Abstract
Background
Although early monitoring of BK virus infection in renal transplant patients has led to improved outcomes over the past decade, it remains unclear whether monitoring for viremia is the best screening tool for BK virus nephropathy (BKVN).
Methods
We conducted a retrospective review of the medical records of 368 renal transplant recipients who had a minimum of 18 months of posttransplantation follow-up. The relationship between the presence of BK viruria and a composite end point of BK viremia/BKVN was established, and the predictive value of high-grade BK viruria for development of viremia/BKVN was determined.
Results
High grade of BK viruria was present in 110 (30.1%) of the renal transplant recipients. BK viremia/BKVN was present in 64 (17.4%) patients and was 50 times more likely to be present in patients with high-grade BK viruria. The risk of developing BK viremia/BKVN was 3 times higher in high-grade viruria patients, and viruria preceded viremia by nearly 7 weeks.
Conclusion
The presence of high-grade viruria is an early marker for developing BK viremia/BKVN. Detection of high-grade viruria should prompt early allograft biopsy and/or preemptive reduction in immunosuppression.
Keywords: BK virus, BK virus nephropathy, Renal transplant, Viruria
Introduction
Along with cytomegalovirus, polyoma BK virus (BKV) remains a common cause of posttransplantation viral infections in renal transplant recipients. Although the first report of the virus was in 1971 [1], its prevalence in renal transplant recipients was not appreciated until the 1990s when more potent immunosuppressive agents were used for immunosuppression. Polyoma BKV is a small and nonenveloped DNA virus; the virus is ubiquitous, and as much as 80–90% of the general population is seropositive [2]. In immune-competent individuals, BKV reactivation and viruria occur in 15–40% of the general population [3], [4], [5], about 5–15% of renal transplant recipients become viremic, and 20–40% become BK viruric. It causes nephropathy in 1–10% of renal transplant recipients with most cases occurring during the first year [6], [7], [8], [9].
An early identification of renal transplant recipients at risk of BK virus nephropathy (BKVN) and an early diagnosis ensure appropriate evaluation and initiation of treatment. Several obstacles such as lack of standardized assays for polyomavirus and the lack of consensus on effective antiviral therapy remain [10], [11], [12]. Although Babel et al [13] showed in 2009 that sustained BK viruria was a reliable marker for development of BKV-associated nephropathy, checking for viruria using quantitative polymerase chain reaction (PCR) has not been widely adopted. Current guidelines recommend monitoring for viremia [14], [15].
As it is known that viruria precedes viremia in all renal transplant patients who eventually develop BKVN, our investigation was to see if the detection of high-grade viruria is a good screening test.
Methods
Study design
We retrospectively reviewed the medical charts of all renal transplant patients who were transplanted at the University of Chicago Medical Center (UCMC) between December 2001 and August 2009. This study was approved by the Institutional Review Board of the University of Chicago. Data regarding the transplant recipient demographics and HLA type, donor demographics and HLA type, urine and whole-blood/plasma BK PCR values, and results of transplant renal biopsies were collected. We defined BK viremia as >2,000 copies/mL in the blood sample.
Inclusion criteria
All patients who received a renal transplant at the UCMC were followed up at the UCMC for at least 18 months after transplantation and also had at least 1 urine specimen examined for the presence of BKV by quantitative PCR.
Exclusion criteria
We excluded renal transplant patients who suffered the loss of allograft immediately after transplantation because of graft thrombosis or other complications, were lost to follow-up, moved to a different place immediately after transplantation, died in the early posttransplantation period, or had no urine test done for BKV monitoring.
BKV monitoring
All renal transplant recipients have their urine samples checked for BKV by PCR at least once a month for the first 6 months and then every 2–3 months for additional 6 months. If a high-grade viruria (i.e., viral load greater than the upper limit of the detectable range) [9], [16] is detected, a second urine sample is obtained in 2 weeks. If 2 consecutive urine samples show high-grade viruria, whole-blood/plasma BK PCR is ordered.
