Abstract
Rationale
Activation of Nox1 initiates redox-dependent signaling events crucial in the pathogenesis of vascular disease. Selective targeting of Nox1 is an attractive potential therapy but requires a better understanding of the molecular modifications controlling its activation.
Objective
To determine the whether posttranslational modifications of Nox1 regulate its activity in vascular cells.
Methods and Results
We first found evidence that Nox1 is phosphorylated in multiple models of vascular disease. Next, studies using mass spectroscopy and a pharmacological inhibitor demonstrated that protein kinase C-beta1 (PKC-βI) mediates phosphorylation of Nox1 in response to tumor necrosis factor-α (TNF-α). siRNA-mediated silencing of PKC-βI abolished TNF-α-mediated reactive oxygen species (ROS) production and vascular smooth muscle cell (VSMC) migration. Site-directed mutagenesis and isothermal titration calorimetry indicated that PKC-βI phosphorylates Nox1 at T429. Moreover, Nox1 T429 phosphorylation facilitated the association of Nox1 with the NoxA1 activation domain and was necessary for NADPH oxidase complex assembly, ROS production, and VSMC migration.
Conclusions
We conclude that PKC-βI phosphorylation of T429 regulates activation of Nox1 NADPH oxidase.
Keywords: NADPH oxidase, vascular smooth muscle cells, atherosclerosis, neointima, TNF-α, PKCβ
INTRODUCTION
Nox1 serves as the catalytic core of a multi-subunit NADPH oxidase enzyme complex, which assembles in response to signaling cascades initiated by mechanical stress, cytokines and growth factors. Nox1 is a transmembrane protein expressed in multiple tissues including vascular smooth muscle cells (VSMCs), brain, gastrointestinal epithelium, and prostate tumor cells.1–4 Nox1 plays a critical role in the development of cardiovascular disease, amyotropic lateral sclerosis, gastrointestinal disease, immunological disorders, and multiple forms of cancer.5–13
Since its discovery in 1999, multiple studies have provided evidence that activation of Nox1 is a multi-step process that requires assembly of a complex of proteins.14 Nox1 associates with the transmembrane protein p22phox for stability and membrane localization. The recruitment of cytosolic proteins to the membrane forms a complex which allows electron transfer from NADPH to oxygen to form superoxide.15–17 When activated, the organizer cytosolic protein p47phox or its homolog NoxO1 tethers to p22phox.18–21 Recruitment of the activator p67phox or its homolog NoxA1 is mediated via tail-to-tail binding to the organizer protein.22, 23 Mutation of the “activation domain” of NoxA1 abrogates Nox1-generated reactive oxygen species (ROS).24 However, the molecular interaction of Nox1 with the activation domain of NoxA1 is not known. Phosphorylation of NoxA1 allows for dissociation from Nox1 and is one mechanism to terminate enzyme activity.25 Whether post-translational modifications of Nox1 regulate its activation has not been explored.
The goal this study was to examine molecular mechanisms of Nox1 activation. Our data reveal that protein kinase C-βI (PKC-βI) phosphorylation of Nox1 at T429 is necessary for its interaction with NoxA1 activation domain, complex assembly, and generation of superoxide. Furthermore, mutation of T429 prevents Nox1-mediated VSMC migration. These findings identify a novel regulatory mechanism by which Nox1 is activated.
METHODS
Phosphorylation of Nox1 and its role in Nox1 activation was assessed in cultured aortic VSMCs (C57Bl6/J mouse, Nox1-/y mouse, A7r5 rat) and in Cos7 cells that stably express p22phox or p22phox with p47phox and p67phox (CosPhox). For details on animal models, cell models and transfection protocol, detection of Nox1 phosphorylation, measurement of reactive oxygen species, construction of Nox1 mutants, detection of NADPH oxidase complex assembly, isothermal titration calorimetry assays, circular dichroism analysis, and computational modeling, an expanded methods section is available in the online Supplemental Material.
RESULTS
Nox1 phosphorylation is increased in multiple models of vascular disease
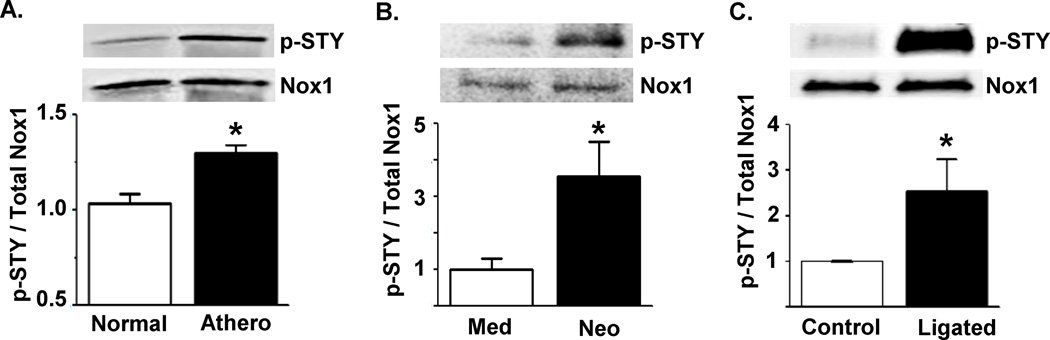
Phosphorylation is a common mechanism for post-translational regulation of protein activity. Using an antibody that detects phosphorylation at serine, threonine and tyrosine residues (anti-STY), we examined whether Nox1 is phosphorylated under conditions known to be associated with increased Nox1 activity. Specifically, we immunoprecipitated with anti-p22phox and blotted with anti-STY or anti-Nox1 in three models of vascular disease. First, we detected increased levels of Nox1 phosphorylation in aorta from monkeys fed an atherogenic diet as compared to normal diet (Figure 1A, Online Figure I). Next, we found elevated Nox1 phosphorylation in cultured VSMCs derived from the neointima of balloon-injured rat aorta as compared to medial VSMCs (Figure 1B). Using a murine carotid injury model known to induce neointimal hyperplasia, Nox1 phosphorylation was significantly increased as compared to contralateral non-injured arteries (Figure 1C). These results provide evidence of Nox1 phosphorylation in response to vascular injury.
Figure 1. Nox1 phosphorylation is increased in multiple models of vascular disease.
Nox1 phosphorylation was assessed by subjecting lysates to immunoprecipitation with anti-p22phox followed by Western blotting with either anti-STY or anti-Nox1. (A) Aorta from monkeys fed a normal or atherogenic (Athero) diet. (B) Cultured medial (Med) and neointimal (Neo) VSMCs derived from rat aorta 14 days following balloon injury. (C) Murine carotid artery 3 days postligation. n=3–5 per group. *p<0.05 as compared to non-diseased.
