Abstract
The type III secretion system effector protein NleE from enteropathogenic Escherichia coli plays a key role in the inhibition of NF-κB activation during infection. NleE inactivates the ubiquitin chain binding activity of host proteins TAK1-binding proteins 2 and 3 (TAB2 and TAB3) by modifying the Npl4 zinc finger domain through S-adenosyl methionine-dependent cysteine methylation. Using yeast two-hybrid protein interaction studies, we found that a conserved region between amino acids 34 and 52 of NleE, in particular the motif 49GITR52, was critical for TAB2 and TAB3 binding. NleE mutants lacking 49GITR52 were unable to methylate TAB3, and wild type NleE but not NleE49AAAA52 where each of GITR was replaced with alanine restored the ability of an nleE mutant to inhibit IL-8 production during infection. Another NleE target, ZRANB3, also associated with NleE through the 49GITR52 motif. Ectopic expression of an N-terminal fragment of NleE (NleE34–52) in HeLa cells showed competitive inhibition of wild type NleE in the suppression of IL-8 secretion during enteropathogenic E. coli infection. Similar results were observed for the NleE homologue OspZ from Shigella flexneri 6 that also bound TAB3 through the 49GITR52 motif and decreased IL-8 transcription through modification of TAB3. In summary, we have identified a unique substrate-binding motif in NleE and OspZ that is required for the ability to inhibit the host inflammatory response.
Keywords: Escherichia coli (E. coli), infection, inflammation, NF-κB (NF-κB), substrate specificity
Introduction
Many bacterial pathogens, including enteropathogenic Escherichia coli (EPEC),4 enterohemorrhagic E. coli, and Shigella, utilize a type III secretion system (T3SS) to deliver multiple virulence proteins directly into host cells that subvert a diverse range of normal cellular functions (1). During infection, EPEC and enterohemorrhagic E. coli remain extracellular and attach intimately to the apical surface of enterocytes, forming attaching and effacing (A/E) lesions. A/E lesions are characterized by localized microvillus effacement and the accumulation of host cytoskeletal proteins beneath the adherent bacteria (2). A/E lesion formation requires the EPEC T3SS and secreted effector proteins, which are encoded on a genomic pathogenicity island called the locus of enterocyte effacement. EPEC also delivers a repertoire of non-locus of enterocyte effacement-encoded (Nle) effector proteins into host cells, some of which dampen the inflammatory response during infection and allow EPEC to evade early detection by the host immune system (3–10).
Recently, several T3SS effectors have been described as novel enzymes that target innate immune signaling pathways for the benefit of bacterial survival and dissemination. For example, the EPEC effector NleB1 is a novel glycosyltransferase that modifies death domain proteins with a single GlcNAc residue and inhibits death receptor-mediated apoptosis, thereby promoting the survival of enterocytes during EPEC infection (11, 12). The EPEC effector NleC is a zinc metalloprotease that directly cleaves NF-κB Rel proteins, including p65 and p50, as well as p300 (3, 4, 7, 10, 13, 14). OspF is a T3SS effector of Shigella classified as a phospholyase that represses the expression of proinflammatory cytokine genes by dephosphorylating activated mitogen-activated protein kinases and thereby blocking histone 3 phosphorylation at serine position 10 (15).
Another EPEC effector, NleE, is a novel S-adenosyl-l-methionine (AdoMet)-dependent methyltransferase that modifies a cysteine residue in the Npl4 zinc finger (NZF) domain of the host signaling adaptor proteins TAK1-binding proteins 2 and 3 (TAB2 and TAB3) (16). NleE is conserved across all A/E pathogens and has a homologue in Shigella spp., termed OspZ (17, 18). TAB2 and TAB3 are redundant proteins that are essential for signaling via the Toll-like, IL-1, and TNF receptors. Upon activation, the NZF domains of TAB2/3 bind Lys63-linked polyubiquitin chains on target proteins, such as the receptor-associated ubiquitin ligases TRAF6 and TRAF2. Polyubiquitin chain binding by TAB2/3 allows TAK1 to form a complex with IκB kinase and subsequently phosphorylate IκB kinase β. This leads to the phosphorylation and degradation of IκB and subsequent activation of NF-κB. NleE abolishes the ubiquitin chain binding capacity of the NZF domains of TAB2/3 and thereby disrupts NF-κB signaling (16). More recently, the NZF domain-containing protein ZRANB3 was identified as an NleE substrate, and when methylated, the NZF domain of ZRANB3 also lost the ability to bind polyubiquitin chains (19). ZRANB3 has been described previously as a translocase or annealing helicase that interacts with Lys63-linked polyubiquitinated proliferating cell nuclear antigen (19).
Despite the comprehensive work that has defined the novel enzymatic activities of NleE and other T3SS effectors, knowledge of effector target recognition and binding regions is often lacking. Here, we identified a highly conserved motif in the N terminus of NleE that is critical for the recognition of targets in host. We also investigated the propensity of OspZ to bind and methylate NleE host targets as well as the role of OspZ in the inhibition of NF-κB activation and IL8 expression during Shigella flexneri 6 infection.
Results
Identification of a TAB3-binding Domain in NleE
Although the 208IDSYMK214 motif is essential for NleE activity (6), the contribution of this motif to enzyme function is unknown. To test whether the 208IDSYMK214 motif was involved in target recognition, we used the yeast two-hybrid system to investigate binding of NleE to its host target, TAB3. Saccharomyces cerevisiae AH109 was co-transformed with NleE and TAB3 yeast expression constructs, and protein-protein interactions were assessed by growth of the yeast on selective quadruple dropout medium. Full-length NleE from EPEC E2348/69 interacted with TAB3 as did NleE6A, a derivative of NleE where each amino acid in the 208IDSYMK214 motif was substituted with alanine (Fig. 1A). Hence despite the fact that NleE6A is unable to inhibit NF-κB activation (6), this mutant derivative still bound to TAB3, suggesting the existence of a substrate recognition domain distinct from the 208IDSYMK214 motif.
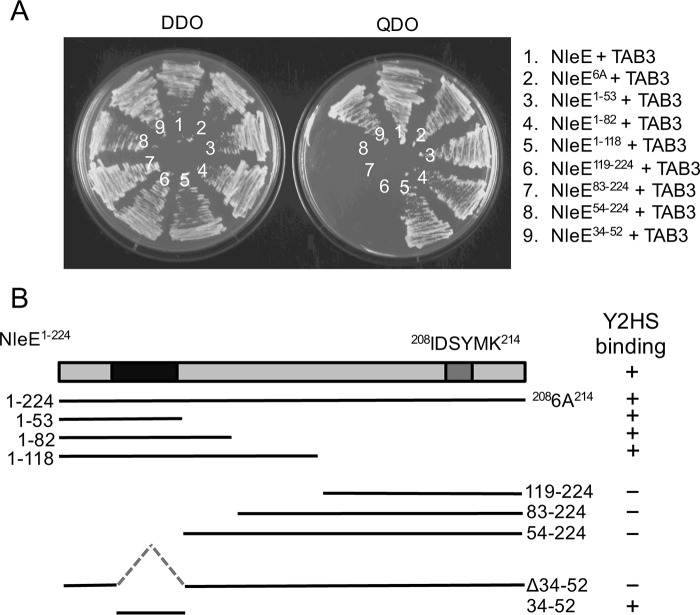
FIGURE 1.
Mapping of NleE-TAB3 interaction domains. A, growth of S. cerevisiae AH109 on medium lacking histidine, adenine, leucine, and tryptophan (QDO) to select for protein-protein interactions (right panel) or medium lacking leucine and tryptophan (DDO) to select for plasmid maintenance only (left panel). Yeast are co-expressing NleE and TAB3 (1), NleE6A and TAB3 (2), NleE1–53 and TAB3 (3), NleE1–82 and TAB3 (4), NleE1–118 and TAB3 (5), NleE119–224 and TAB3 (6), NleE83–224 and TAB3 (7), NleE54–224 and TAB3 (8), and NleE34–52 and TAB3 (9). B, schematic representation of NleE and various deletion profiles with TAB3 binding capacity indicated in the right panel. Y2HS, yeast two-hybrid system.
To narrow down the TAB3-binding region within NleE, we performed a sequential deletion analysis of NleE and determined the capacity of the NleE truncations to bind TAB3 in the yeast two-hybrid system (Fig. 1, A and B). The results suggested that the N-terminal 53 amino acids of NleE were crucial for the interaction with TAB3 (Fig. 1, A and B). Within this 53-amino acid sequence, amino acids 34–52 are highly conserved among NleE/OspZ proteins from EPEC, enterohemorrhagic E. coli, Citrobacter rodentium, and Shigella (Fig. 2A). Upon transformation of S. cerevisiae AH109 with constructs expressing TAB3 and just the fragment comprising amino acids 34–52 (NleE34–52), growth was observed on quadruple dropout medium, suggesting that amino acids 34–52 within NleE were sufficient to sustain an interaction with TAB3 (Fig. 1, A and B). To further confirm the interaction, purified GST-NleE and GST-NleE34–52 were used to pull down TAB3-FLAG from cell lysates of transfected HEK293T cells (Fig. 2B). GST alone showed no interaction with TAB3-FLAG.
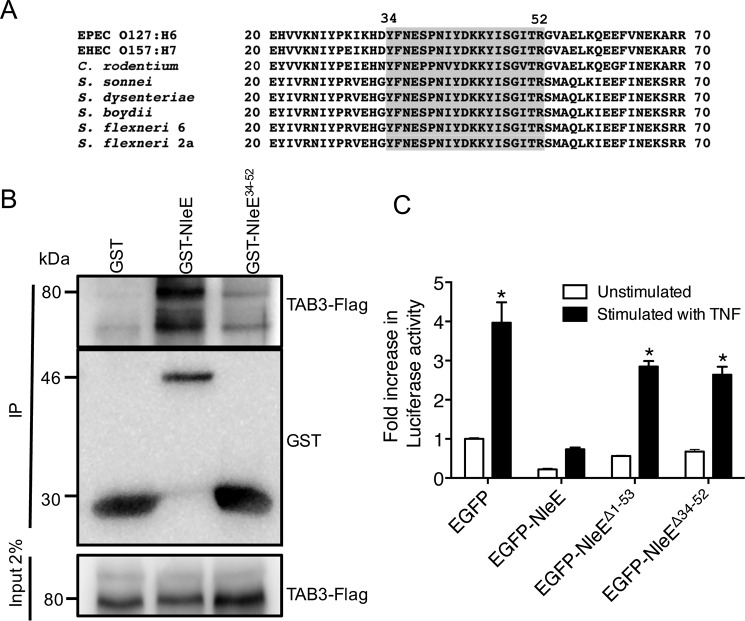
FIGURE 2.
