Abstract
Agricultural soil is the largest source of nitrous oxide (N2O), a greenhouse gas. Soybean is an important leguminous crop worldwide. Soybean hosts symbiotic nitrogen-fixing soil bacteria (rhizobia) in root nodules. In soybean ecosystems, N2O emissions often increase during decomposition of the root nodules. Our previous study showed that N2O reductase can be used to mitigate N2O emission from soybean fields during nodule decomposition by inoculation with nosZ++ strains [mutants with increased N2O reductase (N2OR) activity] of Bradyrhizobium diazoefficiens. Here, we show that N2O emission can be reduced at the field scale by inoculation with a mixed culture of indigenous nosZ+ strains of B. diazoefficiens USDA110 group isolated from Japanese agricultural fields. Our results also suggested that nodule nitrogen is the main source of N2O production during nodule decomposition. Isolating nosZ+ strains from local soybean fields would be more applicable and feasible for many soybean-producing countries than generating mutants.
Agricultural soil is the single largest source of global anthropogenic nitrous oxide (N2O) emission1, accounting for approximately 59% of anthropogenic emissions2. N2O is a greenhouse gas that is also detrimental to the ozone layer2. The global warming potential of N2O is ~300-fold higher than that of CO2 on a molar basis, and the concentration of N2O has increased at a rate of 0.73 ppb yr−1 over the last three decades2.
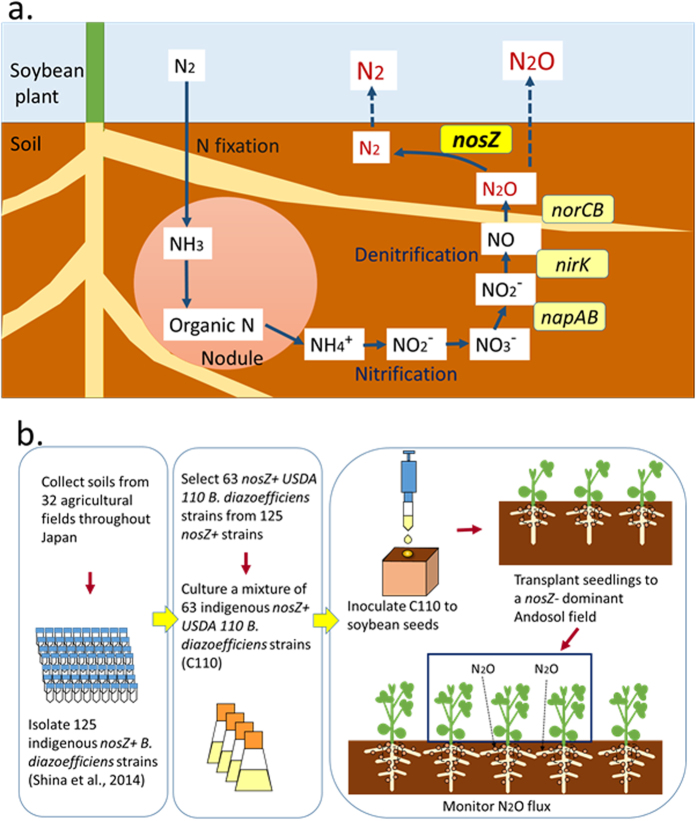
Soybean (Glycine max [L.] Merr.) is one of the most important crops in the world. Soybean is grown on 6% of the world’s arable land, and its production has dramatically increased from 26 Mt in 1961 to 308 Mt in 2014 (ref. 3). The soybean production area is expected to increase more than that of other crops4. As a leguminous crop, soybean hosts symbiotic nitrogen-fixing soil bacteria (rhizobia) that can also produce N2O in root nodules5. In soybean ecosystems, increase of N2O emission during decomposition of the root nodules has often been reported6. Organic nitrogen inside the decomposing nodules is mineralized to NH4+ followed by nitrification and denitrification that produce N2O (Fig. 1a)7,8. N2O is then emitted into the atmosphere or is further reduced to N2 by N2O reductase (N2OR), which is encoded by the nosZ gene. Bradyrhizobium diazoefficiens is a nitrogen-fixing rhizobium that also possesses a denitrification pathway8. Both B. diazoefficiens nosZ+ (strains that have the nosZ gene) and nosZ− (strains that do not have the nosZ gene) strains are found in soil9. Denitrification by nosZ− strains produces N2O because they lack nosZ, whereas nosZ+ strains can reduce N2O to N2 (Fig 1a).
Figure 1.