Nox1 is activated by protein kinase C-βI phosphorylation
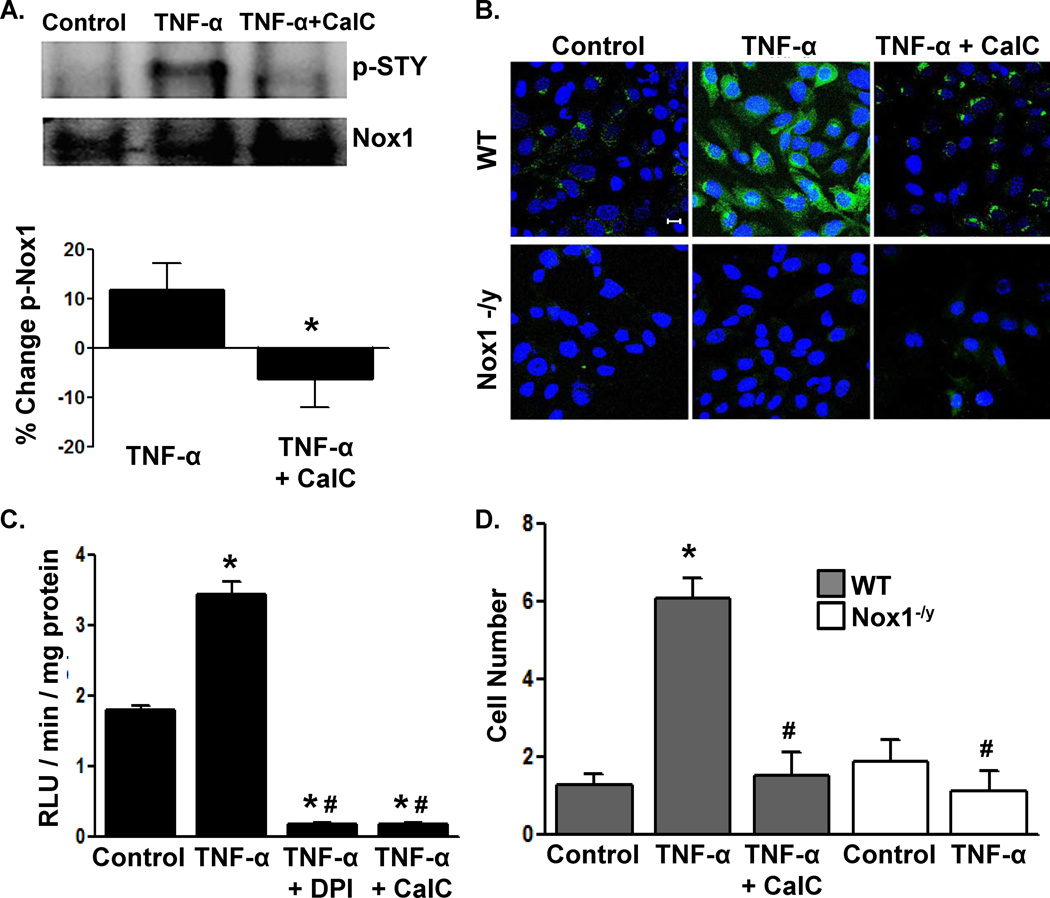
We next used NetPhosK sequence analysis software to identify putative kinases that phosphorylate Nox1. Of the top 10 predicted phosphorylation sites, PKC was the predicted kinase for 7 of those sites (Online Figure II). Therefore, we examined whether inhibition of PKC with Calphostin C (CalC) 26 modifies Nox1 phosphorylation following stimulation with tumor necrosis factor-α (TNF-α), which is a known activator of Nox1.27, 28 Treatment of cultured A7r5 rat aortic VSMCs with TNF-α increased Nox1 phosphorylation (Figure 2A) similar to levels seen in vivo (Figure 1). CalC inhibited TNF-α-stimulated Nox1 phosphorylation. Consistent with reports that multiple agonists are capable of Nox1 activation, we observed that, in addition to TNF-α, phosphorylation of VSMC Nox1 occurs following treatment with PDGF-BB and PMA (Online Figure III). It also appears that the timing of phosphorylation may be agonist dependent (Online Figure III).
Figure 2. PKC inhibition abolishes TNF-α-mediated Nox1 phosphorylation, ROS production and VSMC migration.
The effect of Calphostin C (CalC) pretreatment on TNF-α-mediated (A) Nox1 phosphorylation in rat VSMCs by Western blotting with anti-STY as in Figure 1, (B) CMH 2DCF fluorescence (green) in murine VSMCs, (C) lucigenin-enhanced chemiluminescence (RLU: relative light units) in rat VSMCs, and (D) migration of murine VSMCs. In (B), nuclei were counterstained with ToPro3; scale bar = 10 µm. n=3 in A and 5–15 in C, D. *p<0.05 vs. control, #p<0.05 vs. WT TNF-α-treated.
Next, TNF-α caused robust ROS production in WT but not Nox1-/y VSMCs (Figure 2B), confirming that the TNF-α-dependent generation of ROS is Nox1-dependent.27, 28 CalC abrogated Nox1-dependent ROS production in WT VSMCs (Figure 2B). Analysis of VSMC membrane fractions from WT cells demonstrated that TNF-α pre-treatment primes NADPH oxidase activity (Figure 2C). This effect was completely abolished by either the flavoenzyme inhibitor DPI or CalC (Figure 2C). Thus, PKC activity is required for Nox1 generation of ROS.
Nox1 has been implicated in VSMC migration to multiple agonists. 29–32 Using Nox1-/y VSMCs, we first established that VSMC migration to TNF-α requires Nox1 (Figure 2D). Similar to the effects of PKC inhibition on ROS production, migration of WT VSMCs was blocked with CalC (Figure 2D). Taken together, these data demonstrate that PKC is necessary for TNF-α-mediated redox-dependent migration.
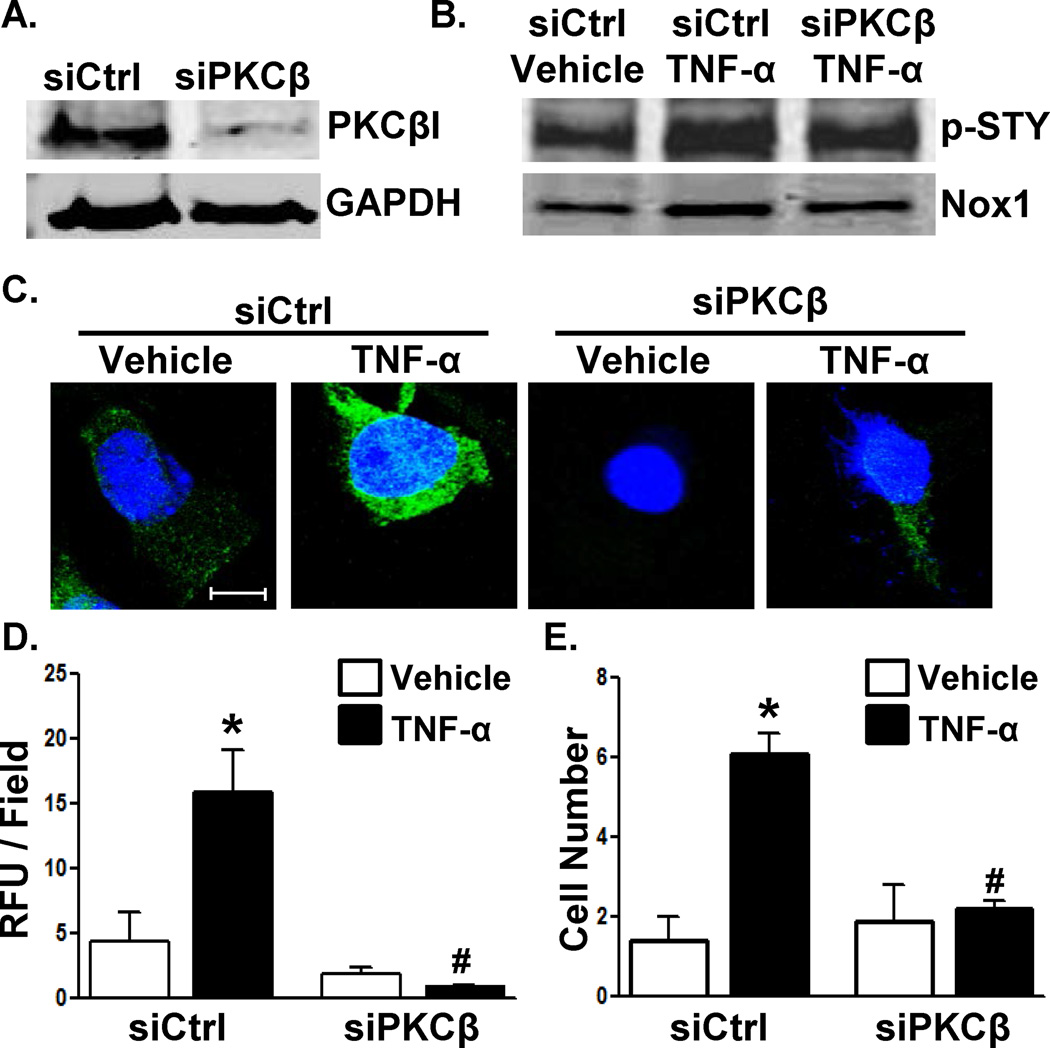
Mass spectrometry identified the interaction of PKC-βI with the Nox1-p22phox complex in response to TNF-α treatment of VSMCs (data not shown). PKC-βI and II are splice variants from the same gene and are both reported to be expressed in mice and humans.33 Western blotting demonstrated expression of PKC-βI but not PKC-βII in WT VSMCs (Online Figure IV). Using an siRNA against PKC-β (siPKC-β), we achieved significant knockdown of PKC-βI expression in WT VSMCs (Figure 3A). Silencing PKC-β resulted in partial inhibition of TNF-α-induced Nox1 phosphorylation (Figure 3B) and near complete abrogation of ROS production (Figure 3C), NADPH oxidase activity (Figure 3D), and VSMC migration (Figure 3E). These finding suggest that PKC-βI is the kinase that regulates Nox1 NADPH oxidase activation.