Functional analysis of the TAB3-binding domain of NleE. A, alignment of the N-terminal regions of NleE and OspZ from A/E pathogens and Shigella. Amino acids 34–52 are shaded. B, pulldown of TAB3-FLAG by immobilized GST, GST-NleE, and GST-NleE34–52. C, -fold increase in NF-κB-dependent luciferase activity in HeLa cells expressing EGFP, EGFP-NleE, EGFP-NleEΔ1–53, or EGFP-NleEΔ34–52 and left unstimulated or stimulated with TNF for 8 h where indicated. Results are the mean ± S.E. (error bars) of three independent experiments carried out in duplicate. *, significantly different from unstimulated HeLa cells expressing EGFP only (p < 0.0001, unpaired two-tailed t test). EHEC, enterohemorrhagic E. coli; IP, immunoprecipitation.
Contribution of the NleE34–52 TAB3-binding Region to the Inhibition of NF-κB Activation
Based on the observation that the region between amino acids 34 and 52 of NleE was sufficient for binding to TAB3, we tested whether this region was required for inhibition of NF-κB activation by NleE. Using a Dual-Luciferase reporter system to measure NF-κB activation (6), we found that NleE lacking the N-terminal 53 amino acids (NleEΔ1–53) or the region between amino acids 34 and 52 (NleEΔ34–52) was unable to inhibit NF-κB activation (Fig. 2C). HeLa cells expressing EGFP alone were used as a positive control for activation. This suggested that the region between amino acids 34 and 52 was essential for the function of NleE.
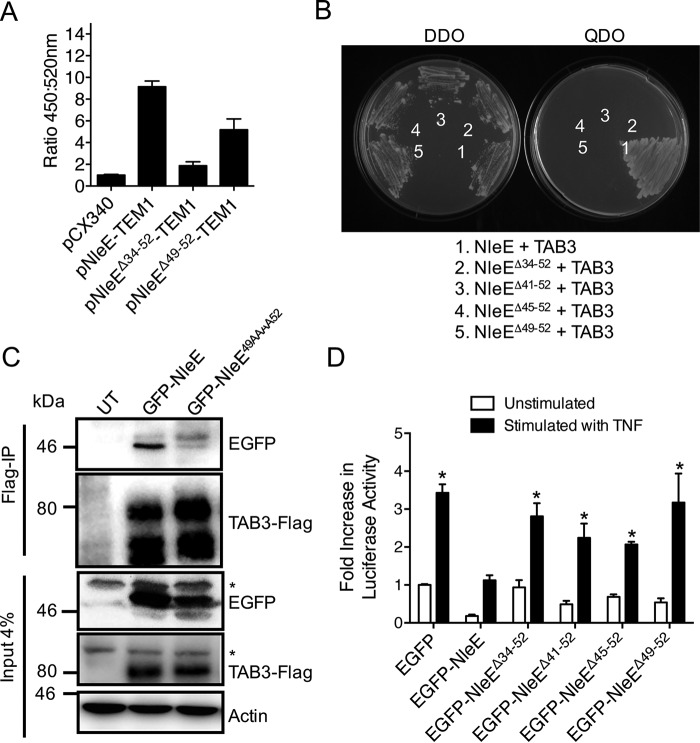
We predicted that amino acids 34–52 would overlap the T3SS secretion and translocation signal, making analysis of this region of NleE difficult in EPEC. To test this, we used a system that utilizes translational fusions to TEM1 β-lactamase (20). Equivalent expression of the TEM1-fused NleE derivatives in EPEC E2348/69 was detected with β-lactamase antibodies (data not shown). Not surprisingly, a derivative of NleE lacking amino acids 34–52 was not translocated into host cells (Fig. 3A). To narrow down the TAB3-binding domain of NleE further, we screened three NleE deletion mutants, NleEΔ41–52, NleEΔ45–52, and NleEΔ49–52, for their ability to bind TAB3. None of the NleE mutants tested bound to TAB3 in the yeast two-hybrid system, suggesting that the region between amino acids 49 and 52 was important for NleE-TAB3 interactions (Fig. 3B). Co-immunoprecipitation experiments in cultured epithelial cells confirmed this observation as full-length green fluorescent protein (GFP)-NleE immunoprecipitated with TAB3-FLAG, whereas no binding was observed between TAB3-FLAG and a derivative of NleE where each of 49GITR52 was replaced with alanine (GFP-NleE49AAAA52) (Fig. 3C). Despite equivalent expression (data not shown), none of the NleE deletion mutants (NleEΔ41–52, NleEΔ45–52, and NleEΔ49–52) were able to inhibit NF-κB activation as efficiently as full-length NleE (Fig. 3D), further suggesting that the 49GITR52 region was important for NleE function. HeLa cells expressing EGFP alone were used as a positive control for activation. We then tested the ability of EPEC to translocate NleEΔ49–52 and observed that NleEΔ49–52-TEM1 was translocated into cells (Fig. 3A), making analysis of the 49GITR52 region possible in the EPEC background.
FIGURE 3.
Translocation and function of NleE mutants lacking the TAB3-binding domain. A, translocation of NleE-TEM1, NleEΔ34–52-TEM1, and NleEΔ49–52-TEM1 fusions in HeLa cells. The ratio of blue fluorescence at 450 nm and green fluorescence at 520 nm is presented. HeLa cells were infected with EPEC E2348/69 (pCX340), EPEC E2348/69 (pNleE-TEM1), EPEC E2348/69 (pNleEΔ34–52-TEM1), or EPEC E2348/69 (pNleEΔ49–52-TEM1). Results are the mean ± S.E. (error bars) of three independent experiments carried out in triplicate. *, significantly different from EPEC E2348/69 carrying pCX340 only (p < 0.0001, unpaired two-tailed t test). B, growth of S. cerevisiae AH109 on medium lacking histidine, adenine, leucine, and tryptophan (QDO) to select for protein-protein interactions (right panel) or medium lacking leucine and tryptophan (DDO) to select for plasmid maintenance only (left panel). Yeast are co-expressing NleE and TAB3 (1), NleEΔ34–52 and TAB3 (2), NleEΔ41–52 and TAB3 (3), NleEΔ45–52 and TAB3 (4), and NleEΔ49–52 and TAB3 (5). C, immunoprecipitation (IP) of TAB3-FLAG and detection of EGFP-NleE and EGFP-NleE49AAAA52 binding in HEK293T cells. Actin is a loading control. * indicates nonspecific bands. D, -fold increase in NF-κB-dependent luciferase activity in HeLa cells expressing EGFP, EGFP-NleE, EGFP-NleEΔ34–52, EGFP-NleEΔ41–52, EGFP-NleEΔ45–52, or EGFP-NleEΔ49–52 and stimulated with or without TNF for 8 h where indicated. Results are the mean ± S.E. (error bars) of three independent experiments carried out in duplicate. *, significantly different from unstimulated HeLa cells expressing EGFP only (p < 0.02, unpaired two-tailed t test). UT, untransfected.
Contribution of the NleE 49GITR52 Motif to TAB3 Cysteine Methylation and Inhibition of IL-8 Secretion during EPEC Infection
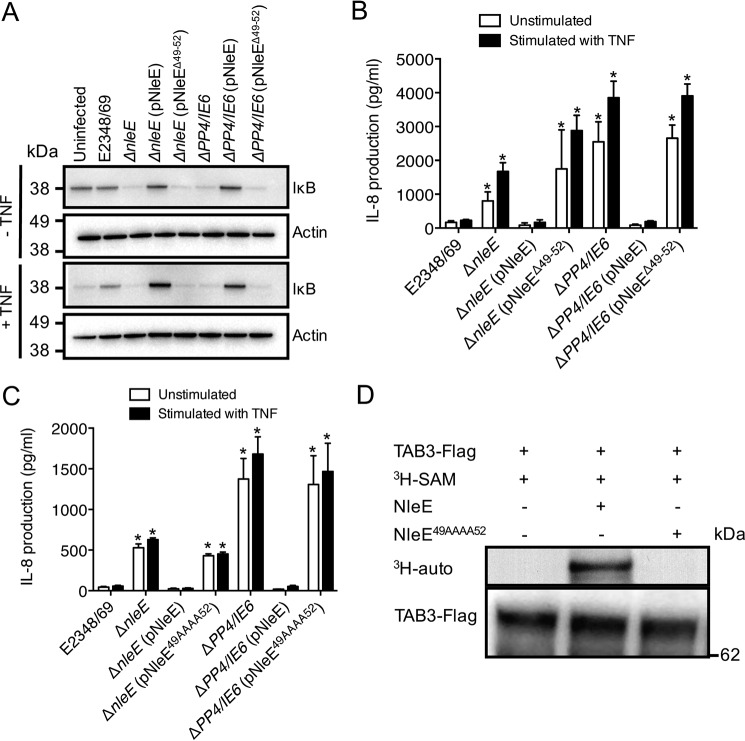
Because EPEC has been reported to inhibit IκB degradation (21) and NleE is necessary for this inhibition (6), we examined the effect of NleEΔ49–52 on IκB degradation. As observed previously, cells infected with an EPEC nleE deletion mutant or the PP4/IE6 double island deletion mutant, which lacks seven effector genes (6), were unable to inhibit IκB degradation in response to TNF stimulation, an effect that could be reversed upon complementation with full-length nleE (Fig. 4A) (6). In contrast, reintroduction of nleEΔ49–52 to these mutant backgrounds was unable to restore the inhibition of IκB degradation in response to TNF (Fig. 4A) or the inhibition of IL-8 secretion during EPEC infection (Fig. 4B). Similarly, exchange of each of 49GITR52 in NleE with alanine (NleE49AAAA52) generated a non-functional derivative of NleE, similar to NleEΔ49–52 (Fig. 4C).
FIGURE 4.
