Abstract
Rationale
Disorders of behavioral regulation, including attention deficit hyperactivity disorder (ADHD) and drug addiction, are in part due to poor inhibitory control, attentional deficits, and hyper-responsivity to reward-associated cues.
Objectives
To determine whether these traits are related, we tested genetically variable male and female heterogeneous stock rats in the choice reaction time (CRT) task and Pavlovian conditioned approach (PavCA). Sex differences in the response to methylphenidate during the CRT were also assessed.
Methods
In the CRT task, rats were required to withhold responding until one of two lights indicated whether responses into a left or right port would be reinforced with water. Reaction time on correct trials and premature responses were the operational definitions of attention and response inhibition, respectively. Rats were also pre-treated with oral methylphenidate (0, 2, 4 mg/kg) during the CRT task to determine whether this drug would improve performance. Subsequently, during PavCA, presentation of an illuminated lever predicted the delivery of a food pellet into a food-cup. Lever-directed approach (sign-tracking) and food-cup approach (goal-tracking) were the primary measures, and rats were categorized as “sign-trackers” and “goal-trackers” using an index based on these measures.
Results
Sign-trackers made more premature responses than goal-trackers, but showed no differences in reaction time. There were sex differences in both tasks, with females having higher sign-tracking, completing more CRT trials, and making more premature responses after methylphenidate administration.
Conclusions
These results indicate that response inhibition is related to reward-cue responsivity, suggesting that these traits are influenced by common genetic factors.
Keywords: Autoshaping, Associative Learning, Conditioned Response, Action Impulsivity, Motivation, Stimulant Drugs, Classical Conditioning, Reward Stimuli, Incentive Salience, Feeding Behavior
Introduction
In humans and in animals, individuals must regulate their behavior in response to stimuli (“cues”) and other environmental challenges. However, there is enormous individual variation in behavioral regulation and cue responsivity (Meyer et al. 2012; Richards et al. 2013). In particular, deficits in behavioral regulation and hyper-responsivity to cues are implicated in impulsive, maladaptive behaviors that are related to attention deficit hyperactivity disorder (ADHD), obsessive compulsive disorder, pathological gambling, and drug addiction (de Wit 2009; Tavares and Gentil 2007; Charach et al. 2011; Semrud-Clikeman et al. 2010; Fillmore and Rush 2002; Oosterlaan et al. 1998; Penades et al. 2007). Impulsivity has been assessed in rodent models using behavioral paradigms that measure an animal's ability to inhibit responding to experimental contingencies (Bari and Robbins 2013; Richards et al. 2013; Robbins 2002; Eagle and Baunez 2010). One such paradigm is the choice reaction time (CRT) task, where attentional control can be inferred by measuring choice accuracy and reaction times while rats make a choice in response to a stimulus. In addition, failure to withhold the initiation of a response until stimulus presentation is used as a measure of “action” impulsivity (Evenden 1999). Action impulsivity is involved in a number of drug-related behaviors, including self-administration acquisition and compulsive drug seeking in rodents, and also subjective drug responses in humans (Diergaarde et al. 2008; Weafer and de Wit 2013; Perry and Carroll 2008; Murray et al. 2013; Economidou et al. 2009; Belin et al. 2008; Leeman et al. 2014).
Action impulsivity and other forms of behavioral regulation are particularly influenced by the presence of cues associated with food and other rewards. In some individuals, these cues acquire incentive salience, thereby eliciting approach, reinforcing new behaviors, and motivating reward seeking (Milton and Everitt 2010; Bindra 1978; Robinson et al. 2014). In this manner, cues engage and bias attention, influencing individuals' choices, sensitivity to distractors, and how quickly they make decisions (Lloyd et al. 2012; Richards et al. 2013; Robinson et al. 2014). These three properties are related; animals that approach a food-associated cue (“sign-trackers”; Hearst and Jenkins 1974; Meyer et al. 2012; Tomie et al. 2012) in a Pavlovian conditioned approach (PavCA) task also show greater conditioned reinforcement and are more sensitive to the effects of cues on motivated behavior (Saunders et al. 2013; Yager and Robinson 2010; Saunders and Robinson 2010; Robinson and Flagel 2009), compared to rats that approach the food-delivery location (“goal-trackers”; Boakes 1977). These incentive cues can therefore affect behavioral regulation, and in pathological states they can instigate maladaptive behavior, including craving and relapse into addiction (Childress et al. 2008; Franklin et al. 2011).
A previous study suggests a link between sign-tracking and action impulsivity, as measured by a 2-choice serial reaction time task and a differential reinforcement of low rates of responding task (Lovic et al. 2011). The purpose of the current study was to further characterize this relationship between attention, impulsivity, and attribution of incentive salience by utilizing a genetically and phenotypically diverse population of rats and to also measure these traits in males and females. To this end, we compared attentional performance and premature responding during a CRT task with responsivity to food cues during a PavCA task, and determined whether performance on these tasks would depend on sex. Further, we tested whether methylphenidate would improve performance on the CRT task in a sex dependent manner. For these studies, we utilized heterogeneous stock rats (HS; formerly N:NIH). Compared to commercially available outbred strains that have been subjected to breeding bottlenecks, HS rats are especially useful for dissociating the effects of linked genes from phenotypes, because they have approximately one centiMorgan average distance between recombination events per individual (Parker et al. 2013; Mott et al. 2000).
Methods
2.1.1 Subjects
Subjects were 24 male and 24 female HS rats (provided by Dr. Leah Solberg Woods, Medical College of Wisconsin). The HS strain was established at the NIH using eight inbred founder strains from separate lineages. These subjects are currently maintained in the laboratory of Dr. Solberg-Woods at the Medical College of Wisconsin. Specifically, 64 breeder pairs are used in a random breeding scheme that minimizes inbreeding and maximizes recombination of genetic loci across each litter. This strategy is particularly useful because it reduces the likelihood of genetic drift and fixation, and thus maintains the genetic heterogeneity of the population.
The rats arrived at the Research Institute on Addictions in Buffalo, NY, at approximately 35 days of age. CRT testing began at 60 days of age in the Richards lab at the Research Institute on Addictions. After CRT testing, the rats were tested in PavCA and conditioned reinforcement as outlined below, in the Psychology department at the University at Buffalo. At the beginning of testing, the average weight of females was 197 g, and the average weight of males was 315 g. All rats were pair-housed with a member of the same sex for the duration of the experiment, in Plexiglas cages (45 length × 24cm width × 20cm height). Cages were lined with bedding (Aspen Shavings) and kept in a temperature controlled environment (22±1°C). Rats were maintained on a reverse light/dark cycle (lights on at 8:00 or 9:30 AM for CRT and PavCA testing, respectively). Testing began at least one hour after the beginning of the light cycle. Standard lab chow was freely available except during testing. There was no environmental enrichment in the home cages throughout the experiment. For CRT training, access to water was restricted beginning the week prior to testing, in which rats had access to water for 20 min following testing. During PavCA and conditioned reinforcement testing, rats were not food or water restricted. All procedures were approved by the University at Buffalo Institutional Animal Care and Use Committee.
