Abstract
In this issue of Molecular Cell, Dango and Mosammaparast et al. discover that the human oxidative demethylase ALKBH3 functions in complex with a DNA helicase to eliminate N3-methylcytosine lesions from ssDNA, and that specific cancer cell lines are dependent on this activity for proliferation.
The endogenous methyl donor, S-adenosylmethionine, and exogenous alkylating agents react non-enzymatically with DNA nucleobases to generate toxic and mutagenic alkyl adducts, which lead to heritable diseases and genome instability. Particularly deleterious are modifications to Watson-Crick base-pairing positions since they can interfere with replication, transcription and translation. The most common of these lesions are N1-methyladenine (1mA) and N3-methylcytosine (3mC), which in humans are repaired mainly by two demethylases: ALKBH2 and ALKBH3 (Sedgwick et al., 2007). The mechanisms by which these enzymes integrate into cellular DNA transactions to maintain integrity of the genome are largely unknown. In this issue of Molecular Cell, Dango and Mosammaparast et al. discover that repair of 3mC in vivo depends on a collaboration between ALKBH3 and a DNA helicase, and establish an important link between overexpression of ALKBH3 and cancer (Dango et al., 2011).
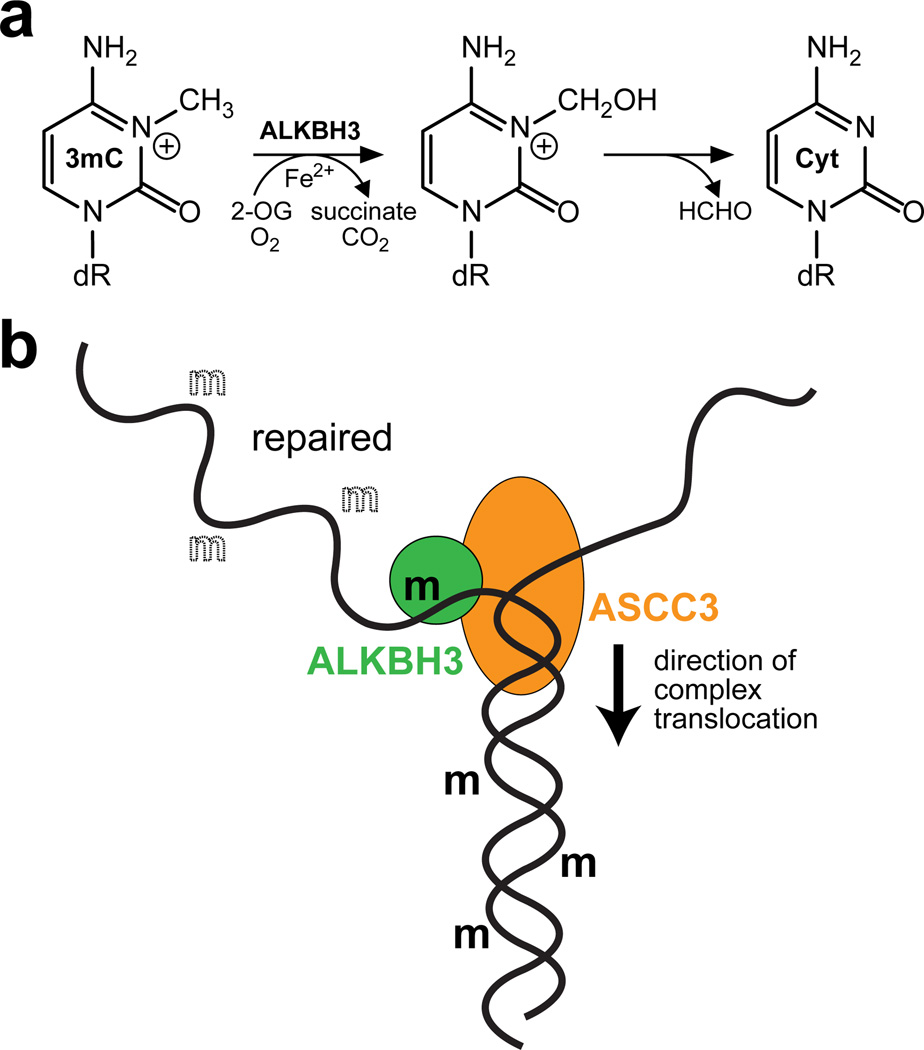
A decade ago, E. coli AlkB was found to repair 1mA and 3mC by oxidative demethylation, an error-free, single-step enzymatic reaction that removes the methyl group to restore the original base (Figure 1a) (Falnes et al., 2002; Trewick et al., 2002). AlkB homologues (ALKBH) were subsequently found in multicellular organisms, underscoring the fundamental importance of this repair mechanism for nucleic acid homeostasis (Sedgwick et al., 2007). Humans contain 9 ALKBH genes, three of which—ALKBH1, ALKBH2 and ALKBH3—are known to perform in vitro DNA demethylation analogous to AlkB. Whereas ALKBH2 is specific for dsDNA, ALKBH3 has a preference for single-strand templates and is the only oxidative demethylase known to repair both DNA and RNA substrates with similar efficiency in vitro (Sedgwick et al., 2007). Importantly, alterations in expression of ALKBH2, 3 and 8 have been described in diverse tumors, and ALKBH2 and ALKBH3 expression has been linked to positive effects on proliferation of tumor-derived cell lines, providing indirect evidence for a possible role of these genes in DNA homeostasis (Gao et al., 2011; Tasaki et al., 2011). In mice, however, ALKBH3 appeared to be dispensable for 1mA and 3mC repair, questioning the role of this enzyme in genome maintenance (Ringvoll et al., 2006).
