Abstract
B cell activating factor of the TNF family (BAFF), also known as B lymphocyte stimulator, is a ligand required for the generation and maintenance of B lymphocytes. In this study, the ability of different monoclonal antibodies to recognize, inhibit, or activate mouse BAFF was investigated. One of them, a mouse IgG1 named Sandy-2, prevented the binding of BAFF to all of its receptors, BAFF receptor, transmembrane activator and calcium modulating ligand interactor, and B cell maturation antigen, at a stoichiometric ratio; blocked the activity of mouse BAFF on a variety of cell-based reporter assays; and antagonized the prosurvival action of BAFF on primary mouse B cells in vitro. A single administration of Sandy-2 in mice induced B cell depletion within 2 weeks, down to levels close to those observed in BAFF-deficient mice. This depletion could then be maintained with a chronic treatment. Sandy-2 and a previously described rat IgG1 antibody, 5A8, also formed a pair suitable for the sensitive detection of endogenous circulating BAFF by ELISA or using a homogenous assay. Interestingly, 5A8 and Sandy-5 displayed activities opposite to that of Sandy-2 by stimulating recombinant BAFF in vitro and endogenous BAFF in vivo. These tools will prove useful for the detection and functional manipulation of endogenous mouse BAFF and provide an alternative to the widely used BAFF receptor-Fc decoy receptor for the specific depletion of BAFF in mice.
Keywords: antibody, cytokine, immunology, lymphocyte, receptor, BAFF
Introduction
The ligand B cell activating factor of the TNF family (BAFF),4 also known as B lymphocyte stimulator, and its close relative a proliferation-inducing ligand (APRIL) play important roles in the generation and maintenance of B cells (1). BAFF and APRIL share two receptors, transmembrane activator and calcium modulating ligand interactor (TACI) and B cell maturation antigen (BCMA), whereas BAFF additionally binds to BAFF receptor (BAFFR; also known as BR3). BAFF acts on BAFFR to support survival and fitness of transitional and mature B cells in the periphery, a conclusion supported by the observation that BAFF-deficient and BAFFR-deficient mice have few mature peripheral B cells, whereas the reverse is observed in BAFF transgenic mice (for a review, see Ref. 1). BCMA is expressed late in the B cell differentiation process (2) and participates in the maintenance of plasma cells in vivo (3). The function of TACI appears to be dual: on the one hand, it is required for the generation of T-independent antibody responses in vivo and to promote survival of B cells stimulated through the BCR in vitro; on the other hand, TACI-deficient mice have significantly more B cells (4, 5). At least in marginal zone B cells that express TACI highly, simultaneous engagement of Toll-like receptors and TACI prime these cells to killing by the Fas ligand-Fas apoptotic pathway (6). This negative function of TACI on the mature B cell pool may explain why depletion of BAFF or of BAFF and APRIL in humans using anti-BAFF monoclonal antibody or a TACI-Ig decoy receptor first induces an increase in memory or mature B cells (that would be due to the release of the inhibitory function of TACI) followed only later by B cell depletion (that would be caused by the blockade of the prosurvival function of BAFFR) (7–9).
BAFF and APRIL both crystallize as homotrimers that can recruit three monomeric receptors per trimer (10, 11). Trimers may not represent the optimal active unit, and a higher status of multimerization might be required for efficient signaling (12). BAFF has an intrinsic multimerization site distinct from the receptor-binding site, whereas APRIL could oligomerize by binding to proteoglycans (13, 14). Blockade of BAFF and/or APRIL is expected to benefit autoimmune patients with excess B cell activation and antibody production. A function-blocking anti-BAFF antibody has shown some efficacy for the treatment of systemic lupus and was approved in 2011 (for a review, see Ref. 15). A TACI-Ig decoy receptor also showed encouraging results for the treatment of the same disease (16). Inhibition of BAFF and APRIL in mice is usually achieved with the decoy receptor TACI-Ig or with BCMA-Ig, whereas selective blockade of BAFF relies on the decoy receptor BAFFR-Ig or on 10F4, a non-commercial hamster anti-mouse monoclonal antibody (17–20). In this study, we describe additional murine antibodies that block or activate mouse BAFF in vitro and in vivo.
Results and Discussion
A Sensitive Assay to Detect Endogenous Circulating Levels of Mouse BAFF
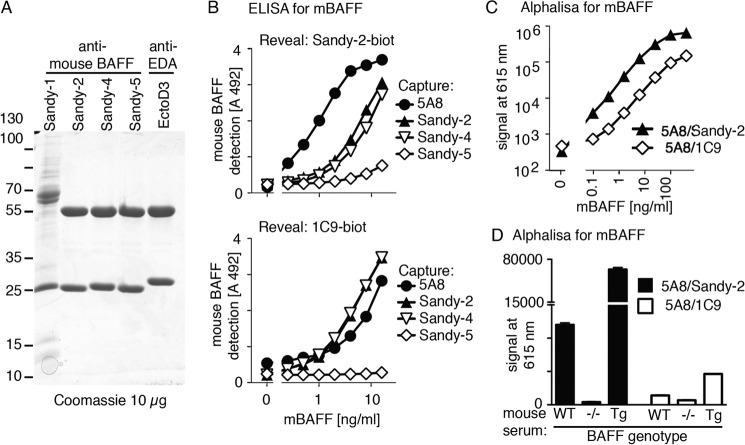
A previously characterized pair of rat IgG1 anti-mouse BAFF antibodies (5A8 and 1C9) is suitable for the detection of endogenous mouse BAFF (21, 22). Here, three monoclonal mouse IgG1 anti-mouse BAFF antibodies, Sandy-2, Sandy-4, and Sandy-5, were purified by affinity purification on protein A (Fig. 1A). A fourth antibody of mouse IgM isotype, Sandy-1, did not bind to protein A or G but was partially purified by size exclusion chromatography of concentrated hybridoma supernatants (Fig. 1A). We compared various combinations of sandwich ELISA and found that 5A8 as capture antibody with Sandy-2 for revelation was the best, allowing a more sensitive detection of mouse BAFF than the 5A8/1C9 couple (Fig. 1B). This was confirmed in a homogenous AlphaLISA assay in which signal is generated when donor beads come in close contact to acceptor beads upon binding of both antibodies to the same antigen (Fig. 1C). With the 5A8/Sandy-2 pair, endogenous BAFF in serum of WT mice or BAFF overexpressed in BAFF transgenic mouse serum were convincingly detected in 0.5 μl of serum with a favorable signal to noise ratio (Fig. 1D). This indicates that the 5A8 and Sandy-2 antibodies, both of which are commercially available, are more sensitive than the 5A8/1C9 pair for the detection of endogenous mouse BAFF.
FIGURE 1.
Detection of endogenous levels of BAFF with anti-mouse BAFF 5A8 and Sandy-2 mAbs. A, Coomassie Blue staining of the indicated monoclonal antibodies (10 μg/lane) under reducing conditions. B, sandwich ELISA using four anti-mouse BAFF mAbs to capture mouse BAFF and two biotinylated anti-mouse BAFF mAbs to reveal it as indicated. C, homogenous sandwich assay (AlphaLISA) for recombinant mouse BAFF using two pairs of anti-mouse BAFF mAbs performed in the presence of BAFF−/− serum diluted 1:10. D, homogenous mouse BAFF assay (AlphaLISA) performed with two pairs of mAbs on sera of WT, BAFF KO, and BAFF transgenic mice diluted 1:10. Error bars represent S.E.
