Abstract
Context
Worldwide studies show that the type 1 diabetes (T1D) incidence is increasing by 3% annually.
Objectives
We investigated the recent incidence of T1D in a US Midwestern county to determine whether this increase has been sustained and compare to celiac disease (CD) incidence. The prevalence of (CD), an associated autoimmune disease, within the cohort was also investigated.
Design
A broad search strategy was used to identify all cases of T1D in Olmsted County, Minnesota, between January 1,1994 and December 31, 2010 using the Rochester Epidemiology Project. Diagnosis and residency status were confirmed through the medical record. Incidence rates were directly standardized to the 2010 United States population. Poisson regression was used to test for a change in incidence rate. Clinical charts were reviewed to confirm case status.
Setting
Population-based study in Olmsted County, Minnesota.
Main Outcome Measure
The trend in T1D incidence in a population-based study in a Midwestern US county.
Results
There were 233 incident cases of T1D. Directly adjusting for age and sex with respect to the 2010 US white population, the overall annual incidence of T1D was 9.2 (95% CI, 8.0-10.4) per 100,000 people per year among all ages and 19.9 (95% CI, 16.6-23.2) per 100,000 per people per year for those younger than 20 years. There was no significant increase in the incidence of T1D over time (P=.45). Despite the overall stability in annual incidence, there was an initial increasing trend followed by a plateau. Of the 109 T1D patients (46%) tested for CD, 12% had biopsy-proven CD.
Conclusions
The incidence of T1D has stopped increasing in Olmsted County, Minnesota, in the most recent decade. Further studies are needed to confirm this finding and explore reasons for this plateau.
Keywords: type 1 diabetes, incidence, celiac disease
Recent studies describe an increasing incidence of T1D by an average of 3% annually worldwide (1), along with increases in various populations with varying degrees of genetic susceptibility. The rates at which the incidence is increasing vary depending on geographic location (1): incidence rates ranged from 20.0 to 57.2 per 100,000 in Sweden from 2006 to 2011 (2), whereas China had the lowest reported incidence rate of 3.0 per 100,000 but also saw the mean incidence rate increase by about 14% annually from 1997 to 2011 (3). Some, but not all, studies show sex differences in incident cases. One difficulty in these reports is the varying eligibility criteria for populations enrolled in studies. Varying ages and age cutoffs have been used. Numerous European studies have used an age cutoff of <15 years (4, 5, 6) while the US used age < 20 years (5). Population-based studies are difficult to perform in the United States because of the lack of population databases. One large US study showed the incidence of T1D to increase from 2002 to 2009 (7).
The environmental drivers behind the rising incidence remain unclear. To date, many theories implicate infectious causes (8), improved sanitation, widespread use of antibiotics, increased cesarean deliveries (9), low vitamin D levels (10), and increased gluten consumption and timing of its introduction (11, 12).
The relationship among T1D incidence, gluten exposure, and comorbid celiac autoimmunity is of interest for several reasons. Patients with T1D are at increased risk for CD, another common immune-mediated disease, characterized by destruction of small bowel mucosa with gluten exposure (13, 14, 15). The incidence of CD also has been rapidly increasing globally at all ages (16, 17) and is most likely driven by some potent environmental factor(s) interacting with similar human genetic susceptibilities. A closer relationship between these diseases was recently suggested; detection of CD and early treatment with mass screening have decreased the subsequent incidence of T1D in children (18).
In this study, we analyzed the annual incidence of T1D in Olmsted County, Minnesota, from January 1,1994 to December 31, 2010 by sex and age to determine trends in incidence, determine the proportion of the cohort with CD, and compare the T1D incidence trends to previously reported Olmsted County CD incidence trends (16).
Methods
This study was approved by the institutional review boards of Mayo Clinic and Olmsted Medical Center, Rochester, Minnesota. The Rochester Epidemiology Project (REP) is a database that allows for population-based studies in Olmsted County, Minnesota (19). According to the 2010 US census, there were 144,248 Olmsted County residents. Two health systems, Mayo Clinic and Olmsted Medical Center, provide almost all the medical care in the county, including outpatient, inpatient, and emergency department settings. All residents of the county who receive any care at either institution are entered into the REP database, which links medical charts and indexes demographics, diagnoses, surgical interventions, and medications. The reliability and validity of the REP have been described elsewhere (19).
