Abstract
Gastroenterologists are still unable to differentiate between some of the most ordinary disorders of the gut and consequently patients are misdiagnosed. We have developed a swallowable gas sensor capsule for addressing this. The gases of the gut are the by-product of the fermentation processes during digestion, affected by the gut state and can consequently provide the needed information regarding the health of the gut. Here we present the first study on gas sensor capsules for revealing the effect of a medical supplement in an animal (pig) model. We characterise the real-time alterations of gastric-gas in response to environmental heat-stress and dietary cinnamon and use the gas profiles for understanding the bio-physiological changes. Under no heat-stress, feeding increases gastric CO2 concentration, while dietary cinnamon reduces it due to decrease in gastric acid and pepsin secretion. Alternatively, heat-stress leads to hyperventilation in pigs, which reduces CO2 concentration and with the cinnamon treatment, CO2 diminishes even more, resulting in health improvement outcomes. Overall, a good repeatability in gas profiles is also observed. The model demonstrates the strong potential of real-time gas profiler in providing new physiological information that will impact understanding of therapeutics, presenting a highly reliable device for monitoring/diagnostics of gastrointestinal disorders.
Currently, gastroenterology is still mired with patients’ problems stemming from misdiagnosis1,2,3,4. In many cases, due to the lack of direct physiological evidences, gastroenterologists can only rely on physical symptoms rather than associated hard data to diagnose the diseases and disorders of the gastrointestinal tract5,6. Conventional medical devices including acoustic and electromagnetic based systems and endoscopy are only able to provide diagnostic imaging on limited types of gastrointestinal disorders such as ulcer, tumour related illnesses and inflammable bowel diseases (IBD)7,8. However, diagnostic imaging is ineffective for many other very frequent disorders of the gut such as irritable bowel syndromes (IBS)6.
It is now widely accepted that gastrointestinal gas profiles provide key information regarding the gut’s state-of-health9,10. Based on indirect measurements using commonly implemented breath analysis and direct measurements dates back to 1980s or earlier, the concentration and types of gases produced in the gut are affected by gastrointestinal tract physiological functions, and may be involved in the pathogenesis of some gastrointestinal tract disorders9,10. Gases presented in the gut, commonly H2, CH4, CO2, NOx and H2S as well as many volatile organic compounds, are mainly the result of the fermentation processes and other activities by the intestinal microbial community and may appear in the metabolic pathways9,10. So far, many in vivo and in vitro studies have shown that there are strong links between the intestinal gases and different types of gastrointestinal disorders. For instance, H2 and CH4 are used for detecting and characterising carbohydrate malabsorption11. CH4 has been found to play an important role in the peristaltic control of the gut and its presence is closely correlated to constipation-type IBS10,12. H2S and NOx have been identified as gasotransmitters that are important for the intestinal motility and secretory functions. Both of them are also considered as typical markers for IBD13,14,15,16.
Nevertheless, the accuracies and reliabilities of the current intestinal gas measurements have been of great concerned. Breath analysis is currently the most common indirect method which relies on the measurement of intestinal gases that are first absorbed onto the gut walls, then recirculated to the lung through the blood stream and eventually excreted by respiration11,17,18. Misleading diagnostic information may be given due to interfering gases produced by the interfering parts of the human body and activities which are highly dependent on the metabolic and physiological nature of individuals and also environmental effects9,19. Another common indirect approach is to measure the headspace gas produced from in vitro fermentation of faecal samples20,21,22,23. This method results in more accurate assessment outcomes but limits to the information of the microbial community of the distal colon area. As such, it is critical to measure intestinal gases directly from where they are produced in the gastrointestinal tract for providing more accurate information regarding the physiological abnormities of the gut.
Early attempt of direct and accurate intestinal gas measurements have been realised by inserting tubes into patients’ oral cavity or anus to collect gas samples24,25. However, such approaches are no longer applied in nowaday gastroenterology community due to their invasive and inconvenient nature.
Recently, we have successfully developed a non-invasive and direct gas profiling tool called “gas sensor capsule” (Fig. 1). The capsule is swallowable but indigestible, which has enabled real-time and precise gas measurements in different sections of the gastrointestinal tract in animal models26. The emergence of such a novel tool can therefore potentially provide a new approach in vivo for the investigation of gut under various disease and disorder conditions. The gas capsule can also be a unique tool in assessing therapeutics and their impacts on the gut. However, such capabilities have not been shown yet.
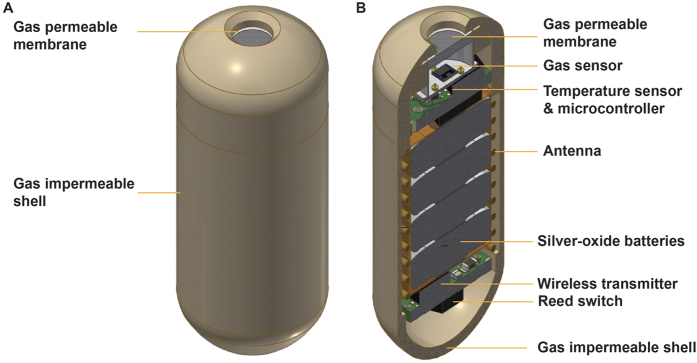
Figure 1.
Schematic of a gastric gas profiler (a modified version of gas sensor capsule with higher density): (A) whole capsule; (B) cross sectional view.