BKV PCR
The BKV quantitative PCR assay at the UCMC is a multiplex assay that detects both BK and JC virus (JCV) DNA. It was initially validated for whole blood (EDTA) and urine specimens, as well as for cerebrospinal fluid (qualitative JCV results only). DNA extraction is performed using the MagNA Pure LightCycler (LC) (Roche Diagnostics, Indianapolis, IN, USA). An initial volume of 200 μL of patient sample is extracted and concentrated in 50 μL of eluate, using the MagNA Pure LC Total Nucleic Acid Isolation Kit. Two different known concentrations of positive samples containing BKV target, as well as a negative control containing bacterial DNA, are processed in each sample run to verify the accuracy of extraction.
Beginning December 14, 2009, processing was changed to plasma instead of whole blood. In addition, extraction was performed on an initial volume of 200 μL of patient sample and concentrated in 100 μL of eluate, using the MagNA Pure LC DNA Isolation Kit I. These changes improved the reliability of detection of low concentrations of BKV from blood specimens.
The assay is specifically adapted for PCR in glass capillaries using the LC Instrument from Roche Diagnostics. A 219-base pair (bp) fragment of the BKV and a 174-bp fragment of the JCV genome are amplified with specific primers and detected with probes labeled with LightCycler Red 705 (JCV) or with LightCycler Red 640 (BKV). An additional PCR product of 278 bp is formed from the internal positive control DNA to verify the absence of amplification inhibitors in negative samples. Primers and probes were purchased from TIB MOLBIOL (Berlin, Germany):
| Primer | Probe base sequence |
|---|---|
| 5BKfor | ACAgCAAAgCAggCAAgg |
| BK rev | GgAgTCCTggTggAgTTCC |
| 5JCfor | CTgAggAATgCATgCAgATCTA |
| JC rev | ggAATCCTggTggAATACA |
| Anchor | TTTTgCCATgAAgAAATgTTTgCCAgTAgATgA-FL |
| BKV | LC 640-AAgCAACAgCAgATTCTCAACACTCAACA-PH |
| JCV | LC 705-AAAACACAggATCCCAACACTCTACCCC-PH |
| IPC F | ATgCCACgTAAgCgAAACA |
| IPC R | gCATAAACgAAgCAgTCgAgT |
| IPC SS | CACTTCCCgAATAAC-FL |
| IPC 705 | LC 705-CggATATTTTTgATCTgACCgAAgCg-PH |
BKV, BK virus; IPC, internal positive control; JCV, JC virus.
The target is the gene for large T antigen. Master mix is prepared using LC FastStartPLUS DNA Master Hybridization Probes from Roche Diagnostics. The reaction mix is 5 μL of patient eluate added to 15 μL of master mix. The protocol includes 45 cycles of PCR.
Up to 6 dilutions of a stock solution of cloned target DNA are processed in each run and used to generate a standard curve to determine the absolute quantification of DNA present in positive patient samples. Quantification was reported in a range from 2,500 to 25 million copies/mL of patient sample; positives outside the range are reported as greater than (>) or less than (<). Beginning on December 14, 2009, the upper limit of the reportable range was extended to 50 million copies/mL of patient sample. The detection limit for the assay was validated as equivalent to 500 copies/mL of patient sample (10 copies per reaction). For positive samples, melting curve analysis is performed to verify the identification of the amplified product.
Renal allograft biopsy
An increase in the serum creatinine level of 0.4 mg/dL or higher without a probable cause led to an ultrasound-guided renal allograft biopsy. An 18-gauge spring-loaded biopsy gun was used to obtain 2–3 cores, and the samples were prepared and reviewed under light microscopy and immunofluorescence. BKVN was defined by the presence of typical viral cytopathic findings, stained with hematoxylin and eosin and periodic acid–Schiff methods and positive polyomavirus SV40 large T antigen (Tag) expression in tubular epithelial nuclei by standard immunohistochemistry (Ab-2; Oncogene Research Products, Cambridge, MA, USA).
Statistical analysis
The primary objective of this study was to determine the relationship between the presence of BKV replication in the urine (viruria) and concurrent or subsequent BK viremia or nephropathy. The composite event of interest, BK+, was defined as either positive whole-blood or plasma BKV DNA by quantitative PCR, or a positive biopsy test for BKVN. BK+ date was defined as the date when BKV was first detected in the blood or biopsy. For patients who had no evidence of BKV in any of the blood or biopsy tests (or BK–), the reference test date was defined as that of the last blood test or biopsy date.