Importance of the TAB3-binding domain to NleE function during infection. A, immunoblot of IκB degradation in HeLa cells infected with derivatives of EPEC E2348/69 for 2 h and left unstimulated or stimulated with TNF for 30 min. B and C, IL-8 production from HeLa cells infected with derivatives of EPEC E2348/69 as indicated for 2 h and left unstimulated or stimulated with TNF for 8 h. Results are the mean ± S.E. (error bars) of three independent experiments carried out in duplicate. *, significantly different from unstimulated HeLa cells or HeLa cells stimulated with TNF and infected with EPEC E2348/69 (p < 0.02, unpaired two-tailed t test). D, [3H]AdoMet (SAM) labeling of full-length TAB3 immunopurified from HEK293T cells and incubated with either purified GST-NleE or GST-NleE49AAAA52 in the presence of [3H]AdoMet. [3H]AdoMet labeling was determined by 3H autoradiography (auto).
Given that NleE disrupts NF-κB signaling by transferring a methyl group onto the conserved zinc-coordinating cysteine located in the NZF domain of TAB2 and TAB3 (16), we investigated whether the 49GITR52 motif was important for target methylation by NleE. TAB3-FLAG was immunoprecipitated from cell lysates of transfected HEK293T cells and then incubated with either purified wild type GST-NleE or GST-NleE49AAAA52 together with [3H]AdoMet. [3H]AdoMet labeling of full-length TAB3 was detected by 3H autoradiography. Wild type NleE effectively catalyzed the transfer of [3H]methyl from [3H]AdoMet onto TAB3, whereas NleE49AAAA52 failed to mediate TAB3 cysteine methylation (Fig. 4D). This was presumably due to the requirement of the 49GITR52 motif for NleE substrate recognition and interaction with TAB3, although influence of the mutation on other aspects of enzyme activity cannot be ruled out.
Competitive Inhibition of Native NleE during Infection by Ectopic Expression of NleE34–52
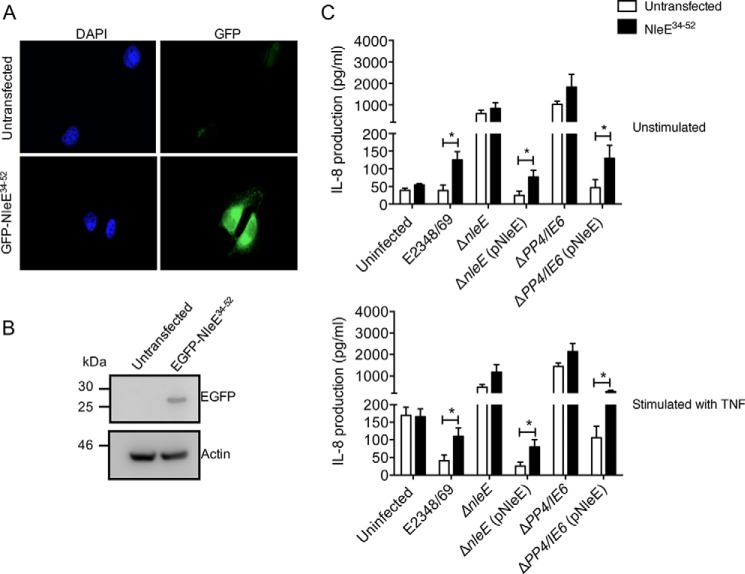
We reasoned that if NleE34–52 bound TAB3 then a fragment of this region alone would act as a competitive inhibitor for native NleE. HeLa cells stably expressing GFP-NleE34–52 were utilized to determine whether expression of the NleE TAB3-binding fragment diminished the efficacy of full-length NleE during infection. Suppression of IL-8 production by EPEC was used as a measure of native NleE activity. HeLa cells or HeLa cells stably expressing GFP-NleE34–52 (Fig. 5, A and B) were infected with wild type EPEC E2348/69, an nleE deletion mutant, ΔnleE complemented with full-length nleE, the PP4/IE6 double island mutant, or ΔPP4/IE6 complemented with full-length nleE. As observed previously, wild type EPEC inhibited IL-8 secretion in HeLa cells stimulated with TNF, whereas infection of HeLa cells with ΔnleE or ΔPP4/IE6 induced a significant increase in IL-8 production in both stimulated and unstimulated cells (Fig. 5C) (6). In HeLa cells stably expressing GFP-NleE34–52, IL-8 secretion was significantly increased during wild type EPEC infection compared with HeLa cells alone. Similarly, GFP-NleE34–52-expressing cells infected with ΔnleE or ΔPP4/IE6 and complemented with full-length nleE showed significantly higher IL-8 levels than HeLa cells alone (Fig. 5C). Overall, this suggested that the NleE34–52 fragment could block the activity of full-length NleE during infection.
FIGURE 5.
Inhibition of NleE activity during infection by overexpression of NleE34–52. A, representative immunofluorescence fields showing HeLa cells or HeLa cells expressing the GFP-NleE34–52 fusion protein (green). Cell nuclei were counterstained with DAPI (blue). B, immunoblot of untransfected HeLa cells or HeLa cells stably expressing the EGFP-NleE34–52 fusion protein using antibodies to GFP. Actin is a loading control. C, IL-8 production from HeLa cells or HeLa cells stably expressing EGFP-NleE34–52 infected with derivatives of EPEC E2348/69 for 2 h and left unstimulated (top panel) or stimulated with TNF (bottom panel) for 8 h. Results are the mean ± S.E. (error bars) of three independent experiments carried out in duplicate. *, EGFP-NleE34–52-expressing HeLa cells significantly different from HeLa cells when infected with E2348/69 or mutants complemented with pNleE (p < 0.05, unpaired two-tailed t test).
The 49GITR52 Motif of NleE Is Important for Cysteine Methylation of All Identified Host Substrates
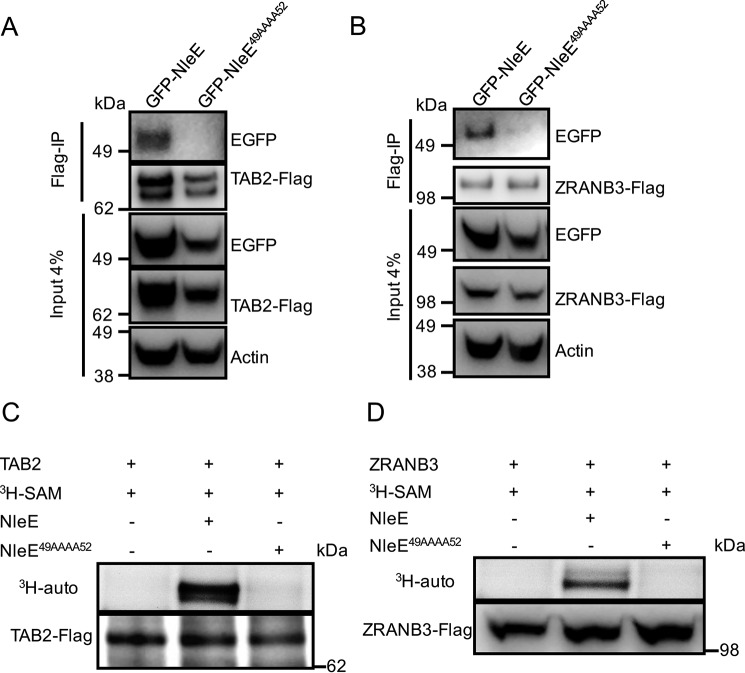
To determine whether the 49GITR52 motif of NleE was also needed for the interaction of NleE with TAB2 and ZRANB3, we performed FLAG immunoprecipitation on HEK293T cells co-expressing GFP-NleE derivatives and TAB2-FLAG or ZRANB3-FLAG. GFP-NleE co-immunoprecipitated with TAB2-FLAG and ZRANB3-FLAG, whereas no interaction was observed between GFP-NleE49AAAA52 and TAB2-FLAG or ZRANB3-FLAG (Fig. 6, A and B). We next investigated whether the 49GITR52 motif of NleE was important for TAB2 and ZRANB3 cysteine methylation. TAB2-FLAG or ZRANB3-FLAG were immunoprecipitated from cell lysates of transfected HEK293T cells, respectively, and then incubated with either purified wild type GST-NleE or GST-NleE49AAAA52 together with [3H]AdoMet. [3H]AdoMet labeling of full-length TAB2 or ZRANB3 was detected by 3H autoradiography. Wild type NleE effectively catalyzed the transfer of [3H]methyl from [3H]AdoMet to TAB2 and ZRANB3, whereas NleE49AAAA52 failed to induce the methylation of TAB2 and ZRANB3 (Fig. 6, C and D).
FIGURE 6.
Binding of NleE to TAB2 and ZRANB3. A, immunoprecipitation of TAB2-FLAG and detection of EGFP-NleE and EGFP-NleE49AAAA52 binding in HEK293T cells. Actin is a loading control. B, immunoprecipitation (IP) of ZRANB3-FLAG and detection of EGFP-NleE and EGFP-NleE49AAAA52 binding in HEK293T cells. Actin is a loading control. C and D, [3H]AdoMet (SAM) labeling of full-length TAB2-FLAG or ZRANB3-FLAG as indicated immunopurified from HEK293T cells and incubated with purified GST-NleE or GST-NleE49AAAA52 in the presence of [3H]AdoMet. [3H]AdoMet labeling was determined by 3H autoradiography (auto).
Interaction of OspZ with Identified Host Targets of NleE
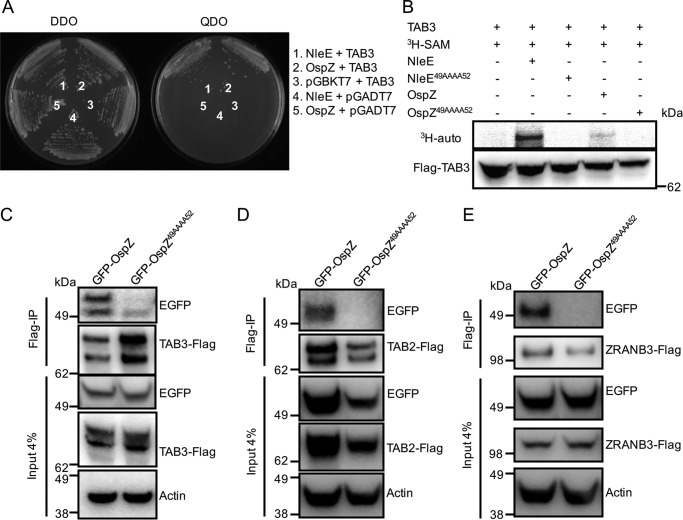
Given that the amino acid sequences of OspZ from S. flexneri and EPEC NleE show a high degree of similarity, we investigated whether OspZ targets the same host proteins as NleE. Using the yeast two-hybrid system, we observed an interaction between OspZ and TAB3 (Fig. 7A). OspZ also showed an ability to methylate TAB3, and this required the 49GITR52 motif (Fig. 7B). Co-immunoprecipitation experiments using HEK293T cells expressing GFP-OspZ confirmed the interaction between OspZ and TAB3 and showed that OspZ also interacted with TAB2 and ZRANB3 (Fig. 7, C, D, and E). Similar to NleE, binding of OspZ to TAB2, TAB3, and ZRANB3 required the 49GITR52 motif because GFP-OspZ49AAAA52 did not co-purify with any of TAB3-FLAG, TAB2-FLAG, or ZRANB3-FLAG (Fig. 7, C, D, and E). However, we could not detect methylation of TAB2 and ZRANB3 by OspZ (data not shown).