(a) Microbial pathway involved in N2O production from decomposition of root nodules. During decomposition of nodules, nitrogen becomes available for soil microorganisms. Using this nitrogen, B. diazoefficiens nosZ+ strains sequentially reduce nitrogen oxides during denitrification (NO3− → NO2− → NO → N2O → N2), with each step catalyzed by specific reductases encoded by denitrifying genes: napA (periplasmic nitrate reductase), nirK (copper-containing nitrite reductase), norCB (nitric oxide reductase), and nosZ (nitrous oxide reductase), respectively. However, denitrification by B. diazoefficiens nosZ− strains produces N2O because they lack the nosZ gene that encodes N2O reductase (N2OR). Both nosZ+ and nosZ− strains are found in the soil. (b) Design of the experiment. First, soils were collected from 32 agricultural fields throughout Japan. Then, 125 indigenous nosZ+ B. diazoefficiens strains were isolated (Shina et al.)9. From these 125 strains, 63 indigenous nosZ+ strains of B. diazoefficiens USDA110 group were selected (C110), because nitrogen fixation in USDA110 is higher than that in other strains (Itakura et al.)18. C110 was cultured and inoculated onto soybean seeds in biodegradable pots. For control plots, soybean seeds were inoculated with soil from the experimental field. Soybean seedlings were grown for 10 days in a greenhouse and then transplanted into a nosZ- dominant Andosol field. Annual N2O flux was monitored and mitigation of N2O production by soybean nodules of inoculated strains was evaluated.
Enhancing microbial N2OR activity has been suggested as an N2O mitigation option10, and mitigation of N2O using nosZ has been demonstrated on the laboratory scale. Pure culture and vermiculite pot experiments showed lower N2O emission by nosZ+ strains11 and nosZ++ strains (mutants with increased N2OR activity)12,13 of B. diazoefficiens than by nosZ− strains. A pot experiment using soil confirmed these results14. In addition to the use of B. diazoefficiens, transgenic plants expressing N2OR following introduction of soil bacterial nosZ were generated to reduce N2O emission15. However, no field-scale study has been reported except our previous study, which showed that N2OR can be used to mitigate N2O emission by inoculation with nosZ++ strains of B. diazoefficiens13. Although it is an effective approach, generating nosZ++ mutants requires time, cost, and technical skills, and the field use of genetically modified microbes is regulated in many countries. In contrast, isolating indigenous strains from field soil is easy and cost-effective in comparison with generating mutants. Moreover, isolated indigenous strains may be more competitive than mutants with native field strains. Here we report the mitigation of N2O emission from a soybean field by inoculation with a mixed culture of indigenous nosZ+ strains of B. diazoefficiens isolated from agricultural fields, without the use of a mutant (Fig. 1b).
Results and Discussion
Construction of cell mixture of indigenous USDA110 group isolates
Although (brady)rhizobia have been used as inoculants for legume crop production worldwide, rhizobial inoculation is often ineffective in the presence of indigenous rhizobia in soils because of the problem of so-called competition between inoculants and (brady)rhizobial populations indigenous to field soils16,17. Many genomic variations have been found even in isolates in a B. deazoefficiens collection9,18 (Itakura et al. unpublished results), suggesting that field inoculation with a mixture of B. deazoefficiens isolates could overcome the competition problem. We accordingly prepared a cell mixture (C110) of native B. diazoefficiens as an inoculant (Table S1). Shiina et al.9 isolated 125 native nosZ+ B. diazoefficiens from 32 field soils in Japan. Because B. diazoefficiens strains belonging to the USDA110 group showed high ability to fix N2 in soybean nodules18,19, we selected 63 of the 125 isolates whose 16S–23S rRNA ITS sequences were identical to that of strain USDA110 (Table S1). The cell mixture C110 derived from these 63 isolates was used in a field experiment as an inoculant (Fig. 1b). We expected that field inoculation efficiency could be increased if more-competitive isolates were included in C110.
Inoculation efficiency and gene expression in the field experiment
We conducted a two-year field experiment to test the effectiveness of C110 inoculation in reducing N2O emission in an Andosol field dominated by nosZ− strains. In our previous study, postharvest N2O emission was significantly reduced by nosZ++ (mutants with increased N2OR activity) inoculation, whereas the proportion of nosZ++ nodules in the field experiment was only 23% (ref. 13). We expected that increasing the proportion of inoculated strains in nodules might reduce more N2O from decomposition of nodules. In addition to the construction of C110, we improved our germination and inoculation methods to increase the proportion of inoculated strains of nodules. To increase the proportion of cotyledon emergence, soybean seeds were germinated in trays filled with moist vermiculite for one day instead of being seeded in soil-filled pots as in our previous study13. With this change, the proportion of cotyledon emergence increased from approximately 30% for soil to 95% for vermiculite germination. The low proportion of cotyledon emergence for soil may have been observed because maintaining optimal soil water content for germination is much more difficult for small and water-permeable biodegradable pots filled with soil than for large trays filled with vermiculite. After soybean seeds were germinated in moist vermiculite for a day, they were transferred to biodegradable pots filled with soil. For the pots, soil were collected from a nearby Andosol orchard that showed a lower nod C copy number than the Andosol soil of the experimental field (Fig. S1), instead of using soil from the experimental field as in Itakura et al.13. Immediately after transfer to the pots, the seeds were inoculated with the mixed culture C110 (nosZ+) or soil from the experimental field (native). The seedlings were grown for 10 days in a greenhouse and then were transplanted to the Andosol field. As a result of these changes in methods, the proportion of nosZ+ nodules in nosZ+ inoculated plots in this study were 71.4% to 82.8% from August to October (Table 1), much higher than the 23% for inoculated strain in our previous study13. Our results also showed that the proportion of nosZ+ nodules remained high from vegetative to full maturity stage in nosZ+ inoculated plots (Table 1). Furthermore, the proportion of nosZ+ outside pots in nosZ+ inoculated plots were 38–68%, significantly higher than that in native plots (P < 0.001) on all sampling dates. This result indicated that C110 was able to infect soybean roots outside of pots where native rhizobia populations were high. In addition, C110 was more competitive with native strains than nosZ++, which showed 0% of inoculated strain outside of the pots in our previous field experiment13.