Anti-mouse BAFF mAbs Sandy-1, Sandy-2, and Sandy-4, but Not Sandy-5, Prevent Binding of Mouse BAFF to All Canonical Receptors
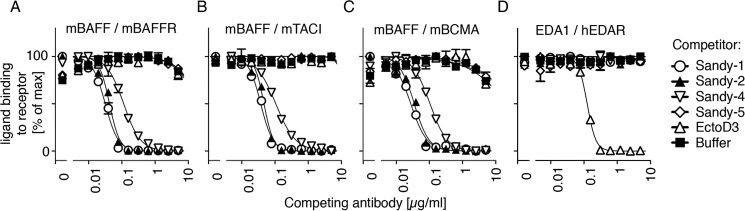
The extracellular domains of mouse BAFFR, TACI, or BCMA fused to the Fc portion of an immunoglobulin, when immobilized in an ELISA plate, all bound recombinant FLAG-mouse BAFF, whereas the control receptor EDAR-Fc bound FLAG-EDA1 (Fig. 2). Sandy-5 did not inhibit the binding of mBAFF to its receptors, whereas Sandy-1, Sandy-2, and to a lesser extent Sandy-4 specifically inhibited the binding of FLAG-mBAFF to BAFFR, TACI, and BCMA but not of EDA1 to EDAR. As expected, the anti-EDA antibody EctoD3 abolished the EDA1-EDAR interaction but did not affect mouse BAFF (Fig. 2). Molar ratios of antibody to trimeric FLAG-mBAFF at EC50 were about 0.5 for Sandy-1 and Sandy-2, indicating an inhibition at close to stoichiometric ratio, and 1.7 for Sandy-4 (Table 1). EctoD3 inhibited trimeric EDA with a slightly lower ratio of 0.3 (Table 1), which is not so far away from the previously reported value of 1.1 (23). As it is not trivial to precisely determine protein concentrations, this difference can probably be attributed to an overestimation of the FLAG-EDA1 concentration or to variations in the specific activity of different ligand preparations. In any case, this direct inhibition assay differentiated antibodies that prevent binding of ligand to their receptor(s) (Sandy-1, Sandy-2, Sandy-4, and EctoD3) from those that do not (Sandy-5).
FIGURE 2.
Sandy-1, Sandy-2, and to a lesser extent Sandy-4 inhibit the binding of mouse BAFF to BAFFR, TACI, and BCMA. A fixed, non-saturating concentration of FLAG-mouse BAFF (33 ng/ml) or FLAG-mEDA1 (180 ng/ml) was preincubated with serial dilutions of anti-mouse BAFF (Sandy-1, Sandy-2, Sandy-4, and Sandy-5) or anti-EDA (EctoD3) mAbs and then applied to their cognate coated receptors-Fc. A, mBAFFR-Fc. B, mTACI-Fc. C, mBCMA-Fc. D, hEDAR-Fc. Binding of FLAG-mouse BAFF or FLAG-mouse EDA1 to receptors was revealed via the FLAG tag, and inhibition of binding resulted in a decreased signal. Error bars represent S.E. of triplicate measures. The experiment was performed twice with similar results.
TABLE 1.
Number of antibody molecules per ligand 3-mer at EC50 in the ELISA
Data are based on data shown in Fig. 2. n.i., no inhibition.
| Ligand | Receptor | Sandy-1 | Sandy-2 | Sandy-4 | Sandy-5 | EctoD3 |
|---|---|---|---|---|---|---|
| FLAG-mBAFF | mBAFFR-Fc | 0.4 | 0.6 | 1.7 | n.i. | n.i. |
| FLAG-mBAFF | mTACI-Fc | 0.5 | 0.6 | 1.6 | n.i. | n.i. |
| FLAG-mBAFF | mBCMA-Fc | 0.4 | 0.5 | 1.7 | n.i. | n.i. |
| FLAG-mEDA1 | hEDAR | n.i. | n.i | n.i. | n.i. | 0.3 |
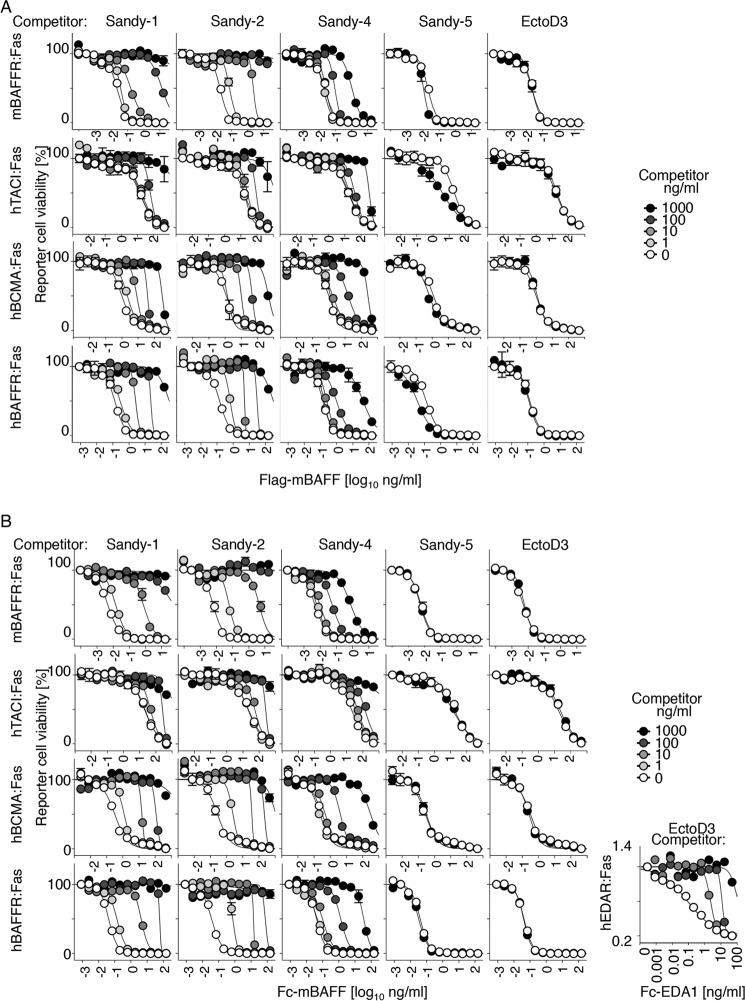
Sandy-1, Sandy-2, and to a Lesser Extent Sandy-4 Inhibit the Agonist Activity of Mouse BAFF in Reporter Cell Assays
The antibodies were tested in an activity assay for BAFF in which the extracellular domains of BCMA, TACI, or BAFFR are fused to the transmembrane and intracellular domains of the death receptor Fas. In these cell lines, multimerization of surface-expressed chimeric receptors by BAFF triggers the surrogate Fas apoptotic signaling pathway and kills reporter cells (24). As already observed in the binding assay (Fig. 2), Sandy-1 and Sandy-2 blocked the activity of recombinant FLAG-mouse BAFF and Fc-mouse BAFF, whereas the irrelevant antibody EctoD3 did not (Fig. 3A). The antibody to ligand ratio at EC50 was again close to 1 (0.4–3.9) when the assay was performed on cell lines expressing chimeric receptors containing human TACI, BCMA, or BAFFR but was somewhat higher (2.3–16) when assayed in mouse BAFFR:Fas reporter cells (Fig. 3 and Table 2). The reason why a higher ratio of inhibitory antibodies is required to neutralize the exact same mouse BAFF preparations when assayed on mBAFFR reporter cells might be due to a higher affinity of mBAFF for mBAFFR than for the other receptors tested and/or to the fact that mBAFFR:Fas reporter cells being the most sensitive to BAFF, a greater percentage of BAFF blockade is required to achieve a given effect in these cells. Sandy-4 inhibited FLAG-mBAFF and Fc-mBAFF activities at higher concentrations compared with Sandy-1 or Sandy-2, especially in BAFFR:Fas reporter cells (Table 2).
