Abstract
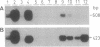
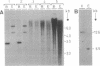
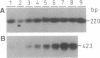
A genomic clone of one member of the Arabidopsis thaliana (L.) Heynh. 1-aminocyclopropane-1-carboxylate (ACC) synthase (S-adenosyl-L-methionine methylthioadenosine-lyase, EC 4.4.1.14) gene family (AT-ACC1) was isolated and sequenced. A region of homology was found in the 5'-untranslated region with the promoter of a zucchini and a tomato ACC synthase gene. Comparison of its primary structure with other ACC synthases revealed conservation of seven peptide regions as well as similarity with 11 amino acids of the catalytic site of aminotransferases. Genomic DNA gel blotting suggested the existence of an ACC synthase multigene family in Arabidopsis, possibly with three other members, none of which is very closely related to AT-ACC1. The existence of at least one other gene was confirmed by the isolation of a cDNA (AT-ACC2) from a flower-specific cDNA library. The AT-ACC1 gene was mapped on the Arabidopsis restriction fragment length polymorphism map and is located on the top of chromosome 1. This position does not correspond to any known mutation on the genetic map. Expression of the AT-ACC1 gene was studied by reverse transcription-PCR on total RNA. Messenger accumulation was strong in young leaves and flowers. The gene was not induced by wounding of young leaves or in seedlings in the presence of auxin. Ethylene exposure of mature plants led to an induction of AT-ACC1 gene expression. It is suggested that AT-ACC1 protein has a role in developmental control of ethylene synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell T. K., Kretzner L., Blackwood E. M., Eisenman R. N., Weintraub H. Sequence-specific DNA binding by the c-Myc protein. Science. 1990 Nov 23;250(4984):1149–1151. doi: 10.1126/science.2251503. [DOI] [PubMed] [Google Scholar]
- Botella J. R., Schlagnhaufer C. D., Arteca R. N., Phillips A. T. Identification and characterization of three putative genes for 1-aminocyclopropane-1-carboxylate synthase from etiolated mung bean hypocotyl segments. Plant Mol Biol. 1992 Feb;18(4):793–797. doi: 10.1007/BF00020022. [DOI] [PubMed] [Google Scholar]
- Buck K. J., Harris R. A., Sikela J. M. A general method for quantitative PCR analysis of mRNA levels for members of gene families: application to GABAA receptor subunits. Biotechniques. 1991 Nov;11(5):636–641. [PubMed] [Google Scholar]
- Csank C., Taylor F. M., Martindale D. W. Nuclear pre-mRNA introns: analysis and comparison of intron sequences from Tetrahymena thermophila and other eukaryotes. Nucleic Acids Res. 1990 Sep 11;18(17):5133–5141. doi: 10.1093/nar/18.17.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Tamaki S., Dunsmuir P., Favreau M., Katayama C., Dooner H., Bedbrook J. mRNA transcripts of several plant genes are polyadenylated at multiple sites in vivo. Nucleic Acids Res. 1986 Mar 11;14(5):2229–2240. doi: 10.1093/nar/14.5.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delidow B. C., Peluso J. J., White B. A. Quantitative measurement of mRNAs by polymerase chain reaction. Gene Anal Tech. 1989 Nov-Dec;6(6):120–124. doi: 10.1016/0735-0651(89)90002-2. [DOI] [PubMed] [Google Scholar]
- Giuliano G., Pichersky E., Malik V. S., Timko M. P., Scolnik P. A., Cashmore A. R. An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7089–7093. doi: 10.1073/pnas.85.19.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goblet C., Prost E., Whalen R. G. One-step amplification of transcripts in total RNA using the polymerase chain reaction. Nucleic Acids Res. 1989 Mar 11;17(5):2144–2144. doi: 10.1093/nar/17.5.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiltinan M. J., Marcotte W. R., Jr, Quatrano R. S. A plant leucine zipper protein that recognizes an abscisic acid response element. Science. 1990 Oct 12;250(4978):267–271. doi: 10.1126/science.2145628. [DOI] [PubMed] [Google Scholar]