Table 1. Nodule number and nodule occupancy in the field experiment in 2013 and 2014.
| Sampling date | Treatment | Nodule number (plant−1) |
Nodule occupancy by nosZ+ (%) |
||||
|---|---|---|---|---|---|---|---|
| Inner | Outer | Total | Inner | Outer | Total | ||
| 2013 | |||||||
| July 23 | Native | 47 ± 9 | NA | 47 ± 9 | 2.4 ± 5.4 | NA | 2.4 ± 5.4 |
| nosZ+ | 32 ± 3 | NA | 32 ± 3 | 89.1 ± 6.7 | NA | 89.1 ± 6.7 | |
| Statistical significance | P < 0.05 | P < 0.05 | P < 0.001 | P < 0.001 | |||
| August 7 | Native | 67 ± 18 | 17 ± 5 | 84 ± 20 | 4.9 ± 6.7 | 6.7 ± 10.1 | 5.1 ± 5.5 |
| nosZ+ | 56 ± 29 | 29 ± 11 | 84 ± 16 | 89.4 ± 13.8 | 38.3 ± 22.1 | 71.4 ± 14.5 | |
| Statistical significance | ns | P < 0.001 | ns | P < 0.001 | P < 0.001 | P < 0.001 | |
| October 1 | Native | 75 ± 31 | 32 ± 13 | 108 ± 41 | 1.7 ± 6.2 | 7.5 ± 13.5 | 3.1 ± 4.8 |
| nosZ+ | 165 ± 52 | 39 ± 31 | 204 ± 71 | 86.5 ± 16.2 | 65.0 ± 18.4 | 82.8 ± 13.4 | |
| Statistical significance | P < 0.001 | ns | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |
| 2014 | |||||||
| August 19 | Native | 92 ± 14 | 66 ± 26 | 159 ± 31 | 26.6 ± 16.1 | 12.5 ± 15.1 | 21.0 ± 11.4 |
| nosZ+ | 113 ± 38 | 49 ± 25 | 162 ± 56 | 78.8 ± 15.5 | 68.3 ± 13.6 | 75.7 ± 13.6 | |
| Statistical significance | ns | ns | ns | P < 0.001 | P < 0.001 | P < 0.001 | |
| October 1# | Native | 68 ± 32 | 44 ± 32 | 113 ± 57 | 12.5 ± 12.5 | 6.3 ± 10.1 | 10.8 ± 10.1 |
| nosZ+ | 102 ± 23 | 36 ± 14 | 138 ± 30 | 82.5 ± 15.0 | 63.8 ± 17.2 | 78.3 ± 11.9 | |
| Statistical significance | P < 0.05 | ns | ns | P < 0.001 | P < 0.001 | P < 0.001 | |
Soybean seeds were inoculated with a mixed culture of 63 Bradyrhizobium diazoefficiens strains C110 (nosZ+) or native strains (Native; nosZ− dominant). “Inner” describes nodules on parts of roots inside the pots; “outer” describes nodules on parts of roots that extended outside the pots. Values are means ± SD (n = 15 or 10#).
Statistical significance was tested using the t-test (two-sided).
NA: data were not available because nodules were collected only from inside of pots. ns: not significant.
Gene expression analysis showed that nosZ expression was significantly higher in nodules collected from nosZ+ inoculated plots than in those from native plots (Table 2), suggesting that N2OR activity in nosZ+ inoculated plots was higher than that in native plots. In contrast, no significant difference in nirK expression between the two treatments was found (Table 2), suggesting that the denitrification process before N2O reduction did not differ between the treatments.
Table 2. Expression of nirK, nosZ, and sigA genes in soybean nodules in the field experiment in 2013 were quantified by RT-real time PCR.