Here, such hypothesises are validated using an animal model influenced by a representative gut disorder and a dietary therapeutic agent. The model of gut disorder is induced by stressing pigs under cyclical heat. The heat-stress effects on the gut physiological abnormalities are well-known in the literature. When under heat-stress, the shunting of blood from the central circulation to the periphery reduces the perfusion to the viscera, particularly the gastrointestinal tract, which may result in tissue hypoxia and intestinal mucosal injury27. Damage to the integrity of the intestinal wall has been linked to a reduction in permeability and associated with gut inflammation and endotoxemia28,29, which can significantly alter nutrient digestive and absorptive functionalities and hence the intestinal gas profile27,30,31. On the other hand, supplemental cinnamon (cinnamomum verum) is chosen as a representative therapeutic agent due to its anti-inflammatory, anti-oxidant and free radical scavenging properties in the gastrointestinal tract32,33. These advantageous characterises make this spice an ideal candidate for maintaining normal digestive functionalities and intestinal barrier integrity under the heat-stress condition, while at the same time it has relatively minor adverse-effects that cause minimum disturbance on the intestinal microbial community.
In this pilot study, the capability of gas sensor capsule is for the first time investigated through examining gastric gas production in pigs under heat-stress and assessing the effect of oral cinnamon on such gas profiles. The results are interpreted in the light of other complementary physiological and biochemical assessments. The repeatability of the gas profiles is also evaluated in a large group of pigs for revealing the effectiveness of this novel medical tool.
Results
Thermoneutral conditions
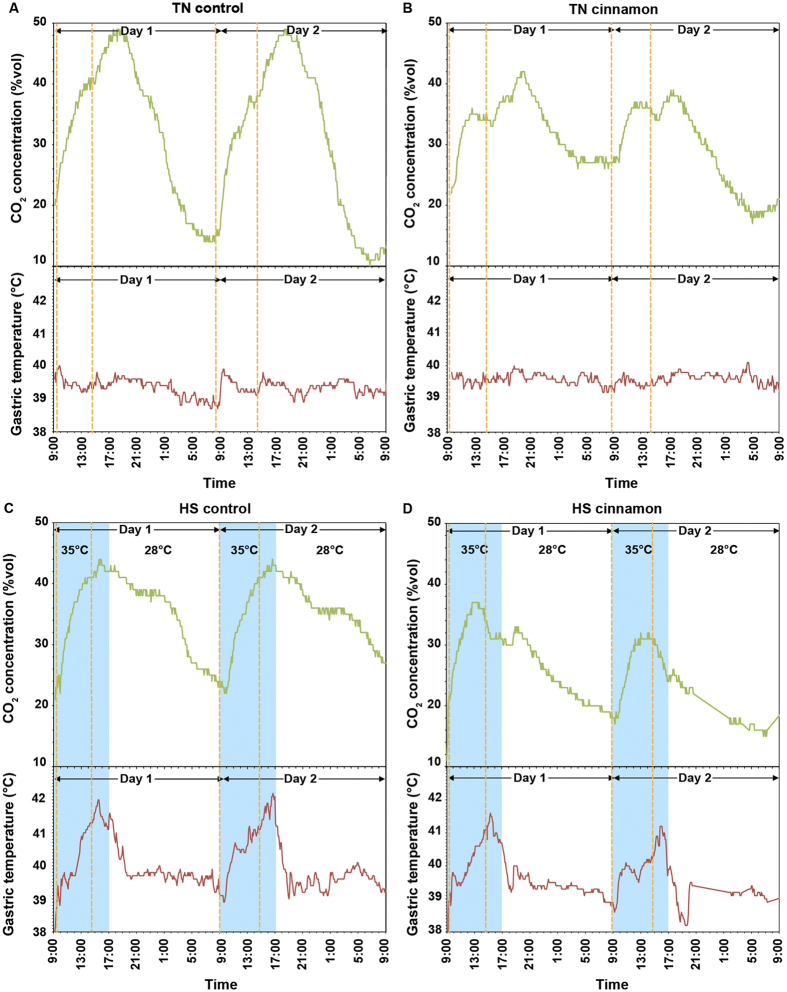
The real-time gastric CO2 gas profile of the thermoneutral control pig (no cinnamon in the diet) was measured by the gastric gas profiler (a modified version of gas sensor capsule with higher density) and shown in Fig. 2A. The first feeding of the day resulted in an increase of CO2 level by ~21% in ~3–4 h, while there was a ~8% increase of CO2 concentration at ~3 h after the second feeding. Simultaneously, a rise of gastric temperature of up to 0.8 °C corresponding to each feeding was observed. A rapid decline of CO2 gas level was seen after reaching the maximum of the day.
Figure 2.
Gastric CO2 gas and temperature profiles of: (A) thermoneutral (TN) control pig; (B) thermoneutral cinnamon-treated pig; (C) heat-stress (HS) control pig; and (D) heat-stress cinnamon-treated pig. The profiles are obtained using high-density gastric gas profilers. The dotted orange lines indicate the pig feeding times.
The gastric CO2 gas pattern and production kinetics in the thermoneutral cinnamon-treated pig (Fig. 2B) were distinctly different from that observed in the control pig. In particular, the CO2 concentration level increased only by ~13% and ~6% after the first and second feeding, respectively, which were markedly lower than that of thermoneutral control and were consistent with the decrease of the blood CO2 levels presented in Table 1. It was also noted that a decrease in CO2 concentration was seen immediately after the second feeding, which was associated with a ~50% reduction of the metabolic heat production (temperature fluctuation was less than 0.4 °C that was just above the noise floor of the temperature sensor).