2.1.2 Drugs
Methylphenidate HCl (0, 2, or 4mg/kg; Research Triangle Institute, Research Triangle Park, NC) was dissolved into a 10% sucrose solution in cherry Kool-Aid, and injected onto a small cracker. Rats were then observed to confirm consumption of the cracker prior to testing.
2.1.3 Apparatus
Choice reaction time task (CRT)
Behavioral measures were obtained using locally constructed testing chambers (Richards et al. 2013; Sabol et al. 2003). Two water dispensers were located on either the left or right side of a center snout poke hole, with two stimulus lights mounted above the two water dispensers and center hole. The equipment was arranged to be level with the rat's eyes. Entries into the dispensers and center hole were monitored using infrared photobeams. Med associates syringe pumps were used to deliver water into the dispensers (PHM-100; MED Associates, East Fairfield, VT, USA). All chambers were contained in light and sound attenuating boxes. Data from testing chambers were collected using MED-PC IV software.
Pavlovian conditioned approach (PavCA) and conditioned reinforcement
Testing occurred in 16 modular test chambers (20.5×24.1 cm floor area, 29.2 cm high; MED-Associates Inc., St. Albans, VT) located inside sound attenuating cubicles equipped with vent fans for noise masking (A&B Display Systems, Bay City, MI). A pellet dispenser delivered 45mg banana pellets into a food-cup equipped with an infrared head entry detector. Retractable backlit levers (2 cm long, 6 cm above floor) were adjacent to the food-cup on either the left or right side. A red light was located high (27 cm) on the opposite wall. For conditioned reinforcement, the food-cup was removed and the retractable lever was moved to the center of the wall in its place. Two nose poke holes with head-entry detectors were placed on the left and right side of the lever. All data were collected using MED-PC IV software.
Procedures
2.2.1 Choice reaction time task (CRT)
The CRT task consists of a series of trials that are initiated when a rat pokes its snout into a center hole and remains inserted until either a left or right stimulus light is activated (imperative stimulus). The period of time between snout insertion and imperative stimulus light activation is the hold time. Activation of the imperative stimulus indicated availability of a water reinforcer in the respective left or right feeder hole. Reinforcer availability was confined to a 3 s window, where the rat must correctly pick the appropriate hole paired with the stimulus light in order to receive the reinforcer. After 3 s, the trial ended. The only illumination came from the stimulus lights adjacent to the center hole. Testing occurred 6 days of the week over the course of 36 sessions. Each session ended either after 100 trials or 30 min.
Rats underwent a total of 72 training and test sessions. Acquisition training occurred from sessions 1-56. During training, the magnitude of the water reinforcer was alternated between 10 and 30 μL of water, and the hold time was progressively increased from a minimal value of 0.1 to 5.0 s. Once the 5 s hold time was reached, a variable hold time was imposed, ranging from 0.06 s to 10.5 s with a mean of 5 s. The hold time was cumulative. For example, a target hold time of 10 s could be reached through two separate 5-s pokes. The purpose of the variable hold time was to make the onset of the imperative stimulus less predictable. In this within-subjects design there was no formal training stability criterion. There is some variability in training rates, in that some rats have more difficulty than others meeting the hold time criterion. Therefore, we required all of the rats to complete at least 10 trials per session before increasing the hold time to the next level.
Beginning at session 50, the rats were fed a single cracker injected with cherry Kool-Aid/sucrose solution 30 min before testing for 5 sessions in order to habituate them to this procedure before drug testing began. Beginning with session 55, the cracker contained 0, 2, and 4 mg/kg doses of methylphenidate, and was given 15 min before testing. The drug dose was given in ascending order returning to 0 after the 4 mg dose was administered. During methylphenidate administration, the reinforcer magnitude continued to alternate between 10 and 30 μL. Each subject was tested under each of six possible conditions (3 methylphenidate doses × 2 reinforcer volumes). Doses and reinforcer volumes were given in the same sequence to each animal to equalize any sustained pharmacokinetic effects of the oral administration of this drug.
2.2.2 CRT Measures
Reaction time (RT) is the elapsed time between presentation of the imperative stimulus and snout withdrawal from the center hole, averaged across trials. Premature responses were defined as a withdrawal from the center hole followed by an insertion into one of the feeder holes before onset of the imperative stimulus. Total trials were the total number of trials initiated by the subject, and correct trials were the number of initiated trials that were successfully completed. Proportion correct was measured by dividing the total number of correct trials by the total number of trials. An incorrect response occurred when the rat entered the wrong feeder hole during the 3 second availability, resulting in termination of the trial without the reinforcer. No response in either feeder hole within the 3 s imperative stimulus window was considered an omission.
2.3.1 Pavlovian Conditioned Approach
Rats were transferred to the University at Buffalo and given one week of handling prior to testing. In the two days preceding testing, rats received home cage exposure to banana-flavored food pellets (∼25 pellets per day; Bio-Serv, Flemington, NJ, #F0059). Rats then received one day of food-cup training in the conditioning chamber to habituate subjects to the testing procedure. During food-cup training, rats first underwent a 5-min period to habituate to the chamber with the houselight off. The houselight was then illuminated and rats received 25 pellets delivered into the food-cup on a VI-30 s schedule (1-60 s range). The session ended after 25 pellets were delivered. The lever remained retracted throughout food-cup training,
Following food-cup training, rats were then tested for 10 daily sessions in the PavCA procedure. Each session contained 25 trials in which an illuminated lever was presented for 8 s, followed by the retraction of the lever and delivery of the food pellet. While lever presses were measured, they had no programmed consequences in this Pavlovian procedure. Trials were separated on a VI-90 schedule (30 – 150 s range); each session thus lasted approximately 37.5 min.
2.3.2 PavCA Measures
Repeated lever-pellet pairings produce two major conditioned responses: lever directed approach (as measured by lever contacts), and goal-directed approach (as measured by entries into the food-cup). Lever and food-cup interactions were further quantified by 1) number of occurrences, 2) latency to first occurrence, and 3) number of occurrences in the inter-trial interval versus the CS period.
We have previously identified three measures of conditioned approach (Meyer et al., 2012), which were used in the present study: 1) The probability differential of a contact with the lever or food-cup in a given CS period (average probability of a lever press on a given CS trial – average probability of a food-cup entry on a given CS trial), 2) the overall response bias for either the lever or the food-cup CS (# lever contacts - # food-cup contacts / # lever + # food-cup contacts), and 3) average latency to contact either lever or food-cup in the CS period (food-cup latency – lever latency / 8). The average of these three measures produces a PavCA index ranging from -1 to 1 and was used to classify sign-trackers (ST) and goal-trackers (GT) based on the average of the PavCA index on days 4 and 5, see Meyer et al., 2012). Specifically, ST (n = 15) had PavCA index scores ranging from 0.5 to 1.0, while goal-trackers (n = 25) had scores from -1 to -0.5. Rats with scores between -0.5 and 0.5 were classified as intermediates (n = 8).
2.4 Conditioned reinforcement test and measures
The conditioned reinforcement test evaluates the reinforcing properties of the food-associated lever-cue following conditioning. The chambers were reconfigured with two nose poke holes, one active and one inactive on either the left or right side of the chamber. Between the two nose-poke holes was the retracted lever. Active nose pokes into a designated hole resulted in deployment of the lever-cue for 3 s, and entries into the inactive port had no programmed consequences. The same floors, house light, and fans used during PavCA were also used in this task. Sessions lasted 40 min. The number entries into the active and inactive ports, the number of lever presentations, and the number of lever deflections were recorded during the task.