Figure 3. Knockdown of PKCβ prevents TNF-α-mediated VSMC activation.
(A) Validation of PKCβI silencing at the protein level. WT murine VSMCs were treated with control (Ctrl) or PKCβ siRNA followed by treatment with TNF-α and assessment of (B) Nox1 phosphorylation (C,D) CM-H2DCF fluorescence (RFU: relative fluorescent units; n=4–7; scale bar = 10 µm), and (E) migration (n=10–15) as in Figure 2. In (C), nuclei were counter-stained with ToPro3 (blue). *p<0.05 vs. vehicle; #p<0.05 vs. siCtrl TNF-α-treated.
PKC-βI phosphorylation of T429 is necessary for Nox1 activation
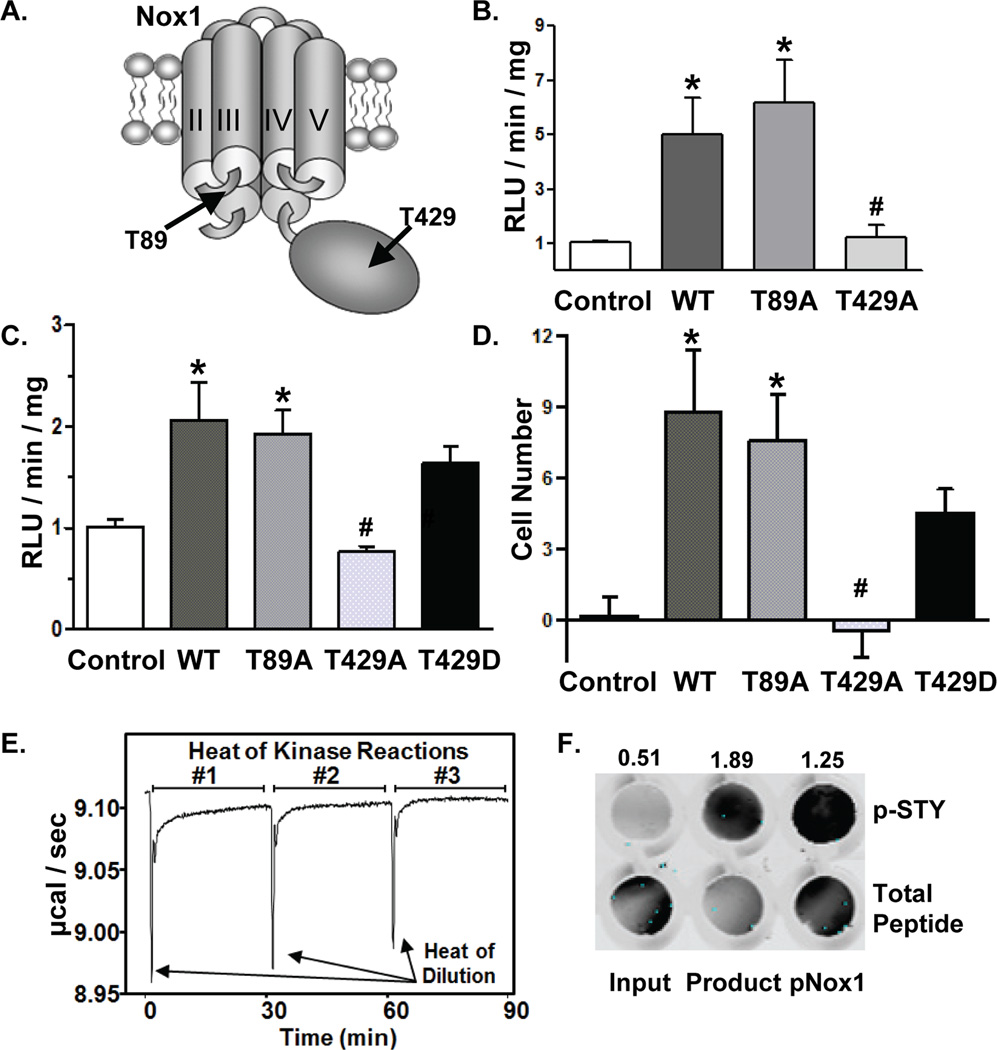
NetPhosK prediction algorithm identified several PKC consensus phosphorylation sites in Nox1 (Online Figure II). Based on the NetPhosK score, the conservation of the putative phosphorylation sites between mouse, rat, and human (Online Figure V), and their location within intracellular regions (Online Figure V and Figure 4A), we evaluated T89 and T429. T89 is located in the first intracellular loop between transmembrane domains I and II, whereas T429 is in the C-terminal region (Figure 4A).
Figure 4. Phosphorylation of Nox1 T429 by PKCβI is required for TNF-α-induced ROS production and VSMC migration.
(A) Relative locations of T89 and T429 in Nox1. (B) CosPhox cells or (C) Nox1-/y VSMCs expressing the indicated Nox1 mutants were treated with TNF-α, followed by measurement of ROS in membrane fractions by NADPH-stimulated chemiluminescence. n=4 independent experiments. (D) Migration of Nox1-/y VSMCs expressing the indicated Nox1 mutants to TNF-α. The number of cells migrating under non-stimulated conditions was subtracted from TNF-α-stimulated migration for each group. n=5–10 independent experiments. *p<0.05 vs. mock-transfected (control) cells. #p<0.05 vs. WT-transfected cells. (E) ITC measurement of in vitro kinase reaction of recombinant PKC-βI with Nox1 peptide (KLKTQKIYF). (F) To confirm PKC-Βi phosphorylation of the Nox1 peptide, dot blot analysis of in vitro kinase assay input and product was performed using anti-p-STY. Phosphorylated Nox1 peptide (pNox1, KLKT*QKIYF). Total peptide levels were determined by Ponceau staining. Data were quantitated as the ratio of p-STY to total peptide using Odyssey imaging software and are indicated above the dot blot.