| Sampling day | Treatment | nirK | nosZ | sigA | nirK expression | nosZ expression |
|---|---|---|---|---|---|---|
| (copy number g dry nodule weight−1) | (nirK/sigA) | (nosZ/sigA) | ||||
| 2013 | ||||||
| July 29 | Native | 2.3 × 108 ± 2.1 × 108 | 3.0 × 106 ± 2.8 × 106 | 9.5 × 107 ± 6.2 × 107 | 3.16 ± 1.62 | 0.03 ± 0.02 |
| nosZ+ | 4.3 × 107 ± 2.2 × 107 | 1.6 × 108 ± 3.8 × 107 | 1.2 × 108 ± 3.2 × 107 | 0.43 ± 0.27 | 1.45 ± 0.6 | |
| Statistical significance | ns | P < 0.001 | ns | ns | P < 0.05 | |
| August 12 | Native | 2.6 × 109 ± 1.7 × 109 | 3.7 × 106 ± 2.0 × 106 | 2.4 × 108 ± 1.4 × 108 | 12.81 ± 5.40 | 0.02 ± 0.00 |
| nosZ+ | 1.7 × 109 ± 3.0 × 108 | 4.0 × 108 ± 9.5 × 107 | 4.3 × 108 ± 1.5 × 108 | 4.31 ± 1.00 | 1.00 ± 0.27 | |
| Statistical significance | ns | P < 0.001 | ns | ns | P < 0.001 | |
| October 3# | Native | 4.9 × 108 ± 4.3 × 108 | 1.1 × 106 ± 1.0 × 106 | 5.9 × 107 ± 5.0 × 107 | 7.12 ± 5.37 | 0.12 ± 0.18 |
| nosZ+ | 2.3 × 108 ± 1.6 × 108 | 2.7 × 108 ± 2.0 × 108 | 1.0 × 108 ± 9.9 × 107 | 5.22 ± 4.38 | 4.27 ± 2.41 | |
| Statistical significance | ns | P < 0.05 | ns | ns | P < 0.05 | |
Soybean seeds were inoculated with a mixed culture of 63 Bradyrhizobium diazoefficiens strains C110 (nosZ+) or native strains (Native; nosZ− dominant). In quantification of nosZ mRNA, some samples showed values below the minimum limit of determination by real time PCR. These values were assigned the copy number corresponding to the minimum limit of determination when the averages were calculated. Values are means ± SD (n = 3 or 5#).
Statistical significance was tested using the t-test (two-sided).
N2O emissions in the field experiment
Nodule decomposition begins during the late growth period7. N2O fluxes increased after fertilizer application and the nodule decomposition period (end of August to mid-November) in 2013 (Fig. S2) and in 2014 (Fig. S3). In some studies, nodule decomposition and the consequent N2O emission were observed from late growth period until after harvest13,20. N2O emissions during the nodule decomposition period were larger than those after fertilizer application in both years. N2O fluxes from the nosZ+ inoculated plots were lower than those from native plots during the nodule decomposition period in both years. Consequently, cumulative N2O emission during the nodule decomposition period in nosZ+ inoculated plots was significantly lower than that of native plots based on a mixed linear model using two years of field data (Table 3; P < 0.05). In this study, significant mitigation of N2O by nosZ+ inoculation was observed during nodule decomposition period; that is, before and after harvest, whereas only postharvest N2O emission showed a significant decrease following nosZ++ inoculation in our previous study13.
Table 3. Cumulative N2O emission in the field experiment.
| Annual N2O emission | Nodule decomposition period N2O emission | Reduction rate | |
|---|---|---|---|
| (kgN ha−1) | (kgN ha−1) | (%) | |
| 2013 | (from March 18, 2013 to March 17, 2014) | (from Aug 29 to Nov 15, 2013) | |
| Native | 0.287 ± 0.104 | 0.180 ± 0.076 | — |
| nosZ+ | 0.260 ± 0.115 | 0.130 ± 0.045 | 28 |
| 2014 | (from March 3, 2014 to March 2, 2015) | (from Aug 29 to Nov 15, 2014) | |
| Native | 0.246 ± 0.078 | 0.121 ± 0.034 | — |
| nosZ+ | 0.235 ± 0.098 | 0.084 ± 0.033 | 30 |
| Statistical significance* | ns | P < 0.05 |
Soybean seeds were inoculated at sowing with a mixed culture of B. diazoefficiens strains C110 (nosZ+) or native strains (Native; nosZ− dominant).
*Statistical significance for N2O emission was tested using a mixed linear model based on two years of field data.
Increased N2O emission in soybean ecosystems during the harvest period has been reported13,20,21,22. Uchida and Akiyama6 reviewed N2O emissions from soybean fields and reported that 0–13.4% of soybean residual N was emitted as N2O after harvest (average: 1.3% ± 2.7%) in previous studies. Although cumulative N2O emissions in our field experiments were relatively low, N2O emission from a soybean field during nodule decomposition can reach as high as 5 kg N ha−1 (ref. 22).
