Abstract
Depressive disorders that onset in the juvenile years have been linked to far reaching adverse consequences, making it imperative to elucidate key mechanisms and contributory factors. Excessive use of regulatory responses that exacerbate sadness (maladaptive mood repair) or insufficient use of regulatory responses that reduce it (adaptive mood repair) may reflect behavioral mechanisms of depression risk. Cardiac vagal control, indexed by patterns of respiratory sinus arrhythmia (RSA), has received attention as a putative physiological risk factor for depression. Although mood repair and RSA are related, the nature of this relationship is not well characterized in the context of depression risk. Therefore, we tested alternative models of the relationships between RSA patterns (at rest and in response to a sad film), trait mood repair, and the effectiveness of a mood repair response in the laboratory (state mood repair) among adolescents with depression histories (n=210) and emotionally healthy peers (n=161). In our data, a mediation model best explained the association between the key constructs: Adolescents with normative RSA patterns exhibited lower levels of depression and trait maladaptive mood repair, and benefited more from instructed (state) mood repair in the laboratory. By contrast, adolescents with atypical RSA patterns exhibited higher levels of depression and dispositional maladaptive mood repair, which, in turn, mediated the relations of RSA patterns and depression symptoms. Atypical RSA patterns also predicted reduced benefits from laboratory mood repair.
Keywords: depression, emotion regulation, mood repair, respiratory sinus arrhythmia, adolescence
Depressive disorders, which onset in the juvenile years, have been linked to greater functional impairment later in life (Zisook et al., 2007), higher rates of episode recurrence (Lewinsohn, Clarke, Seeley, & Rohde, 1994), and increased risk for nonaffective disorders (Birmaher et al., 1996; Zisook et al., 2007) relative to depressions that first onset in adulthood. Experiencing a clinical depression during childhood or adolescence interferes with normative development in multiple functional domains and predicts worse subsequent educational and economic achievement (Birmaher et al., 1996; Fergusson, Boden, & Horwood, 2007; Zisook et al., 2007), as well as impaired social and marital relationships (Birmaher et al., 1996; Gotlib, Lewinsohn, & Seeley, 1998). Given the generally chronic course and wide reaching adverse consequences of juvenile-onset depressions, it is vital to elucidate related mechanisms and contributory factors. Indeed, several behavioral and physiological processes have already been identified as contributors to early onset depression (e.g., Goodman, 2007; Stewart, Bismark, Towers, Coan & Allen, 2010), but there is scant information about how such processes interact, which is critical to a better understanding of psychopathology (Cuthbert & Insel, 2010). Thus, the central goal of the present study was to test alternative interactive models of behavioral (mood repair) and physiological (cardiac vagal control) processes as putative mechanism in juvenile onset depressive disorders.
Mood repair, the processes whereby individuals attenuate sadness and dysphoria (Josephson, Singer & Salovey, 1996)1, has received increased attention as a behavioral mechanism central to depression (Gross & Munoz, 1995; Joormann, Cooney, Henry, & Gotlib, 2012; Kovacs, Joormann & Gotlib, 2008; Kovacs & Yaroslavsky, 2014). Problems with mood repair have indeed been documented in depressed youths, those remitted from prior depression, and those at high familial risk of future depression (Bylsma et al., 2015; Garber, Braafladt & Weiss, 1995; Silk, Shaw, Skuban, Oland, & Kovacs, 2006; Thompson et al., 2010). Studies that used self-report measures (which typically quantify usual or “trait” response tendencies) have revealed that one aspect of problematic mood repair is the excessive use of regulatory responses such as depressive rumination that prolong or worsen distress (maladaptive responses) rather than the underuse of adaptive responses that can attenuate it (Bylsma et al., 2015; Ehring, Fischer, Schnülle, Bösterling, & Tuschen-Caffier, 2008; Garber, Braafladt, & Weiss, 1995; Gentzler, Santucci, Kovacs, & Fox, 2009; Kovacs, Rottenberg, & George, 2009; Thompson et al., 2010). Notably, maladaptive mood repair not only distinguishes depression-prone and emotionally well controls, but also predicts recurrence of depressive episodes both among youths and adults with juvenile onset depression histories (Kovacs, Rottenberg, & George, 2009; Stone, Hankin, Gibb, & Abela, 2011). Thus, maladaptive mood repair is a plausible mechanism in depressive psychopathology.
Conversely, the expectation that employing regulatory responses that attenuate sadness, such as neutral reinterpretation of depressogenic stimuli (adaptive mood repair), should protect against depression is generally not born out in previous studies. A growing literature suggests that depressed, high-risk, and emotionally healthy youths do not differ in their deployment of adaptive mood repair responses (Bylsma et al., 2015; Kovacs & Yaroslavsky, 2014; Thompson et al., 2010), nor does adaptive mood repair predict risk of depression symptoms and disorders over time (Aldao & Nolen-Hoeksema, 2012; Kovacs et al., 2009). Further, according to laboratory studies, in which individuals are instructed to implement a specific adaptive mood repair response after sadness induction, depression-prone youths or young adults and healthy controls often report similar subjective benefits (Joormann et al., 2012; Kanske, Heissler, Schonfelder, & Wessa, 2012). Thus, there is currently scant support for a mechanistic role for habitual (trait) or experimentally manipulated (state) adaptive mood repair in affective psychopathology. Nevertheless, as a central therapeutic goal of cognitive-behavioral interventions is to increase depressed individuals’ adaptive mood repair repertoires, it is important to identify contextual factors, such as physiological states, that may influence the effectiveness of adaptive mood repair responses.
Cardiac vagal control, one of the physiological parameters that has been implicated in depression risk (Rottenberg, 2007), has received attention in studies of young vulnerable offspring and already depressed youths (e.g., Blom, Olsson, Serlachius, Ericson, & Ingvar, 2010; Jones et al., 1998). Cardiac vagal control is a widely used index of the functioning of the parasympathetic nervous system; it reflects individual differences in internal physiological reserves along with physiological flexibility (Porges, 2007). More specifically, activation of the vagal nerve, which has a direct input into the sinoatrial node of the heart (Berntson et al., 1997), serves to slow heart rate. High resting levels of cardiac vagal control is desirable and reflects the ability to conserve energy (Porges, 2007). In response to most external challenges, vagal control typically is decreased (withdrawn), which allows heart rate to increase as needed and to direct internal resources to responding to the stimulus (Porges, 2007).
Cardiac vagal control is typically quantified as the magnitude of heart rate variability at the respiratory frequency (respiratory sinus arrhythmia or RSA) during resting states or in response to an external stimulus (Berntson et al., 1997). High resting RSA, as well as moderate RSA withdrawal to sadness induction together represent normative RSA activity (see Beauchaine, 2001; Overbeek, van Boxtel, & Westerink, 2012), and are associated with fewer depression symptom in children and adolescents (Blom et al., 2010; Gentzler et al., 2009; but see Byrne et al., 2010; Crowell et al., 2005). Conversely, low resting RSA, blunted RSA reactivity, or increased RSA (RSA augmentation) to certain prompts are associated with higher levels of depression symptoms in children (Gentzer et al., 2009; Graziano & Derefinko, 2013; Jones et al., 1998). More recently, atypical RSA patterns, such as combinations of RSA augmentation in response to negative mood prompts and high resting RSA (or low resting RSA and RSA withdrawal during negative mood prompts) were associated with heightened risk for familial depression and elevated depression symptom trajectories across childhood and adolescence (Yaroslavsky, Rottenberg, & Kovacs, 2014). Atypical RSA patterns also predict internalizing symptoms during childhood and adolescence (Hinnant & El-Sheikh, 2009; 2013). These findings extend to older ages, with atypical RSA patterns in depression-prone adults signaling higher levels of depressive symptoms and a history of juvenile onset depression (Yaroslavsky et al., 2013a; 2013b). Thus, atypical RSA characteristics constitute a plausible physiological mechanism that facilitates depression symptoms and disorders.
Affect regulation and RSA have been linked both in theory (Beauchaine, 2001, 2015; Porges, 2007; Rottenberg, 2007; Thayer & Lane, 2009) and empirical studies (Geisler, Kubiak, Siewert, & Weber, 2013; Ingjaldsson et al., 2003; O’Connor, Allen, & Kaszniak, 2005; Sloan & Epstein, 2005; Volokhov & Demaree, 2010). For example, adults with normative RSA are more likely to use adaptive mood regulatory responses, including attention refocusing, reappraisal, distraction, and seeking social support (Geisler et al., 2013; Volokhov & Demaree, 2010) and obtain greater subjective benefit from their use (O’Connor et al., 2005; Sloan & Epstein, 2005). In turn, adults with atypical RSA activity are characterized by extensive use of maladaptive regulatory strategies both in the laboratory (Ingjaldsson et al., 2003) and in daily life (Geisler et al., 2013). Such associations are plausible given that RSA has been connected closely to the experience and regulation of affect (see Kreibig, 2010; Thayer & Lane, 2009). Further, RSA processes have been linked to neural circuits associated with the experience of emotions (limbic regions) and their cognitive regulation (prefrontal regions) (Allen, Jennings, Gianaros, Thayer, & Manuck, 2015; Beauchaine, 2015; Porges, 2007; Thayer, Åhs, Fredrikson, Sollers, & Wager, 2012; Thayer & Lane, 2009). Although RSA and mood repair are clearly associated, the exact nature of their interrelationship in the context of depression is not well characterized, particularly in the juvenile years. Consequently, little is known about how various patterns of association between RSA and RSA reactivity might increase or attenuate the risk of depression symptoms among youth. The goal of our study was to fill these critical gaps.