Repair of N3-methylcytosine (3mC) by human ALKBH3. a, ALKBH3 catalyzes the oxidative demethylation of 3mC. In an Fe(II)-dependent reaction, the N3-methyl group is hydroxylated by molecular oxygen to yield an N3-hydroxymethyl intermediate that is spontaneously released as formaldehyde to produce cytosine (Cyt) base. A 2-oxoglutarate (2-OG) co-substrate is converted to succinate and carbon dioxide in the process. b, ALKBH3 repairs 3mC lesions (bold m) from ssDNA by forming a complex with the ASCC3 helicase. 3mC is frequently found in dsDNA, which is unwound by ASCC3 to provide ALKBH3 with its preferred ssDNA substrate. In the figure, a clear m denotes 3mC lesions that have been demethylated to cytosine.
In an effort to address the relevance of ALKBH3 in mammalian cells, Dango and Mosammaparast et al. make the significant discovery that ALKBH3 physically associates with a 3′–5′ DNA helicase known as the Activating Signal Co-integrator Complex Subunit 3 (ASCC3), and show that ASCC3’s DNA unwinding activity provides the ssDNA substrate for ALKBH3 (Figure 1b) (Dango et al., 2011). Coupling ALKBH3 repair and DNA unwinding activities enables ALKBH3 to process 3mC within double-stranded regions of DNA. The ALKBH3-ASCC3 complex, like those of other DNA repair enzymes, may also serve to recruit additional repair factors to sites of DNA damage or to establish crosstalk between multiple pathways involved in genome maintenance. In fact, ALKBH2 was previously shown to associate with PCNA (Gilljam et al., 2009), suggesting that partnership with proteins involved in DNA homeostasis may be a general feature of the ALKBH family.
Interestingly, the ASCC complex was previously characterized as a transcription co-activator of nuclear receptors, raising the possibility that ALKBH3 demethylation may be coupled to transcription and/or chromatin remodeling. Indeed, the superfamily 2 (SF2)-type helicases to which ASCC3 belongs are typically involved in chromatin remodeling through their ATP-dependent DNA translocase activities. It is interesting to note that E. coli AlkB has been postulated to play a role in transcription-coupled DNA repair (TCR), which is initiated by the multifunctional SF2 helicase, Mfd (Wrzesinski et al., 2010). It is tantalizing to speculate that AlkB also functions in conjunction with a helicase given its substrate preference for ssDNA. Furthermore, while the ALKBH3-ASCC3 interaction nicely explains the preference of ALKBH3 for ssDNA, the rationale for its activity toward RNA substrates is not as clear given the generally high turnover rate of RNA. A link to transcription may serve to explain this dilemma.
In the current work, knockdown of either ALKBH3 or ASCC3 in specific tumor cell lines led to an increase in 3mC adducts and to the formation of γH2AX and 53BP1 foci, consistent with activation of the DNA damage response. Most significantly, loss of ALKBH3 and ASCC3 significantly reduced cell proliferation in these cell lines (Dango et al., 2011). This observation suggests that ALKBH3 acts as a “gatekeeper” to prevent 3mC accumulation in tumors and raises the exciting possibility that inhibition of ALKBH3, either alone or in combination with DNA damaging agents, may be of therapeutic value for cancer treatment. In contrast, loss of ALKBH2 in gastric tumors was reported by another study to result in increased proliferation (Gao et al., 2011), suggesting an additional role of ALKBH2 as a replication checkpoint, possibly through its interaction with PCNA.