A positive urine test (viruria) was defined as urine BK PCR count of ≥ 25 million copies/mL. Urinary BK PCR counts were further classified as none (0), low (< 25 million copies/mL), or high (≥ 25 million copies/mL).
We first examined the association between the reference BK+ test and the presence of BKV in the patients' urine test closest to it in time (within 30 days). A chi-square test was used to test for association between BK+ and viruria, and an Armitage trend test was used to determine whether the proportion of BK+ increased across the none/low/high viruria groups.
The association between viruria and BK+ status was also examined among all same-day urine and blood/biopsy tests. We used a generalized linear mixed model where BK+ was the outcome variable, urine BK count was the predictor, and patient random effect was included to account for serial tests within the patient. A receiver operating characteristic (ROC) curve was constructed based on the fitted generalized linear mixed model, and the area under the ROC curve (AUC) was calculated [17].
To assess whether a positive urine test increases the risk of developing BK+ in the future, we used the Cox proportional hazards model with time-varying covariate (urine positivity).
Time to first BK+ was defined as the time from the date of transplantation to the first positive blood or biopsy test. Patients without a positive test were censored at the date of their last available blood/biopsy date as described previously. The Kaplan–Meier method was used to estimate the survival curve.
Results
We identified 368 patients who underwent renal transplantation at the UCMC between December 2001 and August 2009 and met the inclusion criteria of our study. The baseline characteristics of these patients are shown in Table 1. The average age of recipients was 47.8 years (range, 0.9–79), and 133 (36%) were female. The average age of the donors was 39.3 years (range, 0.5–76). Two hundred thirty (62.5%) of 368 patients received a deceased-donor kidney, whereas 109 (29.6%) patients received a living-donor transplant. Of the 230 deceased donor renal transplants, 13 were second renal transplants and 2 were third renal transplants. The remaining 29 patients received multiorgan transplants (e.g., simultaneous pancreas kidney transplant, liver–kidney, heart–kidney, liver–heart–kidney, or heart–pancreas–kidney). Primary kidney diseases included diabetes (31.0%), hypertension (16.6%), unknown (13.0%), focal segmental glomerulosclerosis (6.3%), polycystic kidney disease (5.7%), systemic lupus erythematosus (4.1%), congenital kidney disease (4.1%), and IgA nephropathy (3.5%). There was no ABO-incompatible or desensitized renal transplantation. Two hundred thirty-three (63.3%) of 368 recipients were induced with thymoglobulin, whereas 123 (33.4%) received interleukin 2 receptor antagonists as their induction agent. Three hundred forty-three of 368 patients (93.2%) received a maintenance regimen consisting of tacrolimus, mycophenolate, and prednisone. Sixty-nine of 368 patients (18.8%) were considered at high risk for rejection (i.e., repeat transplantation, highly sensitized [panel-reactive antibody > 50%], multiorgan [excluding liver–kidney], and bilateral renal transplants; data not shown).
Table 1.
Baseline characteristics of patients receiving renal transplant between January 1, 2004 and December 31, 2009 (N = 368)
| Patient characteristics | No. of patients or mean ± SD |
|---|---|
| Donor type | |
| Deceased-donor renal transplant | 230 (62.5) |
| Living-donor kidney (related or unrelated) | 109 (29.6) |
| Multiorgan | 29 (7.9) |
| Donor age (y) | 39.3 ± 14.7 (range, 0.5–16) |
| Primary kidney disease | |
| Diabetes mellitus | 114 (31.0) |
| Hypertension | 61 (16.6) |
| Unknown | 48 (13.0) |
| FSGS | 23 (6.3) |
| PKD | 21 (5.7) |
| Congenital kidney diseases | 15 (4.1) |
| SLE and other CT disorders | 15 (4.1) |
| IgA nephropathy | 13 (3.5) |
| Other GNs (PSGN, MGN, etc) | 20 (5.4) |
| Miscellaneous | 38 (10.3) |
| Recipient gender | |
| Female | 133 (36.1) |
| Male | 235 (63.9) |
| Recipient age at transplant (y) | 47.7 ± 14.4 (range, 0.9–79) |
| Recipient race | |
| Caucasians | 121 (32.9) |
| African Americans | 165 (44.8) |
| Hispanic | 33 (9.0) |
| Asian | 9 (2.4) |
| Unknown | 40 (10.9) |
| HLA mismatches | 4.2 ± 1.7 |
| Induction | |
| Anti–thymocyte globulin | 233 (63.3) |
| IL-2 receptor antagonist | 123 (33.4) |
| Other/unknown | 12 (3.3) |
| Maintenance immunosuppression | |
| FK/MMF or MPS/pred | 343 (93.2) |
| CsA/MMF or MPS/pred | 7 (1.9) |
| Bela/MMF/pred | 5 (1.4) |
| CsA or FK/sirolimus/pred | 6 (1.6) |
| Other | 7 (1.9) |
Data are presented as n (%) or mean ± SD.