FIGURE 3.
Sandy-1, Sandy-2, and to a lesser extent Sandy-4 inhibit activity of mouse BAFF. A, different reporter cell lines expressing the extracellular domains of BAFFR, TACI, or BCMA (or EDAR as a control) fused to the transmembrane and intracellular domains of Fas (mBAFFR:Fas, hTACI:Fas, hBCMA:Fas, hBAFFR:Fas, and hEDAR:Fas) were exposed to titrated amounts of FLAG-mouse BAFF in the presence of the indicated fixed concentrations of different anti-mBAFF or anti-EDA (EctoD3) mAbs. After 16 h of culture, cell viability was monitored with the phenazine methosulfate/3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay. Blocking antibodies protect reporter cells from death. B, same as A except that Fc-mouse BAFF (or Fc-EDA for EDAR:Fas reporter cells) was used to trigger death of reporter cells. Error bars represent S.E. of triplicate measures (except for EDAR:Fas, monoplicates). The experiment was performed twice (once in monoplicate) with similar results.
TABLE 2.
Number of antibody molecules per ligand 3-mer at EC50 in the cell-based assay
Data are based on those shown in Fig. 3. Fc ligands were considered to contain two trimers. n.i., no inhibition; n.i. (3.5×); no inhibition, instead ligand was activated 3.5-fold.
| Ligand | Cells | Sandy-1 | Sandy-2 | Sandy-4 | Sandy-5 | EctoD3 |
|---|---|---|---|---|---|---|
| FLAG-mBAFF | hBAFFR:Fas | 1.8 | 1 | 32 | n.i. (3.5×) | n.i. |
| FLAG-mBAFF | hBCMA:Fas | 1.1 | 0.8 | 2.3 | n.i. (1.8×) | n.i. |
| FLAG-mBAFF | hTACI:Fas | 0.7 | 0.5 | 1.3 | n.i. (5.5×) | n.i. |
| FLAG-mBAFF | mBAFFR:Fas | 16 | 3.9 | 450 | n.i. (1.9×) | n.i. |
| Fc-mBAFF | hBAFFR:Fas | 3.9 | 1.1 | 53 | n.i. | n.i. |
| Fc-mBAFF | hBCMA:Fas | 0.8 | 0.4 | 14 | n.i. | n.i. |
| Fc-mBAFF | hTACI:Fas | 0.4 | 0.4 | 0.8 | n.i. | n.i. |
| Fc-mBAFF | mBAFFR:Fas | 7.8 | 2.3 | 1600 | n.i. | n.i. |
| Fc-hEDA1 | hEDAR:Fas | n.i. | n.i. | n.i. | n.i. | 4.2 |
Sandy-1, Sandy-2, and Sandy-4 Partially Inhibit BAFF-rich Heteromers
Human single chain BAFF-BAFF-BAFF (BBB), APRIL-APRIL-APRIL (AAA), and BAA and ABB heteromers have previously been shown to signal in BCMA:Fas reporter cells (25). Single chain mouse APRIL and BAFF homomers and heteromers (mAAA, mBAA, mBBA, and mBBB) were tested on BCMA:Fas reporter cells in the presence of various inhibitors. TACI-Fc inhibited all forms of BAFF and APRIL, and BAFFR-Fc inhibited BAFF but none of the APRIL-containing constructs, whereas Sandy-1, Sandy-2, and Sandy-4 inhibited BAFF but not mBAA or mAAA (Fig. 4). mBBA used at a non-saturating concentration was only partially inhibited by Sandy-1, Sandy-2, and Sandy-4 (Fig. 4). In summary, Sandy-1 and Sandy-2 inhibit BAFF-rich heteromers to some extent but not APRIL-rich heteromers.
FIGURE 4.
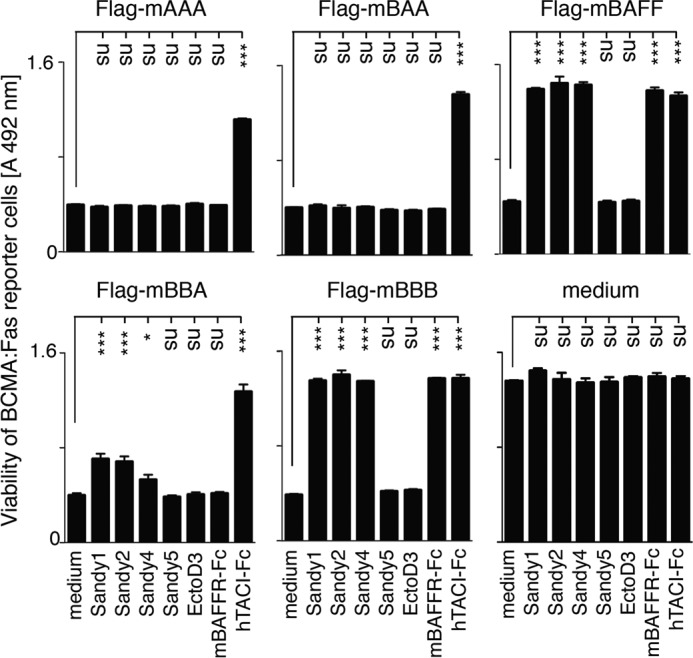
Sandy-1, Sandy-2, and Sandy-4 partially inhibit BAFF-rich, but nor APRIL-rich, heteromers. BCMA:Fas reporter cells were exposed to FLAG-mBAFF at 50 ng/ml or to unknown but lethal and non-saturating concentrations of single chain mouse BAFF (mBBB), mouse APRIL (mAAA), or mouse BAFF/APRIL heteromers (mBAA and mBBA) in the presence of the indicated inhibitors at a concentration of 2 μg/ml. After 12 h of culture, cell viability was monitored with the phenazine methosulfate/3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay. Error bars represent S.E. of triplicate measures. The experiment was performed three times with similar results. One-way analysis of variance comparing medium with inhibitors was performed: *, p < 0.05; ***, p < 0.001; ns, not significant.