Although there are various possible interrelations between RSA and mood repair, for heuristic purposes, we focused on two that appear relatively plausible pathways to depression. First, RSA may be linked to depression indirectly via problematic mood repair. That is, atypical RSA may potentiate problematic mood repair, which, in turn increases the risk of depression symptoms. Second, RSA may directly moderate the consequences of mood repair responses and thereby influence depression risk. That is, atypical RSA may enhance the depressogenic effects of maladaptive mood repair. The just noted associations represent alternate mediation and moderation models linking the three constructs of interest, which may compete, or be complementary.
To test the mediation model, we focused on dispositional (trait) mood repair. We hypothesized that atypical RSA patterns will predict lower levels of trait adaptive and higher levels of maladaptive mood repair repertoires that, in turn, will mediate the effects of atypical RSA on depression. To test the moderation model, we focused first on mood repair as a disposition (trait). We hypothesized that atypical RSA patterns will enhance the negative effects of trait maladaptive mood repair, and attenuate the potentially beneficial effects of adaptive mood repair on depression symptoms. We then examined a moderated-mediation model involving trait mood repair that combined the two models described above. Finally, as an alternative way to examine moderation, we focused on the subjective consequences of instructed mood repair in the laboratory (state mood repair). We hypothesized that atypical RSA patterns will reduce the subjective benefits of deploying an adaptive mood repair response in the laboratory.
Methods
Participants
Trait mood repair mediation and moderation models used data from 371 school-age youths in Hungary (M = 16.50 years, SD = 1.85), 64% male and 97% Caucasian (3% were multi-racial, Roma, or “other” racial category). A subset of this sample was previously diagnosed with Major Depressive Disorder (probands, n = 210). Remaining subjects were free of life-time major mental disorders (controls, n = 161). Probands were on average 1 year older, t(369) = 6.20, p < .001) but did not differ from controls in sex distribution. Eighty-two probands (39%) had a history of anxiety disorders, and 37% had a history of externalizing disorders. At the time of this study, 14% of probands were in a depressive episode and 7% had a current anxiety disorder. Three-percent (3%) were prescribed psychotropic medications (n=1 antidepressants, n=2 anxiolytics, n=2 stimulants, n=1 antipsychotic).
The state mood repair moderation model used laboratory data from those subjects (n=183) who were assigned to complete an instructed attention refocusing mood repair task. Of these participants, n = 57 did not report sadness after the negative mood induction (described below). Given that some dysphoria must be present in order to examine mood repair, these participants were excluded from analyses, yielding a final sample of n = 126 .2 Probands and controls in this subsample did not significantly differ in reported levels of post-film dysphoria, Probands M = 1.43 SD = 1.05; Controls M = 1.79 SD =1.13, t (124) = 1.82, p < .07.
Recruitment, clinical assessment, and diagnostic procedures have been described in detail elsewhere (Bylsma et al., 2015; Rottenberg et al., 2014; Kovacs et al., 2015). Briefly, probands were originally recruited from 23 child mental health and guidance facilities across Hungary for a prior genetic study on childhood depression (Kiss et al., 2007); controls for the current study were selected from medium size public elementary and secondary schools in the 3 cities where most probands resided (Budapest, Pecs, and Szeged). Subjects underwent a stringent assessment procedure that included: a) standardized psychiatric diagnostic evaluations using a semi-structured interview (each involving the child and a parent informant) by trained interviewers who generated DSM-IV mood-disorder diagnoses and b) independent verification of the diagnosis by pairs of trained child psychiatrists, using “best estimate” diagnostic consensus guidelines (Maziade et al., 1992).
Overall Procedures
Study visits included a psychiatric-psychosocial evaluation by a clinician, the completion of self-rated questionnaires by parents and children, and a psychophysiological protocol. All procedures, schedules, rating scales, and instruments were first developed in English, translated into Hungarian, and then back-translated by bilingual child psychiatrists and clinical psychologists: an iterative procedure was used to resolve any discrepancies.
Clinical Evaluation
Youths were evaluated by trained child psychologists and psychiatrists via the Interview Schedule for Children and Adolescents: Diagnostic version (ISCA-D), a DSM-IV-based, semi-structured diagnostic interview, which has been shown to have good symptomatic interrater reliability (Kiss et al., 2007). The ISCA-D requires separate interviews with the parent and offspring about the offspring; the final rating for each symptom reflects the interviewer’s judgment based on both sources of information. For this study, clinically impairing (threshold) major depressive disorder (MDD) symptoms during the month prior to the assessment, including the assessment day, were aggregated as one indicator of the latent depression factor (Weighted Kappa = .84). Parents also responded to a structured interview, which included questions about offspring’s cigarette smoking and psychotropic medication use.
Self-Rated Questionnaires
Youths completed the Children’s Depression Inventory-2nd edition (CDI-2), a validated and reliable measure (α=.89 in this study) of symptoms during the prior 2 weeks (Kovacs & MHS Staff, 2011). The CDI-2 score served as the second indicator of youths’ latent depression levels. Physical activity was measured using the Physical Activity History questionnaire (adapted from the Youth Risk Behavior Surveillance-United States, Centers for Disease Control and Prevention), which has good psychometric properties (Rottenberg et al., 2014).
The Feelings and Me/Feelings and My Child (FAM) questionnaires survey a wide range of common responses to sadness (Kovacs et al., 2009). Both the youth and parent-rated versions contain the same 54-items (Bylsma et al., 2015; Tamas et al., 2007), each of which is rated on 3-point scale to indicate the extent to which use of the target response characterizes the individual. The two major FAM scores reflect adaptive responses (e.g., “when I am sad, I look for a teacher or other adult to talk to”) and maladaptive responses (e.g., “when I am sad, I throw, kick, or hit things”); each includes 3 sub-scores mirroring cognitive, behavioral, and social response domains. Information on validity and reliability of the FAM scores has been reported elsewhere (e.g., Bylsma et al., 2015; Tamas et al., 2007). The FAM is associated with remission status (Tamas et al., 2007), and distinguishes controls from youths with depression histories (Bylsma et al., 2015). The scales displayed good-to-adequate internal consistency in this sample (Child report: Maladaptive cognitive α=.79, behavioral, α=.58, & social α=.64; Adaptive cognitive α=.75, behavioral, α=.72, & social α=.73; Parent report: Maladaptive cognitive α=.83, behavioral, α=.64, & social α=.70; Adaptive cognitive α=.78, behavioral α=.67, & social α=.68).
Psychophysiological procedures
As summarized elsewhere (Kovacs et al., 2015), our protocol probed physiological and psychological reactions to various stimuli/tasks, lasted about 1 hour, and included sadness induction followed by mood repair tasks. Potential order effects were minimized by counterbalancing task order. In this article, we report on the portions of the protocol that included a baseline rest period, sad mood induction, and subsequent instructed mood repair using an attention-refocusing task. The resting baseline was a 180 second task during which respiration was paced at 12 breaths per minute. Sadness was induced via a 164 second clip from the Champ (dubbed in Hungarian) (Gross & Levenson, 1995), which was pilot tested with Hungarian youth (see Kovacs et al., 2015). The mood repair task, which lasted about 2.5 minutes, involved looking through a kaleidoscope and was adapted from a pediatric study (Carlson, Broome, & Vessey, 2000). After the subject was handed the kaleidoscope and asked to keep turning it, the experimenter posed standardized questions (e.g., “What shapes do you see?) to facilitate task engagement. Prior work demonstrates the importance of cognitive engagement for attention re-focusing tasks (e.g., Erber & Tesser, 1992; Van Dillen & Koole, 2007).
Affect ratings were collected at baseline, after sad mood induction, and after the mood repair task, using Likert scales from 0 (not at all) to 7 (very much). Affects rated included sad, blue, and happy, interspersed with items such as interested, upset, and angry. Our dysphoria index was the average of the ratings of the “sad” and “blue” items, similar to the approach taken by others (e.g., Joormann, Siemer, & Gotlib, 2007).
RSA data acquisition and reduction
Physiological data were recorded continuously via electrocardiogram (ECG) using Mindware BioLab software. The ECG signal was acquired according to published guidelines (Berntson et al., 1997) using Ag/AgCl electrodes that were placed in a modified Lead II configuration on the chest. R-waves were sampled online at 1000Hz using the Mindware Bionex system (MindWare Technologies, Ltd., Gahanna, OH). RSA was calculated using MindWare HRV 3.0.21 software (MindWare Technologies, Ltd., Gahanna, OH). R-wave markers in the ECG signal were processed with the MAD/MED artifact detection algorithm, and signals were manually inspected and suspected artifacts were corrected (Berntson et al., 1997). The interbeat interval (IBI) series was resampled, linearly detrended, and tapered using a Hanning window. Heart rate variability (HRV) was calculated using Fast Fourier transformation analysis of the IBI series, with spectral power values determined in ms2/Hz (Berntson et al., 1997). Our index of cardiac parasympathetic activity, RSA, was defined as the log transformed high frequency (HF) power band of HRV (.15-.40 Hz range; see Berntson et al., 1997). Hereafter we refer to HF-HRV as RSA, since HF-HRV is the power band of HRV that occurs in the typical range of respiration.
The paced breathing baseline and sad film clip were processed in single 180 and 164 second epochs. Mean heart rate during the paced breathing baseline was 74.43 (SD = 11.52) and 69.70 (SD = 10.30) during the sad film, respectively. Resting RSA was derived from the paced breathing task. RSA reactivity to the sad film mood induction task was computed by subtracting the task RSA values from the paced breathing RSA values. Thus, positive RSA reactivity values represent RSA Withdrawal (RSA was lower during the sad film) and negative values represent RSA Augmentation (RSA was higher during the sad film). RSA patterns reflect the statistical interaction between zero-centered resting RSA and RSA reactivity levels.