CsA, cyclosporine; CT, connective tissue; FK, tacrolimus; FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; HLA, human leukocyte antigen; IgA, immunoglobulin A; IL-2, interleukin 2; MGN, membranous; MMF, mycophenolate mofetil; MPS, mycophenolate sodium; PKD, polycystic kidney disease; PSGN, post-streptococcal glomerulonephritis; SLE, systemic lupus erythematosus.
Prevalence of BK viruria and BK viremia/BKVN
Of the 368 patients who underwent testing for BKV in their urine, 216 (59.2%) had nonzero BKV counts. A high level of BK viruria (≥ 25 million copies/mL) was found in 110 (30.1%) patients. At least 1 blood or biopsy BK test was available for all patients. Blood tests for BKV DNA were available for 361 patients, and 52 (14.4%) were positive. Transplant renal biopsies were performed in 248 patients, and 46 (18.6%) stained positive for SV40 large T antigen. The combined incidence of BK viremia and nephropathy was 17.4% (64 BK+ results).
Relationship between BK viruria and BK viremia/BKVN
A urine test done within 30 days of the reference blood or biopsy test was available for 318 (86.4%) patients. Of these, 274 (74.4%) patients had same-day tests, and 31 and 13 patients had a urine test within 30 days before or after the reference blood or biopsy test date, respectively. Sixty-two of the 318 patients (19.5%) were BK+. Median urine BK counts were 0 (range, 0–36.2 million copies/mL) among BK− patients and 25 million copies/mL (range, 0–25 million copies/mL) among BK+ patients. The proportion of BK+ patients was higher among patients with a positive urine test (77.1% vs. 5.8%, P < 0.0001). When BK urine presence was categorized as none (0 count), low (< 25 million), and high (≥ 25 million), the proportion of BK+ patients across these groups was 1.1%, 19.7%, and 77.1%, respectively (P < 0.0001, trend test).
Next, we considered all concurrent (same-day) urine and blood/biopsy tests. There were 2,724 instances when both types of tests were performed among 358 patients (the number of test instances ranged from 1 to 34 per patient). Agreement was high between the blood/biopsy and urine tests (88%) when a positive urine test was defined as ≥ 25 million (Table 2), and only a small proportion of cases were positive both on blood/biopsy and urine (5.7%). Agreement was lower when a positive urine test was defined as > 0 (62.75%). Among the urine-negative tests, only 20 of 2,262 (0.7%) were BK+, whereas a substantial proportion of urine-positive tests was BK− (307 of 462, 66.5%). The sensitivity and specificity of high-level BK viruria for diagnosing BK viremia/BKVN were 88.57% and 87.96%, respectively. The positive and negative predictive values were 33.55% and 99.12%, respectively.
Table 2.
Concordance between concurrent urine and blood/biopsy tests, using different cut points for defining viruria (N; percent of all 2,724 concurrent tests)
| Urine viruria cut point | BK− | BK+ | Total |
|---|---|---|---|
| Urine ≥ 25 million | |||
| No | 2,242 (82.3) | 20 (0.7) | 2,262 (83.0) |
| Yes | 307 (11.3) | 155 (5.7) | 462 (17.0) |
| Urine > 0 | |||
| No | 1,537 (56.4) | 3 (0.1) | 1,540 (56.5) |
| Yes | 1,012 (37.2) | 172 (6.3) | 1,184 (43.5) |
Data are presented as n (%).