Table 1. Blood parameters of pigs under both thermoneutral and heat-stress conditions.
| Parameters | Thermoneutral | Heat-stress | ||
|---|---|---|---|---|
| Control | Cinnamon | Control | Cinnamon | |
| pH | 7.41 | 7.40 | 7.42 | 7.42 |
| pCO2 (mm Hg) | 55.5 | 53.5 | 50.1 | 50.2 |
| cHCO3− (mmoL/L) | 35.5 | 32.9 | 33.3 | 32.7 |
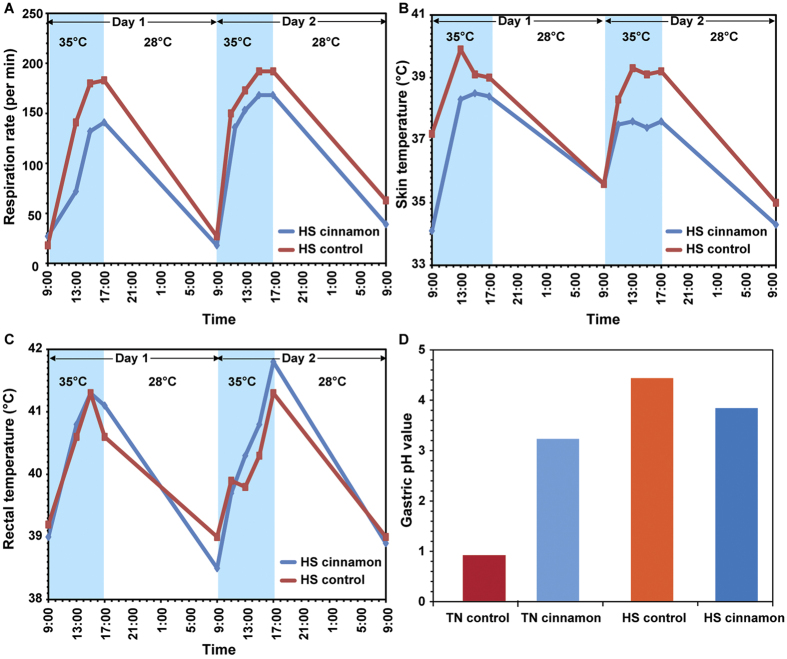
Addition of cinnamon in the diet significantly increased the gastric pH from ~1 to ~3.2 (Fig. 3D), with the anticipated concomitant decrease in blood bicarbonate level (Table 1) as it is concurrently released together with gastric acid during the formation of the alkaline tide. Raman spectroscopy was used for assessing the pepsin level in the stomach of the thermoneutral pigs. In the Raman spectrum of gastric digesta, distinct peaks representing the unique markers of pepsin34,35 were observed (Fig. 4). In particular, the Raman peaks at ~1450, 1555, 1610 and 1675 cm−1 could be ascribed to the stretching modes of C-H, amide NH, C=C and amide CH bonds, respectively. In the pig treated with cinnamon, the normalised intensities of these Raman peaks were much lower than those of control, indicating less pepsin concentration in the gastric digesta.
Figure 3.
Comparisons of (A) respiration rate, (B) skin temperature and (C) rectal temperature between controlled and cinnamon-treated pigs under heat-stress (HS). (D) Gastric pH values of pigs under thermoneutral (TN) and heat-stress conditions.
Figure 4. Raman spectra of gastric digesta of dissected pigs under both thermoneutral (TN) and heat-stress (HS) conditions.
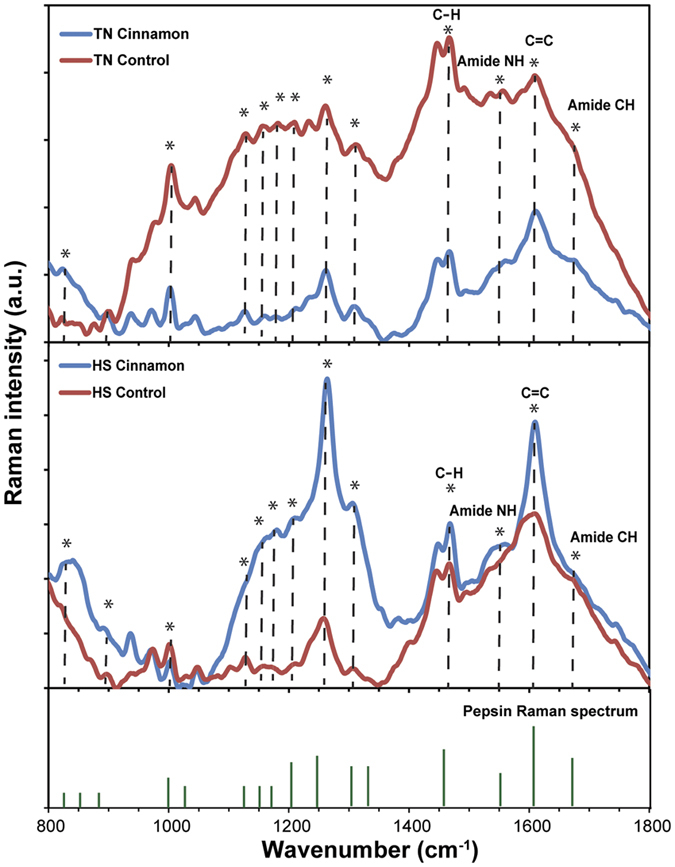
Annotation *indicates the pepsin Raman signature.