FIGURE 7.
OspZ binding to TAB2, TAB3, and ZRANB3 in vitro. A, growth of S. cerevisiae AH109 on medium lacking histidine, adenine, leucine, and tryptophan (QDO) to select for protein-protein interactions (right panel) or medium lacking leucine and tryptophan (DDO) to select for plasmid maintenance only (left panel). Yeast are co-expressing NleE and TAB3 (1), OspZ and TAB3 (2), pGBKT7 and TAB3 (3), NleE and pGADT7 (4), and OspZ and pGBKT7 (5). B, [3H]AdoMet (SAM) labeling of full-length TAB3 immunopurified from HEK293T cells and incubated with purified GST-NleE, GST-NleE49AAAA52, GST-OspZ, or GST-OspZ49AAAA52, respectively, in the presence of [3H]AdoMet. [3H]AdoMet labeling was determined by 3H autoradiography (auto). C, FLAG immunoprecipitation (IP) of TAB3-FLAG and detection of EGFP-OspZ and EGFP-OspZ49AAAA52 in HEK293T cells using anti-GFP antibodies. TAB3-FLAG was detected with anti-FLAG antibodies. Actin is a loading control. D, FLAG immunoprecipitation of TAB2-FLAG and detection of EGFP-OspZ and EGFP-OspZ49AAAA52 in HEK293T cells using anti-GFP antibodies. TAB3-FLAG was detected with anti-FLAG antibodies. Actin is a loading control. E, FLAG immunoprecipitation of ZRANB3-FLAG and detection of EGFP-OspZ and EGFP-OspZ49AAAA52 in HEK293T cells using anti-GFP antibodies. TAB3-FLAG was detected with anti-FLAG antibodies. Actin is a loading control.
OspZ Inhibits NF-κB Activation and IL-8 Production during Shigella Infection
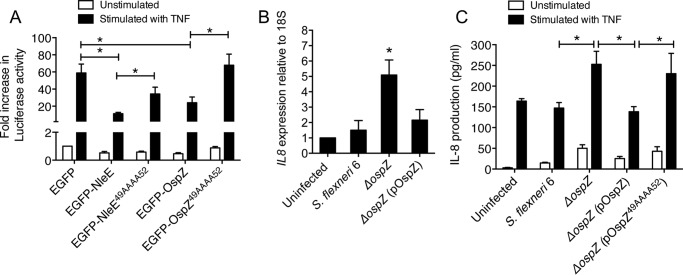
To confirm whether OspZ from S. flexneri 6 could inhibit NF-κB activation, we generated a GFP-OspZ fusion to compare with GFP-NleE. Expression of the GFP fusions was confirmed by immunoblotting using anti-GFP antibodies (data not shown). HeLa cells expressing GFP-NleE or GFP-OspZ were left unstimulated or stimulated with TNF for 2 h. NleE from EPEC E2348/69 and full-length OspZ from S. flexneri 6 inhibited NF-κB activation in response to TNF, although the inhibitory effect of OspZ appeared weaker than that of NleE. Mutation of the 49GITR52 motif to generate GFP-OspZ49AAAA52 abolished the ability of OspZ to block NF-κB activation, similar to NleE. This suggested that the 49GITR52 substrate-binding motif was essential for the full function of NleE and OspZ (Fig. 8A).
FIGURE 8.
OspZ mediated inhibition of NF-κB activation and IL-8 production during Shigella infection. A, -fold increase in NF-κB-dependent luciferase activity in HeLa cells transfected with pEGFP-C2, pEGFP-NleE, pEGFP-NleE49AAAA52, pEGFP-OspZ, or pEGFP-OspZ49AAAA52 and left unstimulated or stimulated with TNF for 8 h where indicated. Results are the mean ± S.E. (error bars) of three independent experiments carried out in duplicate. *, p < 0.05, unpaired two-tailed t test. B, reverse transcription-quantitative PCR analysis of IL8 expression in HT-29 cells 1 h after infection. Results are expressed as mRNA expression levels that were normalized relative to 18S and are the mean ± S.E. (error bars) of three independent experiments carried out in duplicate. *, significantly different from uninfected HT-29 cells or HT-29 cells infected with wild type S. flexneri 6 or ΔospZ (pOspZ) (p < 0.05, unpaired two-tailed t test). C, IL-8 production from HT-29 cells infected with derivatives of S. flexneri 6 as indicated for 2 h and left unstimulated or stimulated with TNF for 2 h. Results are the mean ± S.E. (error bars) of three independent experiments carried out in duplicate. *, p < 0.05, unpaired two-tailed t test.
Given that OspZ inhibited TNF-induced NF-κB activation, we determined whether OspZ affected the expression of NF-κB-regulated genes during Shigella infection. To address this, we generated an ospZ mutant of S. flexneri 6 where ospZ was replaced with a kanamycin cassette by allelic exchange using the λ Red recombinase system. We then compared IL8 transcription levels in HT-29 cells infected with wild type S. flexneri 6, the ospZ deletion mutant, or ospZ mutant complemented with full-length ospZ. Cell monolayers were infected for 1 h, and IL8 mRNA levels were examined by quantitative PCR. IL8 expression was significantly higher in cells infected with the ospZ deletion mutant compared with those infected with wild type S. flexneri 6 and the ospZ mutant complemented with full-length ospZ (Fig. 8B). HT-29 cells were also utilized to determine whether the 49GITR52 motif was important for OspZ-mediated inhibition of IL-8 production during S. flexneri 6 infection. Following infection for 2 h, the ospZ mutant strain showed a diminished ability to inhibit IL-8 production compared with wild type S. flexneri 6, and this effect was restored upon complementation with a copy of wild type ospZ but not with ospZ49AAAA52 (Fig. 8C). Overall, OspZ showed a similar ability to inhibit IL-8 production during infection, and the 49GITR52 motif was important for this function.
Discussion
A number of EPEC T3SS effectors have been implicated in the inhibition of inflammatory cytokine production during EPEC infection (1). Of these, NleE is a potent inhibitor of NF-κB activation through its recognition and modification of the adaptor proteins TAB2 and TAB3 (16). Derivatives of EPEC lacking nleE are defective in their ability to suppress IL-8 secretion even at early time points after epithelial cell infection (5–7). NleE is a novel AdoMet-dependent cysteine methyltransferase that methylates Cys673 in the NZF domain of TAB2 and Cys692 of TAB3, thereby preventing NZF-mediated recognition of polyubiquitinated TRAF2 and TRAF6 and thus TRAF-induced TAK1 activation (16). Hence, in the presence of NleE, NF-κB activation in response to TNF, IL1-β, or Toll-like receptor stimulation is blocked.
Although the enzymatic activity of NleE is now well described, how the enzyme recognizes TAB2 and TAB3 as substrates for modification is unknown. Previously, we identified the 209IDSYMK214 motif in the C terminus of NleE as critical for activity. A derivative of NleE lacking the 209IDSYMK214 motif was unable to inhibit NF-κB signaling or p65 nuclear translocation (6). Zhang et al. (16) further showed that NleE lacking the 209IDSYMK214 motif was unable to modify TAB2/3 and block ubiquitin chain sensing by TAB2/3, and Yao et al. (19) recently demonstrated that the 209IDSYMK214 motif was associated with AdoMet binding. Here we ruled out involvement of the 209IDSYMK214 motif in TAB2/3 recognition using the yeast two-hybrid system to examine NleE-TAB3 interactions. A derivative of NleE in which each residue of 209IDSYMK214 was substituted with alanine (NleE6A) bound to TAB3, similar to native NleE. Further mapping and mutagenesis of NleE identified an N-terminal region from amino acids 34 to 52, in particular 49GITR52, as critical for TAB2/TAB3 binding. NleE lacking 49GITR52 or an NleE derivative where each residue of 49GITR52 was substituted with alanine was unable to inhibit NF-κB activation or suppress IL-8 secretion during EPEC infection. Hence, the TAB2/TAB3-binding domain of NleE was as important for NleE function as the 209IDSYMK214 motif.
Recently, another NZF domain-containing protein, ZRANB3, was identified as a substrate of NleE (19). ZRANB3, an SNF2 ATPase, localizes at the site of DNA replication and plays a significant role in the DNA damage tolerance pathway (24, 25). Previous studies have shown that DNA repair systems are activated to antagonize DNA damage induced by chronic inflammation, which has been well characterized during Helicobacter pylori and Salmonella enterica infections (22, 26). In addition, DNA repair mechanisms are suppressed by chronic infection with H. pylori (22). Given its activity, NleE may also interfere with DNA damage repair systems during EPEC infection. Here, we determined that NleE recognized ZRANB3 through the same motif as TAB2/3. Whereas wild type NleE co-immunoprecipitated with ZRANB3-FLAG, this interaction was abrogated when each amino acid of the 49GITR52 motif was substituted with alanine. Overall, our results suggested that the 49GITR52 motif of NleE is important for recognition and binding of all currently identified host substrates.
NleE homologues are also found in Shigella species where they are termed OspZ. Here we found that full-length OspZ bound the same host substrates of NleE, namely TAB2, TAB3, and ZRANB3. However, OspZ exhibited only weak cysteine methyltransferase activity toward TAB3 and did not modify TAB2 or ZRANB3. It is possible that OspZ has an alternative substrate to NleE, and further work is needed to determine which host proteins are targeted by OspZ. Interestingly, naturally occurring deletion mutants of NleE and OspZ are maintained in some EPEC and Shigella genomes. S. flexneri serotype 2a carries a truncated version of OspZ that lacks the C-terminal 36 amino acids encompassing the 209IDSYMK214 motif (6), whereas EPEC E2348/69 harbors a second copy of NleE (NleE2) that carries an internal deletion between amino acids 48 and 106 encompassing the 49GITR52 motif (5). NleE2 is not translocated into cells (5) unlike the alanine-substituted 49GITR52 mutant of NleE created here. However, even if NleE2 were translocated, it would not be functional. The maintenance of these inactive versions of NleE and OspZ is curious and may point to an evolving advantage in allowing the inflammatory response to progress under certain conditions and with certain strains.