The diagnosis and classification of diabetes has changed over the past 60 years, transitioning from classification by treatment method to classification by clinical and etiologic grounds (20). Because of these changes and subsequent potential for incorrect coding (eg, diabetes mellitus or T2D rather than T1D), a broad search was first performed through several databases to identify incident cases of T1D from 1994 through 2010. The REP database served as the main database to identify patients using the terms “diabetes” and “insulin use” within 1 year of diabetes diagnosis. In addition, the REP database was searched using diagnosis codes 250.01, 250.03, 250.1, 250.11, 250.13, 250.33, 250.91, and 250.93. The lists were merged, and the charts of identified patients were then reviewed. Patients who received oral hypoglycemic agents for at least 1 year were excluded.
Mayo Clinic Life Science Systems Advanced Cohort Explorer and prior studies (13, 21) were also used to identify any residual incident cases of T1D that may have been overlooked in the electronic search in Olmsted County. The Advanced Cohort Explorer is a search engine that allows for rapid searching of text within laboratory results, all aspects of clinical notes including the past medical history, problem lists, and diagnostic codes in the clinical notes of the electronic medical record system at Mayo Clinic. The corresponding demographic data are also available. For this database, the terms “type 1 diabetes” and “type 1” AND “diabetes” were searched within the past medical history, primary diagnosis, diagnoses, and secondary diagnoses clinical notes sections from 1994 through 2010. These lists were then merged to remove redundancy.
All identified charts were then reviewed individually to exclude patients with diabetes other than T1D in accordance with the American Diabetes Association's classification and diagnosis of diabetes (20). T1D was defined as ketosis (ketoacidosis or greater than ketonuria), catabolic symptoms at diagnosis, insulin use from diagnosis and continued use at 1 year, and no use of oral hypoglycemic medications for longer than 1 year. Some young adults may have an inaccurate diagnosis of T2D, but after subsequent failure of oral hypoglycemic therapy, they may subsequently be realized to have had T1D (20). These patients were included based on clinical diagnosis by us or results of specific testing (ie, C-peptide or GAD autoantibodies associated with T1D) (20). Patients who did not clearly fit the classification of T1D were included based on clinical review on the basis of glucose variability (intraday hypo- and hyperglycemia documented serving as a surrogate for T1D) documented in the laboratory section of the medical record (22). Patients with development of diabetes after longstanding chronic pancreatitis or pancreatectomy were excluded.
Latent autoimmune diabetes in adults (LADA) was recognized in the 1970s (23) as a form of diabetes that combined features of both T1D and T2D. Although the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus does not distinguish LADA from T1D diabetes, more recent publications suggest that there are genetic, clinical, and laboratory differences between them (20, 24, 25). Thus, patients with LADA were excluded. LADA was defined as presence of a positive glutamic acid decarboxylase antibody, age of onset older than 35 years, and use of oral hypoglycemic agents for at least 6 months. Because these criteria are not sufficient to distinguish LADA from T1D or T2D, additional criteria were also used: nonobese or lean body habitus, less likely than T1D to initially present in ketosis, rapid progression to insulin use, and C-peptide levels higher than expected for T1D yet lower than expected for T2D. If the distinction between T1D and LADA was not as clear, intermediate C-peptide levels were the main determining factor (25).
We also analyzed presence of CD among the identified patients with T1D. CD was defined as positive anti-tissue transglutaminase serologies and endoscopic biopsy with at least partial villous atrophy as defined by modified Marsh grade (26). For those diagnosed with CD in another county, reference to the above criteria within clinical documentation and response to a gluten free diet (normalized anti-tissue transglutaminase antibodies and symptomatic improvement) was regarded as a a CD diagnosis.
For all included patients, Olmsted residency status was confirmed. According to Minnesota law, only patients who consented to participate in research were included in the study. Table 1 displays the methodological approach and identification of T1D incident cases.
Table 1. Methodology of Identifying Type 1 Diabetes Cases in Olmsted County, Minnesota, 1991-2010a.
| Search Strategy | ||
|---|---|---|
| Rochester Epidemiology Project | Mayo Clinic Life Science Systems Advanced Cohort Explorer | Prior Studies |
| Search terms “diabetes” and “insulin use within 1 year of diagnosis” | Search past medical history, primary diagnosis, diagnoses, and secondary diagnoses of clinical documentation for ‘type 1 diabetes’ and ‘type 1’ AND ‘diabetes’ | Participation in Mahmud et al (13) and Dyck et al (21) |
| Search diagnostic codes 250.01, 250.03, 250.1, 250.11, 250.13, 250.33, 250.91, 250.93 | ||
| Confirm Type 1 Diabetes Diagnosis | ||
| Symptoms | Medications | Laboratory tests |
| Ketosis | Insulin use at diagnosis and continued use for 1 year | C-peptide |
| Catabolic symptoms | No use of oral hypoglycemic agents for >1 year | Glucose variability |
| Confirm Residency Status | ||
| Confirm Celiac Disease Diagnosis | ||
An exhaustive search was followed by review of clinical documentation to confirm T1D diagnosis. Those with latent autoimmune diabetes in adults (LADA) were excluded on the basis of recent studies suggesting a difference in pathogenesis and epidemiology between T1D and LADA.