Statistical Analyses Overview
Descriptive analyses were conducted in SAS version 9.3 software (SAS Institute Inc., 2013), and latent variable and regression models were fit in Mplus v. 7.11 software (Muthén & Muthén, 1998 - 2012). Robust Full Information Maximum Likelihood was used to adjust parameter estimates for missing values on outcome variables that comprised less than 2% of the sample. Following Hu and Bentler (1999), CFI values of .95 or greater and RMSEA of .06 or lower indicated excellent model fit, and CFI greater than .90 and RMSEA below .07 was indicative of acceptable fit (Browne & Cudeck, 1993; Steiger, 2000). Smoking, physical activity, and psychotropic medication use were explored as covariates given their known effects on RSA (Rottenberg, 2007).
Models Involving Trait Mood Repair
Measurement Models
Latent Depression
To reduce measurement error (see Bollen, 1989), a latent depression factor was estimated using CDI-2 scores and ISCA-D depression symptom counts. Given that most subjects were free of threshold depression symptoms at the time of the study procedures (78%), ISCA-D depression symptom counts were modeled using a zero-inflated negative binomial distribution. While the use of symptom counts precluded an evaluation of relative model fit indices (CFI & RMSEA), high factor loadings from the latent factor to the two indicators suggested good model fit (λ = .78-1.00, p < .001).3
Latent Trait Mood Repair
A latent multi-trait multi-method (MTMM) approach was taken to estimate latent adaptive and maladaptive mood repair factors from parents’ and youths’ FAM cognitive, social and behavioral subscale scores. Latent MTMM models can account for measurement error and method effects associated with self- and parental reports of youths’ characteristics (e.g., Najman et al., 2001). Residual variance of youths’ and parents’ reports were allowed to covary to accommodate reporting bias associated with each informant (correlated uniqueness model, see Kenny & Kashy, 1992). This model displayed an acceptable fit to the data (CFI = .97, RMSEA = .07). Our use of two rather than a single mood repair factor reflects substantial work showing low-to-no associations between measures of adaptive and maladaptive mood repair responses (Gentzler et al., 2009; Tamas et al., 2007; Yaroslavsky et al., 2013b)
Structural Models
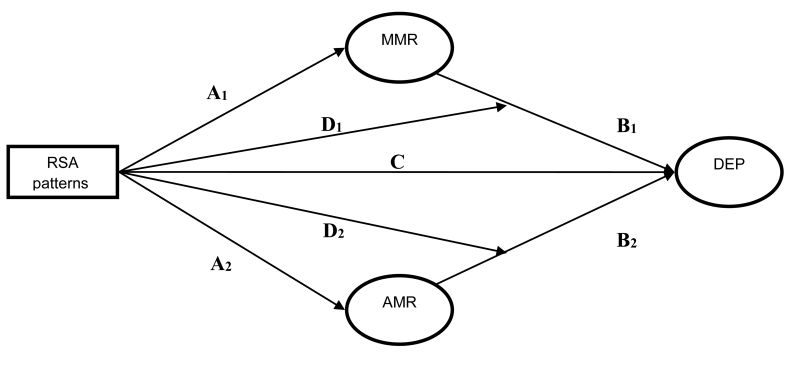
Nested structural equation models (SEMs) were fit to test the hypothesized mediation and moderation models. These models reflected (1) the direct effect of RSA patterns on depression symptoms (Figure 1, path C), (2) the mediated (indirect) effects of RSA patterns on depression via trait adaptive and maladaptive mood repair factors (Figure 1, paths A1-2 & B1-2), and (3) RSA patterns’ moderation of trait adaptive and maladaptive mood repair on depression (Figure 1 Paths D1-D2). The model containing all A-D paths is a moderated-mediation model within which our hypothesized mediation and moderation models are nested. This model tests the combination of moderation and mediation relationships between RSA patterns, trait mood repair, and depression symptoms. The nesting structure of this model allows us to directly compare the fits of the mediation and moderation models to the moderated-mediation model via likelihood ratio tests, as well as to indirectly compare the fit of the mediation and moderation models by examining their respective Akaike Information Criteria (AIC) and Bayesian Information Criteria (BIC).
Figure 1.
Conceptual model of the effects of RSA patterns on depression (DEP) via adaptive (AMR) and maladaptive mood repair (MMR). RSA patterns = second-order interaction of resting RSA & RSA reactivity, and take into account the first-order effects of the two RSA indices. C = direct effect of RSA patterns on depression symptoms, A = direct effects of RSA patterns on mood repair, B = direct effects of mood repair on depression symptoms, A*B = indirect effects of RSA patterns on depression symptom via mood repair. D = RSA pattern moderation of mood repair effects on depression symptoms.
Confidence intervals around significant indirect effects were estimated using the PRODCLIN program (MacKinnon, Fritz, Williams, & Lockwood, 2007), which is robust in moderate sample sizes (Fritz & MacKinnon, 2007). Moderation effects were tested via Latent Moderated Structural (LMS) equations to accommodate the use of latent variables in the interaction (see Klein & Moosbrugger, 2000). These models tested three-way interactions between resting RSA, RSA reactivity, and each mood repair factor. Incremental value of the three-way interaction terms were evaluated through Wald χ2 tests. Following Aiken and West (1991) resting RSA and RSA reactivity were zero-centered.
Models Involving State Mood Repair
The effectiveness of experimentally manipulated mood repair (state mood repair) was quantified as the difference in dysphoria rating after the sad film clip versus after the kaleidoscope task. Higher scores indicated greater (more effective) mood repair. Two regression models were fitted to test first- and second-order effects of resting RSA and RSA reactivity on mood repair effectiveness. Significant second-order effects of the two RSA indices would indicate that RSA patterns modify the degree to which subjects reduced their distress while engaging in attention refocusing. RSA indices were zero-centered, as before, when predicting mood repair scores (Aiken & West, 1991).
Results
Sample Characteristics and Covariates
Descriptive statistics and bivariate associations among study variables are presented in Table 1. As noted previously (Bylsma et al., 2015), girls reported using more mood repair strategies than did boys, partly mirroring parental reports of their children’s mood repair responses. Proband youths reported using fewer adaptive but a higher number of maladaptive mood repair responses relative to control youths.
Table 1.
Descriptive statistics and correlations among demographic, RSA, symptom, and mood repair measures (N=366-371).
| Measures |
M
(SD) |
2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | 16. | 17. | 18. | 19. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sex | --- | −.01 | .02 | −.09 | −.03 | −.13* | −.03 | −.17** | −.28** | −.27** | −.10 | −.02 | −.09 | −.15** | −.11* | −.24** | −.16** | −.04 | −.17** |
| 2. Prob | --- | .12* | .44** | .34** | .01 | .04 | .18** | .21** | .22** | .24** | .32** | .30** | −.28** | −.18** | −.17** | −.33** | −.20** | −.18** | |
| 3. Rx | --- | .13* | .31** | .02 | .15** | .11* | .07 | .00 | .13* | .17** | .19** | −.07 | .01 | .10 | −.13* | −.10* | −.02 | ||
| 4. CDI-2 | 7.98 (6.82) |
.50** | .05 | .02 | .54** | .45** | .48** | .39** | .40** | .34** | −.20** | −.18** | −.23** | −.19** | −.08 | −.10* | |||
| 5. MDDSx | .67 (1.66) |
.01 | .12* | .38** | .33** | .29** | .36** | .32** | .37** | −.16** | −.06 | −.09 | −.13* | −.08 | −.07 | ||||
| 6. RSA | 7.26 (1.03) |
.38** | .09 | .14** | .08 | .10* | .01 | .02 | .01 | .06 | .13* | −.01 | −.03 | .05 | |||||
| 7. ΔRSA | .76 (.84) |
.02 | .00 | −.02 | .08 | −.01 | .09 | .03 | .01 | .04 | −.06 | −.06 | −.06 | ||||||
| 8. Y-Mcog | 3.55 (3.20) |
.51** | .53** | .24** | .20** | .14** | .18** | .06 | .07 | −.05 | .01 | .01 | |||||||
| 9. Y-Mbeh | 3.39 (2.56) |
.58** | .31** | .36** | .30** | .02 | .09 | .03 | −.12 | −.06 | −.02 | ||||||||
| 10. Y-Msoc | 2.36 (2.02) |
.24** | .26** | .25** | −.10 | −.03 | −.18** | −.10 | −.03 | −.08 | |||||||||
| 11. P-Mcog | 3.28 (3.29) |
.60** | .60** | −.05 | −.06 | −.06 | −.04 | −.01 | .04 | ||||||||||
| 12. P-Mbeh | 2.82 (2.51) |
.66** | −.11* | −.11* | −.08 | − 14** | −.02 | −.06 | |||||||||||
| 13. P-Msoc | 2.32 (2.12) |
−.15* | −.11* | −.13* | −.13* | −.08 | −.14** | ||||||||||||
| 14. Y-Acog | 7.51 (3.54) |
.50** | .45** | 19** | .13* | .18** | |||||||||||||
| 15. Y-Abeh | 10.12 (4.53) |
.52** | .18** | .22** | .11* | ||||||||||||||
| 16. Y-Asoc | 3.89 (2.89) |
.15** | .14** | .31** | |||||||||||||||
| 17. P-Acog | 5.50 (3.21) |
.58** | .53** | ||||||||||||||||
| 18. P-Abeh | 8.86 (3.99) |
.47** | |||||||||||||||||
| 19. P-Asoc | 4.68 (2.70) |
--- |
Sex = high value represents males, Prob = high value represents proband group membership, Rx = high values represent psychotropic medication use, CDI-2 = self-report Child Depression Inventory–2, MDDSx = Major Depressive Disorder symptom counts, P- = parent report, Y- = youth self-report, Mcog-Msoc = Feelings and Me Maladaptive Cognitive, Behavioral and Social mood repair subscales, Acog-Asoc = Feelings and Me Adaptive Cognitive, Behavioral, and Social mood repair subscales, RSA = RSA during paced breathing, ΔRSA = change score from paced breathing RSA to RSA during the sad film.
p < .05,
p < .01
Participants on average reported greater dysphoria after watching the sad film clip, MΔsad/blue= .73, SD = 1.23, t(370) = 11.39, p <.001, Cohen’s d = .61. The sad clip also elicited vagal withdrawal for the majority of participants, MΔRSA =.76, SD =.84, t(366) = 17.40, p < .001, Cohen’s d = 1.82, although 19% (n=70) of subjects showed increased RSA over resting RSA levels (vagal augmentation).