Effects of heat-stress
When under heat-stress, gastric pH increased (Fig. 3D) and the gastric CO2 pattern was changed compared to those of the thermoneutral control pig. As shown in Fig. 2C, a CO2 concentration maximum was observed at ~4 h after the first feeding for the heat-stress controlled pig (no cinnamon supplement) with the value comparable to that in the thermoneutral control animal. At the same time, a significant increase in the gastric temperature by ~3 °C was observed. However, the second feeding resulted in a very small increase in the CO2 concentration, which was only ~2% in comparison to ~8% in the thermoneutral condition. This was associated with a decrease of blood CO2 and carbonate concentrations compared to that under thermoneutral controlled condition, while their blood pH values were similar (Table 1).
During heat-stress, physiological indices of the pig including respiratory rate, skin and rectal temperatures were all increased, as expected from the literature (Fig. 3A–C)36,37. In particular, the respiration rate increased from ~30/min to ~140/min at 13:00 and reached a maximum of ~180/min at 15:00 (Fig. 3A), while the skin temperature plateaued much earlier at 13:00 (Fig. 3B). After maximal respiration rate, there was a rapid decrease in the gastric temperature from the peak of ~42 °C down to ~41 °C at the end of the heat-stress period (17:00).
Cinnamon ingestion under heat-stress conditions was associated with a considerably altered CO2 gas pattern (Fig. 2D). From Fig. 2D, after the first feeding with cinnamon supplement, the CO2 concentration was increased by ~17% which was 3% lower than that in the heat-stress control subject. The influence of cinnamon on the CO2 gas profile was more pronounced after 13:00. For the heat-stress cinnamon-treated subject, a rapid drop by 7% in the CO2 concentration was observed and the second feeding of the day did not result in the usual immediate increase in CO2 production as seen in Fig. 2C. Metabolic heat production corresponding to the feeding was also noticed (Fig. 2D). The abnormalities of pH (Fig. 3D) and pepsin levels (Fig. 4) were attenuated in the cinnamon-treated heat-stress pig compared with its corresponding control.
Likewise, the rise of gastric (Fig. 2D) and skin temperatures (Fig. 3B) due to heat-stress reduced from average ~3.3 °C to ~2.5 °C, and ~3.8 °C to ~3.2 °C, respectively, compared to those in the heat-stress control. Despite the conspicuous effect of the cinnamon supplement on the gastric indices, no distinct difference in rectal temperature profile was observed (Fig. 3C).
Evaluation of the repeatability of gastric gas profiles
Figure 2 demonstrated that all timepoint measurements of CO2 were within a narrow band of variation within the overall curve of the graphs produced. Additionally the CO2 patterns were remarkably similar for Day 1 and 2 indicating the repeatability of gas profiles generated for all four test conditions. For both thermoneutral and heat-stress conditions of the control subjects, the overall CO2 concentrations across the first and second day showed less than 1% variance. There was a slight decrease of ~3% and ~5% on the second day for the cinnamon-treated subjects under thermoneutral and heat-stress, respectively.
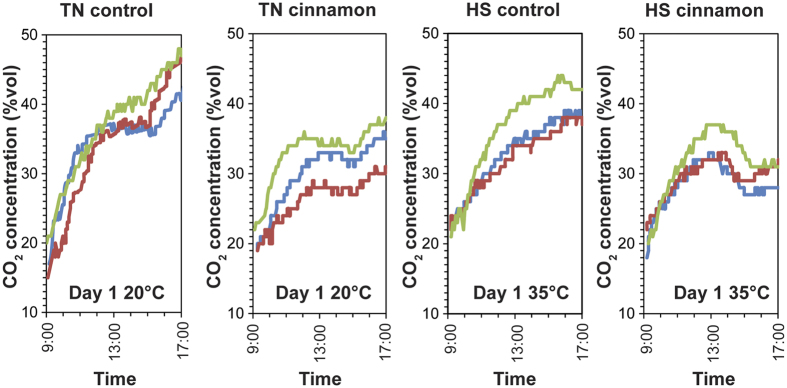
Repeatability was further tested through the use of two additional pigs in each test group using normal-density gas sensor capsules that left the pigs’ bodies within a normal bowel transit time. The profile obtained by the normal-density capsules showed very strong consistency with those obtained by high-density gastric gas profilers that were immobilized in the pigs’ stomach (Fig. 5). As such, during the first 8 h after the oral administration, the normal-density gas sensor capsules were identified to be in the pigs’ stomach.
Figure 5. Comparison of gastric CO2 gas profiles obtained by using the normal-density gas sensor capsules (blue and red lines) and the high-density gastric gas profilers (green line) in the first 8 h of the experiment for the thermoneutral controlled (TN control), thermoneutral cinnamon-treated (TN cinnamon), heat-stress controlled (HS control) and heat-stress cinnamon-treated (HS cinnamon) subjects.
For the measurements using normal-density gas sensor capsules under the thermoneutral condition, the produced CO2 concentrations corresponding to the first and second feeding were averaged at ~20% and ~7%, for the control and ~12% and ~3% for the cinnamon-treated pigs, respectively. These numbers were all within the 1–2% variance range compared to those obtained using high-density gastric gas profilers. For the heat-stress condition, the produced CO2 concentrations were slightly lower but not in contradiction to those observed using the high-density gas profilers.