N2O production rates in the field experiment
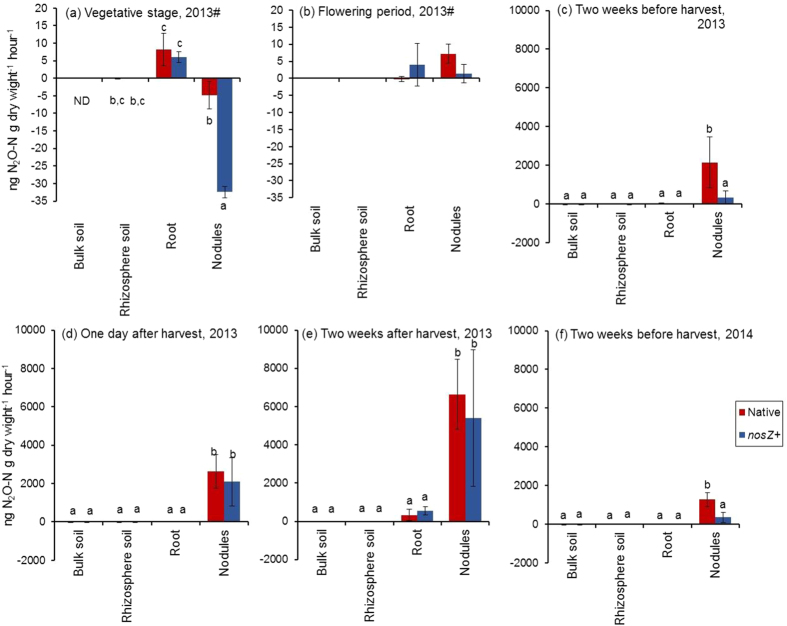
N2O production rates from soil, root, and nodule samples collected from the experimental field were measured at different growth stages in 2013 (Fig. 2a–e). At the vegetative stage, N2O was absorbed by nodules in both treatments, and the N2O uptake rate was significantly higher in the nosZ+ treatment than in the native treatment (Fig. 2a; P < 0.05). Sameshima-Saito et al.11 also reported N2O uptake by nodules from USDA110 (nosZ+)-inoculated plants, but no N2O uptake by nodules from nosZ−mutant-inoculated plants at the vegetative stage. Nodule N2O production rates increased dramatically during the nodule decomposition period (Fig. 2c,d,e), and that in the nosZ+ treatment was significantly lower than that in the native treatment in the two weeks before harvest (Fig. 2c; P < 0.05), whereas no differences were found in other periods (Fig. 2d,e). In contrast, N2O production rates of bulk soil, rhizosphere soil, and root remained low in all growth stages (Fig. 2a–e). In 2014, N2O production rates were measured two weeks before harvest, and the results confirmed that nodule N2O production rates were much higher than those of bulk soil, rhizosphere soil, and roots (Fig. 2f). As in 2013, the nodule N2O production rate of the nosZ+ treatment in 2014 was significantly lower than that of the native treatment two weeks before harvest (P < 0.05). These results suggested that nodules were the main source of N2O emission from the soybean field during the nodule decomposition period. Although our previous study13 also suggested the importance of nodules as a N2O source, N2O production rates from soil and nodules were not measured in that study. Moreover, a lower nodule N2O production rate from nosZ+ treatment than from the native treatment two weeks before harvest (Fig. 2d,f) suggested that the field-scale reduction of N2O emission in the nosZ+ plot (Table 3) was due to a lower N2O production rate from the nosZ+ nodules.
Figure 2. N2O production rates from bulk soil, rhizosphere soil, and root and nodule samples collected from the experimental field at different growth stages in 2013 and 2014.
(a) Vegetative stage (five weeks after inoculation, July 29, 2013), (b) flowering period (seven weeks after inoculation, August 12, 2013), (c) two weeks before harvest (October 3, 2013), (d) one day after harvest (October 18, 2013), (e) two weeks after harvest (October, 29 2013), and (f) two weeks before harvest (October 1, 2014). Soybean seeds were inoculated at sowing with a mixed culture of B. diazoefficiens strains (nosZ+) or native (nosZ− dominant) (n = 3# or 5, see text).
Soil and nodule inorganic N contents in the field experiment
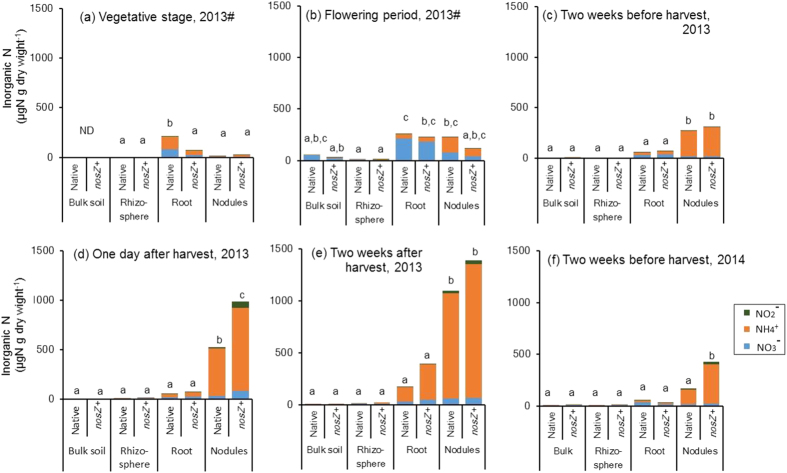
Soil and nodule inorganic N contents also suggested that nodules were the main N source of N2O emission during the nodule decomposition period in the soybean field. Nodule inorganic N content, mostly NH4+, remained low from the vegetative stage to flowering (Fig. 3a,b). It began to increase two weeks before harvest (Fig. 3c), and then dramatically increased just before harvest and two weeks after harvest in 2013 (Fig. 3d,e). In contrast, inorganic N content in bulk soil, rhizosphere soil, and roots remained low in all periods (Fig. 3a–f). As in 2013, nodule inorganic N, mainly NH4+ content, was higher than those of bulk soil, rhizosphere soil, and roots at two weeks before harvest in 2014 (Fig. 3f).
Figure 3. Inorganic N content in bulk soil, rhizosphere soil, and root and nodule samples collected from the experimental field at different growth stages in 2013 and 2014.
(a) Vegetative stage (five weeks after inoculation, July 29, 2013), (b) flowering period (seven weeks after inoculation, August, 12 2013), (c) two weeks before harvest (October 3, 2013), (d) one day after harvest (October 18, 2013), (e) two weeks after harvest (October 29, 2013), and (f) two weeks before harvest (October 1, 2014). Soybean seeds were inoculated at sowing with a mixed culture of B. diazoefficiens strains (nosZ+) or native (nosZ− dominant) (n = 3# or 5, see text).