Predictive value of BK viruria for the presence of BK viremia/BKVN
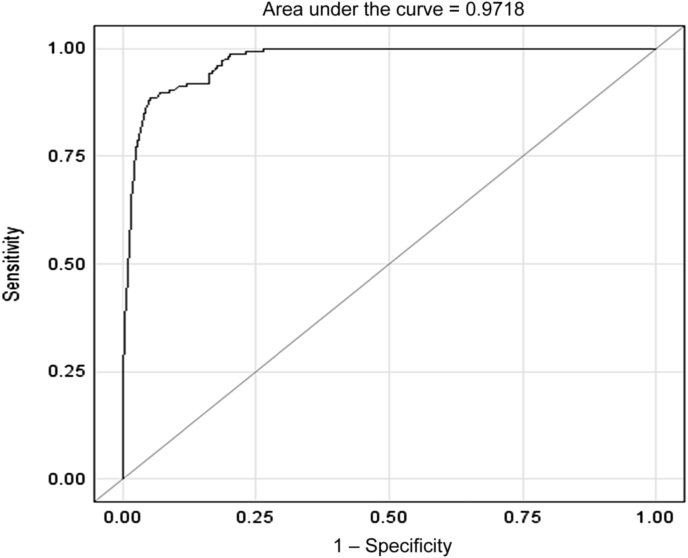
We further analyzed the value of the presence and degree of BK viruria as a predictor of blood/biopsy positivity using the ROC analysis. The generalized linear mixed-effects model was fitted with urine BK count as the predictor, and the resulting AUC was 0.97, indicating that concurrent presence of BK in the urine is a very strong predictor of the presence of viremia and/BKVN. In a separate model, we found that those who had 25 million or higher BK count in the urine were nearly 50 times more likely to also have a positive blood/biopsy result than those who had < 25 million counts (odds ratio, 50.33; 95% confidence interval, 28.6–88.5; P < 0.0001).
Predictive value of BK viruria for the development of BK viremia/BKVN
We explored whether the presence of high levels of BKV in the urine is associated with an increased risk of subsequent detection of BKV in blood or biopsy. The majority of patients (358, 97.3%) had at least 1 urine test available before the reference blood/biopsy evaluation, and 72 (20.1%) of these urine tests were positive (≥ 25 million copies/mL). Among patients with a positive urine test, 24 (33.33%) subsequently developed BK+, whereas only 33 of 286 (11.5%) patients with all negative urine tests subsequently developed BK+.
Time to the development of BK viremia/BKVN
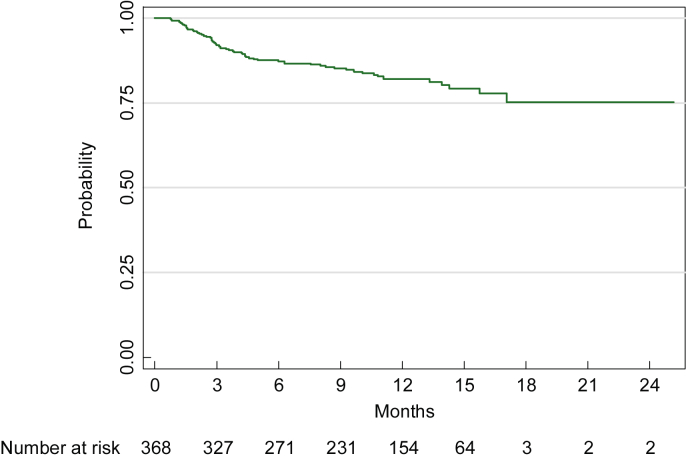
Among the 368 patients, 64 developed BK viremia or nephropathy (or BK+) during 18 months after transplantation. Time to BK+ was estimated using the Kaplan–Meier method, and patients who did not develop viremia or nephropathy (i.e., BK−) were censored at the time of the last blood/biopsy test. BK+ rates at 6, 12, and 18 months were 12.8%, 18%, and 24.8%, respectively (Figure 1, Figure 2). Median follow-up time for patients who did not develop viremia/BKVN was 11.9 months (range, 0.3–25.2).
Figure 1.
Time to first evidence of viremia or BKVN estimated using the method of Kaplan–Meier.
BKVN, BK virus nephropathy.
Figure 2.
ROC curve for BK viruria as a predictor of blood/biopsy positivity.
ROC, receiver operating characteristic.