We first mutated T89 and T429 to alanine to prevent phosphorylation and confirmed that mutation does not disrupt protein expression. Using Flag-tagged constructs (T89A, T429A, or WT Nox1), we validated expression by Western blotting and immunofluorescence in CosPhox cells that express p22phox, p47phox, and p67phox but lack Nox1,34 and in Nox1-/y VSMCs, which express p22phox, p47phox, and NoxA135 (Online Figure VI). However, functional analysis demonstrated that the C-terminal epitope tag interfered with ROS production by WT Nox1 (data not shown). Thus, subsequent studies utilized non-tagged Nox1 mutants.
We next examined whether Nox1 phosphorylation at T89 or T429 is required for Nox1 NADPH oxidase activity following TNF-α stimulation. Expression of WT Nox1 in CosPhox (Figure 4B) or Nox1-/y VSMCs (Figure 4C) resulted in the anticipated NADPH-stimulated superoxide production as measured by lucigenin-enhanced chemiluminescence in membrane-enriched fractions. Whereas superoxide production in cells expressing T89A Nox1 was similar to WT levels, expression of T429A Nox1 returned superoxide to control levels. Next, we determined whether T429 is also required for TNF-α-induced VSMC migration. As with superoxide production, migration was similar in Nox1-/y VSMCs expressing either WT or T89A Nox1 (Figure 4D). By contrast, no migration was observed in cells transfected with T429A Nox1. In addition, expression of a phosphomimetic T429D Nox1 mutant resulted in superoxide production (Figure 4C) and cell migration (Figure 4D), approaching levels observed with WT Nox1. These data are consistent with a negative charge at T429 as necessary for Nox1 enzyme activity and VSMC migration following stimulation with TNF-α.
To directly evaluate whether T429 Nox1 is a bona fide PKC-βI phosphorylation site, we performed an in vitro kinase assay using human recombinant PKC-βI and a Nox1 peptide containing T429 (KLKTQKIYF). We used isothermal titration calorimetry (ITC) to measure the heat generated by phosphorylation of the peptide. The slow return to baseline that occurs following the heat of dilution confirms that the Nox1 peptide is a substrate for PKC-βI (Figure 4E). The reduction in heat produced in subsequent reactions suggests product inhibition, that is inhibition of PKC-βI by the phosphorylated Nox1 peptide (Figure 4F). This substantial product inhibition precluded measurement of kinetic parameters.36 In addition, dot blot analysis of the kinase reaction using p-STY antibody confirms phosphorylation of the Nox1 peptide within the reaction mixture (Figure 4F). These results provide direct evidence that PKC-βI phosphorylates Nox1 at T429.
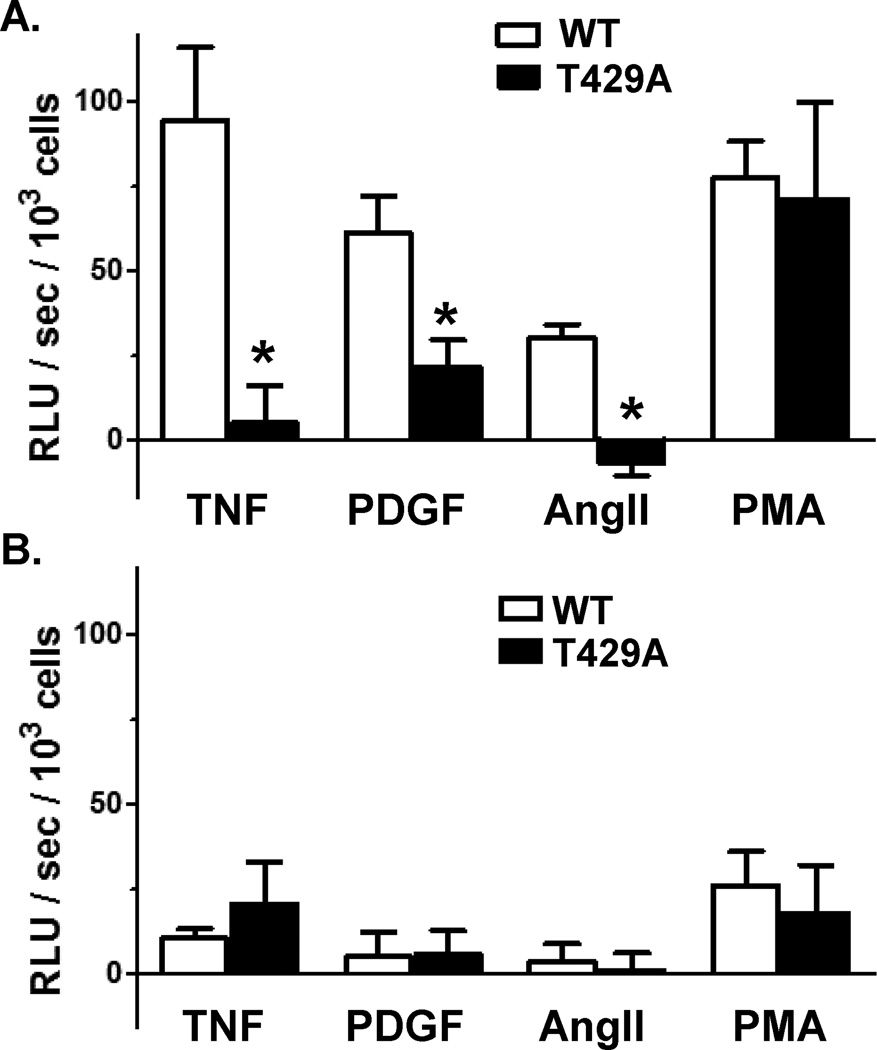
In response to various stimuli, the cytosolic subunit p47phox or its homolog NoxO1 organizes the translocation and association of NoxA1 with the Nox1/p22phox complex at the membrane. We first measured agonist-stimulated superoxide production in intact CosPhox cells expressing p22phox, NoxA1 and p47phox. In cells co-expressing WT Nox1, TNF-α, PDGF-BB, AngII and PMA all increased cellular levels of superoxide (Figure 5A). In the presence of the T429A Nox1 phospho-mutant, superoxide levels in response to TNF-α, PDGF-BB, and AngII were markedly attenuated. Measurements of agonist-dependent cellular superoxide were repeated in CosPhox cells expressing p22phox, NoxA1 and NoxO1. Under these conditions, activation of Nox1 was less than that observed with NoxA1/p47phox-expressing cells (Figure 5B), which is consistent with a previous report.16
Figure 5. Nox1 T429 is required for receptor-mediated ROS production by Nox1.
CosPhox cells were transfected with (A) p47phox and NoxA1 or (B) NoxO1 and NoxA1 and transfected with wild-type (WT) or the T429A mutant Nox1. Superoxide levels were measured in intact cells immediately following stimulation with TNF-α (10 ng/ml), platelet-derived growth factor (PDGF)-BB (10 ng/ml), angiotensin II (AngII, 10-7 M) or phorbol myristate acetate (PMA, 10-6 M). Relative light units (RLU) per second (sec) of the unstimulated sample was subtracted from the agonist treated sample and data normalized to number of cells. n=4 independent experiments. *p<0.05 vs. WT.