In summary, we have identified a region of NleE that is required and sufficient for binding to host substrates TAB2, TAB3, and ZRANB3. Stable ectopic expression of this region in HeLa cells interfered with the function of bacterially delivered NleE, presumably by competitive inhibition of NleE-TAB2/3 interactions. OspZ binds the same host targets of NleE, and the newly identified 49GITR52 motif is involved in substrate binding and the ability of OspZ to inhibit NF-κB signaling.
Experimental Procedures
Bacterial Strains and Oligonucleotide Primers
The bacterial strains and oligonucleotide primers used in this study are listed in Tables 1 and 2, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain/plasmid | Characteristics | Source/Ref. |
|---|---|---|
| EPEC E2348/69 | Wild type EPEC O127:H6 | 23 |
| ΔnleE | EPEC E2348/69 ΔnleE cmR | 18 |
| ΔPP4/IE6 | EPEC E2348/69 ΔPP4/IE6 double island deletion | 6 |
| S. flexneri 6 | Wild type S. flexneri 6 | 6 |
| ΔospZ | S. flexneri 6 ΔospZ kanR | This study |
| S. cerevisiae | S. cerevisiae AH109 | Clontech |
| pTrc99a | Cloning vector for expression of proteins from Ptrc | GE Healthcare |
| pNleE | nleE from EPEC E2348/69 in pTrc99a | 6 |
| pNleEΔ49–52 | nleE from EPEC E2348/69 carrying the GITR deletion in pTrc99a | This study |
| pNleE49AAAA52 | nleE from EPEC E2348/69 carrying alanine substitution for each amino acid in the GITR motif in pTrc99a | This study |
| pEGFP-C2 | GFP expression vector | Clontech |
| pEGFP-NleE | nleE from EPEC E2348/69 in pTrc99a | 18 |
| pEGFP-NleE1–53 | Truncation of nleE from EPEC E2348/69 encoding amino acids 1–53 in pEGFP-C2 | This study |
| pEGFP-NleEΔ34–52 | Full-length nleE from EPEC E2348/69 carrying the GITR deletion in pEGFP-C2 | This study |
| pEGFP-NleE49AAAA52 | nleE from EPEC E2348/69 carrying alanine substitution for each amino acid in the GITR motif in pEGFP-C2 | This study |
| pEGFP-OspZ | ospZ from S. flexneri 6 in pEGFP-C2 | This study |
| pEGFP-OspZ49AAAA52 | ospZ from S. flexneri 6 carrying alanine substitution for each amino acid in the GITR motif in pEGFP-C2 | This study |
| pGEX-6P-1 | GST expression vector | GE Healthcare |
| pEGFP-NleE34–52 | The region between amino acids 34 and 52 of nleE in pEGFP-C2 | This study |
| pGST-NleE34–52 | The region between amino acids 34 and 52 of nleE in pGEX-6P-1 | This study |
| pGST-NleE | nleE from EPEC E2348/69 in pGEX-6P-1 | This study |
| pGST-NleE49AAAA52 | Full-length nleE from EPEC E2348/69 carrying a mutation in the motif 49GITR52 in pGEX-6P-1 | This study |
| pGST-OspZ | ospZ from S. flexneri 6 in pGEX-6P-1 | This study |
| pGST-OspZ49AAAA52 | Full-length ospZ from S. flexneri 6 carrying a mutation in the motif 49GITR52 in pGEX-6P-1 | This study |
| pGBKT7 | Yeast expression vector | Clontech |
| pGBKT7-NleE | nleE from EPEC E2348/69 in pGBKT7 | This study |
| pGBKT7-NleE1–53 | Truncation of nleE from EPEC E2348/69 encoding amino acids 1–53 in pGBKT7 | This study |
| pGBKT7-NleE1–82 | Truncation of nleE from EPEC E2348/69 encoding amino acids 1–82 in pGBKT7 | This study |
| pGBKT7-NleE1–118 | Truncation of nleE from EPEC E2348/69 encoding amino acids 1–118 in pGBKT7 | This study |
| pGBKT7-NleE54–224 | Truncation of nleE from EPEC E2348/69 encoding amino acids 54–224 in pGBKT7 | This study |
| pGBKT7-NleE83–224 | Truncation of nleE from EPEC E2348/69 encoding amino acids 83–224 in pGBKT7 | This study |
| pGBKT7-NleE119–224 | Truncation of nleE from EPEC E2348/69 encoding amino acids 119–224 in pGBKT7 | This study |
| pGBKT7-NleE6A | nleE from EPEC E2348/69 in pGBKT7 carrying alanine substitutions for each amino acid in the IDSYMK motif | This study |
| pGBKT7-NleE34–52 | The region between amino acids 34 and 52 of nleE in pGBKT7 | This study |
| pGBKT7-NleEΔ34–52 | Full-length nleE from EPEC E2348/69 carrying the deletion between amino acids 34 and 52 in pGBKT7 | This study |
| pGBKT7-NleEΔ41–52 | Full-length nleE from EPEC E2348/69 carrying the deletion between amino acids 41 and 52 in pGBKT7 | This study |
| pGBKT7-NleEΔ45–52 | Full-length nleE from EPEC E2348/69 carrying the deletion between amino acids 45 and 52 in pGBKT7 | This study |
| pGBKT7-NleEΔ49–52 | Full-length nleE from EPEC E2348/69 carrying the GITR deletion in pGBKT7 | This study |
| pGBKT7-OspZ | Full-length ospZ from S. flexneri 6 in pGBKT7 | This study |
| pCX340 | Cloning vector for constructing TEM-1 β-lactamase fusions | 20 |
| pCX340-NleE | nleE from EPEC E2348/69 in pCX340 | This study |
| pCX340-NleE54–224 | Truncation of nleE from EPEC E2348/69 encoding amino acids 54–224 in pCX340 | This study |
| pCX340-NleEΔ34–52 | Full-length nleE from EPEC E2348/69 carrying a deletion of amino acids between 34 and 52 in pCX340 | This study |
| pCX340-NleEΔ49–52 | Full-length nleE from EPEC E2348/69 carrying the GITR deletion in pCX340 | This study |
| pcDNA3 | Mammalian cell expression vector | Invitrogen |
| pcDNA3-TAB3–3×FLAG | Full-length human TAB3 with a C-terminal 3 × FLAG tag in pcDNA3 | This study |
| pcDNA3-TAB2–3×FLAG | Full-length human TAB3 with a C-terminal 3 × FLAG tag in pcDNA3 | This study |
| pcDNA3-ZRANB3–3×FLAG | Full-length human ZRANB3 with a C-terminal 3 × FLAG tag in pcDNA3 | This study |
| pGADT7 | Yeast expression vector | Clontech |
| pGADT7-TAB3 | Full-length human TAB3 in pGADT7 | This study |
| pRL-TK | Renilla luciferase vector | Promega |
| pNF-κB-Luc | Vector for measuring NF-κB-dependent luciferase expression | Clontech |
TABLE 2.