Discussion
The purposes of this pilot study are threefold: to gain insights into the physiological alterations that occur under heat-stress conditions; to better define the effects of cinnamon on those physiological changes; and more importantly, to determine the contribution of the new information from continuous monitoring of CO2 concentrations and temperature in the stomach to understanding the physiological processes occurring. The gastric CO2 gas measurements are achieved via a modified version of the gas sensor capsule that, by virtue of changing its density, is immobilised in the pigs’ stomach during the experiment. Swine are chosen as test subjects due to the similarity of their gastrointestinal tract to that of humans, and to the fact that swine are very susceptible to heat-stress that can potentially make the observations more clear28,38,39. Because of their non-functional sweat glands, pigs rely on panting (hyperventilation) as the primary evaporative heat loss mechanism when under heat-stress, which significantly increases respiration rate but causes further redistribution of the gas content in the blood stream and possibly the gastrointestinal tract40. In the present study, observations are limited to the stomach. As the gut is made of different organs that are diverse in their functionalities and physiochemical environments, the investigation of individual organs is essential. The stomach as the area in which significant digestive activities are chemical and biochemical is the first important organ that can be potentially influenced by the ameliorating effect of cinnamon. The stomach is highly sensitive to heat-stress as the health of gastric mucosa is closely related to the gastric mucosal blood flow41, and thus redistribution of blood flow driven by heat-stress may potentiate gastric injury via, for example, ulcerogenic agents42.
Interpretation of the physiological changes that occurred gives insights into the possible therapeutic benefits of diet supplementation. The trend of the changes in CO2 defined by the gastric gas profiler is similar to that obtained from measurements in a respiration chamber43,44, indicating that the gastric CO2 production is mainly attributable to the chemical reactions in the acidic environment of the stomach45. This is also likely to be accompanied by products of aerobic metabolism of amino acids obtained from protein digestion, which are then chemically decarboxylated into CO2 gas46. The concomitant rise in gastric temperature is likely to be attributed to metabolic heat generation from the chemical reaction as a result of the protein digestion47. The marked reduction in CO2 concentration occurring after the second feeding of the day in the thermoneutral cinnamon-treated subject could possibly be related to a chemical reaction of cinnamon in the stomach.
It has been extensively reported in studies both in vivo and in vitro that cinnamon in its extracted form inhibits gastric acid and pepsin secretion33,48. In the present study, this is confirmed by the rise in gastric pH and the reduction of pepsin seen in the Raman spectrum. Such a reduction of gastric acid and pepsin secretion eventually leads to a lowered gastric CO2 and metabolic heat production, because gastric CO2 is mainly produced from the chemical reaction of food in an acidic environment together with the decarboxylation of amino acids upon protein digestion45,46. These are all related to the concentrations of both gastric acid and pepsin.
During heat-stress, the CO2 gas profile looks more like that seen with cinnamon treatment under thermoneutral conditions for the same reason that gastric acid and pepsin secretion falls. Gastric CO2 may also be influenced by hyperventilation and subsequent increases CO2 loss from the lungs and greater uptake of gastric CO2 by the circulation40. The plateauing of the skin temperature in the early afternoon is presumably related to the dominant effect of evaporative heat loss by hyperventilation.
The observation that dietary supplementation with cinnamon appeared to reduce gastric CO2 production under heat-stress conditions is less easily explained. The increase in gastric acid and pepsin secretion (and presumably metabolic production of CO2) relative to those in the heat-stress control pig and relatively reduced respiratory rate associated with heat-stress will tend to increase gastric CO2 content. However, cinnamon increases gastric mucosal blood flow32,49, and this may permit greater CO2 exchange. This is supported by the attenuated rise in gastric temperature that might reflect enhanced radiant heat loss from the mucosa. Skin blood flow might also increase with additional radiant heat loss as suggested by the reduced skin temperature in the cinnamon-treated heat-stress pig. Interestingly, rectal temperature is not reduced and this may indicate rectal mucosal blood flow to be unaffected by cinnamon, an untested but feasible hypothesis.
Hence, this pilot study has demonstrated the potential utility of the real-time gastric gas profiler to enhance understanding of the physiological processes that occur in a model of a gastric disorder and of the mechanisms by which a potential therapeutic agent acts. Thus, for the heat-stress effect, the gastric CO2 level is found to be suppressed due to the combination of reduction of gastric acid and pepsin secretion as well as hyperventilation, providing a novel marker of the physiological disturbances that enhances information from more traditional physiological and biochemical measurements. The addition of cinnamon into the diet inhibits both the gastric acid and pepsin secretion, resulting in a significant decline in the gastric CO2 gas level and alters CO2 gas pattern. When under heat-stress, partial amelioration of disorders signified by physiological indices is observed. The CO2 gas pattern corresponding to the second feeding of the day is greatly altered as the gastric CO2 gas is rapidly depleted during that period, which can be ascribed to the possible enhanced gastric mucosal blood flow in the presence of cinnamon. In addition, the relatively normal gastric acid and pepsin secretion may reflect the potential antioxidant activity of cinnamon against the intestinal mucosal injury induced by tissue hypoxia32,50. Altogether, it is suggested that cinnamon may play an important role in gastric cytoprotection and maintenance of homeostasis in gastric secretion and digestive functionalities.