Seasonal changes in bulk soil NO3− and NH4+ concentrations showed that NH4+ increased just after fertilizer application and consequent increase in NO3− by nitrification (Figs S4 and S5). However, bulk soil inorganic nitrogen concentrations did not increase during the nodule decomposition period and did not differ significantly among treatments in either year, a finding similar to that in our previous study13. These results suggested that nodules were the main N source for N2O emission rather than nitrification and denitrification of soil nitrogen during the nodule decomposition. The nodule N2O production rate (Fig. 2) also suggested that nodules were the main N source for N2O emission during nodule decomposition. Inaba et al.8 reported that N2O emitted during nodule decomposition in a pot experiment was derived from fixed nitrogen in the nodules. They also reported that B. diazoefficiens nosZ+ strains reduced both N2O produced by B. diazoefficiens and N2O produced by other soil microorganisms during nodule decomposition. Although soybean nodules have been proposed as the main N source for N2O emission during nodule decomposition7,8,13,20, the present study is the first to provide evidence that nodule inorganic N content and N2O production rate of nodules increased with N2O flux during nodule decomposition at the field scale.
Conclusion
In our previous report13, we showed that inoculation with the nosZ++ strain of B. diazoefficiens significantly decreased postharvest N2O emission. The nosZ++ strain used in the field study was a genetically unmodified mutant generated using a proofreading-deficient technique12. Although it was an effective approach for reducing N2O emissions from soybean fields, generating nosZ++ mutants requires more time, cost, and technical skill than isolating indigenous nosZ+ strains from soil as in the present study. Also, inoculation of soybean with indigenous strains has a long history and has been practiced commercially in many countries23, whereas use of mutants, especially genetically modified mutants, may need to receive public acceptance before commercial use. In addition, we used a mixed culture of 63 nosZ+ strains of USDA 110 group from agricultural fields from Japan, rather than selecting one strain from the nosZ+ collection. The mixture of many strains provides more diversity and is accordingly expected to be more competitive than a single strain with native strains and also more adaptable to various environments and robust to extreme weather, such as drought, heat, and heavy rainfall. Moreover, using nosZ+ strains isolated from local agricultural fields would have little effect on the ecosystem. Thus, isolating nosZ+ strains from local soybean fields would be more applicable and feasible for many soybean-producing countries than generating mutants.
Crop production needs to increase by approximately 60–100% from 2007 to 2050 to meet global food demand24. The increasing demand for food and biofuel is likely to require increasing N inputs even further, although anthropogenic reactive N input into the biosphere has already exceeded a proposed planetary limit24. Consequently, N2O emission from agriculture is likely to continue to increase25. To reduce N2O emission from agricultural soils, many mitigation options have been proposed, but very few options are available26: they include nitrification inhibitors, polymer-coated fertilizers27, and reducing the input of anthropogenic reactive nitrogen28. No biological method had been demonstrated in the field before our previous study13. The biological approach to reduce N2O emission is still in an early stage of development, but the present study showed that inoculation with indigenous nosZ+ strains has high potential to mitigate N2O emission from soybean ecosystems without the use of mutants. This approach can also be applied to other leguminous crops. Inoculation of alfalfa with the endosymbiont Ensifer meliloti carrying the nosZ gene was recently suggested as a potential mitigation option29. Furthermore, there is potential to mitigate N2O emission by using the nosZ gene in various other soil microbes30.
Methods
Bacterial strains, media, and construction of cell mixture
A cell mixture named C110 was prepared from 63 isolates belonging to a USDA110 group of Bradyrhizobium diazoefficiens that were collected from soybean nodules in 32 agricultural fields of Japan9 (Table S1). The bradyrhizobial isolates were grown individually for five days at 30 °C in HM broth medium31 supplemented with 0.1% L-arabinose (w/v) and 0.025% (w/v) yeast extract. The turbidities of the cultures were adjusted to OD660 = 1 with HM broth medium, and the cultures were mixed in equal amounts. One milliliter of the cell mixture was inoculated into fresh HM broth medium (100 ml) and cultured at 30 °C for five days. The resulting C110 was grown at 30 °C in modified AG medium32 supplemented with 0.3% (w/v) arabinose, 0.3% (w/v) yeast extract, and 0.3% (w/v) sodium gluconate for field inoculation.
Field experiment
The experimental field was located at the Institute for Agro-Environmental Sciences, Japan (36°01′N, 140°07′E). The Andosol field (nosZ−, 98%; nosZ+, 2%) was divided into 6 × 6 m plots. The treatments were inoculation with a mixture of 63 nosZ+ strains of USDA110 group (C110; nosZ+) or with native rhizobia (native) (five replicates of field plots with blocked random design).