List of primers used in this study
| Name | Primer sequences (5`–3`) |
|---|---|
| NleEF | GTGAATTCATGATTAATCCTG |
| NleER | GAGGATCCCTACTCAATTTT |
| NleELacF | CTCATATGATGATTAATCCTG |
| NleELacR | CGAATTCTCCTCAATTTTAGAAAGTTT |
| NleE(1–53)F | GTGAATTCATGATTAATCCTG |
| NleE(1–53)R | GAGGATCCTCCTCTGGTTA |
| NleE(1–82)F | GTGAATTCATGATTAATCCTG |
| NleE(1–82)R | GAGGATCCTTCTGGACATAC |
| NleE(1–118)F | GTGAATTCATGATTAATCCTG |
| NleE(1–118)R | CTGGATCCGTATAAACTATC |
| NleE(54–224)F | GTGAATTCATGGTAGCTAACTAA |
| NleE(54–224)R | GAGGATCCCTACTCAATTTT |
| NleE(83–224)F | GTGAATTCATGGCGTTTGAACC |
| NleE(83–224)R | GAGGATCCCTACTCAATTTT |
| NleE(119–224)F | GTGAATTCATGAATCCTGATTTAC |
| NleE(119–224)R | GAGGATCCCTACTCAATTTT |
| NleE(34–52)F | GAATTCATGTACTTTAATGAATCACCCAATATATATGATAAGAAGTATATATCCGGTATAACCAGAGGATCC |
| NleE(34–542)R | GGATCCTCTGGTTATACCGGATATATACTTCTTATCATATATATTGGGTGATTCATTAAAGTACATGAATTC |
| NleE(49AAAA52)F | GATAAGAAGTATATATCCGCGGCAGCCGCAGGAGTAGCTGAACTAAAACAGGAAG |
| NleE(49AAAA52)R | CTTCCTGTTTTAGTTCAGCTACTCCTGCGGCTGCCGCGGATATATACTTCTTATC |
| NleE(Δ34–52)F | CATGATGGAGTAGCTGAAC |
| NleE(Δ34–52)R | GCTACTCCATCATGTTTAATTTTC |
| NleE(Δ41–52)F | GTCCCAATGGAGTAGCTGAAC |
| NleE(Δ41–52)R | CTTACTCCATTGGGTGATTCAT |
| NleE(Δ45–52)F | CTGATAAGGGAGTAGCTGAAC |
| NleE(Δ45–52)R | CTTACTCCCTTATCATATATATTGG |
| NleE(Δ49–52)F | GTATATCCGGAGTAGCTGAAC |
| NleE(Δ49–52)R | GATACTCCGGATATATACTTCTTATC |
| NleEGSTF | GTGGATCCATGATTAATCCTG |
| NleEGSTR | GAGAATTCCTACTCAATTTT |
| NleE(34–52)GSTF | GGATCCATGTACTTTAATGAATCACCCAATATATATGATAAGAAGTATATATCCGGTATAACCAGATAGGAATTC |
| NleE(34–52)GSTR | GAATTCTATCTGGTTATACCGGATATATACTTCTTATCATATATATTGGGTGATTCATTAAAGTACATGGATCC |
| OspZF | TAGAATTCATGATTAGTCCCATC |
| OspZR | GCGGATCCTTAAGTAACAGGC |
| OspZCF | TAGGATCCGGTTCTGGTTTTCAAATTG |
| OspZCR | TAGTCGACTTAAGTAACAGGCATTCGAGCC |
| OspZ(49AAAA52)F | GACAAGAAGTATATCTCTGCTGCAGCCGCAAGTATGGCACAATTAAAAATAGAGGAG |
| OspZ(49AAAA52)R | CTCCTCTATTTTTAATTGTGCCATACTTGCGGCTGCAGCAGAGATATACTTCTTGTC |
| OspZGSTF | CAGGATCCATGATTAGTCCCATCAAG |
| OspZGSTR | CAGAATTCTTAAGTAACAGGCATTCG |
| ΔospZUPF | TAGGGATAACAGGGTAATGAGCCCCAATTAAGTTATCAAGC |
| ΔospZUPR | GAAGCAGCTCCAGCCTACACAGCCATACTTCTGGTTATACCAG |
| ΔospZDNF | CTAAGGAGGATATTCATATGCAAGGCTCGAATGTCTGTTAC |
| ΔospZDNR | TAGGGATAACAGGGTAATGTAAGCGCTGGGCGTAC |
| ΔospZF | GGGATCACCTGGCATGGACGAGCTGTACAAGTCCGGCCGGACTCAGATCTCGAGCTCAAGCTTCGAATTCTGTGTAGGCTGGAGCTGCTTCG |
| ΔospZR | GTTTCAGGTTCAGGGGGAGGTGTGGGAGGTTTTTTAAAGCAAGTAAAACCTCTACAAATGTGGTATGGCTGATCATATGAATATCCTCCTTA |
| TAB3F | CAGAATTCATGGCGCAAAG |
| TAB3R | GTGGATCCTCAGGTGTACCG |
| TAB33×FLAGF | CAGGATCCATGGCGCAAAG |
| TAB33×FLAGR | CAGAATTCTCACTTGTCGACATCGTCTTTCTAGTCGATGTCATGATCTTTATAATCACCGTCATGGTCTTTGTAGTCGGTGTACCGTGG |
| TAB2F | CAGGATCCATGGCCCAAGGAAGCCAC |
| TAB23×FLAGR | CAGAATTCTCACTTGTCGTCATCGTCTTTGTAGTCGATGTCATGATCTTTATAATCACCGTCATGGTCTTTGTAGTCGAAATGCCTTGGCATCTCACAC |
| ZRANB3F | CACCAGTGTGCTGGATGCCTAGGGTTCATAACC |
| ZRANB33×FLAGR | CAGAATTCTCACTTGTCGTCATCGTCTTTGTAGTCGATGTCATGATCTTTATAATCACCGTCATGGTCTTTGTAGTCCTTCTTTACCAAAAATCGTG |
| IL-8F | GTTTGATACTCCCAGTCTTGTCATTG |
| IL-8R | CTGTGGAGTTTTGGCTGTTTTAATCG |
| 18SF | CGGCTACCACATCCAAGGAA |
| 18SR | GCTGGAATTACCGCGGCT |
Yeast Two-hybrid Assay
A yeast two-hybrid interaction assay was performed according to the manufacturer's instructions and as described previously (Clontech PT4084-1 manual) (12). The S. cerevisiae strain AH109 was co-transformed with pGBKT7 empty vector or pGBKT7 carrying NleE (and derivatives) from EPEC strain E2348/69 (GenBankTM accession number FM180568.1) and pGADT7 empty vector or pGADT7 with human TAB3 derivatives using the lithium acetate method. Transformants were plated onto both double dropout plates (Trp−, Leu−) and quadruple dropout plates (Trp−, Leu−, Ade−, His−) to select for vector expression as well as the interaction of the hybrid proteins encoded by them. The cDNA for human TAB3 (GenBank accession number NM_152787.4) was purchased from OriGene.
Construction of NleE/OspZ and TAB2/TAB3/ZRANB3 Expression Vectors
The nleE gene from EPEC E2348/69 was amplified from the genomic DNA by PCR using the primer pair NleEF/NleER, and the amplicon was inserted into EcoRI/BamHI-digested pGBKT7 vector (Clontech) to generate Gal4 DNA-binding domain fusions to NleE. Similarly, the ospZ gene (GenBank accession number NZ_LAJR01000183.1) from S. flexneri 6 was amplified from genomic DNA by PCR using the primer pair OspZF/OspZR and ligated into EcoRI/BamHI-digested pGBKT7 vector. To create Gal4 DNA-binding domain-fused NleEΔ34–52, the blunt-ended fragments comprising the first 34 bp and the last 172 bp of nleE were amplified by PCR using primer pairs NleEF/NleE(Δ34–52)R and NleE(Δ34–52)F/NleER, respectively. The two fragments were joined together using overlapping PCR to generate a single fragment. The single fragment was digested with EcoRI/BamHI and inserted into pGBKT7. The constructs pGBKT7-NleEΔ41–52, pGBKT7-NleEΔ45–52, and pGBKT7-NleEΔ49–52 were generated using similar methods as for pGBKT7-NleEΔ34–52 with primer pairs NleEF/NleE(Δ41–52)R, NleE(Δ41–52)F/NleER, NleEF/NleE(Δ45–52)R, NleE(Δ45–52)F/NleER, NleEF/NleE(Δ49–52)R, and NleE(Δ49–52)F/NleER, respectively. To create an N-terminal GFP fusion to NleE and OspZ, nleE and ospZ were amplified from the genomic DNA of EPEC E2348/69 or S. flexneri 6 by PCR using the primer pair NleEF/NleER or OspZF/OspZR. The PCR product was digested with EcoRI/BamHI and ligated into pEGFP-C2. To generate NleE truncation constructs, the coding regions for amino acids 1–53, 1–82, 1–118, 119–224, 83–224, 54–224, and 34–52 of NleE were amplified from pEGFP-C2-NleE by PCR using primer pairs NleEF/NleE(1–53)R, NleEF/NleE(1–82)R, NleEF/NleE(1–118)R, NleE(119–224)F/NleER, NleE(83–224)F/NleER, NleE(54–224)F/NleER, and NleE(34–52)F/NleE(34–52)R, respectively. The PCR products were ligated into EcoRI/BamHI-digested pGBKT7 or pEGFP-C2. The plasmids pEGFP-NleEΔ34–52, pEGFP-NleEΔ41–52, pEGFP-NleEΔ45–52, and pEGFP-NleEΔ49–52 were generated with by amplifying nleE from pGBKT7-NleEΔ34–52, pGBKT7-NleEΔ41–52, pGBKT7-NleEΔ45–52, and pGBKT7-NleEΔ49–52 using primer pair NleEF/NleER. The PCR products were digested with EcoRI/BamHI and ligated into pEGFP-C2. To generate the complementing vector, pNleE, nleE was amplified from EPEC E2348/69 genomic DNA by PCR using the primer pair NleEF/NleER and ligated into EcoRI/BamHI-digested pTrc99A. The ospZ gene was amplified from S. flexneri 6 genomic DNA with primer pair OspZCF/OspZCR and ligated into BamHI/SalI-digested pACYC184 to yield complementing vector. To generate TEM1 fusions to NleE, nleE was amplified from EPEC E2348/69 genomic DNA with primer pair NleELacF/NleELacR and ligated into NdeI/EcoRI-digested pCX340 vector. To construct TEM1-fused NleE derivatives, nleEΔ34–52 and nleEΔ49–52 were amplified from pGBKT7-NleEΔ34–52 and pGBKT7-NleEΔ49–52 by PCR using primer pair NleELacF/NleELacR, respectively. The PCR product was inserted into NdeI/EcoRI-digested pCX340 vector. To create GST-NleE and GST-NleE34–52, the coding sequences for NleE and NleE34–52 were amplified using primer pairs NleEGSTF/NleEGSTR and NleE(34–52)GSTF/NleE(34–52)GSTR, respectively. The fragments were digested with EcoRI and BamHI before being ligated into vector pGEX-6P-1. To generate GST-fused NleE49AAAA52, the coding sequence of NleE49AAAA52 was amplified from pEGFP-C2-NleE49AAAA52 with primer pair NleEGSTF/NleEGSTR and inserted into BamHI/EcoRI-digested pGEX-6P-1vector. To create GST fusions to OspZ and OspZ49AAAA52, the coding sequences of OspZ and OspZ49AAAA52 were amplified with primer pair OspZGSTF/OspZGSTR and inserted into BamHI/EcoRI-digested pGEX-6P-1 vector. To generate Gal4 activation domain fusions to TAB3, tab3 was amplified using primer pair TAB3F/TAB3R and inserted into EcoRI/BamHI-digested pGADT7 vector. To create TAB3-FLAG, the coding sequence of TAB3 was amplified from the commercial vector pCMV6-TAB3 by PCR using primer pair TAB33×FLAGF/TAB33×FLAGR and ligated into BamHI/EcoRI-digested pcDNA3. The cDNA for human TAB2 was amplified from a HeLa cDNA library using primer pair TAB2F/TAB2R. The PCR product was ligated into EcoRI/BamHI-digested pGADT7 to generate Gal4 activation domain fusion expression plasmid. The coding sequence of TAB2 was amplified with primer pair TAB2F/TAB2FLAGR and digested with EcoRI and BamHI. The fragment was ligated into pcDNA3 to generate 3×FLAG-fused TAB2. The cDNA of human ZRANB3 was obtained from DNASU (Tempe, AZ). To generate ZRANB3-FLAG, the coding sequence of ZRANB3 was amplified from the commercial vector pDONR201-ZRANB3 by PCR using primer pair ZRANB3F/ZRANB3R. The PCR product was ligated into BstXI/BamHI-digested pcDNA3. All plasmids were verified by DNA sequencing.
