Abstract
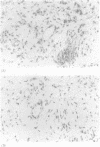
AIMS--Tissue fibrosis is a common and serious consequence of chronic inflammation. The mechanism linking these two processes is poorly understood. The present study has utilised a human in vivo model of a delayed type hypersensitivity (DTH) reaction, the tuberculin Heaf reaction, induced by intradermal tuberculin in BCG immunised subjects, to dissect the relation between these two processes. METHODS--Punch skin biopsy specimens were obtained on day 5, day 13 and six to 16 weeks following the tuberculin Heaf test in 18 subjects with grade 3 or 4 responses. Skin biopsy specimens from six subjects served as controls. The specimens were examined using immunohistochemical staining for type 1 procollagen and transforming growth factor-beta (TGF-beta), as well as in situ hybridisation for type 1 procollagen messenger RNA (mRNA). RESULTS--Immunohistochemical analysis revealed increased deposition of TGF-beta in tissue matrix in the biopsy specimens obtained on day 5 following the tuberculin Heaf test. There was also extensive type 1 procollagen staining in the biopsy specimens obtained as early as day 5. Procollagen-1 staining was maximal on day 13, and was present in biopsy specimens from tuberculin Heaf test sites up to eight weeks after the tuberculin inoculation. The type 1 procollagen was localised within cells surrounding areas of inflammatory infiltrate and in perivascular tissues. The presence of new collagen formation was confirmed by in situ hybridisation using oligonucleotide probes for type 1 procollagen mRNA in cells in sections from biopsy specimens obtained on day 13. CONCLUSIONS--These data from a human in vivo model of a DTH response indicate that the immune response is intimately associated with an increase in the production of growth factors and the initiation of a fibrotic response.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Bravo M. A., Avila R. E., Galanopoulos T., Neville-Golden J., Maxwell M., Selman M. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990 Oct;86(4):1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Abrams J. S., Lu S., Sieling P. A., Rea T. H., Modlin R. L. Patterns of cytokine production by mycobacterium-reactive human T-cell clones. Infect Immun. 1993 Jan;61(1):197–203. doi: 10.1128/iai.61.1.197-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Lu S., Abrams J. S., Wang E., Yamamura M., Modlin R. L. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993 Aug;61(8):3482–3489. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard M. P., Chu M. L., Myers J. C., Ramirez F., Eikenberry E. F., Prockop D. J. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry. 1983 Oct 25;22(22):5213–5223. doi: 10.1021/bi00291a023. [DOI] [PubMed] [Google Scholar]
- Broekelmann T. J., Limper A. H., Colby T. V., McDonald J. A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C. Q., Field M., Andrew E., Haskard D., Feldmann M., Maini R. N. Detection of cytokines at the site of tuberculin-induced delayed-type hypersensitivity in man. Clin Exp Immunol. 1992 Dec;90(3):522–529. doi: 10.1111/j.1365-2249.1992.tb05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders K. C., Thompson N. L., Cissel D. S., Van Obberghen-Schilling E., Baker C. C., Kass M. E., Ellingsworth L. R., Roberts A. B., Sporn M. B. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989 Feb;108(2):653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullmer M. A., Shen J. Y., Modlin R. L., Rea T. H. Immunohistological evidence of lymphokine production and lymphocyte activation antigens in tuberculin reactions. Clin Exp Immunol. 1987 Feb;67(2):383–390. [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Konttinen Y. T., Bergroth V., Visa-Tolvanen K., Reitamo S., Förström L. Cellular infiltrate in situ and response kinetics of human intradermal and epicutaneous tuberculin reactions. Clin Immunol Immunopathol. 1983 Sep;28(3):441–449. doi: 10.1016/0090-1229(83)90111-3. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Broekelmann T. J., Matheke M. L., Crouch E., Koo M., Kuhn C., 3rd A monoclonal antibody to the carboxyterminal domain of procollagen type I visualizes collagen-synthesizing fibroblasts. Detection of an altered fibroblast phenotype in lungs of patients with pulmonary fibrosis. J Clin Invest. 