2.5 Analyses
Repeated-measures analysis of variance (ANOVA), followed by Fisher's LSD post-hoc tests, were conducted to probe significant main effects and interactions. For the CRT, Sex (male, female) was the between-groups factor, while Reinforcer (10μL, 30μL) volume and methylphenidate Dose (0, 2, and 4 mg/kg) were within-subjects factors. The data from five males were lost due to a collection error, which was a result of a technical malfunction in the data export process from the Med-PC software. Data were lost from different test conditions (e.g. one subject lost data for the 0 mg methylphenidate 10 μL reinforcer condition, another lost data for the 2 mg methylphenidate 10 μL condition); we therefore dropped all choice reaction time data for any subject that was missing any data point during this task.
For PavCA, the between-groups factor was Sex, with conditioning Day (1 – 9) as a within-subjects measure. Day 10 was dropped from correlational analyses because data were lost for 16 subjects due to equipment malfunction. Phenotype (sign-tracker, goal-tracker) was added as a between-groups factor when considering the relationship between the CRT task and PavCA, and intermediates (n = 8) were excluded from this analysis only. For conditioned reinforcement, nose-poke Port (Active, Inactive) was also included as a within groups measure, and Student's t-tests were used to compare number of reinforcers and lever presses during this test.
Pearson's correlation was used to analyze comparisons between the CRT task and PavCA. For the example, correlations shown in Fig. 6, day 5 of PavCA was chosen because it coincides with the last day used for calculating the PavCA Index. Statistically significant results were set at p < 0.05. We used Grubb's statistical test for outliers, which is a significance test used to determine whether an outlier exists in a particular distribution of data (Grubbs 1969). Specifically, this test works by detecting data points that exceed a defined critical z-value, which is determined by both the sample size and desired alpha level. This test did not yield significant (p > 0.05) values for our examined measures within lever contacts, food-cup entries, or premature responses.
Results
3.1 Choice Reaction Time
There were no effects of sex (males: n = 19; females: n = 24), reinforcer volume, or methylphenidate dose on the proportion of correct or incorrect responses. For premature responses, reinforcer volume (Fig. 1A) and methylphenidate dose (Fig. 1B) all significantly affected premature responding [F (1, 41) = 69.5, p < 0.05; F (2, 82) = 4.0, p < 0.05; respectively]. Although there was no main effect of sex on premature responding (p = 0.07), methylphenidate dose influenced premature responding differently between males and females [Dose × Sex interaction: F (2, 82) = 3.4, p < 0.05]. Post-hoc tests indicated that 4 mg produced increases in premature responding in females relative to the 0 mg dose (p < 0.05); while methylphenidate had no effect in males (Fig. 1B). Thus females, but not males, made more premature responses following methylphenidate pretreatment.
Fig. 1.
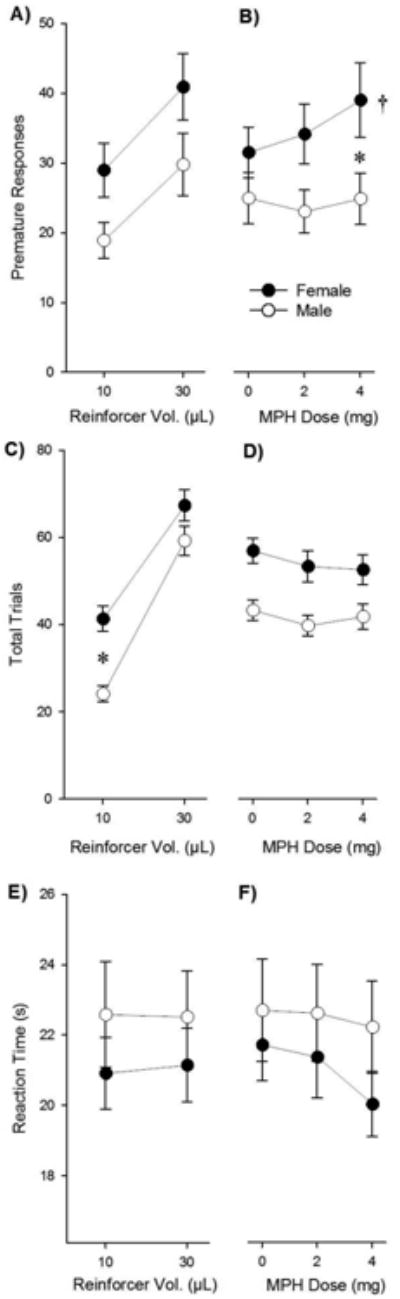
Premature responses, total trials, and reaction time were differentially affected by sex, reinforcer volume, and methylphenidate (MPH) dose. Asterisks (*) denote significant differences between sexes (p < 0.05). Daggers (†) represent differences within-subjects (p < 0.05). (A) Males and females showed similar increases in premature responding for a 30 μL water reinforcer. (B) Females, but not males showed increased premature responding in response to methylphenidate. (C) Females had a tendency to initiate more trials than males, although there were no differences at higher reinforcer volumes and (D) was not altered by methylphenidate. (E) There were no sex differences in reaction time across reinforcer volumes or (F) methylphenidate doses. Although there was a main effect of methylphenidate, the interaction with sex, in which females were more sensitive to methylphenidate, did not reach statistical significance (p = 0.056). Data are presented as means ± SEM, and are collapsed across MPH dose (panels A, C, E) or Reinforcer Volume (B, D, F) measure.
There was a main effect of reinforcer volume on total number of trials initiated [F (1, 41) = 317.6, (p < 0.05)] in which subjects initiated more trials for larger reinforcer volumes. Further, there was a main effect of sex on the total number of trials initiated [F (1, 41) = 7.1, (p < 0.05)] which interacted with reinforcer [Sex × Reinforcer interaction: F (1,41) = 9.8, (p < 0.05]. Post-hoc analysis indicated that females initiated more trials at the 10 μL condition (p < 0.05) (Fig. 1C). We found a similar effect for total number of trials successfully completed [Reinforcer × Sex interaction: F (1,41) = 6.1, (p < 0.05)] despite the fact that there were no overall differences in proportion of correct responses between sexes (p > 0.05, data not shown). Although we found a main effect of methylphenidate treatment [F (2, 82) = 3.4, (p < 0.05)], there were no interactions (p > 0.05) (Fig. 1D). Thus, although females initiated more trials than males at the lower reinforcer condition, sex differences in response to methylphenidate were restricted to premature responding.
Reaction time was significantly reduced by methylphenidate [effect of Dose; F (2, 82) = 8.5, p < 0.05], suggesting an enhancement of attention. ANOVA results indicated a trend for an interaction between Dose and Sex on reaction time [F (2, 82) = 3.0, p = 0.056; Fig. 1E, F]. No other main effects or interactions were found. Thus, methylphenidate increased premature responding in females and enhanced attention overall.
3.2 Pavlovian Conditioned Approach
During conditioning, rats approached the lever CS or the food-cup. Three measures reflect these approach behaviors: probability of approach, number of contacts, and latency to approach (Fig. 2). Approach changed as a function of Day in all of these measures except food-cup contacts [Fs (8, 360) > 2.3, ps < 0.05].