Sandy-5 and 5A8 Activate Trimeric Mouse BAFF in a Reporter Cell Assay, in Primary B Cells in Vitro, and in Vivo
In contrast to Sandy-1, Sandy-2, and Sandy-4, Sandy-5 totally failed to block Fc-mBAFF in a variety of reporter cell lines and even reproducibly stimulated the activity of FLAG-mBAFF 2–5-fold (Fig. 3 and Table 2). This latter result raised the possibility that some anti-BAFF antibodies would cross-link FLAG-mBAFF to stimulate its activity on the reporter cell lines, whereas Fc-mBAFF that is intrinsically cross-linked would benefit less from this effect. To test this hypothesis, hBAFFR:Fas reporter cells were stimulated with size-fractionated FLAG-mBAFF trimers in the presence of various anti-mBAFF antibodies. In this assay, Sandy-5 and 5A8 activated FLAG-mBAFF about 5- and 20-fold, respectively, and 1C9 had no effect, whereas Sandy-1, Sandy-2, and to a lesser extent Sandy-4 inhibited BAFF as observed previously (Fig. 5A). This activating effect was also observed in cultures of primary mouse B splenocytes in which the prosurvival activity of BAFF was stimulated by Sandy-5, 5A8, and to a lesser extent 1C9, whereas BAFF antagonists (Sandy-1, Sandy-2, and Sandy-4) blocked the action of BAFF in this assay (Fig. 5B). When 5A8 was administered weekly at 2 mg/kg in wild type mice for 6 weeks, mature B cells significantly increased in the spleen and in lymph nodes compared with mice treated with the control antibody. Treatment with Sandy-5 also increased mature B cells in lymph nodes (Fig. 5, B and C). Although we did not further explore the mechanism by which 5A8 and Sandy-5 act in vivo, we hypothesize that the activation of the biological activity of BAFF by cross-linking and an increased half-life of BAFF bound to antibodies both contribute to the observed effect. Thus, we have characterized two categories of anti-BAFF reagents: function-blocking antibodies (Sandy-1, Sandy-2, and Sandy-4) that prevent binding of BAFF to its receptors and stimulatory antibodies (Sandy-5 and 5A8) that act as cross-linkers.
FIGURE 5.
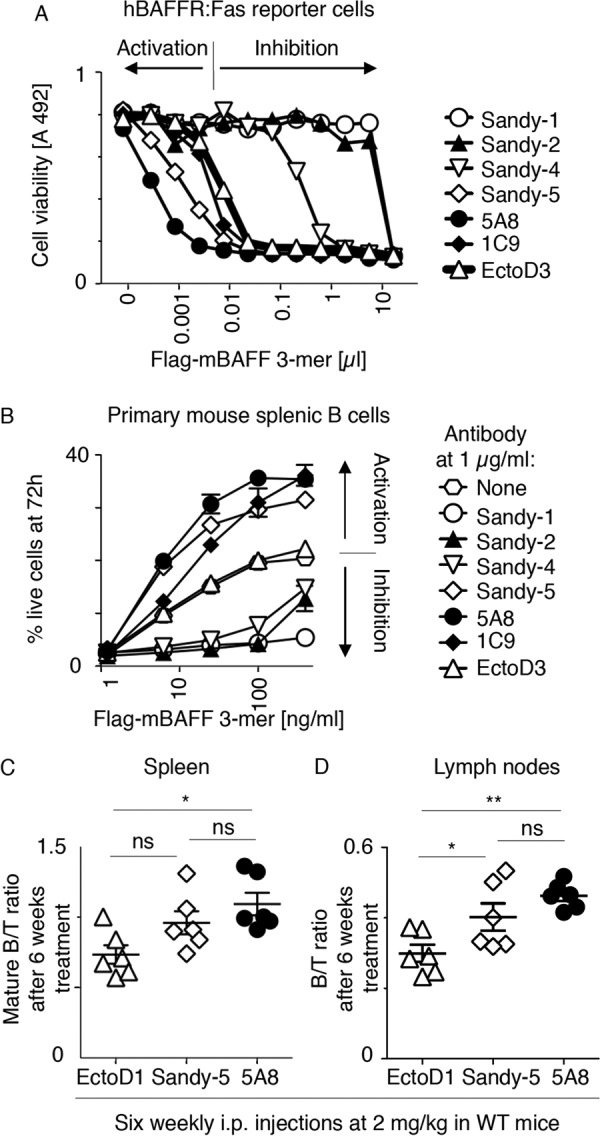
Sandy-5 and 5A8 stimulate trimeric mouse BAFF. A, hBAFFR:Fas reporter cells were exposed to titrated amounts of size-fractionated FLAG-mBAFF trimers in the presence of a fixed concentration (1 μg/ml) of the indicated anti-mBAFF or anti-EDA (EctoD3) mAbs. After 16 h of culture, cell viability was monitored with the phenazine methosulfate/3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay. Antibodies whose combined presence with FLAG-mouse BAFF caused more cell death than FLAG-mouse BAFF with the control EctoD3 antibody were considered to be activating antibodies. B, primary mouse B splenocytes were cultured for 72 h in the presence of the indicated concentrations of size-fractionated trimeric FLAG-mBAFF with or without inhibitors or activators at 1 μg/ml. Cell viability was determined by FACS as described under “Experimental Procedures.” Error bars represent S.E. of triplicate measures. The experiment was performed twice (one in monoplicate) with similar results. C and D, wild type mice were treated weekly during a 6-week period with i.p. injections of control (EctoD1) or anti-mBAFF (Sandy-5 or 5A8) antibodies at 2 mg/kg. At the end of the treatment period (day 42), lymphocytes of the spleen and lymph nodes were analyzed by FACS to determine ratios of mature B cells to T cells in the spleen (C) and of B cells to T cells in lymph nodes (D). Error bars represent S.E. One-way analysis of variance was performed: *, p < 0.05; **, p < 0.01; ns, not significant.
