomallei bpsI, ppk, and rpoS mutant strains. Microbiol Immunol. 2015;59:653–663. doi: 10.1111/1348-0421.12331. [DOI] [PubMed] [Google Scholar]
- Nelson DC, Schmelcher M, Rodriguez-Rubio L, Klumpp J, Pritchard DG, Dong S, Donovan DM. Endolysins as antimicrobials. Adv Virus Res. 2012;83:299–365. doi: 10.1016/B978-0-12-394438-2.00007-4. [DOI] [PubMed] [Google Scholar]
- O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palasatien S, Lertsirivorakul R, Royros P, Wongratanacheewin S, Sermswan RW. Soil physicochemical properties related to the presence of Burkholderia pseudomallei. Trans R Soc Trop Med Hyg. 2008;102(Suppl):S5–9. doi: 10.1016/S0035-9203(08)70003-8. [DOI] [PubMed] [Google Scholar]
- Pibalpakdee P, Wongratanacheewin S, Taweechaisupapong S, Niumsup PR. Diffusion and activity of antibiotics against Burkholderia pseudomallei biofilms. Int J Antimicrob Agents. 2012;39:356–359. doi: 10.1016/j.ijantimicag.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Piuri M, Hatfull GF. A peptidoglycan hydrolase motif within the mycobacteriophage TM4 tape measure protein promotes efficient infection of stationary phase cells. Mol Microbiol. 2006;62:1569–1585. doi: 10.1111/j.1365-2958.2006.05473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotka M, Kaczorowska AK, Morzywolek A, Makowska J, Kozlowski LP. Biochemical characterization and validation of a catalytic site of a highly thermostable Ts2631 endolysin from the Thermus scotoductus phage vB_Tsc2631. PLoS One. 2015;10(9):e0137374. doi: 10.1371/journal.pone.0137374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings ND, Morton FR. The MEROPS batch BLAST: a tool to detect peptidases and their non-peptidase homologues in a genome. Biochimie. 2008;90:243–259. doi: 10.1016/j.biochi.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: a laboratory manual. 3. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Sariya L, Prempracha N, Keelapan P, Chittasophon N. Bacteriophage isolated from Burkholderia pseudomallei causes phenotypic changes in Burkholderia thailandensis. Sci Asia. 2006;32:83–91. doi: 10.2306/scienceasia1513-1874.2006.32.083. [DOI] [Google Scholar]
- Sawasdidoln C, Taweechaisupapong S, Sermswan RW, Tattawasart U, Tungpradabkul S, Wongratanacheewin S. Growing Burkholderia pseudomallei in biofilm stimulating conditions significantly induces antimicrobial resistance. PLoS One. 2010;5:e9196. doi: 10.1371/journal.pone.0009196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer KH, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son B, Yun J, Lim J-A, Shin H, Heu S, Ryu S. Characterization of LysB4, an endolysin from the Bacillus cereus-infecting bacteriophage B4. BMC Microbiol. 2012;12:33. doi: 10.1186/1471-2180-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordpratum U, Tattawasart U, Wongratanacheewin S, Sermswan RW. Novel lytic bacteriophages from soil that lyse Burkholderia pseudomallei. FEMS Microbiol Lett. 2011;314:81–88. doi: 10.1111/j.1574-6968.2010.02150.x. [DOI] [PubMed] [Google Scholar]
- Young I, Wang I, Roof WD. Phages will out: strategies of host cell lysis. Trends Microbiol. 2000;8:120–128. doi: 10.1016/S0966-842X(00)01705-4. [DOI] [PubMed] [Google Scholar]
- Zdobnov EM, Apweiler R. InterProScan–an integration platform for the signature-recognition methods in InterPro. Bioinformatics. 2001;17:847–848. doi: 10.1093/bioinformatics/17.9.847. [DOI] [PubMed] [Google Scholar]