Fig. 2.
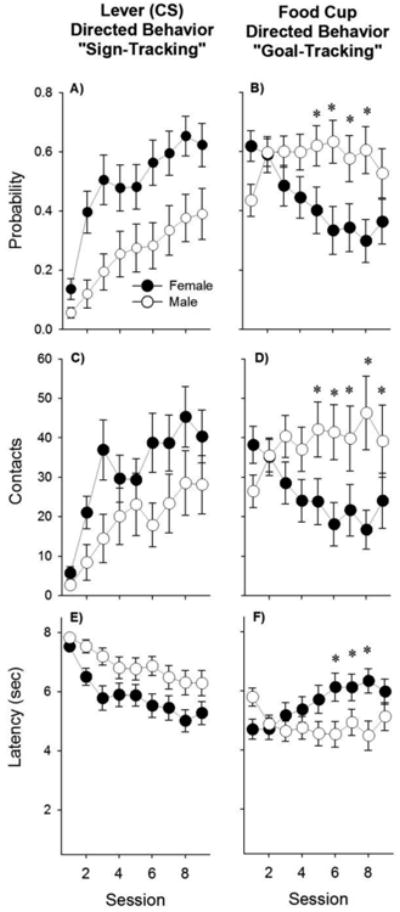
Females approached the lever CS to a greater degree than males, and the food-cup to a lesser degree than males. Probabilities indicate the average number of trials in which a lever contact (A) or food-cup contact (B) occurred. Contacts indicate the average number of lever contacts (C) or food-cup contacts (D) during each session. Latency indicates the average elapsed time (s) before contacting either the lever (E) or the food-cup (F). Asterisks (*) denote differences between sexes on the indicated days (p < 0.05, detected by post-hoc analysis of Sex × Day interactions).
There were main effects of Sex for approach to the lever, as measured by probability of approach and latency to approach [Fs (1, 45) > 6.7, ps < 0.05] with females approaching more often and more quickly than males (ps < 0.05). The interaction between Sex and conditioning Day for all three measures did not reach significance (ps = 0.06 - 0.12). For approach to the food-cup, the main effects of Sex did not reach significance (ps = 0.06 - 0.11), but there were significant interactions between Sex and conditioning Day for all measures [Fs (8, 360) > 5.8, ps < 0.001]. Post hoc analyses indicated that males approached the food-cup more during the last days of conditioning (see Fig. 2; ps < 0.05).
The PavCA Index (Fig. 3A) is constructed by taking the average of the differences in these three measures. This Index changed significantly across conditioning Days [F (8, 360) = 17.3, p < 0.001], and this effect depended on sex [effect of Sex: F (1, 45) = 7.4, p < 0.01; Sex × Day interaction: [F (8, 360) = 4.4, p < 0.001]. Post-hoc analysis revealed that females had larger Index scores than males on days 3 – 9. Females therefore showed an increased propensity to approach the reward cue (i.e., to sign-track). The sexes did not differ on the first two days of conditioning, suggesting that these group differences reflect differences specific to the effects of the conditioning. In addition, there was no effect of sex on the number of inter-trial interval entries into the food-cup outside of the CS period (Fig. 3B), although it did decrease across Days [F (8, 360) = 18.9, p < 0.05]. The individual PavCA Index used to characterize rats as sign-trackers (ST) or goal-trackers (GT) are shown in Fig. 3C. In concordance with the higher mean PavCA Index scores, there were more females categorized as ST than males.
Fig. 3.
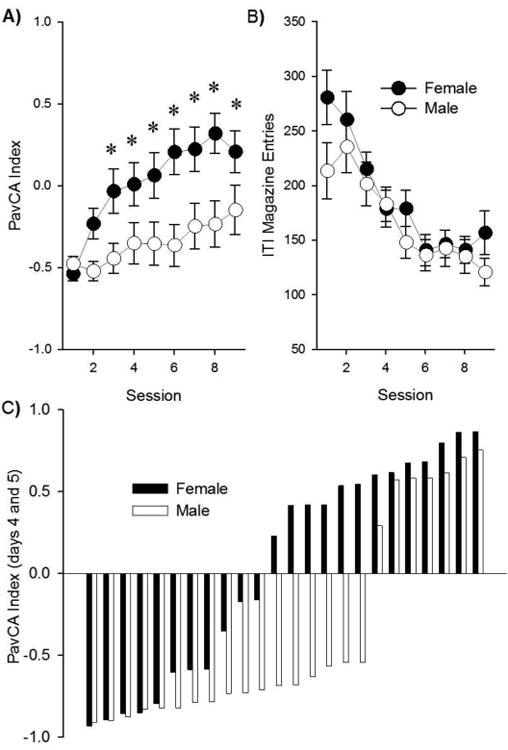
(A) Females had larger PavCA indexes, indicative of an increased propensity to sign-track, relative to males. Asterisks (*) indicate differences between males and females. (B) Food-cup entries during the inter-trial interval (ITI) decreased across sessions, but did not differ between sexes. (C) Individual PavCA index scores show that there were more female than male sign-trackers, and more male than female goal-trackers.
3.4 Conditioned Reinforcement
On the conditioned reinforcement test day (Fig. 4), females responded more for lever CS presentations, as indicated by a Sex × Port interaction [Fig. 4A; F (1, 46) = 32.5, p < 0.001] and post-hoc analysis indicated that females made more active, but not inactive, nose-poke entries than males (p < 0.001). In addition, females received more reinforced nose pokes [t (46) = 5.0, p < 0.001], but made a similar number of lever contacts when corrected for the number of reinforcers.
Fig. 4.
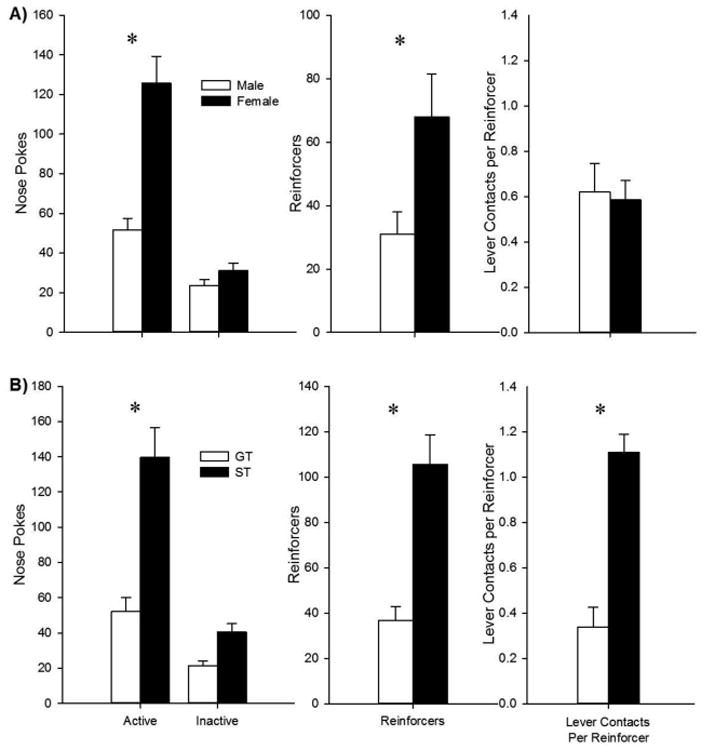
Females and sign-trackers responded more for the CS than males and goal-trackers, respectively. Asterisks (*) indicate differences between males and females or between ST and GT (ps < 0.05). (A) Females made significantly more active nose pokes to present the food-paired cue, but no sex difference in the number of lever presses was observed in this task. (B) ST also responded more for the lever than GT, and contacted the lever more when it was extended (ps < 0.05).