- Huang P. L., Parks J. E., Rottmann W. H., Theologis A. Two genes encoding 1-aminocyclopropane-1-carboxylate synthase in zucchini (Cucurbita pepo) are clustered and similar but differentially regulated. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7021–7025. doi: 10.1073/pnas.88.16.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. T., Silverstone A., Yip W. K., Dong J. G., Yang S. F. Induction of 1-aminocyclopropane-1-carboxylate synthase mRNA by auxin in mung bean hypocotyls and cultured apple shoots. Plant Physiol. 1992 Feb;98(2):465–471. doi: 10.1104/pp.98.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Green P., Abrahamson J., Barlow A., Daly M. J., Lincoln S. E., Newberg L. A., Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987 Oct;1(2):174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Logemann J., Schell J. Nucleotide sequence and regulated expression of a wound-inducible potato gene (wun1). Mol Gen Genet. 1989 Oct;219(1-2):81–88. doi: 10.1007/BF00261161. [DOI] [PubMed] [Google Scholar]
- Myers E. W., Miller W. Optimal alignments in linear space. Comput Appl Biosci. 1988 Mar;4(1):11–17. doi: 10.1093/bioinformatics/4.1.11. [DOI] [PubMed] [Google Scholar]
- Nam H. G., Giraudat J., Den Boer B., Moonan F., Loos WDB., Hauge B. M., Goodman H. M. Restriction Fragment Length Polymorphism Linkage Map of Arabidopsis thaliana. Plant Cell. 1989 Jul;1(7):699–705. doi: 10.1105/tpc.1.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson D. C., White J. A., Edelman L., Harkins R. N., Kende H. Differential expression of two genes for 1-aminocyclopropane-1-carboxylate synthase in tomato fruits. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5340–5344. doi: 10.1073/pnas.88.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. Y., Drory A., Woodson W. R. Molecular cloning of an 1-aminocyclopropane-1-carboxylate synthase from senescing carnation flower petals. Plant Mol Biol. 1992 Jan;18(2):377–386. doi: 10.1007/BF00034964. [DOI] [PubMed] [Google Scholar]
- Rottmann W. H., Peter G. F., Oeller P. W., Keller J. A., Shen N. F., Nagy B. P., Taylor L. P., Campbell A. D., Theologis A. 1-aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol. 1991 Dec 20;222(4):937–961. doi: 10.1016/0022-2836(91)90587-v. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Oeller P. W., Theologis A. The 1-aminocyclopropane-1-carboxylate synthase of Cucurbita. Purification, properties, expression in Escherichia coli, and primary structure determination by DNA sequence analysis. J Biol Chem. 1991 Feb 25;266(6):3752–3759. [PubMed] [Google Scholar]
- Schulze-Lefert P., Dangl J. L., Becker-André M., Hahlbrock K., Schulz W. Inducible in vivo DNA footprints define sequences necessary for UV light activation of the parsley chalcone synthase gene. EMBO J. 1989 Mar;8(3):651–656. doi: 10.1002/j.1460-2075.1989.tb03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford A., Bevan M., Northcote D. Differential expression within a family of novel wound-induced genes in potato. Mol Gen Genet. 1989 Jan;215(2):200–208. doi: 10.1007/BF00339718. [DOI] [PubMed] [Google Scholar]
- Sánchez-Bravo J., Ortuño A. M., Pérez-Gilabert M., Acosta M., Sabater F. Modification by ethylene of the cell growth pattern in different tissues of etiolated lupine hypocotyls. Plant Physiol. 1992 Mar;98(3):1121–1127. doi: 10.1104/pp.98.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Straeten D., Van Wiemeersch L., Goodman H. M., Van Montagu M. Cloning and sequence of two different cDNAs encoding 1-aminocyclopropane-1-carboxylate synthase in tomato. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4859–4863. doi: 10.1073/pnas.87.12.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip W. K., Moore T., Yang S. F. Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2475–2479. doi: 10.1073/pnas.89.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]