Of the variables known to affect RSA (i.e., active depression episodes, physical activity, smoking, and psychotropic medication use), none predicted resting RSA and only psychotropic medication use was associated with greater RSA reactivity, F(1,365) = 8.17, p < .01, η2 = .02. Thus, psychotropic medication use, along with sex and group status (proband vs. control), were included as predictors in all models. Given previously observed relationships between sadness intensity and RSA (e.g., Dywan, Mathewson, Choma, Rosenfeld, & Segalowitz, 2008), a change score in dysphoria ratings from baseline to the sad film clip was added as an additional predictor in the state mood repair models.4 Finally, task order was entered as a covariate in all models.
Are the effects of atypical RSA patterns on depression mediated by trait mood repair?
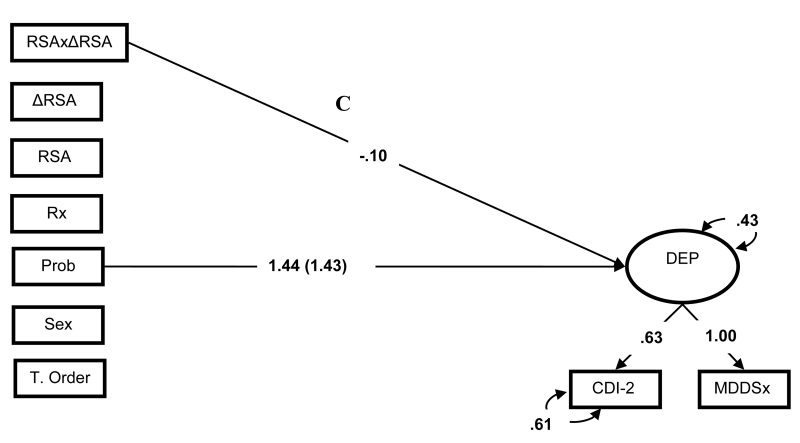
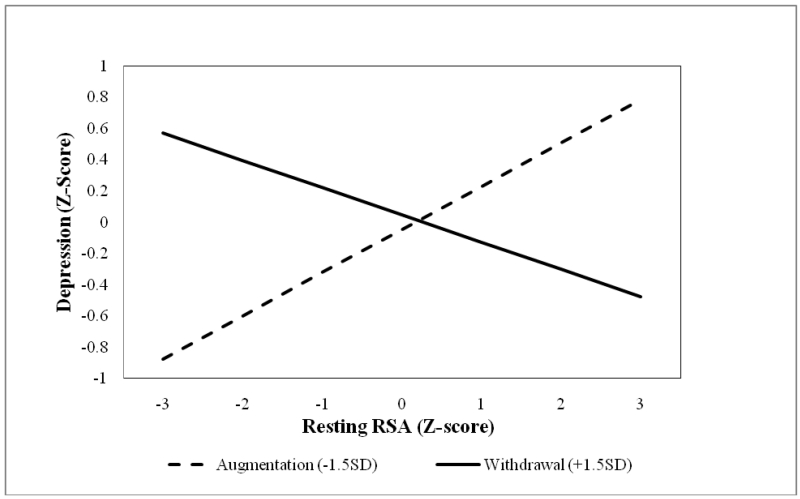
To answer this question, we first examined the effects of RSA patterns on depression in two SEMs, in which the latent depression factor was regressed on the first- and second-order effects of resting RSA and RSA reactivity (Figure 1, path C). In the first-order model, neither resting RSA nor RSA reactivity predicted depressive symptoms. In contrast, in the second-order model, RSA patterns predicted depressive symptoms, βRSA = .03, ns; βΔRSA = −.03, ns; βRSAxΔRSA = −.10, p <.05, ΔR2 = .02 (see Figure 2). Simple slope analyses of the interaction effect revealed that normative RSA patterns predicted lower depressive symptom severity, β = −.12, whereas atypical RSA patterns predicted greater depression symptom severity, β = .18 (see Figure 3). In both models, proband status was a consistent predictor of depression symptoms, β = 1.43-1.44, ps < .001, ΔR2 = .47-.48.
Figure 2.
Standardized First- and Second-order SEMs of RSA patterns’ effects on depression. Effects of categorical predictors standardized with respect to the outcome variable. Parameters within parentheses are from the first-order effects model. Parameters outside parentheses are from the second-order effects model. Bold parameters significant at p < .05. Prob = proband group membership, RSA =RSA during paced breathing, ΔRSA = change score from paced breathing RSA to RSA during the sad film, RSAxΔRSA = second order effects of RSA indices, Rx =prescription medication use, Sex = high value represents males, T. Order = task order, Rx = high values represent psychotropic medication use, CDI-2 = self-report Child Depression Inventory-2, MDDSx = threshold symptom counts on the ISCA-D.
Figure 3.
RSA reactivity moderation of resting RSA effects on latent depression symptoms.
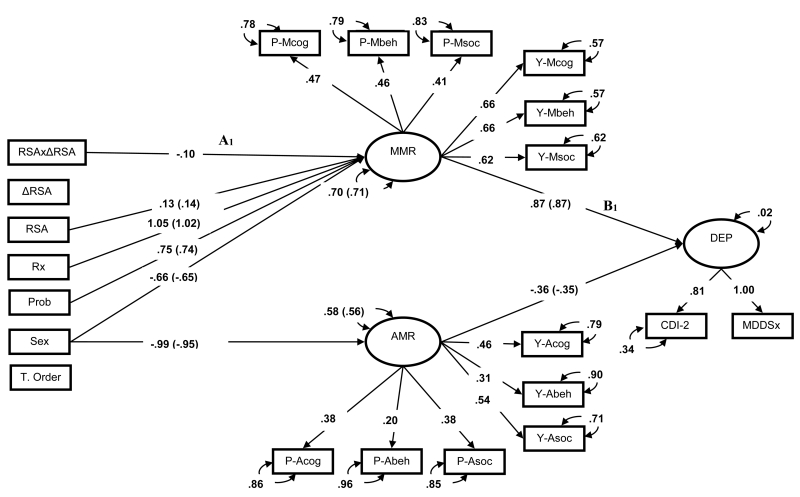
Next, a series of SEMs were estimated to test the effects of RSA patterns on the mood repair factors (Figure 1 paths A1 & A2), and mood repair effects on depression symptoms (Figure 1 paths B1 & B2; Figure 3). The first-order effects model fit the data well (CFI = .93, RMSEA = .06). In the first-order model, neither resting RSA nor RSA reactivity predicted adaptive mood repair. Surprisingly, higher resting RSA levels were associated with greater maladaptive mood repair repertoires, βRSA = .14, p0 < .5, ΔR2 = .02. However, this main effect was qualified by the predicted interaction of resting RSA and RSA reactivity (see below). Of the variables in the model, only adaptive and maladaptive mood repair factors predicted youths’ depression symptoms, βAMR = −.35, p < .05, ΔR2 = .08; βMMR = .87, p < .01, ΔR2 = .81.
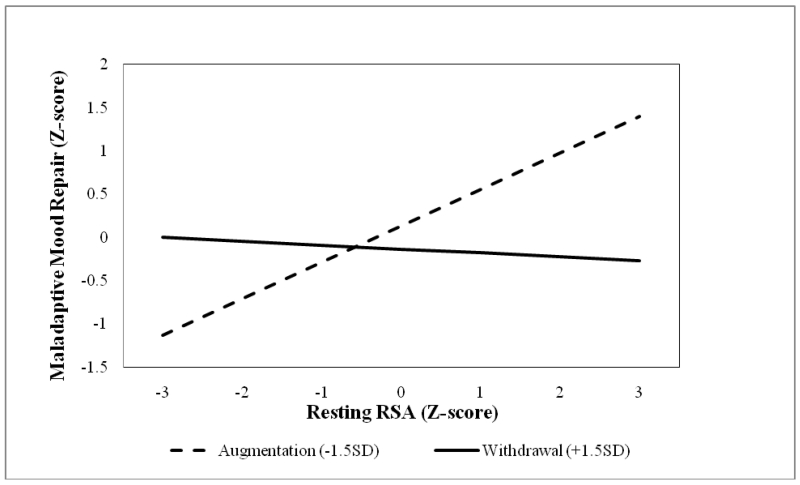
Second-order effects of the two RSA indices were then added to the first-order model to test the incremental utility of RSA patterns on dispositional mood repair. As hypothesized, the second-order model displayed improved fit to the first-order counterpart, which suggests that RSA patterns explained incremental variance in the mood repair factors (CFI=.93, RMSEA=.06, AIC = 23345.39, BIC = 23720.30, Wald χ2(1) = 5.38, p <.05,). The interaction of resting RSA and RSA reactivity predicted the use of dispositional maladaptive mood repair strategies, βRSAxΔRSA = −.10, p =.02, ΔR2 = .01, but not adaptive mood repair responses, βRSAxΔRSA = −.06, p =.40. In other words, RSA patterns were predictive of the frequency with which adolescents deployed maladaptive mood repair responses (Figures 1 & 4, path A1). Simple slopes analyses of the interaction revealed that high resting RSA was associated with an increased use of maladaptive responses as a function of RSA augmentation, and a modestly lower use of these strategies as a function of RSA withdrawal (see Figure 5). These findings support the idea that normative (i.e., high resting RSA + RSA withdrawal) and atypical (i.e., high resting RSA + RSA augmentation) RSA patterns show differential associations with maladaptive mood repair. This pattern of associations set the stage to test whether RSA patterns indirectly predict depression via maladaptive mood repair.