The present work proves that generation of 3mC from endogenous sources can limit cell proliferation, in agreement with the observation that 3mC is generally the preferred substrate in prokaryotic AlkB genes (van den Born et al., 2009). Strong alkylating agents currently in use in chemotherapy preferentially target purine N3 and N7 positions in dsDNA, whereas the base-pairing positions in dsDNA are shielded from alkylation (Sedgwick et al., 2007). New methylating agents favoring the generation of 3mC in ssDNA could potentially be developed to exploit the ALKBH3 dependence of tumors described here. This dependence, however, is restricted to a small subset of tumors that show ALKBH3 and ASCC3 overexpression, and thus new therapeutic approaches based on 3mC cytotoxicity will likely need to be customized to individual tumors showing ALKBH3 dependence. Tumor profiling may be facilitated by the availability of 3mC-specific antibodies and expression markers described in this work. It will be of interest to uncover the mechanisms responsible for the tumor-type specificity of ALKBH3 dependence and for the associated ALKBH3 and ASCC3 up-regulation, as these represent additional potential targets for therapeutic intervention and tumor stratification.
In summary, this article greatly advances our understanding of the role of ALKBH enzymes by furnishing a mechanistic understanding of ALKBH3 function in vivo, in the context of both genome maintenance and transformation. This work also has important translational implications, identifying new, potential therapeutic approaches for the treatment of cancer and providing tools to stratify tumors according to 3mC repair status.
Selected Reading
- Dango S, Mosammaparast N, Sowa ME, Xiong L-J, Wu F, Park K, Rubin M, Gygi S, Harper JW, Shi Y. DNA unwinding by ASCC3 helicase is coupled to ALKBH3 dependent DNA alkylation repair and cancer cell proliferation. Mol Cell. 2011 doi: 10.1016/j.molcel.2011.08.039. XXX, XX-XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falnes PO, Johansen RF, Seeberg E. AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli. Nature. 2002;419:178–182. doi: 10.1038/nature01048. [DOI] [PubMed] [Google Scholar]
- Gao W, Li L, Xu P, Fang J, Xiao S, Chen S. Frequent down-regulation of hABH2 in gastric cancer and its involvement in growth of cancer cells. J Gastroenterol Hepatol. 2011;26:577–584. doi: 10.1111/j.1440-1746.2010.06531.x. [DOI] [PubMed] [Google Scholar]
- Gilljam KM, Feyzi E, Aas PA, Sousa MM, Muller R, Vagbo CB, Catterall TC, Liabakk NB, Slupphaug G, Drablos F, et al. Identification of a novel, widespread, and functionally important PCNA-binding motif. J Cell Biol. 2009;186:645–654. doi: 10.1083/jcb.200903138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringvoll J, Nordstrand LM, Vagbo CB, Talstad V, Reite K, Aas PA, Lauritzen KH, Liabakk NB, Bjork A, Doughty RW, et al. Repair deficient mice reveal mABH2 as the primary oxidative demethylase for repairing 1meA and 3meC lesions in DNA. Embo J. 2006;25:2189–2198. doi: 10.1038/sj.emboj.7601109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick B, Bates PA, Paik J, Jacobs SC, Lindahl T. Repair of alkylated DNA: recent advances. DNA Repair (Amst) 2007;6:429–442. doi: 10.1016/j.dnarep.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Tasaki M, Shimada K, Kimura H, Tsujikawa K, Konishi N. ALKBH3, a human AlkB homologue, contributes to cell survival in human non-small-cell lung cancer. Br J Cancer. 2011;104:700–706. doi: 10.1038/sj.bjc.6606012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewick SC, Henshaw TF, Hausinger RP, Lindahl T, Sedgwick B. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature. 2002;419:174–178. doi: 10.1038/nature00908. [DOI] [PubMed] [Google Scholar]
- van den Born E, Bekkelund A, Moen MN, Omelchenko MV, Klungland A, Falnes PO. Bioinformatics and functional analysis define four distinct groups of AlkB DNA-dioxygenases in bacteria. Nucleic Acids Res. 2009;37:7124–7136. doi: 10.1093/nar/gkp774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzesinski M, Nieminuszczy J, Sikora A, Mielecki D, Chojnacka A, Kozlowski M, Krwawicz J, Grzesiuk E. Contribution of transcription-coupled DNA repair to MMS-induced mutagenesis in E. coli strains deficient in functional AlkB protein. Mutat Res. 2010;688:19–27. doi: 10.1016/j.mrfmmm.2010.02.005. [DOI] [PubMed] [Google Scholar]