Statistical Analysis
All residents of Olmsted County, Minnesota, with a new diagnosis of T1D between January 1,1994 and December 31, 2010 were counted as incident cases. Incidence rates were the number of cases per age-, sex-, and calendar time-specific strata, divided by the corresponding county population based on the annual census. For age, we focused on age < 20 years and age > 20 years to align with that of the US SEARCH study (7). Age- and sex-adjusted incidence rates were standardized against the 2010 US white population; 95% CI was estimated from the Poisson distribution. Although incidence is described with sex-specific rates over intervals of age and calendar years (for brevity), trends in incidence were analyzed with multivariable Poisson regression using a more granular form of the data based on strata of single years of calendar time and age. The nonparametric Loess method, weighted according to population counts, was used to estimate smoothed incidence trends for time and age. To prevent the type 1 error rate from increasing, we tested 2-way interactions among, time, age, and sex with a likelihood ratio test. Statistical analyses were performed with SAS version 9.3 statistical software (SAS Institute Inc), and P<0.05 was considered statistically significant.
Results
Type 1 Diabetes Incidence
After patient exclusions (including 12 with LADA), 233 Olmsted County, Minnesota, residents (134 male, 99 female) were identified with a new diagnosis of T1D during the 17-year study period. An overwhelming majority (88%) of incident cases were Caucasian (n=200; 6 with unknown race were omitted from this estimate). The median age at diagnosis was 15.6 years (range, 1.4-79.5 years), with the majority of cases (60%) being in those younger than 20 years. The overall incidence rate of T1D was 9.2 (95% CI, 8.0-10.4) cases per 100,000 per year, directly adjusting for age and sex with respect to the 2010 US white population. The age-adjusted rate by sex was 10.5 (95% CI, 8.7-12.3) per 100,000 for males and 7.7 (95% CI, 6.2-9.2) per 100,000 for females. For persons younger than 20 years, the age- and sex-adjusted annual incidence rate of T1D was 19.9 (95% CI, 16.6-23.2) per 100,000: age-adjusted to 23.2 (95% CI, 8.7-12.3) per 100,000 for males and 16.5 (95% CI, 6.2-9.2) per 100,000 for females.
Trends
From 1994 through 2010 the average annual incidence rate was 9.2 (95% CI, 8.0-10.4) per 100,000, directly adjusting for age and sex with respect to the 2010 US white population. There was no linear trend in the annual incidence over the entire study period (P=.45), although some fluctuations in the rates were seen (Figure 1A). In particular, the annual incidence rate increased during the first part of the study, peaking at 15.2/100,000 in 2004, and subsequently decreased. The lowest rates were seen in 1994 (6.2/100,000) and years near the end of the study period (7.0 and 7.6 per 100,000 in 2006 and 2009, respectively). Although not hypothesized a priori, we tested whether this pattern reflected a nonlinear trend as described by a quadratic function of calendar year, but the result was statistically non-significant (P=.06). As in the incidence across all ages, there was no significant linear trend in the incidence of T1D among those younger than 20 years (P=.40) (Figure 1A).
Figure 1.