Figure 4.
Standardized First- and Second-order SEMs of latent mood repair mediating RSA patterns effects on depressive symptoms. Residual covariances (correlated uniqueness) for self-report and parent-report FAM scales and non-significant regression paths omitted to improve interpretability. Effects of categorical predictors standardized with respect to the outcome variable. Parameters within parentheses are from the first-order effects models. Parameters outside parentheses are from the second-order effects models. Bold parameters significant at p < .05. Prob = proband group membership, P- = parent report, Y- = youth self-report, Mcog-Msoc = Feelings and Me Maladaptive Cognitive, Behavioral and Social mood repair subscales, Acog-Asoc = Feelings and Me Adaptive Cognitive, Behavioral, and Social mood repair subscales, RSA =RSA during paced breathing, ΔRSA = change score from paced breathing RSA to RSA during the sad film, RSAxΔRSA = second order effects of RSA indices, MMR= latent maladaptive repair factor, AMR= latent adaptive repair factor, Rx =prescription medication use, Sex = high value represents males, T. Order = task order, CDI-2 = self-report Child Depression Inventory-2.
Figure 5.
RSA reactivity moderation of resting RSA effects on latent maladaptive mood repair.
Consistent with expectations, the effects of RSA patterns on depression symptoms were mediated by maladaptive repair, βMMR, RSA × ΔRSA = −.09, 95% CI: −.01 - −.17, ΔR2 = .01. Specifically, indirect effects analysis of the simple slopes revealed that, in the context of RSA augmentation, resting RSA predicted greater levels of maladaptive mood repair and depression, β = .28, whereas RSA withdrawal predicted lower levels of maladaptive mood repair and reduced depression, β = −.03. Thus, atypical RSA patterns were associated with increased maladaptive repair, and with more severe depressive symptoms.5 It is noteworthy that the effects of RSA patterns were independent of proband status, which robustly predicted maladaptive mood repair, β = .75, p < .001, ΔR2 = .47, but not adaptive mood repair.
Do atypical RSA patterns enhance the negative effects of trait maladaptive mood repair, and attenuate the effects of trait adaptive mood repair on depression symptoms?
To answer this question, we added third-order effects of resting RSA, RSA reactivity, and the two mood repair factors to the mediation models described above (D paths in Figure 1), while removing the direct effects of RSA patterns on the two mood repair factors (A paths in Figure 1). Contrary to our expectation, RSA patterns failed to moderate the effects of the two mood repair factors on depression (ps = .16-87).
Are the effects of atypical RSA patterns on trait mood repair best captured by a combined moderated-mediation model?
To test whether RSA patterns predict mood repair and influence its effects on depression, we estimated a moderated-mediation model within which our proposed mediation and moderation models are nested (full model depicted in Figure 1). Thus we were able to compare whether the hypothesized relationships between RSA patterns, mood repair, and depression are best accounted for by a mediation, moderation, or a combined moderated-mediated model. In this moderation -mediation model, the third-order effects between resting RSA, RSA reactivity, and the two mood repair factors were added to the mediation model described above (D paths in Figure 1). According to the results, the moderation effects of RSA patterns on mood repair were not significant (ps = .25-.74), and did not improve the model’s fit to the data relative to the mediation model (ΔLogLikelihood (6) = 1.65, p = .95; Mediation model AIC = 23345.39, BIC = 23720.30 vs. Moderated-Mediation model AIC = 23356.35, BIC = 23754.69). Next, we examined the fit of a “pure” moderation model by removing the predictive effects of RSA patterns on the mood repair factors (A paths in Figure 1). The fit of the “pure” moderation model did not significantly change relative to the moderated-mediation model (ΔLogLikelihood (2) = 3.55, p = .17; Moderation model AIC = 23355.48, BIC = 23746.02 vs. Moderated-Mediation model AIC = 23356.35, BIC = 23754.69). Given this pattern of findings, including lower information criteria, greater parsimony, and no difference in absolute model fit relative to the moderated-mediation models, the mediation model shows a superior ability to account for the functional relationships between RSA patterns, dispositional mood repair, and depression.
Do atypical RSA patterns reduce the beneficial effects of implementing an adaptive mood repair response in the laboratory?
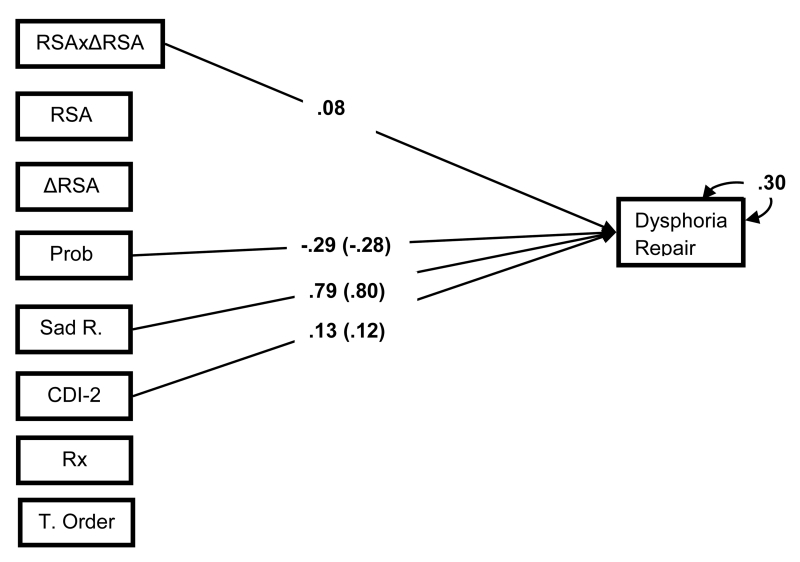
As an alternate test of our trait moderation model, we examined whether in vivo (state) adaptive mood repair outcome is adversely affected by atypical RSA. Mood repair success was defined as the difference in dysphoria rating after mood induction and the instructed mood repair task. These change scores then were regressed on first- and second-order effects of resting RSA and RSA reactivity in a series of regression models that controlled for experimental task order, psychotropic medication use, proband status, depressive symptoms, and subjects’ reactivity to the mood induction relative to their baseline ratings (see Figure 6).
Figure 6.
Standardized First- and Second-order regression models of RSA patterns predicting mood repair during an attention refocusing task. Effects of categorical predictors standardized with respect to the outcome variable. Parameters within parentheses are from the first-order effects models. Parameters outside parentheses are from the second-order effects models. Bold parameters, significant at p < .05; and non-significant effects omitted to improve interpretability. Prob = proband group membership, RSA =RSA during paced breathing, ΔRSA = change score from paced breathing RSA to RSA during the sad film, RSAxΔRSA = second order effects of RSA indices, Sad R. =change from baseline to post-film average sad/blue ratings, CDI-2 = self-report Child Depression Inventory-2, Dysphoria Repair = change from post-film to post repair task average sad/blue ratings, Rx =prescription medication use, T. Order = task order.
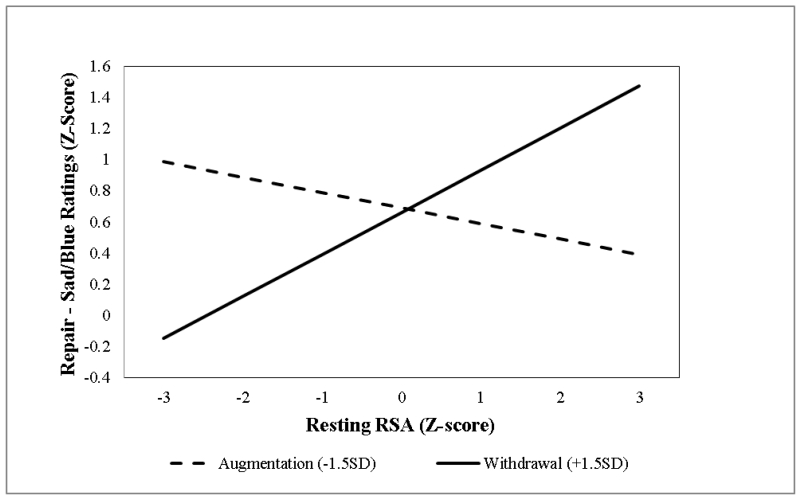
First-order effects of resting RSA and RSA reactivity did not predict mood repair effectiveness. However, consistent with our hypothesis, addition of the second-order effects of the two RSA metrics improved model fit, Wald χ2 (1) = 3.88, p <.05, and predicted the degree of laboratory mood repair, βRSAxΔRSA = .08, p < .05, ΔR2 =.01. Simple slopes analyses of the interaction term revealed that the normative RSA pattern of high resting RSA and RSA withdrawal was associated with more effective laboratory mood repair, β = .18, relative to the atypical RSA pattern of high resting RSA and RSA augmentation, β = −.07 (Figure 7). These effects were independent of proband status, which was associated with lower levels of mood repair, β = −.29, p < .05, ΔR2 = .01.