T429 phosphorylation facilitates the association of NoxA1 activation domain with Nox1
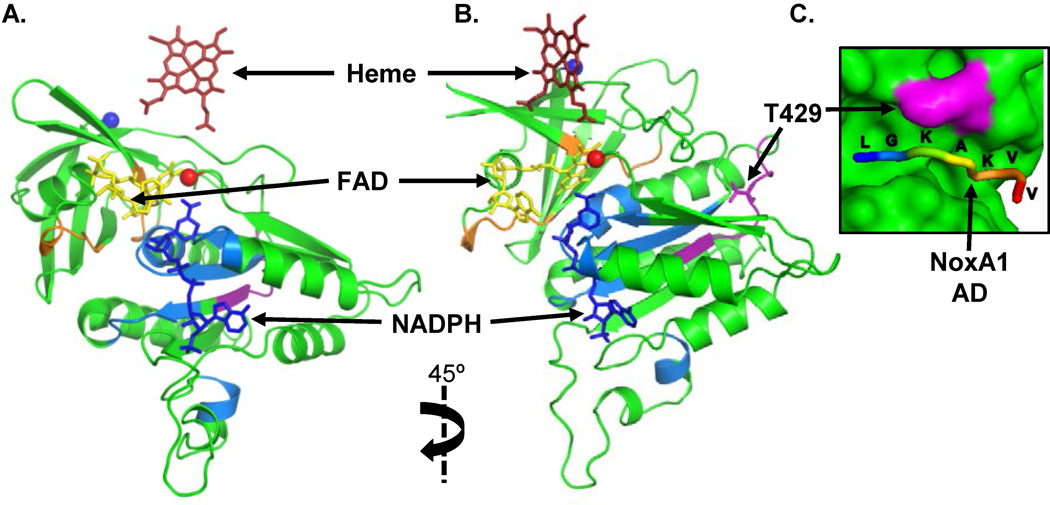
Based on our observation that the combination of p47phox and NoxA1 with Nox1 are dependent on phosphorylation of Nox1 T429, we studied the association of these subunits. This interaction at the membrane involves the association of the NoxA1 activation domain (AD) with the C-terminus of Nox1,18, 23, 34 though the mechanism is incompletely defined. Since the structure of Nox1 has not yet been solved, we used homology modeling to determine whether the position of T429 within the C-terminal domain might facilitate the interaction of Nox1 with cytosolic subunits. Using the cytochrome B5 reductase crystal structure for the FAD domain (PDB ID 2EIX) and the Nox2 crystal structure for the NADPH domain (PDB ID 3A1F) as templates for our model, we found that T429 resides in an unstructured loop on the external surface of the Nox1 cytosolic domain (Figure 6A, B). The position of T429 suggested a potential interaction site with NoxA1. The NoxA1 AD is also in an unstructured loop region as demonstrated by partial crystal structures of NoxA1 that contain the AD.37 Computational docking of the NoxA1 AD peptide with Nox1 consistently demonstrates its occupancy in a long groove near T429 (Figure 6C). Based on these observations, we hypothesized that phosphorylation of Nox1 at T429 is necessary for the interaction with NoxA1 AD.
Figure 6. Homology model of Nox1 cytosolic C-terminus computationally docked with NoxA1 activation domain.
(A, B) Ribbon diagram of Nox1 C-terminus modeled based on the crystal structures of the FAD domain in cytochrome B5 reductase (PDB ID 2EIX) and the NADPH domain in Nox2 (PDB ID 3A1F). Co-factors from template structures positioned within the Nox1 cytosolic C-terminal domain are noted by arrows. The N-terminus that attaches to the transmembrane domain is depicted as a blue sphere, and the extreme C-terminus of Nox1 is depicted as a red sphere. Residues involved in NADPH binding are depicted in blue, and residues involved in FAD binding are depicted in orange. The ribbon diagram in (B) is rotated 45° clockwise to reveal residues used to create the Nox1 peptide (purple) for binding experiments, including T429. (C) Proposed interaction of the NoxA1 AD with Nox1 T429. The NoxA1 AD peptide (shown as the backbone with residues labeled) was computationally docked to the Nox1 cytosolic C-terminal domain (accessible surface area in green with T429 depicted in purple).
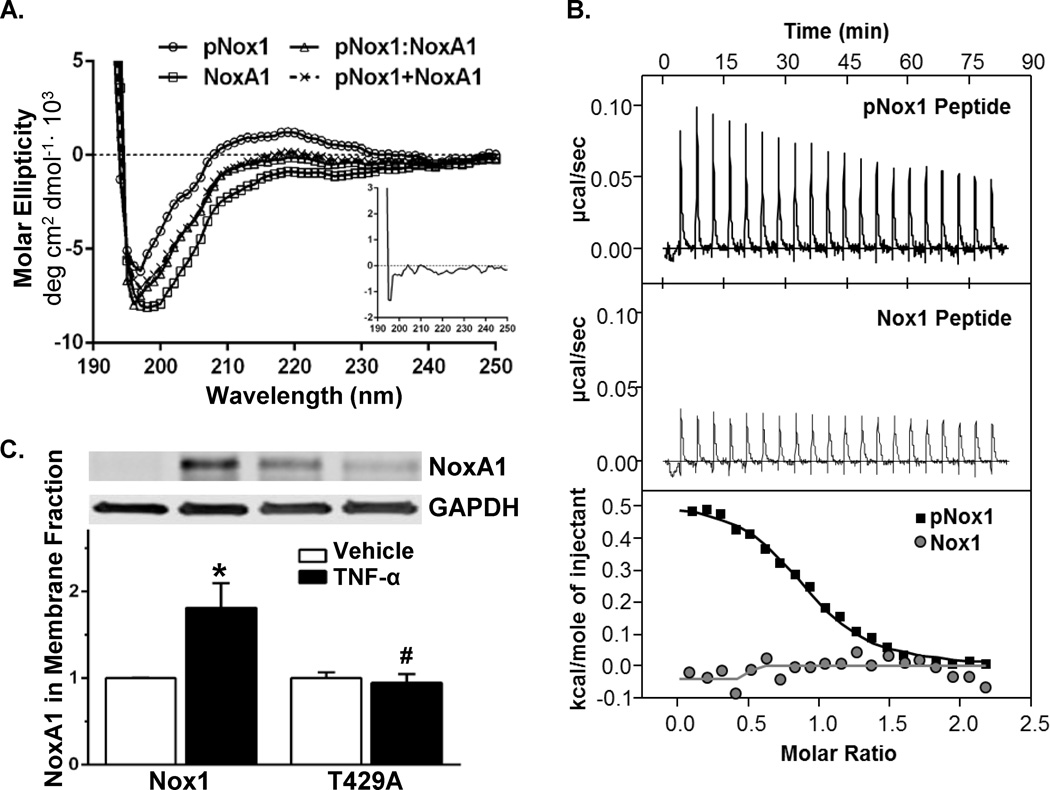
Using peptides containing phosphorylated Nox1 at T429 (pNox1) and the NoxA1 AD, we first demonstrated by circular dichroism (CD) that these peptides are unstructured. Specifically, these peptides lack the characteristic peaks indicative of α-helices and β-sheets (Figure 7A). The presence of a negative peak below 200 nm and near zero shoulders at longer wavelengths (210–240 nm) demonstrates unstructured peptides. Furthermore, analysis of the complex of pNox1:NoxA1 peptides suggests subtle structural changes upon binding without evidence of secondary structure (Figure 7A, inset).
Figure 7. Interaction of phosphorylated T429 of Nox1 with the NoxA1 activation domain is required for NoxA1 membrane recruitment.