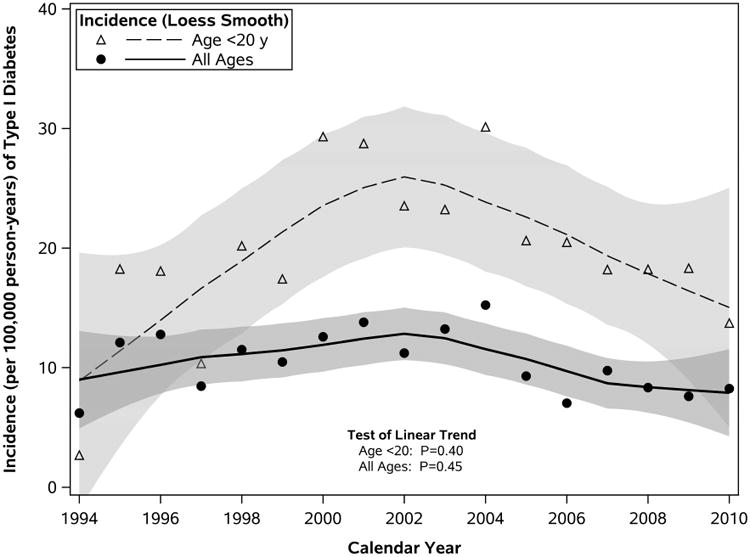
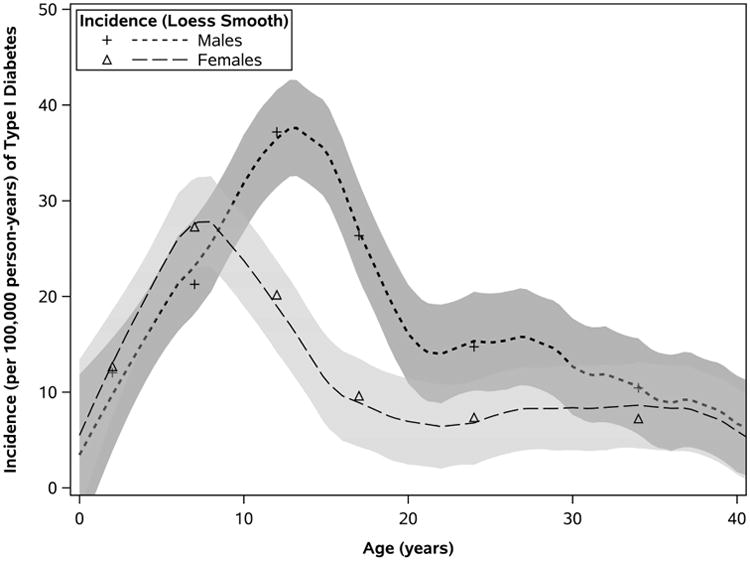
Trends in Incidence of Type 1 Diabetes in Olmsted County, Minnesota, 1994-2010. A, Incidence of T1D overall and in those younger than 20 years. Loess-estimated curves depict smoothed trends in incidence (and 95% confidence bands) from the data defined according to granular strata; symbols reflect annual rates summarized across both sexes and the entire age range. B, T1D incidence by age and sex. Symbols represent sex-specific rates summarized by groupings of age: 0-4, 5-9, 10-14, 15-19, 20-29, and 30-39 years (older age groups were omitted because of very low incidence).
Age and Sex
Incidence of T1D varied with respect to age and sex (Table 2). Incidence was adequately described by a quadratic function of age (P<.001), based on rates increasing over early childhood (0-4 years) and peaking among older children (roughly 5-14 years), then decreasing with age in older adolescents and adults (≥ 15 years). However, this relationship between incidence and age, particularly during childhood, was dependent on sex (test of interaction between quadratic age and sex, P<.001). The early increase in incidence appeared to peak in females just before age 10 years, whereas rates among males continued to increase until mid adolescence (Figure 1B). Expressed by age groups, peak rates of incidence corresponded to females aged 5 to 9 years (27.3/100,000) and to males aged 10 to 14 years (37.2/100,000). The sex-dependent association between incidence and quadratic age were found in all patients younger than 20 years.
Table 2. Incidence of Type 1 Diabetes in Olmsted County, Minnesota, (1994-2010): Incident Cases by Age, Sex, and Calendar Time.
| Variable | Females (n=99) | Males (n=134) | All Patients (N=233) |
|---|---|---|---|
| Age, ya | |||
| 0-4 | 11 (12.7) | 11 (12.0) | 22 (12.4) |
| 5-9 | 22 (27.3) | 18 (21.3) | 40 (24.2) |
| 10-14 | 16 (20.2) | 31 (37.2) | 47 (28.9) |
| 15-19 | 8 (9.6) | 22 (26.4) | 30 (18.0) |
| 20-29 | 14 (7.4) | 24 (14.7) | 38 (10.8) |
| 30-39 | 13 (7.2) | 18 (10.4) | 31 (8.8) |
| ≥40 | 15 (3.3) | 10 (2.5) | 25 (2.9) |
| Yearsb | |||
| 1994-1998 | 7.2 (4.4-10.0) | 9.6 (6.3-12.8) | 8.4 (6.3-10.6) |
| 1999-2002 | 9.8 (6.2-13.4) | 11.1 (7.2-15.0) | 10.5 (7.8-13.1) |
| 2003-2006 | 7.5 (4.4-10.5) | 12.3 (8.4-16.2) | 9.9 (7.5-12.4) |
| 2007-2010 | 6.9 (3.9-9.8) | 8.9 (5.6-12.3) | 8.0 (5.7-10.2) |
| Overallb | |||
| All Agesc | 7.7 (6.2-9.2) | 10.5 (8.7-12.3) | 9.2 (8.0-10.4) |
| Age <20 yd | 16.5 (12.2-20.7) | 23.2 (18.2-28.2) | 19.9 (16.6-23.2) |
Results presented as No. of cases (annual unadjusted incidence rate per 100,000 cases per year).