1986 Nov;78(5):1237–1244. doi: 10.1172/JCI112707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka I., Trapnell B. C., Crystal R. G. Upregulation of platelet-derived growth factor-A and -B gene expression in alveolar macrophages of individuals with idiopathic pulmonary fibrosis. J Clin Invest. 1990 Jun;85(6):2023–2027. doi: 10.1172/JCI114669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Uchida H., Kusumoto Y., Mori Y., Yamamura Y., Hamada S. Increase in tumor necrosis factor alpha- and interleukin-6-secreting cells in peripheral blood mononuclear cells from subjects infected with Mycobacterium tuberculosis. Infect Immun. 1991 Sep;59(9):3021–3025. doi: 10.1128/iai.59.9.3021-3025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Griffin G. L., Senior R. M., Deuel T. F. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989 Jul;109(1):429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Seymour G. J., Duke O., Janossy G., Panayi G. Immunohistological analysis of delayed-type hypersensitivity in man. Cell Immunol. 1982 Dec;74(2):358–369. doi: 10.1016/0008-8749(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Quaglino D., Jr, Nanney L. B., Kennedy R., Davidson J. M. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin. I. Excisional wound model. Lab Invest. 1990 Sep;63(3):307–319. [PubMed] [Google Scholar]
- Ribera E., Español T., Martinez-Vazquez J. M., Ocaña I., Encabo G. Lymphocyte proliferation and gamma-interferon production after "in vitro" stimulation with PPD. Differences between tuberculous and nontuberculous pleurisy in patients with positive tuberculin skin test. Chest. 1990 Jun;97(6):1381–1385. doi: 10.1378/chest.97.6.1381. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Ying S., Taylor I. K., Wangoo A., Mitchell D. M., Kay A. B., Hamid Q., Shaw R. J. Evidence for a Th1-like bronchoalveolar T-cell subset and predominance of interferon-gamma gene activation in pulmonary tuberculosis. Am J Respir Crit Care Med. 1994 Apr;149(4 Pt 1):989–993. doi: 10.1164/ajrccm.149.4.8143065. [DOI] [PubMed] [Google Scholar]
- Shaw R. J., Benedict S. H., Clark R. A., King T. E., Jr Pathogenesis of pulmonary fibrosis in interstitial lung disease. Alveolar macrophage PDGF(B) gene activation and up-regulation by interferon gamma. Am Rev Respir Dis. 1991 Jan;143(1):167–173. doi: 10.1164/ajrccm/143.1.167. [DOI] [PubMed] [Google Scholar]
- Taylor M., Cook T., Pearson C., Risdon R. A., Peart S. Renin messenger RNA localization in congenital mesoblastic nephroma using in situ hybridization. J Hypertens. 1989 Sep;7(9):733–740. [PubMed] [Google Scholar]
- Tsicopoulos A., Hamid Q., Varney V., Ying S., Moqbel R., Durham S. R., Kay A. B. Preferential messenger RNA expression of Th1-type cells (IFN-gamma+, IL-2+) in classical delayed-type (tuberculin) hypersensitivity reactions in human skin. J Immunol. 1992 Apr 1;148(7):2058–2061. [PubMed] [Google Scholar]
- Wangoo A., Taylor I. K., Haynes A. R., Shaw R. J. Up-regulation of alveolar macrophage platelet-derived growth factor-B (PDGF-B) mRNA by interferon-gamma from Mycobacterium tuberculosis antigen (PPD)-stimulated lymphocytes. Clin Exp Immunol. 1993 Oct;94(1):43–50. doi: 10.1111/j.1365-2249.1993.tb05975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton M. J., Ferns S. A., Hughes C. M., Sear C. H., Rudland P. S. Generation of cell types with myoepithelial and mesenchymal phenotypes during the conversion of rat mammary tumor epithelial stem cells into elongated cells. J Natl Cancer Inst. 1987 Jun;78(6):1191–1201. [PubMed] [Google Scholar]
- Watters L. C., Schwarz M. I., Cherniack R. M., Waldron J. A., Dunn T. L., Stanford R. E., King T. E. Idiopathic pulmonary fibrosis. Pretreatment bronchoalveolar lavage cellular constituents and their relationships with lung histopathology and clinical response to therapy. Am Rev Respir Dis. 1987 Mar;135(3):696–704. doi: 10.1164/arrd.1987.135.3.696. [DOI] [PubMed] [Google Scholar]
- Yoshioka K., Takemura T., Murakami K., Okada M., Hino S., Miyamoto H., Maki S. Transforming growth factor-beta protein and mRNA in glomeruli in normal and diseased human kidneys. Lab Invest. 1993 Feb;68(2):154–163. [PubMed] [Google Scholar]
- de Wet W., Bernard M., Benson-Chanda V., Chu M. L., Dickson L., Weil D., Ramirez F. Organization of the human pro-alpha 2(I) collagen gene. J Biol Chem. 1987 Nov 25;262(33):16032–16036. [PubMed] [Google Scholar]