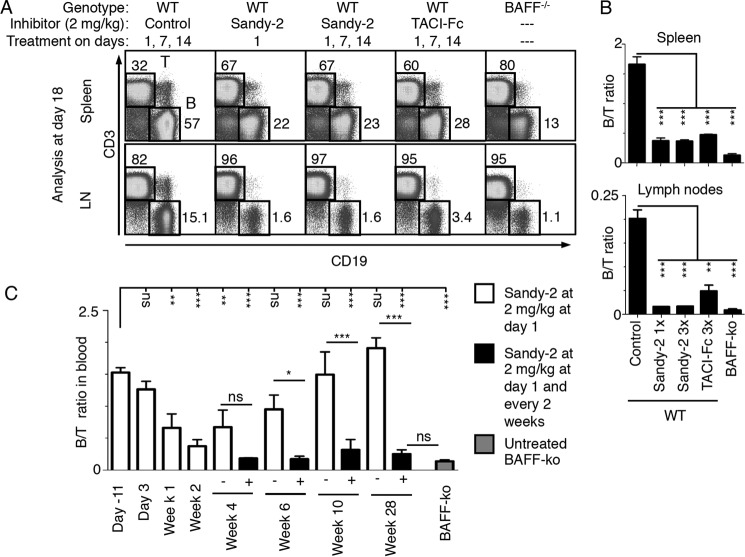
A Single Administration of Sandy-2 Induces Transient B Cell Depletion in Mice, Whereas Chronic Administration Permanently Reduces B Cells
Wild type C57BL/6 mice were treated i.p. with 2 mg/kg Sandy-2 on days 1, 7, and 14 and analyzed at day 18 for B cell content in spleen and lymph nodes. This treatment induced a significant decrease in the B/T cell ratio in both spleen and lymph nodes when compared with an isotype-matched control antibody (anti-EDAR mAb3). B cell depletion was at least as efficient as that obtained with the decoy receptor TACI-Fc at 2 mg/kg (Fig. 6, A and B). In fact, a single injection of Sandy-2 at day 1 induced at day 18 the same B cell depletion as repeated injections. In lymph nodes, treatment with Sandy-2 generated a phenotype close to that observed in BAFF−/− mice (Fig. 6, A and B). Kinetics of B cell depletion and recovery upon treatment with anti-BAFF Sandy-2 were then studied. Upon Sandy-2 administration, a significant decrease of circulating B cells was detected at 1 week and was maximal at 2 weeks, and the effect then slowly vanished over a period of 6–8 weeks (Fig. 6C). This kinetics is in line with the 10-day half-life of a mouse IgG1 administered in mice (26). When treatment was repeated every 2 weeks, circulating B cells were depleted to the level of BAFF KO mice by week 4 and thereafter remained at low levels up to the end of the experiment at 6 months (Fig. 6C).
FIGURE 6.
Sandy-2 blocks the action of endogenous BAFF in vivo. A, wild type mice were treated i.p. with the indicated inhibitors and controls at the indicated dose and times of administration. At day 18, spleens and lymph nodes (LN) were analyzed by FACS for the presence of T (CD3+) and B (CD19+) cells. Untreated BAFF−/− mice were analyzed in parallel. B, quantification of the experiment shown in A performed with two mice per group. Error bars represent S.E. One-way analysis of variance comparing control with treatment was performed: **, p < 0.01; ***, p < 0.001. C, on day 1, wild type mice were treated with anti-BAFF mAb Sandy-2. Blood lymphocytes were prepared at the indicated time points and analyzed by FACS as shown in A to determine the B to T cell ratio. At week 2, two groups of three mice each were either left without further treatment (white bars) or kept under chronic Sandy-2 treatment with administration every 2nd week (black bars). Blood of BAFF−/− mice was similarly analyzed for comparison. Error bars represent S.E. One-way analysis of variance comparing initial condition with time points of treatment or single versus continuous treatment was performed: *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant. The experiment was performed once.
In summary, we showed that the 5A8 rat anti-mouse BAFF and the Sandy-5 mouse anti-mouse BAFF monoclonal antibodies can potentiate the activity of recombinant trimeric mouse BAFF in vitro and that of endogenous mouse BAFF in vivo. We have also characterized Sandy-2, a new mouse anti-mouse BAFF monoclonal antibody that efficiently blocks recombinant and endogenous BAFF in vitro and in vivo. Because Sandy-2 is a fully mouse antibody and given its relatively long functional half-life, it will be an interesting alternative to BAFFR-Fc, which has been widely used so far to achieve selective inhibition of BAFF. Sandy-2 might be especially advantageous for studies requiring long term inhibition of BAFF with low risk of generating neutralizing antidrug responses. Finally, 5A8 and Sandy-2 form an efficient pair for sensitive quantification of endogenous mouse BAFF.
Experimental Procedures
Animals
C57BL/6 WT and BAFF KO were as described (12). Mice were handled according to guidelines and under the authorization of the Swiss Federal Food Safety and Veterinary Office (authorization 1370.6 to P. S.). Serum from BAFF transgenic mice was obtained as described (27).
Antibodies, Recombinant Proteins, and Cell Lines
Anti-mBAFF antibodies Sandy-1 (mouse IgM), Sandy-2 (AG-20B-0063), Sandy-4, and Sandy-5 (all mouse IgG1) were provided by Adipogen Life Sciences (Epalinges, Switzerland). Anti-mBAFF antibodies 5A8 and 1C9 (rat IgG1) (21), anti-EDAR mAb3 (26), and anti-EDA EctoD1 and EctoD3 (23) have been described previously. Anti-FLAG M2 antibody was from Sigma. hTACI (aa 31–110)-hIgG1 Fc (aa 245–470, L258E,A353S,P354S) was provided by Merck, KGaA. FLAG-mBAFF, FLAG-mEDA1, Fc-EDA1, Fc-mBAFF, mBAFFR-Fc, mBCMA-Fc, mTACI-Fc, and hEDAR-Fc were produced essentially as described (24, 28). Reporter cell lines Jurkat-hBCMA:Fas clone 13 (12), Jurkat-JOM2-hBAFFR:Fas clone 21 (25), Jurkat-JOM2-hTACI:Fas clone 112 (29), and Jurkat-JOM2-hEDAR:Fas clone 23 (30) were as described. Jurkat-JOM2-mBAFFR:Fas clone 20 was generated as described (24). Table 3 summarizes sequences of proteins expressed by plasmids used in this study.
TABLE 3.
Plasmids used in this study
FLAG, DYKDDDDK; HA signal, MAIIYLILLFTAVRG; Ig signal, MNFGFSLIFLVLVLKG; CamLinker, PQPQPKPQPKPEPEGS.