Figure 7.
RSA reactivity moderation of resting RSA effects on mood repair effectiveness during attention re-focusing for mood repair.
Discussion
We tested three competing models of the nature of the relationship between RSA patterns and mood repair in the context of depression risk among adolescents. We found that a mediational (rather than a moderational or moderated-mediational) model best represented the association between trait maladaptive mood repair, RSA patterns, and depression symptoms. Youths with atypical RSA patterns (e.g., high resting RSA+RSA augmentation to the sad film) reportedly engaged in more frequent use of maladaptive mood repair responses than youths with normative RSA patterns (e.g., high resting RSA + RSA withdrawal to the sad film). In turn, the extent of maladaptive mood repair accounted for the association between RSA patterns and depression symptoms. However, although RSA patterns were unrelated to trait adaptive mood repair, they moderated the subjective benefits of in vivo adaptive mood repair (state mood repair). In other words, normative RSA patterns predicted greater success at reducing sadness in the laboratory via attention refocusing, while atypical RSA patterns reduced the usual benefits of attention refocusing.
Although our mediation model findings should be viewed cautiously owing to the cross-sectional nature of the data, they may speak to key processes that have been implicated in depression risk. Namely, while atypical RSA patterns are more prevalent among youths at familial risk for depression than among low-risk peers, and such patterns predict elevated depression symptom trajectories in adolescence (Yaroslavsky et al., 2014), the path whereby atypical RSA patterns lead to depression has not yet been elucidated. Together with results from studies showing that maladaptive mood repair is a robust predictor of depression risk (Bylsma et al., 2015; Ehring et al., 2010; Garber et al., 1995; Gentzler et al., 2009; Kovacs et al., 2009; Thompson et al., 2010) and new incidence of depressive episodes (Kovacs et al., 2009; Stone et al., 2011), the present results suggest that problematic mood repair could be one pathway of risk for depression in youths. Indeed, the relationship between atypical RSA patterns and habitual maladaptive mood repair in the present study mirrors results in a sample of adults with and without histories of juvenile onset depression (Yaroslavsky et al., 2013b).
It is noteworthy that we found no support for a mediational model regarding adaptive mood repair. Namely, RSA patterns failed to predict the habitual use of adaptive mood repair responses, also mirroring prior results on adults (Yaroslavsky et al., 2013b). Such findings may reflect that adaptive and maladaptive mood repair are subserved by different psychological and physiological processes and are therefore characterized by different precursors and outcomes. It also may be that the use and consequences of adaptive mood repair strategies are essentially context dependent (Aldao & Nolen-Hoeksema, 2012). Supporting this possibility, we found that the benefits of adaptive mood repair in the laboratory were influenced by RSA patterns: RSA patterns constitute individual differences in physiology that reflect one kind of context.
Intriguingly, RSA did not moderate trait adaptive mood repair, but did predict state adaptive mood repair. One possible explanation of this apparent discrepancy is that our measures of state and trait focused on different facets of mood repair. Our in vivo index of state mood repair reflected the success of a specific adaptive response to reduce sadness in the laboratory, whereas our trait mood repair measures indexed the historical and typical use of a broad range of mood repair responses. By definition, accounts of trait mood repair therefore are divorced from the contexts of their use. Clarifying the relationship between state and trait mood repair should be an important consideration in future models of the role of RSA in mood repair outcomes.
While little is known about the pathways through which atypical RSA patterns relate to mood repair deficits (and hence depression risk), reduced attention flexibility may be one route. Namely, high resting RSA and context-appropriate RSA withdrawal have been linked to the flexible allocation of attention (Park, Van Bavel, Vasey, & Thayer, 2012; Park, Vasey, Van Bavel, & Thayer, 2013) and good executive functioning (Hansen, Johnsen, & Thayer, 2003; Marcovitch et al., 2010). Conversely, excessive RSA withdrawal in response to stimuli, particularly in conjunction with low resting RSA, may reflect autonomic hyperarousal that is likely to interfere with the flexible allocation and control of attention processes (Beauchaine, Gatzke-Kopp, & Mead, 2007). Insufficient vagal withdrawal has also been linked to reduced executive functioning (Marcovitch et al., 2010). Elucidating causal links between attention flexibility, RSA patterns, and mood repair may shed light on key processes implicated in depression risk (see Kovacs & Yaroslavsky, 2014).
It is noteworthy that, in our models, RSA patterns, rather than individual RSA indices, were associated with mood repair and depressive symptoms. These findings suggest that using a single index of a physiological system may not reveal the true extent of inter-individual differences (Lacey, 1959; see also El-Sheikh & Erath, 2011). However, much of the work on RSA patterns has been largely data driven, with relatively little explicit theorizing about the responsible mechanisms (but see Beauchaine, 2015). Thus, there is a need for additional theory as well as further empirical work to connect RSA patterns to self-regulation outcomes (e.g., neural underpinnings).
An important aspect of our findings is that proband status consistently signaled higher levels of depression, more extensive maladaptive mood repair response repertoires, and less adaptive mood repair use and success. However, notwithstanding growing evidence that atypical RSA patterns also are more common among depressed and high-risk individuals than among controls (Yaroslavsky et al., 2014), the effects of RSA patterns in the present adolescent sample were neither unique to, nor accentuated, among subjects with depression histories. Given similar findings on adults (Yaroslavsky 2013a, 2013b), it therefore appears that the potential mechanistic roles of RSA and mood repair in depression are not constrained by age, clinical history, or risk status. In turn, the existence of a biobehavioral construct that spans developmental spectrums should help to better characterize the continuity of juvenile-onset depression into the years of adulthood.
These results therefore have implications for the assessment and treatment of depressive disorders. First, the findings highlight the need to integrate physiologic parameters into a comprehensive characterization of mental disorders (e.g., Cuthbert & Insel, 2010). Second, the results document the empirical utility of cardiac indices of PNS activity (Beauchaine, 2015; Porges, 2007; Thayer & Lane, 2009) among youths with depression histories and other types of internalizing disorders (De Los Reyes & Aldao, 2015). Third, given that atypical RSA patterns predicted maladaptive mood-regulatory responses, “normalization” of RSA may possibly improve mood repair and thereby lower the risk for depressive disorders. Indeed, there is evidence that RSA can be altered through meditation (Tang et al., 2009), and that vagal nerve stimulation has some efficacy in treatment-refractory depression (O’Reardon, Cristancho, & Peshek, 2006). Fourth, our finding that atypical RSA patterns reduced the benefits of an adaptive mood repair response suggests that there may well be a (partial) physiological explanation when depressed patients report limited gains from deploying adaptive mood repair strategies.
Our findings should be interpreted in the context of several limitations. Because we did not control for respiration in our assessment of RSA, we cannot rule out the possibility that respiration influenced the results. This concern is partly minimized by our use of a well-validated, paced breathing task to establish a resting baseline RSA. The fact that we did not consider the effects of psychiatric comorbidity or sympathetic nervous system activity on the association between RSA, mood repair, and depression also limits the findings. Further, as we have previously noted (Kovacs et al., 2015), while we attained, on average, a medium-to-large mood induction effect by Cohen’s standards (Cohen, 1992), a notable portion of our subjects, particularly those with depression histories, failed to respond to the negative mood induction. While the reasons for this are not entirely clear, the non-response rate is in the range reported by an early review of mood induction studies (Gerrards-Hesse, Spies, & Hesse, 1994). Another limitation is the use of cross-sectional data, which constrains our ability to specify causal relationships between RSA patterns, mood repair, and depression. Finally, our experimental findings are limited by focusing only on one form of instructed mood repair (attention refocusing).
However, the strengths of the present study outweigh its limitations. Importantly, we used a multi-trait, multi-method analytic approach, and structural equation modeling, which attenuated measurement error and self-report biases and increased the precision of the findings. Further, this is the first study to combine survey and laboratory measures of mood repair to examine the effects of parasympathetic nervous system activity on emotion and depression outcomes. Finally, to our knowledge, this study was the first to investigate how RSA level and RSA reactivity jointly predict the use and effectiveness of mood regulation strategies in the laboratory, implicating mood repair as one mechanism through which physiological vulnerabilities adversely affect mental health.
General Scientific Statement.
Results of this study suggest that patterns of parasympathetic nervous system (PNS) activity are related to depression symptoms and the use and effectiveness of regulatory responses to dysphoric affect. We found that atypical patterns of PNS activity predict the use of ineffective affect regulatory responses that, in turn, predict depression. Atypical PNS patterns also relate to reduced ability to attenuate sadness in the laboratory.
Acknowledgements
This research was supported by NIH Grants MH-084938, MH-056193 and the Hungarian Scientific Research Fund Grant NN85285. Dr. Kovacs reports receiving royalties from MHS, which publishes the Children’s Depression Inventory (CDI).
Footnotes
While emotion regulation refers to altering the intensity or duration in any direction of any emotion (Thompson, 1994), mood repair focuses specifically on processes by which people attenuate sadness and dysphoria (Josephson, Singer, & Salovey, 1996).
Subjects who were excluded for their non-response to the mood induction procedure were approximately 9 months younger than those who were retained in the analysis (t(181) = 2.57, p <.05), but did not differ in their depression symptoms, dispositional mood repair, or RSA levels.
Since Mplus does not provide relative model fit indices (i.e., CFI & RMSEA) in the presence of count variables, depression factor scores were used as proxies for the latent depression variable to estimate relative fit of measurement and structural models. Depression factor scores were a good approximation of the latent depression factor (r = .97).