The strong similarity and repeatability shown when comparing the gastric gas profiles obtained from the high-density profilers and normal-density gas sensor capsules indicates that the profiles obtained using the transient non-invasive capsules are precise. This suggests that this technology can be reliably used for the interpretation of the gastric gas profiles.
In conclusion, this gastric gas profiler has provided novel, precise and repeatable real-time assessment of gastrointestinal physiological processes that enhance our understanding of a representative gastric disorder induced by heat-stress and the effect of a cinnamon supplement as a dietary remedial model. Since such emerging gas sensor capsule technology can readily be designed to target other parts of the gastrointestinal tract and/or other gas molecules, it presents as a novel medical tool that is capable of providing unique information (particularly via the gas level, pattern and production kinetics) about disorders of the gastrointestinal tract. Such information may permit new insights that will underpin future research into pathogenesis of gastrointestinal disorders, dietary and drug effects, and clinical diagnostic applications.
Methods
Ethics Statement
All experiments involving pigs were undertaken in accordance with the approved guidelines outlined and approved by the University of Melbourne Faculty of Veterinary and Agricultural Sciences Animal Ethics Committee. Ethics Document No. 1513462, approved on the 5th of June 2015.
Feeding and environmental manipulation
Main tests
Four female crossbred pigs (averaged at ~40 kg) were kept at four different conditions. They were fed a commercial ration (18% crude protein, 13.8 MJ digestible energy/kg) supplemented with either 0 or 1.5% wt/wt cinnamon (cinnamomum verum) and housed under either thermoneutral (20 °C) or heat-stress conditions (35 °C from 09:00–17:00 and 28 °C for the reminder of each 24 h period). Swine were acclimatised to diets for 14 days pre-trial and fed twice a day at around 09:00 and 15:00 over the 2-day experiments. Access to feed and water was provided by troughs and nipple drinkers. Heat and humidity were monitored by using temperature and humidity Dataloggers® placed in the thermally controlled rooms.
Repeatability tests
The repeatability of gastric gas profiles were assessed using a group of 12 pigs, which included 4 pigs from the main tests that were administrated with high-density gastric gas profilers and 8 additional pigs that were administrated with normal-density gas sensor capsules. The additional 8 pigs were divided evenly for four different feeding and environmental conditions similar to those in the main tests. This resulted in a total of three pigs for each condition (N = 3).
Fabrication and deployment of the gastric gas profiler
The design of the gastric gas profiler was similar to our previously implemented gas sensor capsules26, with the difference that its density was increased to promote its permanent immobilisation in the pigs’ stomach during the experiment. The high-density gastric gas profiler consisted of gas and temperature sensors, a gas-permeable membrane, micro-electronic circuits, silver-oxide batteries, a reed switch and telecommunication components (including wireless transmitter and antenna), all sealed within a gas impermeable shell (Fig. 1). The gas sensor was based on the thermal conductivity calorimetric technology that has been shown to be sensitive to CO2 gas (the selectivity of the gas sensor was presented in Supplementary Information Note 1). As demonstrated in a post-mortem pig study, CO2 was the only major gas produced in the stomach in response to a normal diet51. The gas profiler was a capsule-shaped device of 2.4 cm length and 1 cm diameter. The resolution of the CO2 gas and temperature sensors was 1%vol and 0.2 °C, respectively. The evaluation of the accuracy of the temperature sensor was presented in Supplementary Information Note 2. Gas and temperature profiles were wirelessly transmitted to an external handheld device that allowed for data storage and analysis. The devices were administered by gavaging into the pigs’ stomach and data was acquired at 5 min intervals.
Physiological measurements
Respiration rate, skin and rectal temperatures were measured at around 09:00, 11:00, 13:00, 15:00 and 17:00 daily. Respiration rate was recorded by observing flank movements and counting the number of breaths per minute. Skin temperature was measured using an infrared thermometer at the skin surface between the leg and torso while rectal temperature was measured using a handheld digital thermometer.
Blood measurements
Before the completion of the experiment, 1 mL of blood was collected from the ear vein of each pig. The samples were then immediately assayed for acid-base indices, including pH, pCO2 (plasma CO2) and cHCO3− (concentration of bicarbonate in plasma), using an automated blood gas analyser (Epoc®, Alere).
Measurement of the gastric digesta
At the completion of the experiment, pigs were euthanized and dissected. During dissection, the gastric digesta were collected and pH values were immediately measured using a calibrated pH meter (Accumet, AB15). Measurements were repeated five times for each sample and the average calculated.
After pH measurements, gastric digesta were mixed with a 5% wt/wt solution of silver nanoparticles (Sigma Aldrich, average particle size < 150 nm) at ratio of 1:1. The silver nanoparticles were used for amplifying Raman spectroscopic signals of chemicals presented in the digesta52. Subsequently, the mixture was cyclically bath-sonicated for 15 min (4 min sonication/1 min incubation at rest, repeated three times). 40 μL of the mixture was then drop-cast onto clean silicon substrates at 60 °C for Raman spectroscopic measurements (Craic 20–30 microspectrophotometer). An excitation wavelength of 785 nm at 1 mW power was used in the experiment (accuracy of ±4 cm−1). The final intensity of the Raman spectrum of each sample was average of four repeated Raman scans with an exposure time of 30 s each. Background luminescent offset, noise removal and baseline adjustment of the Raman spectra were conducted using a Matlab® 10 software package.