| Plasmid | Designation | Protein encoded | Vector |
|---|---|---|---|
| ps548 | FLAG-mEDA1 | HA signal-FLAG-GPGQVQLQVD-mEDA1 (aa 245–391) | PCR3 |
| ps657 | FLAG-mBAFF | HA signal-FLAG-GPGQVQLQVD-mBAFF (aa 127–309) | PCR3 |
| ps837 | mBCMA-Fc | Ig signal-DVT-mBCMA (aa 1–46)-VD-hIgG1 (aa 245–470) | PCR3 |
| ps930 | hEDAR-Fc | hEDAR (aa 1–183)-VD-hIgG1 (aa 245–470) | PCR3 |
| ps1111 | mTACI-Fc | HA signal-LE-mTACI (aa 2–78)-AAAVD-hIgG1 (aa 245–470) | PCR3 |
| ps1219 | Fc-mBAFF | HA signal-LD-hIgG1 (aa 245–470)-RS-CamLinker-GSLQVD-mBAFF (aa 127–309) | PCR3 |
| ps1377 | pMSCS-puro | Modified pMSCV-puro (Clontech) with HindIII-BglII-EcoRI-NotI-XhoI-HpaI-ApaI cloning sites | ps1377 |
| ps1199 | Fc-EDA1 | Signal-hIgG1 (aa 245–470)-hEDA1 (aa 238–391) | PCR3 |
| ps1938 | hEDAR:Fas | hEDAR (aa 1–183)-VD-hFas (aa 169–335) | ps1377 |
| ps2297 | mBAFFR:Fas | HA signal-LD-mBAFFR (aa 2–70)-VD-hIgG1 (aa 245–470) | PCR3 |
| ps2308 | hBAFFR:Fas | HA signal-LE-hBAFFR (aa 2–71)-EFGSVD-hFas (aa 169–355) | ps1377 |
| ps2309 | hBCMA:Fas | Ig signal-VQCEVKLVPRGS-hBCMA (aa 2–54)-VD-hFas (aa 169–335) | ps1377 |
| ps2455 | hTACI:Fas | HA signal-L-hTACI (aa 1–118)-VD-hFas (aa 169–335) | ps1377 |
| ps2922 | mBAFFR:Fas | HA signal-LE-hBAFFR (aa 2–70)-VD-hFas (aa 169–355) | PCR3 |
| ps3618 | FLAG-mAAA | HA signal-FLAG-GPGQVQLQVDLQ-mAPRIL (aa 95–232)-GGGGS-mAPRIL (aa 95–232)-GGGGS-mAPRIL (aa 95–232) | PCR3 |
| ps3637 | FLAG-mBAA | HA signal-FLAG-GPGQVQLQVDLQVD-mBAFF (aa 81–309)-GGGGS-mAPRIL (aa 95–232)-GGGGS-mAPRIL (aa 95–232) | PCR3 |
| ps3638 | FLAG-mBBB | HA signal-FLAG-GPGQVQLQVDLQVD-mBAFF (aa 81–309)-GGGGS-mBAFF (aa 81–309)-GGGGS-mBAFF (aa 81–309) | PCR3 |
| ps3640 | FLAG-mBBA | HA signal-FLAG-GPGQVQLQVDLQVD-mBAFF (aa 81–309)-GGGGS-mBAFF (aa 81–309)-GGGGS-mAPRIL (aa 95–232) | PCR3 |
SDS-PAGE, Coomassie Blue Staining, Western Blotting, and Quantification of Mouse BAFF
SDS-PAGE was performed according to standard procedures. Coomassie blue staining of 10 μg of antibody per lane was performed with a semidry iD Stain System (Eurogentech). FLAG-mBAFF purified by anti-FLAG affinity chromatography was similarly analyzed by SDS-PAGE and Coomassie Blue staining together with a standard curve of 3–0.5 μg of size-fractionated human His-BAFF 60-mer previously quantified by spectrophotometry assuming a molar extinction coefficient at 280 nm of 14,565 (12). Band intensity was quantified using ImageJ, and the concentration of FLAG-mBAFF was determined using the standard curve of His-hBAFF. The concentration of FLAG-mBAFF and FLAG-mEDA1 in conditioned medium was estimated by Western blotting against a standard curve of 10–120 ng of FLAG-mBAFF. Proteins were revealed with anti-FLAG M2 antibody (1 μg/ml) and IRDye 800CW-coupled anti-mouse antibody (65 ng/ml in PBS, 0.5% Tween 20, 1% powdered milk) and detected using a LI-COR Odyssey infrared fluorescence detector (LI-COR Biosciences).
Biotinylations
1 mg of antibody in 300 μl of 0.1 m sodium borate, pH 8.8, was labeled with 100 μg of EZ-Link sulfo-NHS-LC-biotin (Pierce) in 10 μl of dimethyl sulfoxide for 2 h at room temperature prior to terminating the reaction by addition of 10 μl of 1 m NH4Cl and exchanging buffer to PBS.
Receptor-Ligand Interaction ELISA
Purified receptors-Fc (1 μg/ml in PBS) were coated into ELISA plates and incubated with fixed concentrations of FLAG-tagged mBAFF (33 ng/ml) or FLAG-tagged EDA1 (180 ng/ml) in supernatants of transfected cells that had been preincubated with titrated amounts of antibodies of interest. Bound FLAG-tagged ligands were revealed with biotinylated anti-FLAG M2 antibody (Sigma) and horseradish-coupled streptavidin as described (24).
Sandwich ELISA
ELISA plates were coated with capture antibodies (5A8, Sandy-1, Sandy-2, Sandy-4, or Sandy-5) at 3 μg/ml in PBS, then blocked, and exposed overnight to titrated amounts of FLAG-mBAFF. After washing, bound ligand was revealed with 2 μg/ml biotinylated anti-mBAFF antibodies (Sandy-2 or 1C9) followed by horseradish-coupled streptavidin and revelation with ortho-phenylenediamine as described (24).
AlphaLISA
Monoclonal antibody 5A8 (100 μg) was coupled for 24 h at 37 °C to 1 mg of AlphaLISA acceptor beads (PerkinElmer Life Sciences) in 400 μl of 27 mm sodium phosphate, pH 8, 5 mm NaBH3CN, 0.03% Tween 20. The reaction was terminated by addition of 100 μl of carboxymethoxylamine at 65 mg/ml in 0.8 m NaOH for 1 h at 37 °C. Beads were washed twice in 0.1 m Tris-HCl, pH 8, and then stored at 4 °C at a concentration of 5 mg/ml in PBS, 0.05% Proclin-300. Assays were performed in white 384-well plates by mixing 5 μl of sample, usually serum-diluted 1:10 in assay buffer (PerkinElmer Life Sciences) with 20 μl of biotinylated antibody (Sandy-2 at 0.075 μg/ml or 1C9 antibody at 1.8 μg/ml) and 0.5 μg of 5A8 donor beads in assay buffer. After 1-h incubation at room temperature, 1 μg of streptavidin-coupled donor beads in 25 μl of assay buffer was added. Emission at 615 nm after excitation at 680 nm was recorded with an Enspire plate reader (PerkinElmer Life Sciences).
Cytotoxic Assays with Receptor:Fas Reporter Cell Lines
Reporter cells were incubated for 16 h with the indicated concentrations of ligands in the presence of the indicated inhibitors at the indicated concentrations after which time cell viability was measured by the phenazine methosulfate/3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay essentially as described (24).
Mouse B Splenocyte Survival Assay
B cells were purified from spleens of wild type mice using an EasySepTM mouse B cell isolation kit (StemCell Technologies). B cells were washed with PBS; suspended in 5 ml of PBS, 1% FCS, 2 μm CFSE; incubated for 8 min at 37 °C; washed with PBS, 2% FCS; and suspended in complete RPMI (RPMI 1640 medium, 10% FCS, 20 μm 2-mercaptoethanol, 100 units/ml penicillin, 100 μg/ml streptomycin). CFSE-labeled B cells (2.5 × 105) were cultured in triplicates in 200 μl of complete RPMI in round bottomed 96-well tissue culture plates. Cells were stimulated for 72 h at 37 °C in medium with graded concentrations of size-fractionated FLAG-mBAFF trimer in the presence or absence of the indicated antibodies at 1 μg/ml after which time cells were stained with propidium iodide (PI; 100 ng/sample) and analyzed by FACS (see “FACS Analyses”). The percentage of live cells was determined as the number of live cells (CFSE+, PI−) divided by the number of cells (CFSE+) × 100. CFSE profiles indicated that no B cell proliferation occurred under any of the conditions tested.