Results presented as incidence (95% CI) per 100,000 cases per year directly adjusted to the 2010 US white population according to age and sex.
Incidence with respect to an all-age population, based on a total of 233 cases (age range, 1.4-79.5 years).
For reference, incidence was computed for an age-restricted population using the numerator of 139 cases and denominator of age younger than 20 years.
Celiac Disease
Of the 233 incident cases of T1D, 109 patients (46%) underwent serologic testing for CD (eg, anti–tissue transglutaminase antibodies) for various reasons (eg, symptoms, screening, prior research studies). Of those tested (n=109), 13 (12%) ultimately had biopsy-proven CD. Thus, based on the small numbers tested, 5.6% of the cohort has CD.
Discussion
Several findings from our study were unexpected. First, no linear trend was seen in the incidence rate of T1D during January 1, 1994 through December 31, 2010, which is in contrast to the current literature. The incidence during the study years was dynamic, with an initial increase until 2004, followed by a slight decrease in the latter half of the time period (Figure 1). In addition, the peak incidence in T1D occurred during a similar time frame as that of CD incidence in Olmsted County, Minnesota, which suggests a shared environmental trigger beyond genetic predisposition. Diagnostic and clinical characteristics of CD in Olmsted County, Minnesota, have been well described. CD incidence increased in this population from the 1950s until a peak in 2003, remaining stable thereafter (16, 17).
Until now, prior studies showed a worldwide increasing incidence of T1D, which peaked interest in its pathogenesis. The US SEARCH study group (7) estimated the incidence of T1D in those younger than 20 years in different geographic locations, including Washington, California, Colorado, South Carolina, and Ohio, during 2001 through 2004. Through these centers, subjects from Hawaii, New Mexico, and Arizona also participated. Patients were classified as having T1D on the basis of clinical and laboratory data. The study found the incidence to increase from 24.4 to 27.4 per 100,000 during the study years.
The differences in findings between the US SEARCH study and our population-based cohort raise several important points, including the potential effect of geography and the time frame studied. Although prior studies have all noted an increased incidence, the rates of increase varied, and the incidence rates themselves vary significantly worldwide. Others have suggested that geography has a role in T1D incidence (1). These geographic differences are both genetic (eg, HLA subtypes) and environmental. To name a few, degree of a nation's development, antimicrobial use, and gluten ingestion have been implicated as environmental factors. The etiologies at work in our community may differ from those in other countries. Our finding of a nonlinear trend, however, is inconsistent with that of another US study (7). Heterogeneity within the United States is unlikely to be the sole explanation for the differences.
The study period, 1994 to 2010, overlaps with that of the other studies. Thus, it is unlikely that the different results obtained are solely due to differences in the years examined. This unexpected trend should encourage the continued tracking of T1D incidence worldwide, because the previously documented trend may be changing. In addition, the nonlinear trend seen in our study also needs further investigation. The first part of the study revealed a rising trend, followed by a falling trend in the second part. This finding prompts more questions than the overall nonlinear trend in T1D incidence. The question is no longer the lack of increasing incidence but what triggers a shift in the trends. Age may also impact incidence to some extent. One group noted that the T1D incidence increased and those incident cases were younger at diagnosis (4), suggesting that this rise in T1D incidence is greater or more significant within certain subgroups. However, geography and age are unlikely to provide the only answers. We must obtain a broader perspective on factors that may influence T1D incidence, including autoimmune conditions associated with T1D, such as CD.
Comparing the incidence rates of T1D with those of CD in Olmsted County revealed an interesting observation. The peak incidences of the diseases both occurred within a 1-year period. Figure 2 shows the annual incidence of CD superimposed on the overall annual incidence of T1D in Olmsted County, Minnesota (27). Both curves reflect dynamic incidence rates during the study years. Although incidence of T1D was higher than CD over the first few years of the study period, rates appeared to converge during the middle portions (roughly 2000-2002) and peak shortly after that (T1D in 2004, and CD in 2003). Annual incidence of CD surpassed that of T1D in 2003, after which CD rates leveled off yet remained higher compared to the declining rates of T1D over the remainder of the study period. Common environmental factors have potentially influenced the development of these disorders in people with shared genetic risk. The factors may indirectly or directly affect T1D incidence. Our current idea emphasizes the shared genetic predisposition of the two conditions. It is possible, however, that a more direct relationship exists between CD led to a decreased incidence of T1D (18).