To increase the proportion of cotyledon emergence, soybean seeds were germinated for one day in trays of moist vermiculite. Then soybean (Glycine max [L.] Merr., ver. Tachinagaha) seeds were planted in biodegradable Jiffy pots (Jiffy International AS, Kristiansand, Norway) filled with Andosol soil collected from an orchard located approximately 100 m from the experimental field. The orchard soil was chosen because it had a lower population of native soybean bradyrhizobia than, but soil properties similar to those of, the experimental field. Fruit trees had been grown in the orchard for more than 40 years, and thus had experienced no soybean cultivation for at least 40 years. Soybean seeds were inoculated with C110 (nosZ+) or 50 ml of soil from the experimental field (native) on June 26, 2013 and June 18, 2014. Soybean seedlings were then grown in a greenhouse under natural light and then transplanted into the field on July 3, 2013 and June 25, 2014. Basal fertilizer was applied as a compound fertilizer (30 kg N ha–1) one day before transplanting the soybean seedlings. Soybean crops were harvested on October 17, 2013 and October 10, 2014, aboveground residues were removed, and only roots and stubble were left in the field. N2O emission was measured every two to four days using an automated gas sampling system33. N2O concentrations were determined on a gas chromatograph equipped with an electron capture detector (GC-ECD). The effect of nosZ+ on N2O emission based on data from two years of field experiments was evaluated using a mixed linear model.
N2O production rates of soil, roots, and nodules
Bulk soil, rhizosphere soil, and root and nodule samples were collected from the experimental field at five different growth stages in 2013. Samples were also collected two weeks before harvest in 2014 to confirm the results of 2013. N2O production rates of these samples were determined in an incubation experiment. Bulk soil was randomly collected from five points (0 to 5 cm) in each plot and mixed in a plastic bag to produce a composite sample. Root segments growing inside the Jiffy pot were collected along with rhizosphere soil. Field samples were immediately transferred to the laboratory. There, root samples were separated into rhizosphere soil, roots, and nodules. Bulk soil, rhizosphere soil, and root and nodule samples were transferred to glass vials. These were sealed with butyl rubber stoppers and incubated at 25 °C for 30 min. The nodule incubation experiments were started one hour after the field sampling to reflect N2O production rate in the field. Because our pre-experiment results showed that N2O production rate of nodules decline with time after sampling, it was important to incubate nodules as soon as possible after the field sampling, but 1 h was needed for transportation of samples and nodule sample preparation. The root and soil incubation experiments were also performed simultaneously. Gas samples were collected from vials 0, 15, 30 min after sealing. N2O concentrations of the gas samples were determined with the GC-ECD.
Details of all methods are provided in the Supplementary Information.
Additional Information
How to cite this article: Akiyama, H. et al. Mitigation of soil N2O emission by inoculation with a mixed culture of indigenous Bradyrhizobium diazoefficiens. Sci. Rep. 6, 32869; doi: 10.1038/srep32869 (2016).
Supplementary Material
Acknowledgments
This work was supported financially by the Bio-oriented Technology Research Advancement Institution (BRAIN; the Program for Promotion of Basic Research Activities for Innovative Biosciences), by the Funding Program for Next Generation World-Leading Researchers of the Japan Society for the Promotion of Science (JSPS) GS027, and by JSPS KAKENHI 26292184 and 26252065.
Footnotes
Author Contributions K.M., M.H., H.A. and Y.T.H. designed the research. M.I. constructed C110. H.A., Y.T.H., Y.S., K.T., Y.W., A.Y., Y.N. and M.H. conducted the field experiments. H.A., Y.T.H., M.I., M.H. and K.M. wrote the paper. All authors discussed the results and approved the manuscript.
References
- Smith K. A., Crutzen P. J., Mosier A. R. & Winiwarter W. The global nitrous oxide budget: A reassessment. Nitrous Oxide and Climate Change (ed. Smith K. A.) 4–35 (Earthscan, 2010). [Google Scholar]
- Ciais P. et al. Carbon and Other Biogeochemical Cycles. In Stocker T. F., Qin D., Plattner G.-K., Tignor M., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V. & Midgley P. M. (ed) Climate Change 2013. The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA (2013). [Google Scholar]
- Food and Agriculture Organization of The United Nations Statistics Division (FAOSTAT) http://faostat3.fao.org/(accessed on May 9, 2016).