In a separate analysis of rats classified as ST or GT (Fig. 4B), STs responded more for the Lever CS than GTs [Phenotype × Port interaction: F (1, 38) = 25.1, p < 0.001], which was due to STs making more active, but not inactive, nose-poke entries (p < 0.001). As a result, STs received more reinforcers [t (38) = 5.4, p < 0.001], and made more contacts with the lever, even when corrected for the number of reinforcers [t (38) = 6.0, p < 0.001]. Thus, the lever was a more effective reinforcer for sign-trackers compared to goal-trackers.
3.4 Relationship between sign-tracking and premature responses
We compared sign- and goal-trackers on the number of premature responses, total trials completed, and reaction time during the 10 and 30 μL conditions of the CRT task. In order to assess the relationship between traits, all comparisons were made for the 0 mg methylphenidate condition so that differences in drug responsivity between individuals were not included. Our analysis revealed a main effect of Phenotype, with sign-trackers making more premature responses overall than goal-trackers [F (1,36) = 7.8, p < 0.05; Fig. 5A], although this effect did not interact with reinforcer volume. There was a trend towards a main effect of Phenotype on total trials completed (p = 0.051; Fig. 5B). There was no main effect of Phenotype on reaction time (p > 0.05; Fig. 5C), indicating that sign- and goal-trackers responded at similar speeds during the task. Thus, sign-trackers preform more premature responses than goal-trackers during the CRT task, but there were no differences in overall reaction time to the imperative stimulus.
Fig. 5.

(A) Sign-trackers (ST) had more overall premature responses than goal-trackers (GT), but did not differ from GT in (B) total trials initiated or (C) mean reaction time. Asterisk denotes significant ST/GT difference (p < 0.05).
For correlation analyses examining the relationship between the CRT task and PavCA, we focused on the 0 mg dose of methylphenidate, the 10 μL reinforcer volume, and day 5 of PavCA. Sign-tracking was significantly correlated with premature responding (Fig. 6B; r = 0.33, p < 0.05) and goal tracking was negatively correlated with premature responding (Fig. 6C; r = -0.33, p < 0.05). Significant correlations between tasks were observed across multiple PavCA sessions (Fig. 6A). The relationship between PavCA measures and total trials completed was weaker (Fig. 6A) compared to premature responses. No sex differences were detected, so all rats were included in the analysis.
Fig. 6.
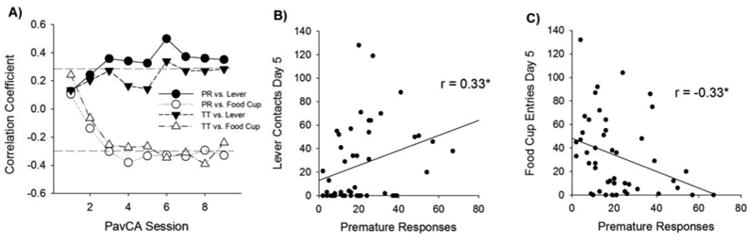
(A) Correlation between lever contacts and food cup entries on Pavlovian conditioned approach to premature responses (PR) and total trials (TT) on the Choice Reaction Time Task. Dotted line indicates threshold for significant correlations across days (p < 0.05). The strongest and most consistent correlations were between premature responding and conditioned approach to the lever and food cup. (B) Example scatterplot with regression line showing the relationship between lever contacts on PavCA day 5 and the premature responses in the choice reaction time task (in the 10 μL, 0 mg condition). Asterisks indicate statistically significant correlations (p < 0.05). (C) In contrast to sign-tracking, tendency to approach the food cup (goal-tracking) was negatively associated with premature responding (p < 0.05).
Discussion
Rodent models of ADHD-related behaviors provide one avenue to examine the immediate biological causes of sex differences observed in human patients (Arnett et al. 2015; Gaub and Carlson 1997; Williamson and Johnston 2015; but see Sharp et al. 1999; Pelham Jr et al. 1989), yet few studies have examined such sex differences in rodents. Here, we show that, during the CRT task, female rats complete more trials without a concomitant increase in premature responding and that that methylphenidate affects premature responding in female but not male rats. We also report that female rats are more prone to attribute incentive salience to reward cues (as indexed by sign-tracking and conditioned reinforcement), and that sign-tracking is associated with premature responding in a heterogeneous population of rats. Together, this suggests that premature responding and incentive salience attribution to reward cues are influenced by common neurobehavioral and genetic processes.
Choice Reaction Time
In studies with ADHD children, attentional measures are affected by individuals' sensitivity to reward, incentive motivation, and stimulant medication (Fosco et al. 2015; Strand et al. 2012). Performance during the CRT task, which is similar to the 5-choice serial reaction time task used in animals and humans (5CSRT; Robbins 2002), is similarly sensitive to reinforcer magnitude, stimulus salience, and psychostimulant (including amphetamine, methamphetamine, and methylphenidate) treatment (Sabol et al. 2003; Spencer et al. 2009; Cole and Robbins 1987). However, in our study, rats engaged in more total trials when the reinforcer magnitude was increased, but their reaction time was not affected. This is in line with theories of positive incentive contrast, which suggest that increasing a reinforcer's magnitude following a smaller one can invigorate motivated behavior (Flaherty and Largen 1975; Flaherty 1982; Bower 1961). This further suggests that, in our paradigm, total trials are more sensitive to differences in motivation and reward than reaction time, while reaction time relates more to attention.
In support of this, in our study, methylphenidate decreased reaction time, and decreased total trials, which is consistent with an effect of methylphenidate on attention rather than motivation (Robbins 2002; Richards et al. 2013; Leth-Steensen et al. 2000; Posner et al. 1980). If methylphenidate did alter motivation, it would be expected to increase the total number trials initiated by the rats, as was the case when reinforcer magnitude was increased. We also found that methylphenidate increased the number of premature responses (i.e., increased impulsivity) in females and reduced reaction time (i.e. increased attention) overall. This is consistent with previous studies using amphetamine (van Gaalen et al. 2006; Cole and Robbins 1987), and methylphenidate (Puumala et al. 1996; Navarra et al. 2008), although Puumala et al. (1996) found that poorly-performing rats had fewer premature responses under methylphenidate. It should also be noted that one experiment (Bizarro et al. 2004) found that amphetamine and methylphenidate decreased premature responses. The source of this discrepancy is unknown, but may have been due to the testing of multiple compounds (including nicotine), the strain tested (male Lister rats), or other procedural details including the route of administration and the temporal predictability of stimulus onset (see Bizarro et al., 2004 for discussion). Further, in our experiment, the increases in premature responses occurred in females, which is also consistent with previous findings with amphetamine in the choice reaction time task (Burton and Fletcher 2012), and in general with a greater sensitivity to psychostimulants in females (Becker et al. 2001; Torres-Reveron and Dow-Edwards 2005; Roeding et al. 2014; Bentley et al. 2015; see Chelaru et al. 2012 for strain-dependent differences).