(A) Structural analysis of NoxA1 AD and phospho- Nox1 (pNox1) peptides by circular dichroism. Peptides for pNox1 and NoxA1 AD displayed characteristic random coil signals (negative dip below 200 nm and flat, near zero shoulders in the 210–220 nm range). Complex formation of the pNox1 peptide with NoxA1 AD peptide (pNox1:NoxA1) did not induce order and the circular dichroism signal is similar to that obtained by addition of the individual peptide signals (pNox1+NoxA1). Inset shows the residual curve of the pNox1:NoxA1 complex after subtraction of the individual components, indicating no major conformational changes of these peptides on complex formation. (B) Affinity and stoichiometry for the interaction of NoxA1 AD with pNox1 (top panel) and an unphosphorylated Nox1 peptide (middle panel) as measured by ITC. Lower panel, analyzed binding data for both pNox1 and Nox1 binding to NoxA1 AD. Kd= >100 µM for unphosphorylated Nox1 peptide (stoichiometry N.D.) and 1.5±0.3 µM for pNox1 peptide (stoichiometry of 0.86±0.06; n=3 independent experiments). (C) Membrane recruitment of NoxA1 was assessed in CosPhox cells expressing p22phox, NoxA1, and WT or T429A Nox1. After treating with TNF-α, membrane fractions isolated and blotted for NoxA1. Whole cell lysates (prior to membrane isolation) were probed with anti-GAPDH. n=4 independent experiments. *p<0.05 vs. Nox1 vehicle; #p<0.05 vs. Nox1 TNF-α-treated.
In order to determine whether Nox1AD directly interacts with Nox1 phosphorylated at T429, we used ITC to compare the affinity of the Nox1AD peptide with either the pNox1 peptide or a corresponding unphosphorylated Nox1 peptide (Figure 7B). NoxA1 had no measurable interaction with the unphosphorylated Nox1 peptide (affinity >100 µM, stoichiometry N.D.), whereas its affinity for the pNox1 peptide was 1.5±0.3 µM with a stoichiometry of 0.86±0.06 (Figure 7B, n=3). These results indicate that phosphorylation of T429 mediates the interaction with the activation domain of NoxA1.
We next validated this interaction in an intact biological system. We assessed NoxA1 localization to the membrane in cells expressing either WT or T429A Nox1. In CosPhox cells expressing p22phox, p47phox, and NoxA1, the expression of WT Nox1 resulted in the anticipated TNF-α-induced recruitment of NoxA1 to the membrane (Figure 7C). By contrast, mutation of T429 to alanine prevented NoxA1 membrane translocation following TNF-α. However, the phosphomimetic Nox1 T429D mutant promoted NoxA1 membrane recruitment under non-stimulated conditions (Online Figure VII). As expected, WT but not T429A Nox1 caused a recruitment of p47phox to the membrane in response to TNF-α treatment (Online Figure VIII). These data provide additional support for NoxA1 binding to phosphorylated T429 Nox1 in the mechanism of NADPH oxidase activation.
DISCUSSION
Activation of Nox1 NADPH oxidase requires association with cytosolic proteins that function to organize the complex and activate the enzyme to produce superoxide. In this study, we identify for the first time that Nox1 activation is regulated by post-translational modification of the C-terminal region of Nox1. Our data demonstrate that phosphorylation of Nox1 at T429 by PKC-βI is necessary for TNF-α-mediated redox signaling and migration. Homology modeling combined with ITC revealed that Nox1 T429 phosphorylation facilitates association with the activation domain of NoxA1. Moreover, inhibition of T429 phosphorylation prevents recruitment of the cytosolic subunits to the membrane. Together with the observation that Nox1 is phosphorylated in multiple models of vascular disease, our findings suggest that strategies to inhibit Nox1 phosphorylation may mitigate its role in the pathogenesis of vascular disease.
Nox1 NADPH oxidase complex assembly is organized by p47phox. The phosphoinositide-binding (PX) domain of p47phox mediates membrane association, the Src homology 3 (SH3) domains interact with p22phox, and the proline rich (PR) domain interacts with the SH3 domain of NoxA1 or p67phox. 38, 39 p47phox and NoxA1 associate in the cytosol under basal conditions. Phosphorylation of p47phox releases binding of an auto-inhibitory domain,18, 19, 38 allowing translocation of the p47phox/NoxA1 complex to the Nox1/p22phox complex, positioning the NoxA1 AD with the C-terminus of Nox1.18, 23, 34 NoxO1, a homolog of p47phox lacking the auto-inhibitory domain, appears to colocalize with Nox1 in resting cells at the membrane via its PX domain.20, 40 Phosphorylation of each of the cytosolic subunits has been implicated in regulating complex assembly.20, 41–47
In contrast to the cytosolic subunits, less is known regarding phosphorylation of the catalytic subunits. Our study provides the first evidence for the phosphorylation of Nox1. The Nox2 C-terminal domain (within residues 321–405 and 466–570) has recently been shown to be phosphorylated at serine and threonine residues.48 Similar to Nox1, phosphorylation of Nox2 was associated with increased ROS production and complex assembly in response to agents that stimulate PKC. However, there appear to be important differences in phosphorylation-mediated activation of Nox1 and Nox2. First, despite significant homology in surrounding residues, the T429 we show to be phosphorylated on Nox1 is not conserved in Nox2. Second, although the specific residue phosphorylated on Nox2 is not identified, the investigators propose that Ser333, Thr509, and Ser550 are the most likely phosphorylation sites. Interestingly, Ser333 and Thr509, but not Ser550, are conserved between Nox2 and Nox1. Third, phosphorylation of Nox5 in the FAD domain (T494/S498) has also been shown to regulates its activity;49 although the mechanism is not clear, it will be distinct from that of Nox1 and Nox2 since Nox5 does not require complex assembly for activation.
Nox1 activation is important in mediating multiple cellular pathways involved in the pathogenesis of vascular disease.14 Our data demonstrate that migration of cultured VSMCs to TNF-α requires Nox1. Cell migration is also regulated by the PKC family of serine/threonine kinases that include PKC-βI,50 suggesting a functional link between Nox1 activation and PKC phosphorylation. Providing direct evidence for PKC-βI phosphorylation of Nox1, we found that recombinant PKC-βI phosphorylates a Nox1 peptide containing T429 in vitro. Moreover, loss of Nox1 phosphorylation at T429 or the knock down of PKC-βI is sufficient to inhibit ROS production and cell migration. In contrast, the knock down of PKC-βI only partially inhibited TNF-α-induced Nox1 phosphorylation, indicative of phosphorylation of Nox1 by other kinases. In support of this interpretation, Nox1 contains residues that are homologous with proposed Nox2 phosphorylation sites.48
Our data provide evidence that Nox1 phosphorylation is a general mechanism underlying its activation in response to multiple agonists. Specifically, we observed increased ROS production in cells expressing WT but not T492A Nox1 following stimulation with TNF-α, PDGF-BB or AngII. Agonist-dependent activation of Nox1 resulted in greater superoxide production in cells co-expressing NoxA1 with p47phox as compared to its homolog NoxO1, which is consistent with previous reports.16 Whereas PMA induces Nox1 phosphorylation, the generation of superoxide does not require phosphorylation of T429. We speculate that this observation is likely results from the different activation mechanisms between the receptor-dependent agonists and PMA.
Until now, the molecular mechanism whereby Nox1 interacts with the NoxA1 AD was not known. Using ITC, we provide evidence that phosphorylation of Nox1 T429 increases the association of this region to the NoxA1 AD by more than a hundred-fold. Furthermore, the T429A mutant is not able to sustain association of the p47phox/NoxA1 complex with the Nox1/p22phox complex, whereas expression of the phosphomimetic T429D mutant results in NoxA1 membrane recruitment under non-stimulated conditions. We propose that assembly of NoxA1 to Nox1 positions its activation domain within a long groove adjacent to the T429 of Nox1. Taken together, our data suggest that a negative change associated with phosphorylation at T429 is the principle mechanism that stabilizes NoxA1 with the membrane complex.