Mouse Treatments
For B cell depletion experiments, Sandy-2, anti-EDAR mAb3 (control), or TACI-Fc were administered i.p. to wild type mice at 2 mg/kg on days 1, 7, and 14 (or on day 1 only). Untreated BAFF KO mice were used as controls. Mice were sacrificed at day 18 for the analysis of spleens and lymph nodes (inguinal, axillary, and brachial). In a separate time course experiment, two cohorts of wild type mice were treated either once at day 1 or repeatedly every 2 weeks with Sandy-2 at 2 mg/kg. ∼100 μl of tail or facial vein blood was collected on 50 μl of Liquemin (500 units/ml) (DrossaPharm, Basel, Switzerland) from the cohort with a single injection at day −11; day 3; and weeks 2, 4, 6, 10, and 28 and from the cohort with repeated injections at day −11 and weeks 1, 4, 6, 10, and 28. Blood from BAFF KO mice was used as a control.
For B cell stimulation experiments, Sandy-5, 5A8, or EctoD3 (control) antibodies were administered i.p. to 8-week-old C57BL/6 mice at 2 mg/kg on days 1, 7, 14, 21, 28, and 35. Mice were sacrificed at day 42 for the analysis of spleens and lymph nodes (inguinal, axillary, and brachial).
FACS Analyses
Secondary lymphoid organs were homogenized; submitted to a red blood cell lysis step for 5 min on ice in 150 mm NH4Cl, 10 mm KHCO3, 10 μm Na2-EDTA; washed in PBS, 2% FCS; and filtered on a nylon mesh. Heparinized blood was diluted into 5 ml of PBS, cells were recovered by centrifugation, and erythrocytes were lysed in 1 ml of red blood cell lysis buffer for 5 min at room temperature. Cells were washed in PBS, and the lysis step was repeated. Cells were incubated with anti-CD16/32 (Fc-block, clone 93) and stained with a mixture of anti-CD19-phycoerythrin.Cy7 (clone eBio1D3; 1:100); anti-CD3-allophycocyanin (clone 17A2; 1:100) (all from eBiosciences); and in some cases biotinylated anti-CD93 (clone mAb493; a kind gift from Antonius Rolink, University of Basel, Switzerland) followed by streptavidin-phycoerythrin.Cy5.5 for 20 min on ice before analysis with an Accuri C6 or FACSCanto flow cytometer (BD Biosciences). Data were analyzed with the FlowJo software (TreeStar, Ashland, OR).
Statistics
For the determination of EC50 values, normalized data expressed as a function of the logarithm of concentration were analyzed using the “log(agonist) versus normalized response, variable slope” function of Prism (GraphPad Software). One-way analysis of variance with Bonferroni's multiple comparison tests was used to compare selected groups using Prism.
Author Contributions
O. D., J.-E. G., and P. S. conceived experiments. C. K.-Q. (Figs. 1, 2, 3, 5, and 6), S. S.-M. (Figs. 1, 5, and 6), L. W. (Figs. 1, 2, 3, and 4), M. V. (Figs. 1, 2, and 5), A. T. (Figs. 5 and 6), C. R. S. (Fig. 1), and P. S. (Figs. 1, 3, and 4) performed experiments. T. S. Z. (Fig. 6), J. G. (Fig. 1), F. M. (Figs. 1 and 5), O. D. (Figs. 1 and 6), and H. H. (Figs. 4 and 6) contributed experimental ideas and key reagents to perform them. P. S. wrote the paper. C. R. S., M. V., and F. M. edited the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We are grateful to Susan Kalled (Biogen, Boston, MA) for providing BAFF−/− mice and to William Figgett (Monash University, Melbourne, Australia) for independent confirmation of the inhibitory activity of Sandy-2.
This work was supported in part by grants from the Swiss National Science Foundation (to P. S.) and the Commission for Technology and Innovation (to P. S. and O. D.). P. S. is supported by a research grant from EMD Serono, a subsidiary of Merck, KGaA. H. H. is an employee of Merck, KGaA. T. S. Z. is an employee of Biogen. O. D. is chief scientific officer at Adipogen Life Sciences.
- BAFF
- B cell activating factor of the TNF family
- APRIL
- a proliferation-inducing ligand
- BAFFR
- BAFF receptor
- TACI
- transmembrane activator and calcium modulating ligand interactor
- BCMA
- B cell maturation antigen
- EDA
- ectodysplasin A
- EDAR
- ectodysplasin A receptor
- m
- mouse
- h
- human
- CFSE
- carboxyfluorescein succinimidyl ester
- aa
- amino acids.
References
- 1. Mackay F., and Schneider P. (2009) Cracking the BAFF code. Nat. Rev. Immunol. 9, 491–502 [DOI] [PubMed] [Google Scholar]
- 2. Gras M. P., Laâbi Y., Linares-Cruz G., Blondel M. O., Rigaut J. P., Brouet J. C., Leca G., Haguenauer-Tsapis R., and Tsapis A. (1995) BCMAp: an integral membrane protein in the Golgi apparatus of human mature B lymphocytes. Int. Immunol. 7, 1093–1106 [DOI] [PubMed] [Google Scholar]
- 3. O'Connor B. P., Raman V. S., Erickson L. D., Cook W. J., Weaver L. K., Ahonen C., Lin L. L., Mantchev G. T., Bram R. J., and Noelle R. J. (2004) BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 199, 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von Bülow G., van Deursen J. M., and Bram R. J. (2001) Regulation of the T-independent humoral response by TACI. Immunity 14, 573–582 [DOI] [PubMed] [Google Scholar]
- 5. Yan M., Wang H., Chan B., Roose-Girma M., Erickson S., Baker T., Tumas D., Grewal I. S., and Dixit V. M. (2001) Activation and accumulation of B cells in TACI-deficient mice. Nat. Immunol. 2, 638–643 [DOI] [PubMed] [Google Scholar]
- 6. Figgett W. A., Fairfax K., Vincent F. B., Le Page M. A., Katik I., Deliyanti D., Quah P. S., Verma P., Grumont R., Gerondakis S., Hertzog P., O'Reilly L. A., Strasser A., and Mackay F. (2013) The TACI receptor regulates T-cell-independent marginal zone B cell responses through innate activation-induced cell death. Immunity 39, 573–583 [DOI] [PubMed] [Google Scholar]
- 7. Stohl W., Hiepe F., Latinis K. M., Thomas M., Scheinberg M. A., Clarke A., Aranow C., Wellborne F. R., Abud-Mendoza C., Hough D. R., Pineda L., Migone T. S., Zhong Z. J., Freimuth W. W., Chatham W. W., BLISS-52 Study Group, and BLISS-76 Study Group (2012) Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 64, 2328–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tak P. P., Thurlings R. M., Rossier C., Nestorov I., Dimic A., Mircetic V., Rischmueller M., Nasonov E., Shmidt E., Emery P., and Munafo A. (2008) Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum. 58, 61–72 [DOI] [PubMed] [Google Scholar]