At first glance, the increase in premature responses induced by methylphenidate in our paradigm seems at odds with studies in humans showing that methylphenidate improves performance on attention-based tasks in ADHD patients (e.g., Rosch et al. 2016; Bubnik et al. 2015; Ashare et al. 2013). However, as is the case for animal studies, the nature of the effects of psychostimulants in humans depends on dose, age, sex, time since administration, and on the outcome measured (Broos et al. 2012; Berridge et al. 2006; Gunther et al. 2010; Sonuga-Barke et al. 2007; Kim et al. 2015; DeVito et al. 2009; Burton and Fletcher 2012; Abikoff et al. 2002). For example, methylphenidate can improve performance in poor preforming animals, and worsen performance in high performing animals during the 5-CSRTT (Robinson 2012; Caprioli et al. 2015; Puumala et al. 1996). Differences between human and animal studies may also be related to individual differences, to the extent that the drug may even have opposite effects depending on factors such as genotype, baseline impulsivity, and certain biological factors. For example, in mice with low dopamine transporter levels, d-amphetamine reduced hyperactivity, while inducing hyperactivity in normal mice (Zhuang et al. 2001). While the effect of dopamine transporter genotype in humans is controversial (Kambeitz et al. 2014), one study found that methylphenidate was more effective in individuals with the 10/10 and 10/9 dopamine transporter genotypes, compared to those with the 9/9 genotype (Stein et al. 2014). In addition, the effectiveness of stimulants on ADHD symptoms was correlated with cerebrospinal levels of homovanillic acid (HVA; a dopamine metabolite) such that higher HVA levels predicted better drug responses (Castellanos et al. 1996). Taken together, these studies emphasize that effect of methylphenidate may depend on the underlying symptomatology and biological causes of ADHD among individuals.
Pavlovian Conditioned Approach
In this study we also found that females were more likely to attribute incentive salience to a reward cue than males, as measured by approach to the lever CS (i.e. sign-tracking) during PavCA and by responding for the lever CS presentation during conditioned reinforcement. A recent study using a similar PavCA procedure reported only minor differences in PavCA in HS and Sprague-Dawley rats; with females displaying more approach to the lever-CS than males, but only during the first three days of conditioning, and not during subsequent test sessions (Pitchers et al. 2015). Apparent differences in conditioned reinforcement were related to differences in activity levels, as measured by inactive nose-poke port entries. Because of these findings, the authors concluded that their results were “probably not due to differences in the propensity to attribute incentive salience to reward cues” (Pitchers et al. 2015). In contrast, the sex differences in the current study were more robust, with differences occurring later in conditioning (Figs. 2-3), more females being identified as ST than males (Fig. 3C), and differences in conditioned reinforcement that could not be explained by differences in general nose-poking activity (i.e., inactive nose-poke port entries, Fig. 4). Thus, we conclude that females were more prone to attribute incentive salience to the food-associated cue. Reasons for the differences between ours and the Pitchers et al. (2015) studies may include 1) our rats underwent the CRT task before PavCA testing, 2) were older as a result, and 3) our HS rats were from different litters (but the same supplier, Dr. Leah Solberg Woods), and thus may harbor genetic variants that interact with sex to produce the sex differences observed in this study. Additionally, exposure to methylphenidate prior to PavCA may have potentially altered the development of the sign-tracking phenotype. Indeed, a history of amphetamine sensitization promotes subsequent sign-tracking to a food CS in female rats (Doremus-Fitzwater and Spear 2011; Robinson et al. 2015), although it is an open question whether other stimulants such as methylphenidate can produce this effect.
We also report an association between incentive salience attribution (as measured by sign-tracking and conditioned reinforcement) and premature responding, consistent with previous studies (Lovic et al. 2011; see also Flagel et al. 2010), in which sign- and goal-trackers were tested in a 2-choice serial reaction time task and a differential reinforcement of low rates of responding task. In contrast to Lovic et al., (2011), PavCA was conducted after CRT testing, which suggests that differences in premature responding are not a direct result of PavCA training. In further contrast to Lovic et al., (2011), our current study used HS rats, which increases the detection of genetic sources of correlation. Previously, this line of subjects has been used to map the genetic loci of traits related to diabetes (Solberg Woods et al. 2010a; Solberg Woods et al. 2012 ; Solberg Woods et al. 2010b) and is currently being used to map the genetic loci of traits for complex drug-related behaviors by an NIH funded center (see http://ratgenes.org/). HS rats are especially valuable because they are more likely to generate conclusions that may better generalize than any single inbred strain. Our results therefore add significant additional external validity to previous findings by including both sexes and by utilizing a more genetically diverse sample.
In conclusion, our data add to a growing body of literature suggesting that action impulsivity and propensity to attribute incentive salience are related traits, and may reflect partially overlapping neurobehavioral processes. In addition, we demonstrate that female rats have more premature responses and are more prone to sign-track reward cues than males, and that these two traits are related in males and females. These data also suggest that deficits in behavioral disinhibition may be particularly apparent in the presence of reward cues, and that this may lead to maladaptive behavioral regulation, such as that seen in ADHD and drug addiction. For example, a recovering cocaine addict may have particular difficulty refraining from drug-taking in the presence of stimuli associated with cocaine. Individuals with higher action impulsivity and incentive salience attribution to reward cues may be particularly prone to relapse in the presence of drug cues. However, future studies are needed to determine whether the genetic and neurobiological substrates of these traits are dissociable, and whether these traits alter behavioral regulation differently between individuals.
Acknowledgments
This work was support by a grant from the National Institute on Drug Abuse (P50DA037844).