Figure 2.
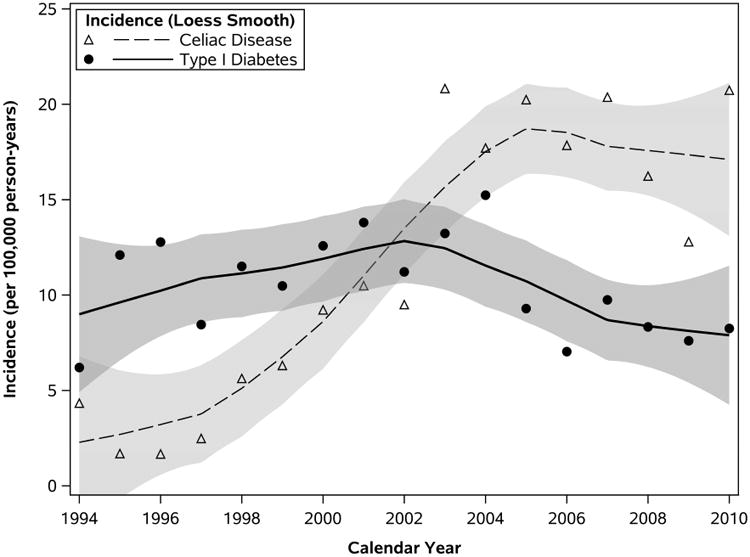
Incidence of Type 1 Diabetes and Celiac Disease in Olmsted County, Minnesota, 1994-2010. Loess-estimated curves depict smoothed trends in incidence (and 95% confidence bands) of T1D (solid line) and CD (dashed line); symbols correspond to year-specific incidence rates summarized across both sexes and all ages.
Risk factors for both T1D and CD are being studied at the animal, genetic, and epidemiologic levels. Current knowledge suggests that a gluten-free diet may affect dendritic cells and the subsequent development of diabetes (28). HLA markers may help determine the risk of CD in patients with T1D (29, 30). The role of perinatal environment and the microbiome is also under examination. Children born by cesarean delivery and during summer months have been reported to be at increased risk for both T1D and CD (31).
The same group noted that females had an increased risk of both T1D and CD (31). Hormones may also have a role, but there is a clear gap in knowledge of the role of sex in development of these related autoimmune conditions. Considering the role of sex in each condition makes its role less clear. Females are more likely than males to have CD, but males and females are almost equally affected by T1D. Furthermore, studies of T1D incidence disagree the most regarding the effect of sex. In our study, a statistically significant increase in T1D incidence was seen only in male patients, a finding not supported by all prior studies. Clinical research teams must consider the intricate relationships among autoimmune diseases as a clue to help identify which factors influence progression of disease.
We recognize the limitations of our study, including clinically defining T1D and residency status. The evolving definition of T1D in the early to mid 1990s limited the study years, precluding the study of incident cases prior to 1994. While residency status is clearly defined for purposes of population-based studies and the REP, it has several practical challenges. Residents of southeastern Minnesota may move in and out of the county during their lifetime, and several towns span Olmsted County lines. Furthermore, while a prior study demonstrated that Olmsted County, Minnesota is representative of the non-Hispanic white US population, we recognize the small sample size and the impact this has on applying our findings to other areas in the US (19). The prevalence of CD cannot be adequately assessed in the cohort due to the low proportion of subjects tested.
Conclusion
Our study, which considered similar years as in prior studies, showed no significant linear trend in T1D incidence between 1994 and 2010. Although a gradual increase in incidence appears evident for roughly the first half of this period, the rates do not continue to increase, and perhaps even decline, during the latter half of the time frame. This observation is in marked contrast to prior studies, which underscores the importance of additional studies, continued monitoring over time, and identification of factors that may have altered the trajectory in incidence trends. The peak incidence of T1D in our study was temporally related to that of CD, which spurs us to further investigate the connection between these genetically and possibly environmentally related autoimmune diseases, especially because the rate of diagnosis per year is higher for T1D than for CD. Identification of such environmental factors can then guide how these environmental triggers are addressed to influence development of T1D.
Acknowledgments
Support: This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also acknowledge NIDDK funding DK85516 (YCK).