Transfection and Immunoprecipitation
HEK293T cells were grown in 10-cm dishes (Corning) and co-transfected with pEGFP-NleE, pGFP-NleE49AAAA52, pEGFP-OspZ, or pEGFP-OspZ49AAAA52 together with pcDNA3-TAB3-3×FLAG, pcDNA3-TAB2–3×FLAG, or pcDNA3-ZRANB3-3×FLAG using FuGENE 6 (Promega, Madison WI). TAB3-FLAG and TAB2-FLAG consistently appear as two bands, which presumably result from the breakdown of overexpressed protein. Immunoprecipitation with anti-FLAG M2 magnetic beads (Sigma-Aldrich) was performed according to the manufacturer's instructions. Briefly, 16–24 h after transfection, cells were washed twice with cold phosphate-buffered saline (PBS) and lysed in 800 μl of cold lysis buffer (50 nm Tris-HCl, pH 7.4, 1 mm EDTA, 150 mm NaCl, 1% Triton X-100). Cell debris was pelleted, and an equal volume of supernatant was collected. 800 μl of cell lysate was applied to equilibrated FLAG M2 beads and incubated on a rotating wheel at 4 °C overnight. Beads were magnetically separated, and the supernatant was kept as non-bound protein. The beads were washed three times with cold lysis buffer, and the protein was eluted with 100 μg ml−1 FLAG peptide (Sigma-Aldrich). The beads were magnetically separated, and the supernatant was subjected to NuPAGE protein gels (Thermo Fisher Scientific). Proteins were transferred onto nitrocellulose membranes and probed by incubating with mouse anti-GFP (Roche Applied Science) or mouse anti-FLAG (Sigma-Aldrich) diluted 1:1000 in TBS (50 mm Tris-HCl, pH 7.5, 150 mm NaCl) supplemented with 5% bovine serum albumin (BSA; Sigma-Aldrich) and 0.05% Tween 20 (Sigma-Aldrich). Proteins were detected using anti-mouse IgG horseradish peroxidase (HRP)-conjugated secondary antibodies diluted 1:3000 in TBS with 5% BSA and 0.05% Tween 20 and developed with enhanced chemiluminescence (ECL) Western blotting reagent (Amersham Biosciences). Proteins were detected using the DNR MF-ChemiBIS Bio Imaging System.
NF-κB Reporter Assay
NF-κB activity was determined by using a Dual-Luciferase Reporter Assay System. Transfection was performed using FuGENE 6. HeLa cells were cultured in DMEM supplemented with 10% FBS, 100 units ml−1 penicillin, and 100 μg ml−1 streptomycin in 5% CO2 at 37 °C. 24 h before transfection, cells were seeded into 24-well trays at a density of 105 cells/well. Cells were transfected with 200 ng of pNF-κB-luc (Clontech), 40 ng of pRL-TK (Promega), and 200 ng of pEGFP derivatives. After 24 h, cells were left untreated or treated with 20 ng ml−1 TNF for another 8 h before assaying the luciferase activation. Firefly and Renilla luciferase levels were detected according to the manufacturer's protocols (Promega part number TM040). Samples were measured on a FLUOstar Omega microplate reader (BMG Labtech).
Site-directed Mutagenesis
The pTrc99A-NleE49AAAA52, pEGFP-NleE49AAAA52, and pEGFP-OspZ49AAAA52 were created using the Stratagene QuikChange Lighting site-directed mutagenesis kit. NleE49AAAA52 and OspZ49AAAA52 were amplified from pTrc99A-NleE, pEGFP-NleE, and pEGFP-OspZ, respectively, by PCR using the primer pair NleE(49AAAA52)F/NleE(49AAAA52)R or OspZ(49AAAA52)F/OspZ(49AAAA52)R. Plasmids were digested with DpnI at 37 °C overnight before transformation into the appropriate E. coli strain.
β-Lactamase Translocation Assay
The secretion and translocation of effector proteins from EPEC were measured using translational fusions to TEM1 β-lactamase (20). 24 h before infection, HeLa cells were seeded in 96-well trays at a density of 4 × 104 cells/well. For infection, EPEC strains were cultured in Luria broth (LB) for ∼16 h at 37 °C. The bacterial cultures were subinoculated 1:50 into DMEM supplemented with 5% HEPES and incubated at 37 °C with 5% CO2. 2.5 h later, the bacterial cultures were induced with isopropyl β-d-thiogalactopyranoside for 30 min at a final concentration of 1 mm. In the meantime, cell monolayers were washed three times with PBS and covered with 100 μl of DMEM plus 10% HEPES and 20 μl of 6× CCF2/AM loading solution prepared from the CCF2/AM kit (Invitrogen). The cells were incubated at room temperature for 1 h in darkness and then infected with 100 μl of induced bacterial culture (A600 = 0.2) for 2 h at 37 °C with 5% CO2. The blue emission fluorescence (450 nm) and green emission fluorescence (520 nm) were measured on a FLUOstar Omega microplate reader, and the translocation signal was shown as the ratio between blue emission fluorescence and green fluorescence.
Detection of IκB Degradation by Immunoblotting
HeLa cells were seeded into 24-well trays at a density of 105 cells/well 24 h before infection. Wild type EPEC and derivatives were cultured in LB for 16 h before being subinoculated 1:50 into DMEM for another 3-h incubation at 37 °C with 5% CO2. The bacterial cultures were induced with isopropyl β-d-thiogalactopyranoside at a final concentration of 1 mm during the last 30 min of incubation. HeLa cells were infected with EPEC derivative cultures (A600 = 0.02) for 2 h after which the cells were washed three times with PBS and the medium was replaced with DMEM supplemented with 100 μg ml−1 gentamicin. Cells were incubated at 37 °C with 5% CO2 for 30 min with or without the stimulation with TNF at 20 ng ml−1. 20 μl of cell lysate was loaded onto NuPAGE protein gels followed by transfer onto nitrocellulose membranes. Proteins were probed with mouse polyclonal anti-IκBα (Cell Signaling Technology) diluted 1:1000 in TBS with 5% BSA and 0.05% Tween 20 or mouse monoclonal anti-β-actin (AC-15) (Sigma-Aldrich) diluted 1:5000 in TBS with 5% BSA and 0.05% Tween 20. Proteins were detected using anti-mouse IgG HRP-conjugated secondary antibodies diluted 1:3000 in TBS with 5% BSA and 0.05% Tween 20 and developed with ECL Western blotting reagent.
IL-8 Secretion Assay
HeLa cells were seeded into 24-well trays at a density of 105 cells/well 24 h before infection. Wild type EPEC and derivatives were cultured in LB 16 h before being subinoculated 1:50 into DMEM for another 3-h incubation at 37 °C with 5% CO2. The bacterial cultures were induced with isopropyl β-d-thiogalactopyranoside at a final concentration of 1 mm during the last 30 min of incubation. HeLa cells were infected with EPEC derivatives cultures (A600 = 0.02) for 2 h after which the medium was replaced with DMEM supplemented with 100 μg ml−1 gentamycin with or without 20 ng ml−1 TNF. Cell culture supernatant was collected for analysis of IL-8 secretion after 8-h incubation at 37 °C with 5% CO2. IL-8 secretion was measured using the human IL-8 ELISA kit (Biolegend) according to the manufacturer's instruction.
Affinity Purification
GST-tagged proteins were purified using the GST·Bind kit (Novagen) according to the manufacturer's instructions. Purified GST-tagged proteins (80 μg) were dialyzed against TBS before being immobilized onto 25 μl of GST·Bind resin in 500 μl of binding buffer. The resin was incubated with 1 ml of TAB3-FLAG-transfected HEK293T cell lysate for 2 h at 4 °C. The bound proteins were eluted with GST elution buffer and analyzed by immunoblotting.
Stable Cell Line Generation
HeLa cells were grown in 10-cm dishes and transfected with pEGFP-NleE34–52. 48 h later, cells were split into fresh growth medium (DMEM supplemented with 10% fetal calf serum) containing 400 μg ml−1 G418. To obtain stable transfectants, cells were selected with medium containing G418 for 2 weeks, and the culture medium was changed every 3 days during selection. Single colonies were selected and seeded into 24-well trays. The expression of GFP-NleE34–52 in G418-resistant cells was detected by immunoblotting and immunofluorescence microscopy.
Immunofluorescence Microscopy
Untransfected HeLa cells or G418-resistant HeLa cells were fixed in 4% (w/v) formaldehyde (Sigma) in PBS for 20 min at room temperature. After a 20-min blocking in PBS with 0.5% (w/v) BSA, 0.1% (w/v) saponin, and 1% (v/v) fetal calf serum, samples were incubated with mouse anti-GFP antibody. Antibody was used at 1:200 (v/v) diluted in blocking solution for 1 h at room temperature. Alexa Fluor 568 (Invitrogen)-conjugated anti-mouse antibody was used at 1:2000 (v/v) in blocking solution for 1 h in the dark. Following 10 min of incubation in PBS containing 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen), coverslips were mounted onto microscope slides. Images were acquired using a Zeiss confocal laser scanning microscope with a 100×/EC Epiplan-Apochromat oil immersion objective.
Construction of Deletion Mutants in S. flexneri 6
For construction of the S. flexneri 6 ospZ mutant, the ospZ gene was disrupted using the λ Red recombinase system in wild type S. flexneri 6. Briefly, the upstream and downstream flanking regions of the gene ospZ were amplified from the genomic DNA of S. flexneri 6 using primer pairs ΔospZUPF/ΔospZUPR and ΔospZDNF/ΔospZDNR. The kanamycin resistance cassette was amplified from pKD4 using the primers ΔospZF and ΔospZR. The flanking region of OspZ and the kanamycin resistance gene were assembled by doing overlap PCR. PCR products were digested using DpnI and electroporated into S. flexneri 6 carrying the Red recombinase expression plasmid, pKD46. Mutants were selected from LB plate supplemented with kanamycin and chloramphenicol. The replacement of ospZ with kanamycin resistance gene was confirmed by sequencing.
S. flexneri 6 Infection and IL-8 Assay
For Shigella infection, HT-29 cells were seeded into a 24-well plate at a density of 105 cells/well in RPMI 1640 medium supplemented with FCS, 100 units ml−1 penicillin, and 100 μg ml−1 streptomycin in 5% CO2 at 37 °C 48 h before infection. Wild type S. flexneri 6 strain and derivatives were cultured for ∼16 h at 37 °C in LB supplemented with kanamycin (100 μg ml−1) or chloramphenicol (25 μg ml−1) when necessary. HT-29 monolayers were washed three times with PBS and then infected at a multiplicity of infection of 100. Infection was synchronized by centrifuging the plate at 1000 rpm for 10 min. After incubation for 15 min at 37 °C in 5% CO2, the plates were washed three times with PBS and transferred into fresh RPMI 1640 medium containing gentamicin (100 μg ml−1) with or without 20 ng ml−1 TNF for indicated time in 5% CO2 at 37 °C. Shigella-infected cells were washed three times with PBS and transferred into fresh RPMI 1640 medium containing 100 μg ml−1 gentamicin for 2 h in 5% CO2 at 37 °C. Cell culture supernatant was collected for analysis of IL-8 secretion. IL-8 secretion was measured using the human IL-8 ELISA kit according to the manufacturer's instruction.