In conclusion, Nox1 requires phosphorylation at T429 for complex assembly, ROS generation and VSMC migration. Our data support a mechanism by which PKC-βI phosphorylation of Nox1 T429 facilitates interaction and stabilization of the NoxA1 AD with Nox1. Furthermore, we provide the first computational model of the Nox1 C-terminus and propose that the NoxA1 AD is positioned in a long groove near T429. In combination with our findings that Nox1 is phosphorylated in atherosclerosis, VSMC dedifferentiation, and neointimal formation, we identify the phosphorylation of Nox1 as a new target for effective and directed therapy of vascular disease.
Supplementary Material
Novelty and Significance.
What Is Known?
The Nox1-containing NADPH oxidase contributes to the pathogenesis of multiple diseases, including cardiovascular disease.
Activation of the catalytic subunit Nox1 and subsequent generation of superoxide is a multi-step process that requires assembly of a complex of proteins.
What New Information Does This Article Contribute?
Phosphorylation of Nox1 is increased in a variety of animal models of vascular disease.
Phosphorylation of Nox1 by protein kinase C-βI (PKC-βI) at threonine 429 (T429) is necessary for the interaction of Nox1 with NoxA1 activation domain (AD).
In vascular smooth muscle cells, phosphorylation of Nox1 at T429 is necessary for agonist-mediated superoxide generation and cell migration.
Generation of superoxide by the Nox1 NADPH oxidase propagates redox-dependent signaling events essential to the development of hypertension, restenosis, and atherosclerosis. Activation of Nox1 requires interaction with cytosolic proteins. The molecular events necessary for Nox1 activation, in particular the mechanism of interaction of Nox1 with the NoxA1 AD, remain unclear. In this study, we examined the mechanism and consequences of Nox1 phosphorylation. Site-directed mutagenesis and isothermal titration calorimetry (ITC) demonstrate that PKC-βI phosphorylates Nox1 at T429, which resides in an unstructured loop on the external surface of the cytosolic domain. Phosphorylation of Nox1 at T429 is necessary for complex assembly, superoxide production, and VSMC migration. Furthermore, Nox1 T429 phosphorylation facilitates the association of Nox1 with the NoxA1 AD. These data suggest that post-translational modification of the C-terminal region of Nox1 regulates its activity and that PKC-βI-mediated T429 phosphorylation of Nox1 may be a novel target for directed therapy of vascular disease.
Acknowledgments
The authors acknowledge the DNA Core Facility and the Mass Spectrometry Core Facility in the University of Iowa Roy J. and Lucille A. Carver College of Medicine. We thank Kristina W. Thiel for assistance in manuscript preparation.
SOURCES OF FUNDING
This work was supported by the Office of Research and Development, Department of Veterans Affairs [1BX001729 to F.J.M.]; the National Institutes of Health [HL081750 to F.J.M. and CA136729 to J.C.D.H.]; and the American Heart Association [POST7640011 to S.J. and PRE12060526 to J.S.].
Nonstandard Abbreviations and Acronyms
- AD
activation domain
- AngII
angiotensin II
- Athero
atherogenic diet
- CalC
Calphostin C
- CD
circular dichroism
- ITC
isothermal titration calorimetry
- Med
Cultured medial VSMCs
- Neo
Cultured neointimal VSMCs
- PDGF
platelet derived growth factor
- PKC-βI
protein kinase C-beta1
- PMA
phorbol myristate acetate
- pNox1
peptide containing phosphorylated Nox1 at T429
- PR
proline rich domain
- PX
phosphoinositide-binding domain
- RFU
relative fluorescent units
- RLU
relative light units
- ROS
reactive oxygen species
- SH3
Src homology 3 domain
- TNF-α
tumor necrosis factor-α
- VSMCs
vascular smooth muscle cells
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Fukui T, Lassegue B, Kai H, Alexander RW, Griendling KK. Cytochrome b-558 alpha-subunit cloning and expression in rat aortic smooth muscle cells. Biochimica Et Biophysica ACTA. 1995;1231:215–219. doi: 10.1016/0005-2728(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 2.Reinehr R, Gorg B, Becker S, Qvartskhava N, Bidmon HJ, Selbach O, Haas HL, Schliess F, Haussinger D. Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. Glia. 2007;55:758–771. doi: 10.1002/glia.20504. [DOI] [PubMed] [Google Scholar]
- 3.Rokutan K, Kawahara T, Kuwano Y, Tominaga K, Sekiyama A, Teshima-Kondo S. NADPH oxidases in the gastrointestinal tract: a potential role of Nox1 in innate immune response and carcinogenesis. Antioxid Redox Signal. 2006;8:1573–1582. doi: 10.1089/ars.2006.8.1573. [DOI] [PubMed] [Google Scholar]
- 4.Lim SD, Sun C, Lambeth JD, Marshall F, Amin M, Chung L, Petros JA, Arnold RS. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005;62:200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 5.Riboldi G, Nizzardo M, Simone C, Falcone M, Bresolin N, Comi GP, Corti S. ALS genetic modifiers that increase survival of SOD1 mice and are suitable for therapeutic development. Prog Neurobiol. 2011;95:133–148. doi: 10.1016/j.pneurobio.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Rokutan K, Kawahara T, Kuwano Y, Tominaga K, Nishida K, Teshima-Kondo S. Nox enzymes and oxidative stress in the immunopathology of the gastrointestinal tract. Semin Immunopathol. 2008;30:315–327. doi: 10.1007/s00281-008-0124-5. [DOI] [PubMed] [Google Scholar]
- 7.Leto TL, Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal. 2006;8:1549–1561. doi: 10.1089/ars.2006.8.1549. [DOI] [PubMed] [Google Scholar]
- 8.Tominaga K, Kawahara T, Sano T, Toida K, Kuwano Y, Sasaki H, Kawai T, Teshima-Kondo S, Rokutan K. Evidence for cancer-associated expression of NADPH oxidase 1 (Nox1)-based oxidase system in the human stomach. Free Radic Biol Med. 2007;43:1627–1638. doi: 10.1016/j.freeradbiomed.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 9.O'Leary DP, Bhatt L, Woolley JF, Gough DR, Wang JH, Cotter TG, Redmond HP. TLR-4 signalling accelerates colon cancer cell adhesion via NF-kappaB mediated transcriptional up-regulation of Nox-1. PLoS One. 2012;7:e44176. doi: 10.1371/journal.pone.0044176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter BJ, Anklesaria P, Choi S, Engelhardt JF. Redox modifier genes and pathways in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11:1569–1586. doi: 10.1089/ars.2008.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schoneich C, Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marden JJ, Harraz MM, Williams AJ, Nelson K, Luo M, Paulson H, Engelhardt JF. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest. 2007;117:2913–2919. doi: 10.1172/JCI31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Streeter J, Thiel W, Brieger K, Miller FJ., Jr Opportunity nox: the future of NADPH oxidases as therapeutic targets in cardiovascular disease. Cardiovasc Ther. 2013;31:125–137. doi: 10.1111/j.1755-5922.2011.00310.x. [DOI] [PubMed] [Google Scholar]