Abbreviations
- T1D
type 1 diabetes
- US
United States
- CD
celiac disease
- T2D
type 2 diabetes
- LADA
latent autoimmune diabetes in adults
- REP
Rochester Epidemiology Project
- CI
confidence interval
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
Author Contributions: A.K.C. contributed to data abstraction, manuscript preparation and editing.
L.O. contributed to data abstraction.
B.L. performed statistical analysis and manuscript preparation and editing.
B.Y. representing the Rochester Epidemiology Project.
J.A.M. contributed to study design, manuscript preparation and editing.
Y.C.K. contributed to study design, data abstraction, manuscript preparation and editing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borchers AT. The geoepidemiology of type 1 diabetes. Autoimmun Rev. 2010;9(5):A355–65. doi: 10.1016/j.autrev.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Rawshani A. The incidence of diabetes among 0-34 year olds in Sweden: new data and better methods. Diabetologia. 2014;57(7):1374–81. doi: 10.1007/s00125-014-3225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Z. Rapidly rising incidence of childhood type 1 diabetes in Chinese population: epidemiology in Shanghai during 1997-2011. Acta Diabetol. 2014;51(5):947–53. doi: 10.1007/s00592-014-0590-2. [DOI] [PubMed] [Google Scholar]
- 4.Berhan Y, Waernbaum I, Lind T, Mollsten A, Dahlquist G. Thirty years of prospective nationwide incidence of childhood type 1 diabetes: the accelerating increase by time tends to level off in Sweden. Diabetes. 2011;60(2):577–81. doi: 10.2337/db10-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–33. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 6.Bendas A, Rothe U, Kiess W, Kapellen TM, Stange T, Manuwald U, Salzsieder E, Holl RW, Schoffer O, Stahl-Pehe A, Giani G, Ehehalt S, Neu A, Rosenbauer J. Trends in incidence rates during 1999-2008 and prevalence in 2008 of childhood type 1 diabetes mellitus in Germany—model-based national estimates. PLoS One. 2015;10(7):e0132716. doi: 10.1371/journal.pone.0132716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawrence JM. Trends in incidence of type 1 diabetes among non-Hispanic white youth in the US, 2002-2009. Diabetes. 2014;63(11):3938–45. doi: 10.2337/db13-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hober D, Sauter P. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat Rev Endocrinol. 2010;6(5):2790289. doi: 10.1038/nrendo.2010.27. [DOI] [PubMed] [Google Scholar]
- 9.Stene LC, Gale EAM. The prenatal environment and type 1 diabetes. Diabetologia. 2013;56(9):1888–1897. doi: 10.1007/s00125-013-2929-6. [DOI] [PubMed] [Google Scholar]
- 10.Mäkinen M, Simell V, Mykkänen J, Ilonen J, Veijola R, Hyöty H, Knip M, Simell O, Toppari J, Hermann R. An increase in serum 25-hydroxyvitamin D concentrations preceded a plateau in type 1 diabetes incidence. Journal of Clinical Endocinology and Metabolism. 2014;99(11):E2353–6. doi: 10.1210/jc.2014-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyerlein A, Chmiel R, Hummel S, Winkler C, Bonifacio E, Ziegler AG. Timing of Gluten Introduction and Islet Autoimmunity in Young Children: Updated Results from the BABYDIET Study. Diabetes Care. 2014;37(9):e194–e195. doi: 10.2337/dc14-1208. [DOI] [PubMed] [Google Scholar]
- 12.Norris JM, Barriga K, Hoffenberg EJ, Taki I, Miao D, Haas JE, Emery LM, Sokol RJ, Erlich HA, Eisenbarth GS, Rewers M. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of celiac disease. JAMA. 2005;293(19):2343–2351. doi: 10.1001/jama.293.19.2343. [DOI] [PubMed] [Google Scholar]
- 13.Mahmud FH, Murray JA, El-Youssef M, et al. Celiac disease in type 1 diabetes mellitus in a North American community: prevalence, serologic screening, and clinical features. Mayo Clin Proc. 2005;80(11):1429–1434. doi: 10.4065/80.11.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elfström P, Sundström J, Ludvigsson JF. Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes. Aliment Pharmacol Ther. 2014;40(10):1123–32. doi: 10.1111/apt.12973. [DOI] [PubMed] [Google Scholar]
- 15.Gillett PM, Gillett HR, Israel DM, Metzger DL, Stewart L, Chanoine JP, Freeman HJ. High prevalence of celiac disease in patients with type 1 diabetes detected by antibodies to endomysium and tissue transglutaminase. Can J Gastroenterol. 2001;15(5):297–301. doi: 10.1155/2001/640796. [DOI] [PubMed] [Google Scholar]