Median time from the first positive urine test until BK+ (n = 24) was 35 days (range, 12–266), and median follow-up time for those who never developed viremia/BKVN was 198 days (range, 14–490). The median number of urine tests before BK+ diagnosis or end of follow-up period was 5 (range, 1–25). A time-varying covariates Cox model revealed that a positive urine test greatly increased the hazard of subsequent development of BK+ (hazard ratio, 7.0; P < 0.001).
Discussion
The art and science of managing renal transplant recipients centers around the Goldilocks principle: providing immunosuppression that is “just right” to prevent allograft rejection while minimizing the risk of opportunistic infections and malignancies. It requires vigilant monitoring for allograft dysfunction and detection of signs and/or symptoms of opportunistic infections. Screening and diagnosis of BKV infection/BKVN in renal transplant recipients is fraught with many obstacles:
-
1.
Negative renal allograft biopsy does not rule out BKVN because of the focal presence of cytopathic changes [18]—and SV40 staining may not detect all infected cells [19].
-
2.
Quantitative DNA PCR tests are not standardized, and direct comparison of the viral load is not possible [20], [21].
-
3.
Currently, there are no safe and effective antiviral agents available, and most data on the use of antiviral agents are based on small nonrandomized studies [10].
-
4.
Reduction of immunosuppression is the recommended treatment, but this can lead to allograft rejection/loss.
-
5.
Although early diagnosis of BKVN on the biopsy, with subsequent reduction or modification of immunosuppression [22], often leads to resolution of infection and prevents further decline in the kidney allograft function in many cases, the damage from BKVN may not be reversed despite modification of immunosuppression [23], [24]. A large percentage of these patients are left with chronic allograft dysfunction, which leads to a shortened allograft survival [25].
Nevertheless, BK viremia and BKVN are almost always preceded by a period of detectable viruria [13]. Therefore, as compared with the following BKV in the blood, monitoring of BKV in the urine may lead to earlier detection of the viral replication in the allograft. Therefore, in our study, we sought to determine whether a high-level BK viruria was predictive of the presence or development of BK viremia or BKVN.
In the present study, we found high prevalence of BK viruria (59.2%) in our renal transplant patients during the first 18 months after transplantation. Almost half of these patients had high levels of BK viruria (> 25 million copies/mL). The prevalence of viremia alone was 14.4% and when combined with nephropathy was 17.4%. Our findings are in line with those of other investigators [13], [25], [26].
The analyses further showed that when a urine test was performed within 30 days of the BK blood test or biopsy, the median BK urine counts were significantly higher among the patients with viremia/BKVN as compared with those without (25 million copies/mL vs. 0 copies/mL). Conversely, the proportion of patients with positive BK blood test or biopsy was significantly higher in the patients with viruria (77.1% vs. 5.8%, P < 0.0001). Furthermore, the proportion of patients with viremia/BKVN increased as the level of viruria increased (1.1% in no viruria, 19.7% with urine BK viral load < 25 million, and 77.1% with urine BK viral load ≥ 25 million).
When the BK testing in the urine and blood/biopsy was done on the same day, a negative urine value (< 25 million copies/mL) highly corresponded with a negative blood/biopsy result, with only 0.9% of tests being positive for viremia or nephropathy in the absence of high levels of viruria. The presence of high-level viruria was found to be a very strong predictor of concurrent systemic BK infection (AUC = 0.97). Furthermore, patients with high levels of BK viruria (> 25 million) were 50 times more likely to have concomitant viremia/BKVN than those with low levels of BK viruria (< 25 million).
This study also shows that for patients with high levels of viruria in the early posttransplantation period, the risk of subsequently developing BK viremia/BKVN is 3 times higher than those without.
We chose to check the urine PCR because of its ease of sample collection and the high negative predictive value of the test. In addition, monitoring for BK viruria is also potentially beneficial as Masutani et al [27] showed that patients who have frequent viruria are more prone to acute rejection episodes.
We also recognize issues associated with urine PCR tests: (1) a large percentage of patients with viruria may not develop viremia or nephropathy (low positive predictive value) and (2) the lag in viral load reduction on lowering immunosuppression. However, early diagnosis using urine PCR may become more useful when an effective antiviral therapy becomes available, and a prompt intervention can result in eradication of viremia before irreversible tissue damages are done.