- 14.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanna IR, Hilenski LL, Dikalova A, Taniyama Y, Dikalov S, Lyle A, Quinn MT, Lassegue B, Griendling KK. Functional association of nox1 with p22phox in vascular smooth muscle cells. Free Radic Biol Med. 2004;37:1542–1549. doi: 10.1016/j.freeradbiomed.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 17.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 18.Sumimoto H, Kage Y, Nunoi H, Sasaki H, Nose T, Fukumaki Y, Ohno M, Minakami S, Takeshige K. Role of Src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc Natl Acad Sci U S A. 1994;91:5345–5349. doi: 10.1073/pnas.91.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Kleinberg ME. Activation of the phagocyte NADPH oxidase protein p47(phox). Phosphorylation controls SH3 domain-dependent binding to p22(phox) J Biol Chem. 1999;274:19731–19737. doi: 10.1074/jbc.274.28.19731. [DOI] [PubMed] [Google Scholar]
- 20.Debbabi M, Kroviarski Y, Bournier O, Gougerot-Pocidalo MA, El-Benna J, Dang PM. NOXO1 phosphorylation on serine 154 is critical for optimal NADPH oxidase 1 assembly and activation. Faseb J. 2013;27:1733–1748. doi: 10.1096/fj.12-216432. [DOI] [PubMed] [Google Scholar]
- 21.Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 22.Ueyama T, Geiszt M, Leto TL. Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol Cell Biol. 2006;26:2160–2174. doi: 10.1128/MCB.26.6.2160-2174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leto TL, Adams AG, de Mendez I. Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to proline-rich targets. Proc Natl Acad Sci U S A. 1994;91:10650–10654. doi: 10.1073/pnas.91.22.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maehara Y, Miyano K, Yuzawa S, Akimoto R, Takeya R, Sumimoto H. A conserved region between the TPR and activation domains of p67phox participates in activation of the phagocyte NADPH oxidase. J Biol Chem. 2010;285:31435–31445. doi: 10.1074/jbc.M110.161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JS, Diebold BA, Babior BM, Knaus UG, Bokoch GM. Regulation of Nox1 activity via protein kinase A-mediated phosphorylation of NoxA1 and 14-3-3 binding. J Biol Chem. 2007;282:34787–34800. doi: 10.1074/jbc.M704754200. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 27.Miller FJ, Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 28.Miller FJ, Jr, Chu X, Stanic B, Tian X, Sharma RV, Davisson RL, Lamb FS. A differential role for endocytosis in receptor-mediated activation of Nox1. Antioxid Redox Signal. 2010;12:583–593. doi: 10.1089/ars.2009.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassegue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jagadeesha DK, Takapoo M, Banfi B, Bhalla RC, Miller FJ., Jr Nox1 transactivation of epidermal growth factor receptor promotes N-cadherin shedding and smooth muscle cell migration. Cardiovasc Res. 2012;93:406–413. doi: 10.1093/cvr/cvr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmerman MC, Takapoo M, Jagadeesha DK, Stanic B, Banfi B, Bhalla RC, Miller FJ., Jr Activation of NADPH oxidase 1 increases intracellular calcium and migration of smooth muscle cells. Hypertension. 2011;58:446–453. doi: 10.1161/HYPERTENSIONAHA.111.177006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroder K, Helmcke I, Palfi K, Krause KH, Busse R, Brandes RP. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1736–1743. doi: 10.1161/ATVBAHA.107.142117. [DOI] [PubMed] [Google Scholar]
- 33.Kubo K, Ohno S, Suzuki K. Primary structures of human protein kinase C beta I and beta II differ only in their C-terminal sequences. FEBS letters. 1987;223:138–142. doi: 10.1016/0014-5793(87)80524-0. [DOI] [PubMed] [Google Scholar]
- 34.Ambasta RK, Schreiber JG, Janiszewski M, Busse R, Brandes RP. Noxa1 is a central component of the smooth muscle NADPH oxidase in mice. Free Radic Biol Med. 2006;41:193–201. doi: 10.1016/j.freeradbiomed.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 35.Price MO, McPhail LC, Lambeth JD, Han CH, Knaus UG, Dinauer MC. Creation of a genetic system for analysis of the phagocyte respiratory burst: high-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood. 2002;99:2653–2661. doi: 10.1182/blood.v99.8.2653. [DOI] [PubMed] [Google Scholar]
- 36.Todd MJ, Gomez J. Enzyme kinetics determined using calorimetry: a general assay for enzyme activity? Anal Biochem. 2001;296:179–187. doi: 10.1006/abio.2001.5218. [DOI] [PubMed] [Google Scholar]
- 37.Lapouge K, Smith SJ, Walker PA, Gamblin SJ, Smerdon SJ, Rittinger K. Structure of the TPR domain of p67phox in complex with Rac.GTP. Mol Cell. 2000;6:899–907. doi: 10.1016/s1097-2765(05)00091-2. [DOI] [PubMed] [Google Scholar]
- 38.Ago T, Kuribayashi F, Hiroaki H, Takeya R, Ito T, Kohda D, Sumimoto H. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc Natl Acad Sci U S A. 2003;100:4474–4479. doi: 10.1073/pnas.0735712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groemping Y, Lapouge K, Smerdon SJ, Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 40.Cheng G, Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem. 2004;279:4737–4742. doi: 10.1074/jbc.M305968200. [DOI] [PubMed] [Google Scholar]
- 41.Benna JE, Dang PM, Gaudry M, Fay M, Morel F, Hakim J, Gougerot-Pocidalo MA. Phosphorylation of the respiratory burst oxidase subunit p67(phox) during human neutrophil activation. Regulation by protein kinase C-dependent and independent pathways. J Biol Chem. 1997;272:17204–17208. doi: 10.1074/jbc.272.27.17204. [DOI] [PubMed] [Google Scholar]
- 42.Kroviarski Y, Debbabi M, Bachoual R, Perianin A, Gougerot-Pocidalo MA, El-Benna J, Dang PM. Phosphorylation of NADPH oxidase activator 1 (NOXA1) on serine 282 by MAP kinases and on serine 172 by protein kinase C and protein kinase A prevents NOX1 hyperactivation. Faseb J. 2010;24:2077–2092. doi: 10.1096/fj.09-147629. [DOI] [PubMed] [Google Scholar]
- 43.Garcia RC, Segal AW. Phosphorylation of the subunits of cytochrome b-245 upon triggering of the respiratory burst of human neutrophils and macrophages. Biochem J. 1988;252:901–904. doi: 10.1042/bj2520901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed S, Prigmore E, Govind S, Veryard C, Kozma R, Wientjes FB, Segal AW, Lim L. Cryptic Rac-binding and p21(Cdc42Hs/Rac)-activated kinase phosphorylation sites of NADPH oxidase component p67(phox) J Biol Chem. 1998;273:15693–15701. doi: 10.1074/jbc.273.25.15693. [DOI] [PubMed] [Google Scholar]
- 45.Dusi S, Rossi F. Activation of NADPH oxidase of human neutrophils involves the phosphorylation and the translocation of cytosolic p67phox. Biochem J. 1993;296(Pt 2):367–371. doi: 10.1042/bj2960367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forbes LV, Truong O, Wientjes FB, Moss SJ, Segal AW. The major phosphorylation site of the NADPH oxidase component p67phox is Thr233. Biochem J. 1999;338(Pt 1):99–105. [PMC free article] [PubMed] [Google Scholar]
- 47.Lewis EM, Sergeant S, Ledford B, Stull N, Dinauer MC, McPhail LC. Phosphorylation of p22phox on threonine 147 enhances NADPH oxidase activity by promoting p47phox binding. J Biol Chem. 2010;285:2959–2967. doi: 10.1074/jbc.M109.030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raad H, Paclet MH, Boussetta T, Kroviarski Y, Morel F, Quinn MT, Gougerot-Pocidalo MA, Dang PM, El-Benna J. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:1011–1022. doi: 10.1096/fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem. 2007;282:6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- 50.Housey GM, Johnson MD, Hsiao WL, O'Brian CA, Weinstein IB. Structural and functional studies of protein kinase C. Adv Exp Med Biol. 1988;234:127–140. doi: 10.1007/978-1-4757-1980-2_10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.