- 16.Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ., 3rd Trends in the identification and clinical features of celiac disease in a North American community, 1950-2001. Clin Gastroenterol Hepatol. 2003;1(1):19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 17.Ludvigsson JF, Rubio-Tapia A, Van Dyke CT, Melton LJ, 3rd, Zinsmeister AR, Lahr BD, Murray JA. Increasing incidence of celiac disease in a North American population. Am J Gastroenterol. 2013;108(5):818–824. doi: 10.1038/ajg.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korponay-Szabo IR, Szabados K, Pusztai J, Rózsáné Rigó É, Gyimesi J, Hyöty H, Maki M. Primary prevention of type 1 diabetes mellitus by celiac mass screening in children. Oral Presentation International Celiac Disease Symposium 2015; Prague, CZK. 23 June 2015. [Google Scholar]
- 19.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: Half a Century of Medical Records Linkage in a US Population. Mayo Clin Proc. 2012;87(12):1202–13. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Expert committee on the diagnosis and classification of diabetes mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(S1):S62–S69. [Google Scholar]
- 21.Dyck PJ, Kratz KM, Lehman KA, Karnes JL, Melton LJ, 3rd, O'Brien PC, Litchy WJ, Windebank AJ, Smith BE, Low PA, et al. The Rochester Diabetic Neuropathy Study: design criteria for types of neuropathy, selection bias, and reproducibility of neuropathic tests. Neurology. 1991;41(6):799–807. doi: 10.1212/wnl.41.6.799. [DOI] [PubMed] [Google Scholar]
- 22.Kovatchev BP, Otoo E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29(11):2433–8. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 23.Irvine WJ, Gray RS, McCallum CJ. Pancreatic islet-cell antibody as a marker for asymptomatic and latent diabetes and prediabetes. Lancet. 1976;2(7995):1097–102. doi: 10.1016/s0140-6736(76)91084-9. [DOI] [PubMed] [Google Scholar]
- 24.Andersen MK, Lundgre V, Turunen JA, Forsblom C, Isomaa B, Groop PH, Groop L, Tuomi T. Latent autoimmune diabetes in adults differes genetically from classical type 1 diabetes diagnosed after the age of 35 years. Diabetes Care. 2010;33(9):2062–2064. doi: 10.2337/dc09-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pipi E, Maretou M, Tsirogianni A. Distinct clinical and laboratory characteristics of latent autoimmune diabetes in adults in relation to type 1 and type 2 diabetes mellitus. World Journal of Diabetes. 2014;5(4):505–510. doi: 10.4239/wjd.v5.i4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA. Diagnosis and Management of Celiac Disease. American Journal of Gastroenterology. 2013;108:656–676. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludvigsson JF, Rubio-Tapia A, van Dyke CT, Melton LJ, 3rd, Zinmeister AR, Lahr BD, Murray JA. Increasing incidence of celiac disease in a North American population. American Journal of Gastroenterology. 2013;108(5):818–24. doi: 10.1038/ajg.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen J, Weile C, Antvorskov JC, Engkilde K, Nielsen SM, Josefsen K, Buschard K. Effect of dietary gluten on dendritic cells and innate immune subsets in BALB/c and NOD mice. PLoS One. 2015;10(3):e0119618. doi: 10.1371/journal.pone.0118618. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadley D, Hagopian W, Liu E, She JX, Simell O, Akolkar B, Ziegler AG, Rewers M, Krischer JP, Chen WM, Onengut-Gumuscu S, Bugawan TL, Rish SS, Erlich H, Agardh D TEDDY Study Group. HLA-DPB1*04:01 protects genetically Susceptible children from celiac disease autoimmunity in the TEDDY Study. Am J Gastroenterol. 2015;110(6):915–20. doi: 10.1038/ajg.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smigoc Schweiger D, Mendez A, Kunilo Jamnik S, Bratanic N, Bratina N, Battelino T, Brecelj J, Vidan-Jeras B. Genetic risk for co-occurrence of type 1 diabetes and celiac disease is modified by HLA-C and killer immunoglobulin-like receptors. Tissue Antigens. 2015;84(5):471–8. doi: 10.1111/tan.12450. [DOI] [PubMed] [Google Scholar]
- 31.Adlercreutz EH, Wingren CJ, Vincente RP, Merlo J, Agardh D. Perinatal risk factors increase the risk of being affected by both type 1 diabetes and coeliac disease. Acta Paediatr. 2015;104(2):178–84. doi: 10.1111/apa.12836. [DOI] [PubMed] [Google Scholar]