We recognize the limitations of our study—retrospective and single-center study; however, the large sample size and the long duration of monitoring period make its findings—the presence of high level of BK viruria can be used as a surrogate marker for viremia or nephropathy—meaningful and should be followed up either with an allograft biopsy or with a preemptive reduction or modification of immunosuppression.
We propose that BK monitoring should be routinely performed by serial examinations of urinary BK viral loads. At our center, the cutoff for BK levels in urine that indicated high risk was determined at ≥ 25 million copies/mL. However, the cutoff values should be determined by each center as there are different assays that use different probes for detection of BKV. Persistence of high levels of viruria should not be ignored and should be considered a risk factor for development of and potentially a marker for the presence of BKVN.
Conflicts of interest
All authors have no conflicts of interest to declare.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.krcp.2016.05.005.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Gardner S.D., Field A.M., Coleman D.V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;19:1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- 2.Egli A., Infanti L., Dumoulin A., Buser A., Samaridis J., Stebler C., Gosert R., Hirsch H.H. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 3.Knowles W.A., Pipkin P., Andrews N., Vyse A., Minor P., Brown D.W., Miller E. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 4.Polo C., Pérez J.L., Mielnichuck A., Fedele C.G., Niubò J., Tenorio A. Prevalence and patterns of polyomavirus urinary excretion in immunocompetent adults and children. Clin Microbiol Infect. 2004;10:640–644. doi: 10.1111/j.1469-0691.2004.00882.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhong S., Zheng H., Suzuki M., Chen Q., Ikegaya H., Aoki N., Usuku S., Kobayashi N., Nukuzuma S., Yasuda Y., Kuniyoshi N., Yogo Y., Kitamura T. Age-related urinary excretion of BK polyomavirus by nonimmunocompromised individuals. J Clin Microbiol. 2007;45:193–198. doi: 10.1128/JCM.01645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuypers D.R. Management of polyomavirus-associated nephropathy in renal transplant recipients. Nat Rev Nephrol. 2012;8:390–402. doi: 10.1038/nrneph.2012.64. [DOI] [PubMed] [Google Scholar]
- 7.Masutani K. Current problems in screening, diagnosis and treatment of polyomavirus BK nephropathy. Nephrology (Carlton) 2014;19(Suppl 3):S11–S16. doi: 10.1111/nep.12254. [DOI] [PubMed] [Google Scholar]
- 8.Babel N., Volk H., Reinke P. BK polyomavirus infection and nephropathy: the virus-immune system interplay. Nat Rev Nephrol. 2011;7:399–406. doi: 10.1038/nrneph.2011.59. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch H.H., Brennan D.C., Drachenberg C.B., Ginevri F., Gordon J., Limaye A.P., Mihatsch M.J., Nickeleit V., Ramos E., Randhawa P., Shapiro R., Steiger J., Suthanthiran M., Trofe J. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79:1277–1286. doi: 10.1097/01.tp.0000156165.83160.09. [DOI] [PubMed] [Google Scholar]
- 10.Johnston O., Jaswal D., Gill J.S., Doucette S., Fergusson D.A., Knoll G.A. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation. 2010;89:1057–1070. doi: 10.1097/TP.0b013e3181d0e15e. [DOI] [PubMed] [Google Scholar]
- 11.Ramos E., Drachenberg C.B., Wali R., Hirsch H.H. The decade of polyomavirus BK-associated nephropathy: state of affairs. Transplantation. 2009;87:621–630. doi: 10.1097/TP.0b013e318197c17d. [DOI] [PubMed] [Google Scholar]
- 12.Dharnidharka V.R., Abdulnour H.A., Araya C.E. The BK virus in renal transplant recipients—review of pathogenesis, diagnosis, and treatment. Pediatr Nephrol. 2011;26:1763–1774. doi: 10.1007/s00467-010-1716-6. [DOI] [PubMed] [Google Scholar]