RT-PCR Analysis
Shigella-infected HT-29 cells were washed three times with PBS and transferred into fresh RPMI 1640 medium containing 100 μg ml−1 gentamicin for 1 h in 5% CO2 at 37 °C. Total RNA was isolated from Shigella-infected HT-29 cells using TRIsure (Bioline). After treatment with DNase I (Ambion), total RNA (1 μg) was transcribed into cDNA with a iScript cDNA synthesis kit (Bio-Rad) in a reaction volume of 20 μl. After cDNA synthesis, the reaction mixture was diluted 1:10, and 2 μl was utilized for quantitative RT-PCR measurement using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad). Relative mRNA levels were calculated, and data were normalized relative to 18S using primer pairs IL-8F/IL-8R and 18SF/18SR. The equation -fold change = 2−ΔΔCt was applied to calculate relative expression. All measurements were done in duplicate, and the experiments were repeated three times.
In Vitro Methylation Using [3H]AdoMet
HEK293T cells were grown in 10-cm tissue culture dishes and transfected with pcDNA3-TAB2–3×FLAG, pcDNA3-TAB3–3×FLAG, or pcDNA3-ZRANB3–3×FLAG. Transfected cells were lysed the following day, and the ectopically expressed proteins were immunopurified with anti-FLAG M2 magnetic beads. To detect the methylation of TAB2/TAB3/ZRANB3 by NleE or OspZ, immunopurified TAB2/TAB3/ZRANB3 was incubated with or without 200 ng of recombinant purified GST-NleE, GST-NleE49AAAA52, GST-OspZ, or GST-OspZ49AAAA52 at 37 °C for 2 h in the presence of 0.55 μCi of [3H]AdoMet (PerkinElmer Life Sciences). 4× lithium dodecyl sulfate sample buffer was added to the samples and heated at 70 °C for 10 min. Samples were subjected to NuPAGE protein gels and subsequently detected by 3H autoradiography.
Author Contributions
Y. Z. conducted most of the experiments, analyzed the results, and wrote the paper. S. M., C. V. O., and J. S. P. assisted with experiments and analysis of results. E. L. H. conceived the idea for the project, assisted with the analysis of results, and co-wrote the paper with Y. Z.
This work was supported in part by Australian National Health and Medical Research Council Grant APP606788 (to E. L. H.). The authors declare that they have no conflicts of interest with the contents of this article.
- EPEC
- enteropathogenic E. coli
- T3SS
- type III secretion system
- A/E
- attaching and effacing
- Nle
- non-locus of enterocyte effacement-encoded
- AdoMet
- S-adenosyl-l-methionine
- NZF
- Npl4 zinc finger
- TAB
- TAK1-binding protein
- EGFP
- enhanced GFP
- TRAF
- TNF receptor-associated factor
- LB
- Luria broth.
References
- 1. Wong A. R., Pearson J. S., Bright M. D., Munera D., Robinson K. S., Lee S. F., Frankel G., and Hartland E. L. (2011) Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Mol. Microbiol. 80, 1420–1438 [DOI] [PubMed] [Google Scholar]
- 2. Frankel G., and Phillips A. D. (2008) Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal. Cell. Microbiol. 10, 549–556 [DOI] [PubMed] [Google Scholar]
- 3. Baruch K., Gur-Arie L., Nadler C., Koby S., Yerushalmi G., Ben-Neriah Y., Yogev O., Shaulian E., Guttman C., Zarivach R., and Rosenshine I. (2011) Metalloprotease type III effectors that specifically cleave JNK and NF-κB. EMBO J. 30, 221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mühlen S., Ruchaud-Sparagano M. H., and Kenny B. (2011) Proteasome-independent degradation of canonical NFκB complex components by the NleC protein of pathogenic Escherichia coli. J. Biol. Chem. 286, 5100–5107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nadler C., Baruch K., Kobi S., Mills E., Haviv G., Farago M., Alkalay I., Bartfeld S., Meyer T. F., Ben-Neriah Y., and Rosenshine I. (2010) The type III secretion effector NleE inhibits NF-κB activation. PLoS Pathog. 6, e1000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newton H. J., Pearson J. S., Badea L., Kelly M., Lucas M., Holloway G., Wagstaff K. M., Dunstone M. A., Sloan J., Whisstock J. C., Kaper J. B., Robins-Browne R. M., Jans D. A., Frankel G., Phillips A. D., et al. (2010) The type III effectors NleE and NleB from enteropathogenic E. coli and OspZ from Shigella block nuclear translocation of NFκB p65. PLoS Pathog. 6, e1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pearson J. S., Riedmaier P., Marchès O., Frankel G., and Hartland E. L. (2011) A type III effector protease NleC from enteropathogenic Escherichia coli targets NF-κB for degradation. Mol. Microbiol. 80, 219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Royan S. V., Jones R. M., Koutsouris A., Roxas J. L., Falzari K., Weflen A. W., Kim A., Bellmeyer A., Turner J. R., Neish A. S., Rhee K. J., Viswanathan V. K., and Hecht G. A. (2010) Enteropathogenic E. coli non-LEE encoded effectors NleH1 and NleH2 attenuate NF-κB activation. Mol. Microbiol. 78, 1232–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruchaud-Sparagano M. H., Mühlen S., Dean P., and Kenny B. (2011) The enteropathogenic E. coli (EPEC) Tir effector inhibits NF-κB activity by targeting TNFα receptor-associated factors. PLoS Pathog. 7, e1002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yen H., Ooka T., Iguchi A., Hayashi T., Sugimoto N., and Tobe T. (2010) NleC, a type III secretion protease, compromises NF-κB activation by targeting p65/RelA. PLoS Pathog. 6, e1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li S., Zhang L., Yao Q., Li L., Dong N., Rong J., Gao W., Ding X., Sun L., Chen X., Chen S., and Shao F. (2013) Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature 501, 242–246 [DOI] [PubMed] [Google Scholar]
- 12. Pearson J. S., Giogha C., Ong S. Y., Kennedy C. L., Kelly M., Robinson K. S., Lung T. W., Mansell A., Riedmaier P., Oates C. V., Zaid A., Mühlen S., Crepin V. F., Marches O., Ang C. S., et al. (2013) A type III effector antagonizes death receptor signalling during bacterial gut infection. Nature 501, 247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sham H. P., Shames S. R., Croxen M. A., Ma C., Chan J. M., Khan M. A., Wickham M. E., Deng W., Finlay B. B., and Vallance B. A. (2011) Attaching and effacing bacterial effector NleC suppresses epithelial inflammatory responses by inhibiting NF-κB and p38 mitogen-activated protein kinase activation. Infect. Immun. 79, 3552–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giogha C., Lung T. W., Mühlen S., Pearson J. S., and Hartland E. L. (2015) Substrate recognition by the zinc metalloprotease effector NleC from enteropathogenic Escherichia coli. Cell. Microbiol. 17, 1766–1778 [DOI] [PubMed] [Google Scholar]
- 15. Arbibe L., Kim D. W., Batsche E., Pedron T., Mateescu B., Muchardt C., Parsot C., and Sansonetti P. J. (2007) An injected bacterial effector targets chromatin access for transcription factor NF-κB to alter transcription of host genes involved in immune responses. Nat. Immunol. 8, 47–56 [DOI] [PubMed] [Google Scholar]
- 16. Zhang L., Ding X., Cui J., Xu H., Chen J., Gong Y. N., Hu L., Zhou Y., Ge J., Lu Q., Liu L., Chen S., and Shao F. (2012) Cysteine methylation disrupts ubiquitin-chain sensing in NF-κB activation. Nature 481, 204–208 [DOI] [PubMed] [Google Scholar]
- 17. Wickham M. E., Lupp C., Vázquez A., Mascarenhas M., Coburn B., Coombes B. K., Karmali M. A., Puente J. L., Deng W., and Finlay B. B. (2007) Citrobacter rodentium virulence in mice associates with bacterial load and the type III effector NleE. Microbes Infect. 9, 400–407 [DOI] [PubMed] [Google Scholar]
- 18. Zurawski D. V., Mumy K. L., Badea L., Prentice J. A., Hartland E. L., McCormick B. A., and Maurelli A. T. (2008) The NleE/OspZ family of effector proteins is required for polymorphonuclear transepithelial migration, a characteristic shared by enteropathogenic Escherichia coli and Shigella flexneri infections. Infect Immun. 76, 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yao Q., Zhang L., Wan X., Chen J., Hu L., Ding X., Li L., Karar J., Peng H., Chen S., Huang N., Rauscher F. J. 3rd, and Shao F. (2014) Structure and specificity of the bacterial cysteine methyltransferase effector NleE suggests a novel substrate in human DNA repair pathway. PLoS Pathog. 10, e1004522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charpentier X., and Oswald E. (2004) Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 β-lactamase as a new fluorescence-based reporter. J. Bacteriol. 186, 5486–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruchaud-Sparagano M. H., Maresca M., and Kenny B. (2007) Enteropathogenic Escherichia coli (EPEC) inactivate innate immune responses prior to compromising epithelial barrier function. Cell. Microbiol. 9, 1909–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Machado A. M., Figueiredo C., Seruca R., and Rasmussen L. J. (2010) Helicobacter pylori infection generates genetic instability in gastric cells. Biochim. Biophys. Acta 1806, 58–65 [DOI] [PubMed] [Google Scholar]
- 23. Levine M. M., Bergquist E. J., Nalin D. R., Waterman D. H., Hornick R. B., Young C. R., and Sotman S. (1978) Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet 1, 1119–1122 [DOI] [PubMed] [Google Scholar]
- 24. Zeman M. K., and Cimprich K. A. (2012) Finally, polyubiquitinated PCNA gets recognized. Mol. Cell 47, 333–334 [DOI] [PubMed] [Google Scholar]
- 25. Weston R., Peeters H., and Ahel D. (2012) ZRANB3 is a structure-specific ATP-dependent endonuclease involved in replication stress response. Genes Dev. 26, 1558–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dutta U., Garg P. K., Kumar R., and Tandon R. K. (2000) Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am. J. Gastroenterol. 95, 784–787 [DOI] [PubMed] [Google Scholar]