- Hartman G., West E. & Herman T. Crops that feed the World 2. Soybean−worldwide production, use, and constraints caused by pathogens and pests. Food Security 3, 5–17 (2011). [Google Scholar]
- O’Hara G. W. & Daniel R. M. Rhizobial denitrification: A review. Soil Biol. Biochem. 17, 1–9 (1985). [Google Scholar]
- Uchida Y. & Akiyama H. Mitigation of postharvest nitrous oxide emissions from soybean ecosystems: a review, Soil Sci. Plant Nutri. 59, 477–487 (2013). [Google Scholar]
- Inaba S. et al. Nitrous oxide emission and microbial community in the rhizosphere of nodulated soybeans during the late growth period. Microbes Environ. 24, 64–67 (2009). [DOI] [PubMed] [Google Scholar]
- Inaba S. et al. N2O emission from degraded soybean nodules depends on denitrification by Bradyrhizobium japonicum and other microbes in the rhizosphere. Microbes Environ. doi: 10.1264/jsme2.ME12100 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina Y. et al. Correlation between soil type and N2O reductase genotype (nosZ) of indigenous soybean bradyrhizobia: nosZ-minus populations are dominant in Andosols. Microbes Environ. 29, 420–426 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D., Felgate H., Watmough N., Thomson A. & Baggs E. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle—could enzymic regulation hold the key? Trends Biotech. 27, 388–397 (2009). [DOI] [PubMed] [Google Scholar]
- Sameshima-Saito R., Chiba K. & Minamisawa K. Correlation of denitrifying capability with the existence of nap, nir, nor and nos genes in diverse strains of soybean bradyrhizobia. Microbes Environ. 21, 174–184 (2006). [Google Scholar]
- Itakura M. et al. Generation of Bradyrhizobium japonicum mutants with increased N2O reductase activity by selection after introduction of a mutated dnaQ Gene. Appl. Environ. Microbiol. 74, 7258–7264 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura M. et al. Mitigation of nitrous oxide emissions from soils by Bradyrhizobium japonicum inoculation, Nature Clim. Change 3, 208–212 (2013). [Google Scholar]
- Hénault C. & Revellin C. Inoculants of leguminous crops for mitigating soil emissions of the greenhouse gas nitrous oxide. Plant Soil 346, 289–296 (2011). [Google Scholar]
- Wan S., Johnson A. M. & Altosaar I. Expression of nitrous oxide reductase from Pseudomonas stutzeri in transgenic tobacco roots using the root-specific rolD promoter from Agrobacterium rhizogenes, Ecology and Evolution 2, 286–297 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W. & Sadowsky M. J. Genetics of competition for nodulation of legumes. Annu. Rev. Microbiol. 46, 399–428 (1992). [DOI] [PubMed] [Google Scholar]
- Bogino P., Banchio E., Bonfiglio C. & Giordano W. Competitiveness of a Bradyrhizobium sp. strains in soil containing indigenous rhizobia. Curr. Microbiol. 56, 66–72 (2008). [DOI] [PubMed] [Google Scholar]
- Itakura M. et al. Genomic comparison of Bradyrhizobium japonicum strains with different symbiotic nitrogen-fixing capabilities and other Bradyrhizobiaceae members. ISME J. 3, 326–339 (2009). [DOI] [PubMed] [Google Scholar]
- Hungria M., Boddey L. H., Santos M. A. & Vargas M. A. T. Nitrogen fixation capacity and nodule occupancy by Bradyrhizobim japonicaum and B. elkanii strains. Biol. Fertil. Soils 27, 393–399 (1998). [Google Scholar]
- Yang L. & Cai Z. The effect of growing soybean (Glycine max. L.) on N2O emission from soil. Soil Biol. Biochem. 37, 1205–1209 (2005). [Google Scholar]
- Ciampitti I. A., Ciarlo E. A. & Conti M. E. Nitrous oxide emissions from soil during soybean [(Glycine max (L.) Merrill] crop phenological stages and stubbles decomposition period. Biol. Fertil. Soils 44, 581–588 (2008). [Google Scholar]
- Marinho E. V. A., DeLaune R. D. & Lindau C. W. Nitrous oxide flux from soybeans grown on Mississippi alluvial soil. Comm. Soil Sci. Plant Anal. 35, 1–8 (2004). [Google Scholar]
- Catroux G., Hartmann A. & Revellin C. Trends in rhizobial inoculant production and use. Plant Soil 230, 21–30 (2001). [Google Scholar]
- Zhang X. et al. Managing nitrogen for sustainable development, Nature 528, 51–59 (2015). [DOI] [PubMed] [Google Scholar]
- Reay D. S., Davidson E. A., Smith K. A., Smith P., Melillo J. M., Dentener F. & Crutzen P. J. Global agriculture and nitrous oxide emissions. Nature Climate Change 2, 410–416 (2012). [Google Scholar]
- Hu H. W., Chen D. & He J. Z. Microbial regulation of terrestrial nitrous oxide formation: understanding the biological pathways for prediction of emission rates, FEMS Microbiol. Rev. 39, 729–749 (2015). [DOI] [PubMed] [Google Scholar]
- Akiyama H., Yan X. Y. & Yagi K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta-analysis, Global Change Biology 16, 1837–1846 (2010). [Google Scholar]
- Bakken L. R., Bergaust L., Liu B. B. & Frostegard A. Regulation of denitrification at the cellular level: a clue to the understanding of N2O emissions from soils. Philo. Trans. R. Soc. B. 367, 1226–1234 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno E. et al. Anoxic growth of Ensifer meliloti 1021 by N2O-reduction, a potential mitigation strategy, Frontiers in Microbiology 6, 537 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa T. et al. Inoculation with N2-generating denitrifier strains mitigates N2O emission from agricultural soil fertilized with poultry manure, Biol. Fertil. Soils 50, 1001–1007 (2014). [Google Scholar]
- Cole M. A. & Elkan G. H. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob. Agents Chemother. 4, 248–253. (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky M. J., Tully R. E., Cregan P. B. & Keyser H. H. Genetic diversity in Bradyrhizobium japonicaum serogroup 123 and its relation to genotype-specific nodulation of soybean. Appl. Environ. Microbiol. 53, 2624–2630 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H. et al. Automated sampling system for long-term monitoring of nitrous oxide and methane fluxes from soils. Soil Sci. Plant Nutr. 55, 435–440 (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.