- 13.Babel N., Fendt J., Karaivanov S., Bold G., Arnold S., Sefrin A., Lieske E., Hoffzimmer M., Dziubianau M., Bethke N., Meisel C., Grütz G., Reinke P. Sustained BK viruria as an early marker for the development of BKV-associated nephropathy: analysis of 4128 urine and serum samples. Transplantation. 2009;88:89–95. doi: 10.1097/TP.0b013e3181aa8f62. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch H.H., Randhawa P., the AST Infectious Disease Community of Practice BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):179–188. doi: 10.1111/ajt.12110. [DOI] [PubMed] [Google Scholar]
- 15.Kidney Disease Improving Global Outcomes (KDIGO) Transplant Working Group KDIGO clinical practice guidelines for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S44–S46. [Google Scholar]
- 16.Viscount H.B., Eid A.J., Espy M.J., Griffin M.D., Thomsen K.M., Harmsen W.S., Razonable R.R., Smith T.F. Polyomavirus polymerase chain reaction as a surrogate marker of polyomavirus-associated nephropathy. Transplantation. 2007;84:340–345. doi: 10.1097/01.tp.0000275205.41078.51. [DOI] [PubMed] [Google Scholar]
- 17.Therneau T.M., Grambsch P.M. 1st edition. Springer Science & Business Media; New York: 2000. Modeling Survival Data: Extending the Cox Model. [Google Scholar]
- 18.Drachenberg C.B., Papadimitriou J.C., Hirsch H.H., Wali R., Crowder C., Nogueira J., Cangro C.B., Mendley S., Mian A., Ramos E. Histological patterns of polyomavirus nephropathy: correlation with graft outcome and viral load. Am J Transplant. 2004;4:2082–2092. doi: 10.1046/j.1600-6143.2004.00603.x. [DOI] [PubMed] [Google Scholar]
- 19.Seemayer C.A., Seemayer N.H., Dürmüller U., Gudat F., Schaub S., Hirsch H.H., Mihatsch M.J. BK virus large T and VP-1 expression in infected human renal allografts. Nephrol Dial Transplant. 2008;23:3752–3761. doi: 10.1093/ndt/gfn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman N.G., Cook L., Atienza E.E., Limaye A.P., Jerome K.R. Marked variability of BK virus load measurement using quantitative real-time PCR among commonly used assays. J Clin Microbiol. 2008;46:2671–2680. doi: 10.1128/JCM.00258-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bechert C.J., Schnadig V.J., Payne D.A., Dong J. Monitoring of BK viral load in renal allograft recipients by real-time PCR assays. Am J Clin Pathol. 2010;133:242–250. doi: 10.1309/AJCP63VDFCKCRUUL. [DOI] [PubMed] [Google Scholar]
- 22.Brennan D.C., Agha I., Bohl D.L., Schnitzler M.A., Hardinger K.L., Lockwood M., Torrence S., Schuessler R., Roby T., Gaudreault-Keener M., Storch G.A. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5:582–594. doi: 10.1111/j.1600-6143.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 23.Wadei H.M., Rule A.D., Lewin M., Mahale A.S., Khamash H.A., Schwab T.R., Gloor J.M., Textor S.C., Fidler M.E., Lager D.J., Larson T.S., Stegall M.D., Cosio F.G., Griffin M.D. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN) Am J Transplant. 2006;6:1025–1032. doi: 10.1111/j.1600-6143.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 24.Vasudev B., Hariharan S., Hussain S.A., Zhu Y.R., Bresnahan B.A., Cohen E.P. BK virus nephritis: risk factors, timing, and outcome in renal transplant recipients. Kidney Int. 2005;68:1834–1839. doi: 10.1111/j.1523-1755.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 25.Alméras C., Foulongne V., Garrigue V., Szwarc I., Vetromile F., Segondy M., Mourad G. Does reduction in immunosuppression in viremic patients prevent BK virus nephropathy in de novo renal transplant recipients? A prospective study. Transplantation. 2008;85:1099–1104. doi: 10.1097/TP.0b013e31816a33d4. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch H.H., Knowles W., Dickenmann M., Passweg J., Klimkait T., Mihatsch M.J., Steiger J. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Eng J Med. 2002;347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 27.Masutani K., Shapiro R., Basu A., Tan H., Ninomiya T., Randhawa P. Putative episodes of T-cell-mediated rejection in patients with sustained BK viruria but no viremia. Transplantation. 2012;94:43–49. doi: 10.1097/TP.0b013e318253e7a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.