- 9. Wallace D. J., Stohl W., Furie R. A., Lisse J. R., McKay J. D., Merrill J. T., Petri M. A., Ginzler E. M., Chatham W. W., McCune W. J., Fernandez V., Chevrier M. R., Zhong Z. J., and Freimuth W. W. (2009) A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. 61, 1168–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hymowitz S. G., Patel D. R., Wallweber H. J., Runyon S., Yan M., Yin J., Shriver S. K., Gordon N. C., Pan B., Skelton N. J., Kelley R. F., and Starovasnik M. A. (2005) Structures of APRIL-receptor complexes: like BCMA, TACI employs only a single cysteine-rich domain for high affinity ligand binding. J. Biol. Chem. 280, 7218–7227 [DOI] [PubMed] [Google Scholar]
- 11. Liu Y., Hong X., Kappler J., Jiang L., Zhang R., Xu L., Pan C. H., Martin W. E., Murphy R. C., Shu H. B., Dai S., and Zhang G. (2003) Ligand-receptor binding revealed by the TNF family member TALL-1. Nature 423, 49–56 [DOI] [PubMed] [Google Scholar]
- 12. Bossen C., Cachero T. G., Tardivel A., Ingold K., Willen L., Dobles M., Scott M. L., Maquelin A., Belnoue E., Siegrist C. A., Chevrier S., Acha-Orbea H., Leung H., Mackay F., Tschopp J., and Schneider P. (2008) TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood 111, 1004–1012 [DOI] [PubMed] [Google Scholar]
- 13. Ingold K., Zumsteg A., Tardivel A., Huard B., Steiner Q. G., Cachero T. G., Qiang F., Gorelik L., Kalled S. L., Acha-Orbea H., Rennert P. D., Tschopp J., and Schneider P. (2005) Identification of proteoglycans as the APRIL-specific binding partners. J. Exp. Med. 201, 1375–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y., Xu L., Opalka N., Kappler J., Shu H. B., and Zhang G. (2002) Crystal structure of sTALL-1 reveals a virus-like assembly of TNF family ligands. Cell 108, 383–394 [DOI] [PubMed] [Google Scholar]
- 15. Stohl W. (2014) Therapeutic targeting of the BAFF/APRIL axis in systemic lupus erythematosus. Expert Opin. Ther. Targets 18, 473–489 [DOI] [PubMed] [Google Scholar]
- 16. Isenberg D., Gordon C., Licu D., Copt S., Rossi C. P., and Wofsy D. (2015) Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann. Rheum. Dis. 74, 2006–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gross J. A., Johnston J., Mudri S., Enselman R., Dillon S. R., Madden K., Xu W., Parrish-Novak J., Foster D., Lofton-Day C., Moore M., Littau A., Grossman A., Haugen H., Foley K., Blumberg H., Harrison K., Kindsvogel W., and Clegg C. H. (2000) TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature 404, 995–999 [DOI] [PubMed] [Google Scholar]
- 18. Pelletier M., Thompson J. S., Qian F., Bixler S. A., Gong D., Cachero T., Gilbride K., Day E., Zafari M., Benjamin C., Gorelik L., Whitty A., Kalled S. L., Ambrose C., and Hsu Y. M. (2003) Comparison of soluble decoy IgG fusion proteins of BAFF-R and BCMA as antagonists for BAFF. J. Biol. Chem. 278, 33127–33133 [DOI] [PubMed] [Google Scholar]
- 19. Ramanujam M., Bethunaickan R., Huang W., Tao H., Madaio M. P., and Davidson A. (2010) Selective blockade of BAFF for the prevention and treatment of systemic lupus erythematosus nephritis in NZM2410 mice. Arthritis Rheum. 62, 1457–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scholz J. L., Crowley J. E., Tomayko M. M., Steinel N., O'Neill P. J., Quinn W. J. 3rd, Goenka R., Miller J. P., Cho Y. H., Long V., Ward C., Migone T. S., Shlomchik M. J., and Cancro M. P. (2008) BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc. Natl. Acad. Sci. U.S.A. 105, 15517–15522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Batten M., Fletcher C., Ng L. G., Groom J., Wheway J., Laâbi Y., Xin X., Schneider P., Tschopp J., Mackay C. R., and Mackay F. (2004) TNF deficiency fails to protect BAFF transgenic mice against autoimmunity and reveals a predisposition to B cell lymphoma. J. Immunol. 172, 812–822 [DOI] [PubMed] [Google Scholar]
- 22. Bossen C., Tardivel A., Willen L., Fletcher C. A., Perroud M., Beermann F., Rolink A. G., Scott M. L., Mackay F., and Schneider P. (2011) Mutation of the BAFF furin cleavage site impairs B-cell homeostasis and antibody responses. Eur. J. Immunol. 41, 787–797 [DOI] [PubMed] [Google Scholar]
- 23. Kowalczyk-Quintas C., and Schneider P. (2014) Ectodysplasin A (EDA)—EDA receptor signalling and its pharmacological modulation. Cytokine Growth Factor Rev. 25, 195–203 [DOI] [PubMed] [Google Scholar]
- 24. Schneider P., Willen L., and Smulski C. R. (2014) Tools and techniques to study ligand-receptor interactions and receptor activation by TNF superfamily members. Methods Enzymol. 545, 103–125 [DOI] [PubMed] [Google Scholar]
- 25. Schuepbach-Mallepell S., Das D., Willen L., Vigolo M., Tardivel A., Lebon L., Kowalczyk-Quintas C., Nys J., Smulski C., Zheng T. S., Maskos K., Lammens A., Jiang X., Hess H., Tan S.-L., and Schneider P. (2015) Stoichiometry of heteromeric BAFF and APRIL cytokines dictates their receptor-binding and signaling properties. J. Biol. Chem. 290, 16330–16342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kowalczyk C., Dunkel N., Willen L., Casal M. L., Mauldin E. A., Gaide O., Tardivel A., Badic G., Etter A. L., Favre M., Jefferson D. M., Headon D. J., Demotz S., and Schneider P. (2011) Molecular and therapeutic characterization of anti-ectodysplasin A receptor (EDAR) agonist monoclonal antibodies. J. Biol. Chem. 286, 30769–30779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCarthy D. D., Kujawa J., Wilson C., Papandile A., Poreci U., Porfilio E. A., Ward L., Lawson M. A., Macpherson A. J., McCoy K. D., Pei Y., Novak L., Lee J. Y., Julian B. A., Novak J., Ranger A., Gommerman J. L., and Browning J. L. (2011) Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J. Clin. Investig. 121, 3991–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schneider P. (2000) Production of recombinant TRAIL and TRAIL receptor:Fc chimeric proteins. Methods Enzymol. 322, 325–345 [DOI] [PubMed] [Google Scholar]
- 29. Kimberley F. C., van der Sloot A. M., Guadagnoli M., Cameron K., Schneider P., Marquart J. A., Versloot M., Serrano L., and Medema J. P. (2012) The design and characterization of receptor-selective APRIL variants. J. Biol. Chem. 287, 37434–37446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swee L. K., Ingold-Salamin K., Tardivel A., Willen L., Gaide O., Favre M., Demotz S., Mikkola M., and Schneider P. (2009) Biological activity of ectodysplasin A is conditioned by its collagen and heparan sulfate proteoglycan-binding domains. J. Biol. Chem. 284, 27567–27576 [DOI] [PMC free article] [PubMed] [Google Scholar]