Additional Information
How to cite this article: Ou, J. Z. et al. Potential of in vivo real-time gastric gas profiling: a pilot evaluation of heat-stress and modulating dietary cinnamon effect in an animal model. Sci. Rep. 6, 33387; doi: 10.1038/srep33387 (2016).
Supplementary Material
Acknowledgments
The authors would like to thank NHMRC Australia for financially supporting this project via grant #1075568.
Footnotes
Author Contributions K.K. and J.O. generated and implemented the idea. They also participated in all experiments and wrote the text. J.C., N.H., N.P., U.W. and F.D. participated in and executed the animal experiments. N.H., C.H. and K.B. participated in the fabrication and laboratorial characterisation of gas sensor capsules. J.C., C.Y., S.W., D.G., J.M., F.D. and P.G. participated in writing the manuscript and in depth analysis of the data.
References
- Spiegel B. M. R., DeRosa V. P., Gralnek I. M., Wang V. & Dulai G. S. Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: A cost-effectiveness analysis. Gastroenterology 126, 1721–1732 (2004). [DOI] [PubMed] [Google Scholar]
- Stark D., van Hal S., Marriott D., Ellis J. & Harkness J. Irritable bowel syndrome: A review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int. J. Parasitol. 37, 11–20 (2007). [DOI] [PubMed] [Google Scholar]
- Su Y.-C. et al. The association between Helicobacter pylori infection and functional dyspepsia in patients with irritable bowel syndrome. Am. J. Gastroenterol. 95, 1900–1905 (2000). [DOI] [PubMed] [Google Scholar]
- Grazioli B. et al. Giardia lamblia infection in patients with irritable bowel syndrome and dyspepsia: A prospective study. World J. Gastroenterol. 12, 1941–1944 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley N. J. & Spiller R. Irritable bowel syndrome: a little understood organic bowel disease? Lancet 360, 555–564 (2002). [DOI] [PubMed] [Google Scholar]
- Olden K. W. Diagnosis of irritable bowel syndrome. Gastroenterology 122, 1701–1714 (2002). [DOI] [PubMed] [Google Scholar]
- Modlin I. M., Kidd M., Latich I., Zikusoka M. N. & Shapiro M. D. Current Status of Gastrointestinal Carcinoids. Gastroenterology 128, 1717–1751 (2005). [DOI] [PubMed] [Google Scholar]
- Mowat C. et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 60, 571–607 (2011). [DOI] [PubMed] [Google Scholar]
- Ou J. Z. et al. Human intestinal gas measurement systems: in vitro fermentation and gas capsules. Trends Biotechnol. 33, 208–213 (2015). [DOI] [PubMed] [Google Scholar]
- Carbonero F., Benefiel A. C. & Gaskins H. R. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat. Rev. Gastroenterol. Hepatol. 9, 504–518 (2012). [DOI] [PubMed] [Google Scholar]
- Shin W. Medical applications of breath hydrogen measurements Anal. Bioanal. Chem. 406, 3931–3939 (2014). [DOI] [PubMed] [Google Scholar]
- Triantafyllou K., Chang C. & Pimentel M. Methanogens, methane and gastrointestinal motility. Neurogastroenterol. Motil. 20, 31–40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medani M. et al. Emerging role of hydrogen sulfide in colonic physiology and pathophysiology. Inflamm. Bowel Dis. 17, 1620–1625 (2011). [DOI] [PubMed] [Google Scholar]
- Linden D. R. Hydrogen sulfide signaling in the gastrointestinal tract. Antioxid. Redox Signal. 20, 818–830 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. O., Hellström P. M., Fagerhol M. K., Weitzberg E. & Roseth A. G. Technology insight: Calprotectin, lactoferrin and nitric oxide as novel markers of inflammatory bowel disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2, 96–102 (2005). [DOI] [PubMed] [Google Scholar]
- Rivera L. R., Poole D. P., Thacker M. & Furness J. B. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol. Motil. 23, 980–988 (2011). [DOI] [PubMed] [Google Scholar]
- De Lacy Costello B. P. J., Ledochowski M. & Ratcliffe N. M. The importance of methane breath testing: A review. J. Breath Res. 7, 024001 (2013). [DOI] [PubMed] [Google Scholar]
- Rattray N. J. W., Hamrang Z., Trivedi D. K., Goodacre R. & Fowler S. J. Taking your breath away: metabolomics breathes life in to personalized medicine. Trends Biotechnol. 32, 538–548 (2014). [DOI] [PubMed] [Google Scholar]
- Sahakian A. B., Jee S. R. & Pimentel M. Methane and the gastrointestinal tract. Digest. Dis. Sci. 55, 2135–2143 (2010). [DOI] [PubMed] [Google Scholar]
- De Lacy Costello B. et al. An analysis of volatiles in the headspace of the faeces of neonates. J. Breath Res. 2, 037023 (2008). [DOI] [PubMed] [Google Scholar]
- Dixon E. et al. Solid-phase microextraction and the human fecal VOC metabolome. PLoS ONE 6, e18471 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner C. E. et al. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J. 21, 1675–1688 (2007). [DOI] [PubMed] [Google Scholar]
- Probert C. S. J. Role of faecal gas analysis for the diagnosis of IBD. Biochem. Soc. Trans. 39, 1079–1080 (2011). [DOI] [PubMed] [Google Scholar]