We also examined models that did not control for subjects’ reactivity to the sad film. Results of these models did not alter our major findings, and in fact strengthened the association between RSA patterns and mood repair effectiveness (βRSA = .11, ns; βΔRSA = .00, ns; βRSAxΔRSA = .18, p <.01).
These results were replicated when we used adolescents’ self-reports; maladaptive mood repair mediated the effects of RSA patterns on depression symptoms, (RSA patterns → MMR: βRSA = .11, p <. 05; βΔRSA = −.09, ns; βRSAxΔRSA = −.09, p <.05; indirect effect = −.08, 95% CI −.009 - −.13).
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; Newbury Park: 1991. [Google Scholar]
- Aldao A, Nolen-Hoeksema S. When are adaptive strategies most predictive of psychopathology? Journal of Abnormal Psychology. 2012;121:276–281. doi: 10.1037/a0023598. [DOI] [PubMed] [Google Scholar]
- Aldao A, Sheppes G, Gross JJ. Emotion regulation flexibility. Cognitive Therapy and Research. 2015;39(3):263–278. [Google Scholar]
- Allen B, Jennings JR, Gianaros PJ, Thayer JF, Manuck SB. Resting high-frequency heart rate variability is related to resting brain perfusion. Psychophysiology. 2015;52:277–287. doi: 10.1111/psyp.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP. Vagal tone, development, and gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Future directions in emotion dysregulation and youth psychopathology. Journal of Clinical Child And Adolescent Psychology. 2015;44(5):875–896. doi: 10.1080/15374416.2015.1038827. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp LM, Mead HK. Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, Nelson B. Childhood and adolescent depression: A review of the past 10 years, Part I. Journal Of The American Academy Of Child & Adolescent Psychiatry. 1996;35(11):1427–1439. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- Blandon AY, Calkins SD, Keane SP, O’Brien M. Individual differences in trajectories of emotion regulation processes: The effects of maternal depressive symptomatology and children’s physiological regulation. Developmental Psychology. 2008;44:1110–1123. doi: 10.1037/0012-1649.44.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom EH, Olsson EM, Serlachius E, Ericson M, Ingvar M. Heart rate variability (HRV) in adolescent females with anxiety disorders and major depressive disorder. Acta Paediatrica. 2010;99(4):604–611. doi: 10.1111/j.1651-2227.2009.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen KA. Structural Equations with Latent Variables. John Wiley & Sons, Inc.; New York: 1989. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Sage; Newbury Park, CA: 1993. pp. 136–162. [Google Scholar]
- Bylsma LM, Yaroslavsky I, Rottenberg J, Kiss E, Kapornai K, Halas K, Dochnal R, Lefkovics E, Baji I, Vetró A, Kovacs M. Familiality of mood repair responses among youth with and without histories of depression. Cognition & Emotion. 2015 doi: 10.1080/02699931.2015.1025707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ML, Sheeber L, Simmons JG, Davis B, Wu Shortt J, Katz LF, Allen NB. Autonomic cardiac control in depressed adolescents. Depression and Anxiety. 2010;27(11):1050–1056. doi: 10.1002/da.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson KL, Broome M, Vessey JA. Using distraction to reduce reported pain, fear, and behavioral distress in children and adolescents: A multisite study. Journal of the Society of Pediatric Nurses. 2000;5(2):75–85. doi: 10.1111/j.1744-6155.2000.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, McCauley E, Smith CJ, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicide among adolescent girls. Development and Psychopathology. 2005;17(4):1105–1127. doi: 10.1017/s0954579405050522. [DOI] [PubMed] [Google Scholar]
- Cuthbert B, Insel T. The data of diagnosis: New approaches to psychiatric classification. Psychiatry: Interpersonal and Biological Processes. 2010;73(4):311–314. doi: 10.1521/psyc.2010.73.4.311. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, Aldao A. Introduction to the special issue: Toward implementing physiological measures in clinical child and adolescent assessments. Journal of Clinical Child And Adolescent Psychology. 2015;44(2):221–237. doi: 10.1080/15374416.2014.891227. [DOI] [PubMed] [Google Scholar]
- Dywan J, Mathewson K, Choma BL, Rosenfeld B, Segalowitz S. Autonomic and electrophysiological correlates of emotional intensity in older and younger adults. Psychophysiology. 2008;45:389–397. doi: 10.1111/j.1469-8986.2007.00637.x. [DOI] [PubMed] [Google Scholar]
- Ehring T, Tuschen-Caffier B, Schnulle J, Fischer S, Gross JJ. Emotion regulation and vulnerability to depression: Spontaneous versus instructed use of emotion suppression and reappraisal. Emotion. 2010;10:563–572. doi: 10.1037/a0019010. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA. Family conflict, autonomic nervous system functioning, and child adaptation: State of the science and future directions. Development and Psychopathology. 2011;23:703–721. doi: 10.1017/S0954579411000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erber R, Tesser A. Task effort and the regulation of mood: The absorption hypothesis. Journal of Experimental Social Psychology. 1992;28:339–359. [Google Scholar]
- Fabes RA, Eisenberg N. Regulatory control and adults’ stress-related responses to daily life events. Journal of Personality and Social Psychology. 1997;73:1107–1117. doi: 10.1037//0022-3514.73.5.1107. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Recurrence of major depression in adolescence and early adulthood, and later mental health, educational and economic outcomes. The British Journal Of Psychiatry. 2007;191(4):335–342. doi: 10.1192/bjp.bp.107.036079. [DOI] [PubMed] [Google Scholar]
- Fritz MS, MacKinnon DP. Required sample size to detect the mediated effect. Psychological Science. 2007;18(3):233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, Braafladt N, Weiss B. Affect regulation in depressed and nondepressed children and young adolescents. Development and Psychopathology. 1995;7(1):93–115. [Google Scholar]
- Geisler FCM, Kubiak T, Siewert K, Weber H. Cardiac vagal tone is associated with social engagement and self-regulation. Biological Psychology. 2013;93(2):279–286. doi: 10.1016/j.biopsycho.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Gentzler AL, Rottenberg J, Kovacs M, George CJ, Morey JN. Atypical development of resting respiratory sinus arrhythmia in children at high risk for depression. Developmental Psychobiology. 2012;54(5):556–567. doi: 10.1002/dev.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, Fox N. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology. 2009;82:156–163. doi: 10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrards-Hesse A, Spies K, Hesse FW. Experimental inductions of emotional states and their effectiveness: A review. British Journal of Psychology. 1994;85:55–78. [Google Scholar]
- Goodman SH. Depression in mothers. Annual Review Of Clinical Psychology. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Lewinsohn PM, Seeley JR. Consequences of depression during adolescence: Marital status and marital functioning in early adulthood. Journal Of Abnormal Psychology. 1998;107(4):686–690. doi: 10.1037//0021-843x.107.4.686. [DOI] [PubMed] [Google Scholar]
- Graziano P, Derefinko K. Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology. 2013;94(1):22–37. doi: 10.1016/j.biopsycho.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotion elicitation using films. Cognition and Emotion. 1995;9:87–108. [Google Scholar]
- Gross JJ, Muñoz RF. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2:151–164. [Google Scholar]
- Hammen C, Brennan PA, Keenan-Miller D. Patterns of adolescent depression to age 20: The role of maternal depression and youth interpersonal dysfunction. Journal Of Abnormal Child Psychology. 2008;36(8):1189–1198. doi: 10.1007/s10802-008-9241-9. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. International Journal of Psychophysiology. 2003;48:263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Healy B, Treadwell A, Reagan M. Measures of RSA suppression, attentional control, and negative affect predict self-ratings of executive functions. Journal of Psychophysiology. 2011;25(4):164–173. [Google Scholar]
- Hinnant JB, El-Sheikh M. Children’s externalizing and internalizing symptoms over time: The role of individual differences in patterns of RSA responding. Journal of Abnormal Child Psychology. 2009;37:1049–1061. doi: 10.1007/s10802-009-9341-1. [DOI] [PubMed] [Google Scholar]
- Hinnant JB, El-Sheikh M. Codevelopment of externalizing and internalizing symptoms in middle to late childhood: Sex, baseline respiratory sinus arrhythmia, and respiratory sinus arrhythmia reactivity as predictors. Development and Psychopathology. 2013;25(2):419–436. doi: 10.1017/S0954579412001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: Relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54 doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Isen AM. Asymmetry of happiness and sadness in effects on memory in normal college students: Comment on Hasher, Rose, Zacks, Sanft, and Doren. Journal of Experimental Psychology: General. 1985;114:388–391. [Google Scholar]
- Jones AN, Field T, Fox NA, Davalos M, Lundy B, Hart S. Newborns of mothers with depressive symptoms are physiologically less developed. Infant Behavior and Development. 1998;21:537–541. [Google Scholar]
- Joormann J, Cooney RE, Henry ML, Gotlib IH. Neural correlates of automatic mood regulation in girls at high risk for depression. Journal of Abnormal Psychology. 2012;121(1):61–72. doi: 10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Siemer M, Gotlib IH. Mood regulation in depression: Differential effects of distraction and recall of happy memories on sad mood. Journal of Abnormal Psychology. 2007;116:484–490. doi: 10.1037/0021-843X.116.3.484. [DOI] [PubMed] [Google Scholar]
- Josephson BR, Singer JA, Salovey P. Mood regulation and memory: Repairing sad moods with happy memories. Cognition and Emotion. 1996;10:437–444. [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Wessa M. Neural correlates of emotion regulation deficits in remitted depression: The influence of regulation strategy, habitual regulation use, and emotional valence. NeuroImage. 2012;61:686–693. doi: 10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Kashy DA. Analysis of the multitrait-multimethod matrix by confirmatory factor analysis. Psychological Bulletin. 1992;112:165–172. [Google Scholar]