References
- Abikoff HB, Jensen PS, Arnold LL, Hoza B, Hechtman L, Pollack S, Martin D, Alvir J, March JS, Hinshaw S, Vitiello B, Newcorn J, Greiner A, Cantwell DP, Conners CK, Elliott G, Greenhill LL, Kraemer H, Pelham WE, Jr, Severe JB, Swanson JM, Wells K, Wigal T. Observed classroom behavior of children with ADHD: relationship to gender and comorbidity. J Abnorm Child Psychol. 2002;30:349–59. doi: 10.1023/a:1015713807297. [DOI] [PubMed] [Google Scholar]
- Arnett AB, Pennington BF, Willcutt EG, DeFries JC, Olson RK. Sex differences in ADHD symptom severity. Journal of Child Psychology and Psychiatry. 2015;56:632–639. doi: 10.1111/jcpp.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Wileyto EP, Perkins KA, Schnoll RA. The First 7 Days of a Quit Attempt Predicts Relapse: Validation of a Measure for Screening Medications for Nicotine Dependence. Journal of addiction medicine. 2013;7:249–254. doi: 10.1097/ADM.0b013e31829363e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–87. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley J, Snyder F, Brown SD, Brown RW, Pond BB. Sex differences in the kinetic profiles of d- and l-methylphenidate in the brains of adult rats. Eur Rev Med Pharmacol Sci. 2015;19:2514–9. [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–20. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Bindra D. How adaptive behavior is produced: A perceptual-motivation alternative to response reinforcement. Behav Brain Res. 1978;1:41–91. [Google Scholar]
- Bizarro L, Patel S, Murtagh C, Stolerman IP. Differential effects of psychomotor stimulants on attentional performance in rats: nicotine, amphetamine, caffeine and methylphenidate. Behav Pharmacol. 2004;15:195–206. [PubMed] [Google Scholar]
- Boakes R. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian interactions. Lawrence Erlbaum Associates; Hillsdale, NJ: 1977. pp. 67–97. [Google Scholar]
- Bower GH. A contrast effect in differential conditioning. Journal of Experimental Psychology. 1961;62:196. [Google Scholar]
- Broos N, Schmaal L, Wiskerke J, Kostelijk L, Lam T, Stoop N, Weierink L, Ham J, de Geus EJ, Schoffelmeer AN, van den Brink W, Veltman DJ, de Vries TJ, Pattij T, Goudriaan AE. The relationship between impulsive choice and impulsive action: a cross-species translational study. PLoS One. 2012;7:e36781. doi: 10.1371/journal.pone.0036781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubnik MG, Hawk LW, Jr, Pelham WE, Jr, Waxmonsky JG, Rosch KS. Reinforcement enhances vigilance among children with ADHD: comparisons to typically developing children and to the effects of methylphenidate. J Abnorm Child Psychol. 2015;43:149–61. doi: 10.1007/s10802-014-9891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: the role of dopamine and glutamate. Behav Brain Res. 2012;230:21–33. doi: 10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Jupp B, Hong YT, Sawiak SJ, Ferrari V, Wharton L, Williamson DJ, McNabb C, Berry D, Aigbirhio FI, Robbins TW, Fryer TD, Dalley JW. Dissociable rate-dependent effects of oral methylphenidate on impulsivity and D2/3 receptor availability in the striatum. J Neurosci. 2015;35:3747–55. doi: 10.1523/JNEUROSCI.3890-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Elia J, Kruesi MJ, Marsh WL, Gulotta CS, Potter WZ, Ritchie GF, Hamburger SD, Rapoport JL. Cerebrospinal fluid homovanillic acid predicts behavioral response to stimulants in 45 boys with attention deficit/hyperactivity disorder. Neuropsychopharmacology. 1996;14:125–37. doi: 10.1016/0893-133X(95)00077-Q. [DOI] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011;50:9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Chelaru MI, Yang PB, Dafny N. Sex differences in the behavioral response to methylphenidate in three adolescent rat strains (WKY, SHR, SD) Behav Brain Res. 2012;226:8–17. doi: 10.1016/j.bbr.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O'Brien CP. Prelude to Passion: Limbic Activation by “Unseen” Drug and Sexual Cues. PLoS ONE. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology (Berl) 1987;91:458–66. doi: 10.1007/BF00216011. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Blackwell AD, Clark L, Kent L, Dezsery AM, Turner DC, Aitken MR, Sahakian BJ. Methylphenidate improves response inhibition but not reflection-impulsivity in children with attention deficit hyperactivity disorder (ADHD) Psychopharmacology (Berl) 2009;202:531–9. doi: 10.1007/s00213-008-1337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–8. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Amphetamine-induced incentive sensitization of sign-tracking behavior in adolescent and adult female rats. Behavioral Neuroscience. 2011;125:661–667. doi: 10.1037/a0023763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 2010;34:50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–6. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–73. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty CF. Incentive contrast: A review of behavioral changes following shifts in reward. Animal Learning & Behavior. 1982;10:409–440. [Google Scholar]
- Flaherty CF, Largen J. Within-subjects positive and negative contrast effects in rats. J Comp Physiol Psych. 1975;88:653–664. doi: 10.1037/h0076416. [DOI] [PubMed] [Google Scholar]
- Fosco WD, Hawk LW, Jr, Rosch KS, Bubnik MG. Evaluating cognitive and motivational accounts of greater reinforcement effects among children with attention-deficit/hyperactivity disorder. Behav Brain Funct. 2015;11:20. doi: 10.1186/s12993-015-0065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O'Brien CP, Childress AR. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry. 2011;68:516–26. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaub M, Carlson CL. Gender Differences in ADHD: A Meta-Analysis and Critical Review. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:1036–1045. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Grubbs FE. Procedures for Detecting Outlying Observations in Samples. Technometrics. 1969;11:1–21. [Google Scholar]
- Gunther T, Herpertz-Dahlmann B, Konrad K. Sex differences in attentional performance and their modulation by methylphenidate in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2010;20:179–86. doi: 10.1089/cap.2009.0060. [DOI] [PubMed] [Google Scholar]
- Hearst E, Jenkins HM. Sign-tracking: The stimulus-reinforcer relation and directed action. Monograph of the Psychonomic Society 1974 [Google Scholar]
- Kambeitz J, Romanos M, Ettinger U. Meta-analysis of the association between dopamine transporter genotype and response to methylphenidate treatment in ADHD. Pharmacogenomics J. 2014;14:77–84. doi: 10.1038/tpj.2013.9. [DOI] [PubMed] [Google Scholar]
- Kim E, Cheon KA, Joung YS, Kim JY, Song DH. The relationship between symptomatic and functional changes of Korean children and adolescents with attention-deficit/hyperactivity disorder treated with osmotic-controlled release oral delivery system-methylphenidate. Clin Neuropharmacol. 2015;38:30–5. doi: 10.1097/WNF.0000000000000064. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Ralevski E, Limoncelli D, Pittman B, O'Malley SS, Petrakis IL. Relationships between impulsivity and subjective response in an IV ethanol paradigm. Psychopharmacology (Berl) 2014;231:2867–76. doi: 10.1007/s00213-014-3458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leth-Steensen C, King Elbaz Z, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Lloyd DR, Gancarz AM, Ashrafioun L, Kausch MA, Richards JB. Habituation and the reinforcing effectiveness of visual stimuli. Behav Processes. 2012;91:184–91. doi: 10.1016/j.beproc.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav Brain Res. 2011;223:255–61. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci. 2010;31:2308–19. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- Mott R, Talbot CJ, Turri MG, Collins AC, Flint J. A method for fine mapping quantitative trait loci in outbred animal stocks. Proceedings of the National Academy of Sciences. 2000;97:12649–12654. doi: 10.1073/pnas.230304397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Dilleen R, Pelloux Y, Economidou D, Dalley JW, Belin D, Everitt BJ. Increased Impulsivity Retards the Transition to Dorsolateral Striatal Dopamine Control of Cocaine Seeking. Biological Psychiatry. 2013;76(1):15–22. doi: 10.1016/j.biopsych.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;2:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Logan GD, Sergeant JA. Response inhibition in AD/HD, CD, comorbid AD/HD + CD, anxious, and control children: a meta-analysis of studies with the stop task. J Child Psychol Psychiatry. 1998;39:411–25. [PubMed] [Google Scholar]