- McKay L. F., Eastwood M. A. & Brydon W. G. Methane excretion in man - A study of breath, flatus, and faeces. Gut 26, 69–74 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez G. & Brown S. D. Gastric tonometry: A new monitoring modality in the intensive care unit. J. Intensive Care Med. 10, 34–44 (1995). [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K. et al. Intestinal gas capsules: A proof-of-concept demonstration. Gastroenterology 150, 37–39 (2016). [DOI] [PubMed] [Google Scholar]
- Hall D. M. et al. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 280, H509–H521 (2001). [DOI] [PubMed] [Google Scholar]
- Lambert G. P. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J. Anim. Sci. 87, E101–E108 (2009). [DOI] [PubMed] [Google Scholar]
- Krau S. D. Heat-related illness. A hot topic in critical care. Crit. Care Nurs. Clin. North Am. 25, 251–262 (2013). [DOI] [PubMed] [Google Scholar]
- Maseko T. et al. Selenium-enriched Agaricus bisporus mushroom protects against increase in gut permeability ex vivo and up-regulates glutathione peroxidase 1 and 2 in hyperthermally-induced oxidative stress in rats. Nutrients 6, 2478–2492 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. et al. Selenium and Vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat stressed pigs. Exp. Physiol. 101, 801–810 (2016). [DOI] [PubMed] [Google Scholar]
- Tankam J. M., Sawada Y. & Ito M. Regular ingestion of cinnamomi cortex pulveratus offers gastroprotective activity in mice. J. Nat. Med. 67, 289–295 (2013). [DOI] [PubMed] [Google Scholar]
- Ozbayer C. et al. Gastroprotective, cytoprotective and antioxidant effects of Oleum cinnamomi on ethanol induced damage. Cytotechnology 66, 431–441 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin M. C. Raman spectra of crystalline lysozyme, pepsin, and alpha chymotrypsin. Science 161, 68–69 (1968). [DOI] [PubMed] [Google Scholar]
- Rygula A. et al. Raman spectroscopy of proteins: a review. J. Raman Spectrosc. 44, 1061–1076 (2013). [Google Scholar]
- Brown-Brandl T. M., Nienaber J. A. & Turner L. W. Acute heat stress effects on heat production and respiration rate in swine. Trans. ASAE (Am. Soc. Agric. Eng.) 41, 789–793 (1998). [Google Scholar]
- Patience J. F., Umboh J. F., Chaplin R. K. & Nyachoti C. M. Nutritional and physiological responses of growing pigs exposed to a diurnal pattern of heat stress. Livest. Prod. Sci. 96, 205–214 (2005). [Google Scholar]
- Pearce S. C., Sanz-Fernandez M. V., Hollis J. H., Baumgard L. H. & Gabler N. K. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J. Anim. Sci. 92, 5444–5454 (2014). [DOI] [PubMed] [Google Scholar]
- Cottrell J. J. et al. Nutritional strategies to alleviate heat stress in pigs. Anim. Prod. Sci. 55, 1391–1402 (2015). [Google Scholar]
- Renaudeau D. et al. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6, 707–728 (2012). [DOI] [PubMed] [Google Scholar]
- Laine L., Takeuchi K. & Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology 135, 41–60 (2008). [DOI] [PubMed] [Google Scholar]
- Abdel-Salam O. M. E., Czimmer J., Debreceni A., Szolcsányi J. & Mózsik G. Gastric mucosal integrity: Gastric mucosal blood flow and microcirculation. An overview. J. Physiology-Paris 95, 105–127 (2001). [DOI] [PubMed] [Google Scholar]
- Le Goff G., Le Groumellec L., Van Milgen J., Dubois S. & Noblet J. Digestibility and metabolic utilisation of dietary energy in adult sows: Influence of addition and origin of dietary fibre. Br. J. Nutr. 87, 325–335 (2002). [DOI] [PubMed] [Google Scholar]
- Schrama J. W. et al. The energetic value of nonstarch polysaccharides in relation to physical activity in group-housed, growing pigs. J. Anim. Sci. 76, 3016–3023 (1998). [DOI] [PubMed] [Google Scholar]
- Mensink P. B. F., Geelkerken R. H., Huisman A. B., Kuipers E. J. & Kolkman J. J. Effect of various test meals on gastric and jejunal carbon dioxide: A study in healthy subjects. Scand. J. Gastroentero. 41, 1290–1298 (2006). [DOI] [PubMed] [Google Scholar]
- Steer H. W. The source of carbon dioxide for gastric acid production. Anat. Rec. 292, 79–86 (2009). [DOI] [PubMed] [Google Scholar]
- Berg J. M., Tymoczko J. L. & Stryer L. Biochemistry. (W H Freeman, 2002). [Google Scholar]
- Amr A. R. & Maysa M. E. Anti-ulcer effect of cinnamon and chamomile aqueous extracts in rat models. J. Am. Sci. 6, 209–216 (2010). [Google Scholar]
- Akira T., Tanaka S. & Tabata M. Pharmacological studies on the antiulcerogenic activity of Chinese cinnamon. Planta Med. 6, 440–443 (1986). [DOI] [PubMed] [Google Scholar]
- Ranasinghe P. et al. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement. Altern. Med. 13, 275 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B. B. & Jorgensen H. Effect of dietary fiber on microbial activity and microbial gas production in various regions of the gastrointestinal tract of pigs. Appl. Environ. Microbiol. 60, 1897–1904 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrimes A. F. et al. In situ SERS probing of nano-silver coated individual yeast cells. Biosens. Bioelectron. 49, 536–541 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.