- Kiss E, Gentzler AM, George C, Kapornai K, Tamas Z, Kovacs M, Vetro A. Factors influencing mother-child reports of depressive symptoms and agreement among clinically referred depressed youngsters in Hungary. Journal of Affective Disorders. 2007;100:143–151. doi: 10.1016/j.jad.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Moosbrugger H. Maximum likelihood estimation of latent interaction effects with the LMS method. Psychometrika. 2000;65:457–474. [Google Scholar]
- Kovacs M, Joormann J, Gotlib IH. Emotion (dys)regulation and links to depressive disorders. Child Development Perspectives. 2008;2(3):149–155. doi: 10.1111/j.1750-8606.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, MHS Staff . Children’s Depression Inventory (CDI 2), 2nd Edition: Technical Manual. Multi-Health Systems; North Tonawanda, NY: 2011. [Google Scholar]
- Kovacs M, Rottenberg J, George C. Maladaptive mood repair responses distinguish young adults with early onset depressive disorders and predict future depressive outcomes. Psychological Medicine. 2009;39:1841–1854. doi: 10.1017/S0033291709005789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Yaroslavsky I. Practitioner review: Dysphoria and its regulation in child and adolescent depression. Journal of Child Psychology and Psychiatry. 2014 doi: 10.1111/jcpp.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Yaroslavsky I, Rottenberg J, George CJ, Baji I, Benak I, Vetro A. Mood repair via attention refocusing or recall of positive autobiographical memories by adolescents with pediatric-onset major depression. Journal of Child Psychology and Psychiatry. 2015 doi: 10.1111/jcpp.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig SD. Autonomic nervous system activity in emotion: A review. Biological Psychology. 2010;84(3):394–421. doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Lacey JI. Psychophysiological approaches to the evaluation of psychotherapeutic process and outcome. In: Rubinstein EA, Parloff MB, editors. Research in psychotherapy. American Psychological Association; Washington, DC: 1959. pp. 160–208. [Google Scholar]
- Lewinsohn PM, Clarke GN, Seeley JR, Rohde P. Major depression in community adolescents: Age at onset, episode duration, and time to recurrence. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:809–818. doi: 10.1097/00004583-199407000-00006. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behavior Research Methods. 2007;39 doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcovitch S, Leigh J, Calkins SD, Leerks EM, O’Brien M, Nayena Blankson A. Moderate vagal withdrawal in 3.5-year-old children is associated with optimal performance on executive function tasks. Developmental Psychobiology. 2010;52(6):603–608. doi: 10.1002/dev.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziade M, Roy MA, Fournier JP, Cliche D, Merette C, Caron C, Raymond V. Reliability of best-estimate diagnosis in genetic linkage studies of major psychoses: Results from the Quebec Pedigree Studies. American Journal of Psychiatry. 1992;149:1674–1686. doi: 10.1176/ajp.149.12.1674. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Seventh Edition Muthén & Muthén; Los Angeles, CA: 1998-2012. [Google Scholar]
- Najman JM, Williams GM, Nikles J, Spence S, Bor W, O’Callaghan M, Shuttlewood GJ. Bias influencing maternal reports of child behaviour and emotional state. Social Psychiatry and Psychiatric Epidemiology. 2001;36(4):186–194. doi: 10.1007/s001270170062. [DOI] [PubMed] [Google Scholar]
- O’Connor M, Allen JJB, Kaszniak AW. Emotional disclosure for whom? A study of vagal tone in bereavement. Biological Psychology. 2005;68:135–146. doi: 10.1016/j.biopsycho.2004.04.003. [DOI] [PubMed] [Google Scholar]
- O’Reardon JP, Cristancho P, Peshek AD. Vagus Nerve Stimulation (VNS) and Treatment of Depression: To the Brainstem and Beyond. Psychiatry. 2006;3(5):54–62. [PMC free article] [PubMed] [Google Scholar]
- Overbeek TJM, van Boxtel A, Westerink JHDM. Respiratory sinus arrhythmia responses to induced emotional states: Effects of RSA indices, emotion induction method, age, and sex. Biological Psychology. 2012;91(1):128–141. doi: 10.1016/j.biopsycho.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Pang KC, Beauchaine TP. Longitudinal patterns of autonomic nervous system responding to emotion evocation among children with conduct problems and/or depression. Developmental Psychobiology. 2013;55:698–706. doi: 10.1002/dev.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G, Van Bavel JJ, Vasey MW, Thayer JF. Cardiac vagal tone predicts inhibited attention to fearful faces. Emotion. 2012;12(6):1292–1302. doi: 10.1037/a0028528. [DOI] [PubMed] [Google Scholar]
- Park G, Vasey MW, Van Bavel JJ, Thayer JF. Cardiac vagal tone is correlated with selective attention to neutral distractors under load. Psychophysiology. 2013;50(4):398–406. doi: 10.1111/psyp.12029. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijntjes A, Stegge H, Terwogt MM, Kamphuis JH, Telch MJ. Emotion regulation and its effects on mood improvement in response to an in vivo peer rejection challenge. Emotion. 2006;6:543–552. doi: 10.1037/1528-3542.6.4.543. [DOI] [PubMed] [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: A critical analysis. Biological Psychology. 2007;74:200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Yaroslavsky I, Carney RM, Freedland KE, George CJ, Baji I, Kovacs M. The association between major depressive disorder in childhood and risk factors for cardiovascular disease in adolescence. Psychosomatic Medicine. 2014;76:122–127. doi: 10.1097/PSY.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci AK, Silk JS, Shaw DS, Gentzler A, Fox NA, Kovacs M. Vagal tone and temperament as predictors of emotion regulation strategies in young children. Developmental Psychobiology. 2008;50(3):205–216. doi: 10.1002/dev.20283. [DOI] [PubMed] [Google Scholar]
- SAS® 9.3 . System Options: Reference, Second Edition. SAS Institute Inc.; Cary, NC: 2013. [Google Scholar]
- Silk JS, Shaw DS, Skuban EM, Oland AA, Kovacs M. Emotion regulation strategies in offspring of childhood-onset depressed mothers. Journal Of Child Psychology And Psychiatry. 2006;47(1):69–78. doi: 10.1111/j.1469-7610.2005.01440.x. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Epstein EM. Respiratory sinus arrhythmia predicts written disclosure outcome. Psychophysiology. 2005;42:611–615. doi: 10.1111/j.1469-8986.2005.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH. Point estimation, hypothesis testing, and interval estimation using the RMSEA: Some comments and a reply to Hayduck and Glaser. Structural Equation Modeling. 2000;7:149–162. [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JB. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology. 2010;119(3):502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LB, Hankin BL, Gibb BE, Abela JZ. Co-rumination predicts the onset of depressive disorders during adolescence. Journal Of Abnormal Psychology. 2011;120(3):752–757. doi: 10.1037/a0023384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas Z, Kovacs M, Gentzler AL, Tepper P, Gádoros J, Kiss E, Kapornai K, Vetró Á . The relations of temperament and emotion self-regulation with suicidal behaviors in a clinical sample of depressed children in Hungary. Journal of Abnormal Child Psychology. 2007;35:640–652. doi: 10.1007/s10802-007-9119-2. [DOI] [PubMed] [Google Scholar]
- Tang Y, Ma Y, Fan Y, Feng H, Wang J, Feng S, Fan M. Central and autonomic nervous system interaction is altered by short-term meditation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8865–8870. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neuroscience Biobehavioral Review. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thompson RA. Emotion regulation: A theme in search of definition. Monographs Of The Society For Research In Child Development. 1994;59(2-3):25–52. [PubMed] [Google Scholar]
- Thompson RJ, Mata J, Jaeggi SM, Buschkuehl M, Jonides J, Gotlib IH. Maladaptive coping, adaptive coping, and depressive symptoms: Variations across age and depressive state. Behavior Research and Therapy. 2010;48:459–466. doi: 10.1016/j.brat.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dillen LF, Koole SL. Clearing the mind: A working memory model of distraction from negative mood. Emotion. 2007;7:715–723. doi: 10.1037/1528-3542.7.4.715. [DOI] [PubMed] [Google Scholar]
- Verkuil B, Brosschot JF, de Beurs DP, Thayer JF. Effects of explicit and implicit perseverative cognition on cardiac recovery after cognitive stress. International Journal Psychophysiology. 2009;74:220–228. doi: 10.1016/j.ijpsycho.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Volokhov RN, Demaree HA. Spontaneous emotion regulation to positive and negative stimuli. Brain & Cognition. 2010;73:1–6. doi: 10.1016/j.bandc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Bylsma L, Rottenberg J, Kovacs M. Combinations of resting RSA and RSA reactivity impact maladaptive mood repair and depression symptoms. Biological Psychology. 2013b;94:272–281. doi: 10.1016/j.biopsycho.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Kovacs M. The utility of combining RSA indices in depression prediction. Journal of Abnormal Psychology. 2013a;122:314–321. doi: 10.1037/a0032385. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Kovacs M. Atypical patterns of respiratory sinus arrhythmia index an endophenotype for depression. Development and Psychopathology. 2014;26(4):1337–1352. doi: 10.1017/S0954579414001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Cunningham WA. Executive Function: Mechanisms Underlying Emotion Regulation. In: Gross JJ, Gross JJ, editors. Handbook of emotion regulation. Guilford Press; New York, NY, US: 2007. pp. 135–158. [Google Scholar]
- Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani GK, Fava M, Gilmer WS. Effect of age at onset on the course of major depressive disorder. American Journal of Psychiatry. 2007;164:1539–1546. doi: 10.1176/appi.ajp.2007.06101757. [DOI] [PubMed] [Google Scholar]