- Parker CC, Chen H, Flagel SB, Geurts AM, Richards JB, Robinson TE, Solberg Woods LC, Palmer AA. Rats are the smart choice: Rationale for a renewed focus on rats in behavioral genetics. Neuropharmacology. 2013;76:250–258. doi: 10.1016/j.neuropharm.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Jr, Walker JL, Sturges J, Hoza J. Comparative Effects of Methylphenidate on ADD Girls and ADD Boys. Journal of the American Academy of Child & Adolescent Psychiatry. 1989;28:773–776. doi: 10.1097/00004583-198909000-00021. [DOI] [PubMed] [Google Scholar]
- Penades R, Catalan R, Rubia K, Andres S, Salamero M, Gasto C. Impaired response inhibition in obsessive compulsive disorder. Eur Psychiatry. 2007;22:404–10. doi: 10.1016/j.eurpsy.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Pitchers KK, Flagel SB, O'Donnell EG, Solberg Woods LC, Sarter M, Robinson TE. Individual variation in the propensity to attribute incentive salience to a food cue: Influence of sex. Behavioural brain research. 2015;278:462–9. doi: 10.1016/j.bbr.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109:160–74. [PubMed] [Google Scholar]
- Puumala T, Ruotsalainen S, Jakala P, Koivisto E, Riekkinen P, Jr, Sirvio J. Behavioral and pharmacological studies on the validation of a new animal model for attention deficit hyperactivity disorder. Neurobiol Learn Mem. 1996;66:198–211. doi: 10.1006/nlme.1996.0060. [DOI] [PubMed] [Google Scholar]
- Richards JB, Lloyd DR, Kuehlewind B, Militello L, Paredez M, Solberg Woods L, Palmer AA. Strong genetic influences on measures of behavioral-regulation among inbred rat strains. Genes Brain Behav. 2013;12:490–502. doi: 10.1111/gbb.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson ES. Blockade of noradrenaline re-uptake sites improves accuracy and impulse control in rats performing a five-choice serial reaction time tasks. Psychopharmacology (Berl) 2012;219:303–12. doi: 10.1007/s00213-011-2420-3. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Anselme P, Suchomel K, Berridge KC. Amphetamine-Induced Sensitization and Reward Uncertainty Similarly Enhance Incentive Salience for Conditioned Cues. Behav Neurosci. 2015;129:502–511. doi: 10.1037/bne0000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–73. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76(Part B):450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeding RL, Perna MK, Cummins ED, Peterson DJ, Palmatier MI, Brown RW. Sex differences in adolescent methylphenidate sensitization: effects on glial cell-derived neurotrophic factor and brain-derived neurotrophic factor. Behav Brain Res. 2014;273:139–43. doi: 10.1016/j.bbr.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Rosch KS, Fosco WD, Pelham WE, Jr, Waxmonsky JG, Bubnik MG, Hawk LW., Jr Reinforcement and Stimulant Medication Ameliorate Deficient Response Inhibition in Children with Attention-Deficit/Hyperactivity Disorder. J Abnorm Child Psychol. 2016;44:309–21. doi: 10.1007/s10802-015-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol KE, Richards JB, Broom SL, Roach JT, Hausknecht K. Effects of stimulus salience and methamphetamine on choice reaction time in the rat: central tendency versus distribution skew. Behav Pharmacol. 2003;14:489–500. doi: 10.1097/00008877-200311000-00001. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biological psychiatry. 2010;67:730–6. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci. 2013;33:13989–4000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Walkowiak J, Wilkinson A, Butcher B. Executive functioning in children with Asperger syndrome, ADHD-combined type, ADHD-predominately inattentive type, and controls. Journal of autism and developmental disorders. 2010;40:1017–27. doi: 10.1007/s10803-010-0951-9. [DOI] [PubMed] [Google Scholar]
- Sharp WS, Walter JM, Marsh WL, Ritchie GF, Hamburger SD, Castellanos FX. ADHD in Girls: Clinical Comparability of a Research Sample. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:40–47. doi: 10.1097/00004583-199901000-00018. [DOI] [PubMed] [Google Scholar]
- Solberg Woods LC, Holl K, Tschannen M, Valdar W. Fine-mapping a locus for glucose tolerance using heterogeneous stock rats. Physiological genomics. 2010a;41:102–8. doi: 10.1152/physiolgenomics.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods LC, Holl KL, Oreper D, Xie Y, Tsaih SW, Valdar W. Fine-mapping diabetes-related traits, including insulin resistance, in heterogeneous stock rats. Physiological genomics. 2012;44:1013–26. doi: 10.1152/physiolgenomics.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods LC, Stelloh C, Regner KR, Schwabe T, Eisenhauer J, Garrett MR. Heterogeneous stock rats: a new model to study the genetics of renal phenotypes. American journal of physiology Renal physiology. 2010b;298:F1484–91. doi: 10.1152/ajprenal.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Coghill D, Markowitz JS, Swanson JM, Vandenberghe M, Hatch SJ. Sex differences in the response of children with ADHD to once-daily formulations of methylphenidate. J Am Acad Child Adolesc Psychiatry. 2007;46:701–10. doi: 10.1097/chi.0b013e31804659f1. [DOI] [PubMed] [Google Scholar]
- Spencer SV, Hawk LW, Jr, Richards JB, Shiels K, Pelham WE, Jr, Waxmonsky JG. Stimulant treatment reduces lapses in attention among children with ADHD: the effects of methylphenidate on intra-individual response time distributions. J Abnorm Child Psychol. 2009;37:805–16. doi: 10.1007/s10802-009-9316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Waldman I, Newcorn J, Bishop J, Kittles R, Cook EH., Jr Dopamine transporter genotype and stimulant dose-response in youth with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2014;24:238–44. doi: 10.1089/cap.2013.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand MT, Hawk LW, Jr, Bubnik M, Shiels K, Pelham WE, Jr, Waxmonsky JG. Improving working memory in children with attention-deficit/hyperactivity disorder: the separate and combined effects of incentives and stimulant medication. J Abnorm Child Psychol. 2012;40:1193–207. doi: 10.1007/s10802-012-9627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares H, Gentil V. Pathological gambling and obsessive-compulsive disorder: towards a spectrum of disorders of volition. Rev Bras Psiquiatr. 2007;29:107–17. doi: 10.1590/s1516-44462007000200005. [DOI] [PubMed] [Google Scholar]
- Tomie A, Lincks M, Nadarajah SD, Pohorecky LA, Yu L. Pairings of lever and food induce Pavlovian conditioned approach of sign-tracking and goal-tracking in C57BL/6 mice. Behavioural brain research. 2012;226:571–578. doi: 10.1016/j.bbr.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Dow-Edwards DL. Repeated administration of methylphenidate in young, adolescent, and mature rats affects the response to cocaine later in adulthood. Psychopharmacology (Berl) 2005;181:38–47. doi: 10.1007/s00213-005-2221-7. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) 2006;187:73–85. doi: 10.1007/s00213-006-0396-1. [DOI] [PubMed] [Google Scholar]
- Weafer J, de Wit H. Inattention, impulsive action, and subjective response to d-amphetamine. Drug Alcohol Depend. 2013;133:127–133. doi: 10.1016/j.drugalcdep.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D, Johnston C. Gender differences in adults with attention-deficit/hyperactivity disorder: A narrative review. Clinical Psychology Review. 2015;40:15–27. doi: 10.1016/j.cpr.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav Brain Res. 2010;214:30–4. doi: 10.1016/j.bbr.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–7. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


