Abstract
Background:
One of the main reasons why the breast fat grafting was questioned is that there may be lipofilling resorption. In the literature, the resorption rate reported over the 1st year is highly variable (20–90%).
Objective:
The aim of this work was to identify the biochemical and clinical parameters, which increase fat graft maintenance in breast reconstruction.
Materials and Methods:
A sample of 19 patients was treated with fat grafting mixed with platelet-rich plasma. A complete screening of anthropometry, body composition, and blood biochemical parameters was assessed using the standardized equipment. Pre- and post-operative evaluation was performed, which included a complete clinical examination, photographic assessment, nuclear magnetic resonance imaging of the soft tissue, and ultrasound. The follow-up period was 2 years.
Results:
The authors divided the results into two types of patients: “responder” and “not a responder.” In the “responder” group patients with normal weight, gynoid fat distribution, obese, with normal blood biochemical parameters, and atherogenic indices but with high preoperative values of platelet-to-lymphocyte ratio (PLR) (174.49) and neutrophil-lymphocyte ratio (NLR) (2.65) showed a greater increase of fat graft maintenance at 6 and 12 months after the last lipofilling session. In the “not responder group” patients with overweight, android fat distribution, obese, high values of atherogenic indices, but with normal preoperative NLR and PLR ratios showed a lower fat graft maintenance at 6 and 12 months.
Conclusion:
We assume, the problem of fat resorption may be resolved by analysis of body composition and by examine the predictive role of preoperative markers of low-grade inflammation.
Keywords: Breast fat graft, fat graft, low-grade inflammation, neutrophil-lymphocyte ratio, platelet-to-lymphocyte ratio
INTRODUCTION
The accumulation of the adipose tissue (AT) in different anatomic localizations certainly plays a role in the development of obesity comorbidities.
Recent studies have focused on understanding the mechanisms of how specific AT depots or regional fat distribution patterns impact cardiometabolic risk.[1]
Excess adipose mass in the upper parts of the body (also indicated as “android obesity” or “central obesity”) usually constitutes a risk factor for type II diabetes, hypertension, dyslipidemia, and cardiovascular disease, especially in women.[2,3]
On the contrary, the excess of adipose mass in the lower parts of the body (gynoid obesity) seems not to have major metabolic consequences, in fact a number of studies have also demonstrated that greater fat mass distributed in appendicular sites (legs and arms), which is indicative more of a gynoid body shape, appears to be beneficial or protective against cardiovascular disease, and is inversely correlated with metabolic risk factors as compared with the central obesity.[4,5]
Thus, in women, the sites of fat predominance offer an important prognostic marker for many metabolic complications.
Inflammation is a coordinated response to harmful stimuli, with the goal of returning the system back to a normal baseline. The inflammatory response triggered by obesity involves many components of the classical inflammatory response to pathogens and includes systemic increases in circulating inflammatory cytokines and acute phase proteins, recruitment of leukocytes to inflamed tissues, activation of tissue leukocytes, and generation of reparative tissue responses (e.g., fibrosis).[6]
However, the nature of obesity-induced meta-inflammation is unique compared with other inflammatory paradigms (e.g., infection and autoimmune disease) in several key aspects. The chronic nature of obesity produces a tonic low-grade activation of the innate immune system that affects steady-state measures of metabolic homeostasis over time.[7]
Low-grade systemic inflammation due to obesity is considered to be the key link between obesity and obesity-related disorders.
In this regard, we aimed to establish the association between body fat distribution (obesity phenotypes) and transplanted fat resorption percentage after breast autologous fat grafting combined with platelet-rich plasma (PRP).
The hypothesis was tested that body fat distribution together with preoperative alterations of low-inflammatory markers, like neutrophil-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), could be a useful instrument to predict the response to breast autologous fat grafting, regarding resorption percentage.
MATERIALS AND METHODS
Study design and subjects
This descriptive study was conducted on a sample of 19 Italian Caucasian women (aged from 19 to 73 years) affected by breast soft-tissue defects, recruited from ongoing studies at the Division of Clinical Nutrition and Nutrigenomics, Department of Biomedicine and Prevention, University of Rome Tor Vergata (Italy), and then at the Department of Plastic and Reconstructive Surgery, University of Rome Tor Vergata, selected from September of 2009 to May of 2015.
A completed screening of anthropometry, body composition, and blood biochemical parameters was assessed using the standardized equipment.
Participation in the study included a complete medical history to gather information about health status, current medications including supplements of vitamin and mineral, alcohol drinking, smoking, dietary intake, and physical activity.
Preoperative evaluation was performed, which included a complete clinical examination, photographic assessment, nuclear magnetic resonance imaging (MRI) of the soft tissue, and ultrasound.
Postoperative radiological follow-up was performed at 2, 6, 12, and 24 months and then annually. Postoperative clinical follow-up took place at 2, 6, 12, 21, and 36 weeks and then annually.
Exclusion criteria were divided into two types: local and systemic.
The systemic criteria include platelet disorders, thrombocytopenia, anti-aggregating therapy, bone marrow aplasia, uncompensated diabetes, sepsis, and cancer. The local criteria include cancer loss of substance.
Only patients who showed no signs of malignancy were included in this study. All procedures were performed under general anesthesia at least 3 months after surgery and at least 6 months after the end of radiotherapy/chemotherapy.
The study design was clearly written in layperson language and provided to each study subject. A written informed consent was obtained from each patient, and the study protocol conforms to the ethical guidelines of the Declaration of Helsinki.
Anthropometric measurements
Anthropometric parameters for all participants according to standard methods were performed by trained personnel (body weight, height, hip, and waist circumferences), with the participant wearing only light underwear and without shoes. After a 12 h overnight fast, all subjects underwent anthropometric evaluation. Anthropometric measurements for all participants according to the standard methods were carried out.[8] All the individuals were instructed to take off their clothes and shoes before undergoing the measurements.
Waist and hip measures were taken using a flexible steel metric tape to the nearest 0.5 cm, with subjects standing with arms relaxed by their side and balanced on both feet. The tape was held tight to the skin but without compression of tissue. Hip circumference was also measured according to the International Society for the Advancement of Kinanthropometry protocol[9] taken at the greatest posterior protuberance of the buttocks. Waist circumference was measured just above the iliac crest as recommended by the National Institute of Health Guidelines.[10]
Body weight (kg) was measured to the nearest 0.1 kg using a balance scale (Invernizzi, Rome, Italy). Height (m) was measured using a stadiometer to the nearest 0.1 cm (Invernizzi, Rome, Italy).
Body mass index (BMI) was calculated using the formula: BMI = body weight/height2 (kg/m2).
Dual X-ray absorptiometry
The total body composition was assessed by dual X-ray absorptiometry (DXA) (iDXA, G.E. Medical Systems, WI, USA), according to the previously described procedure.[9]
The technique combined a total body scanner, an X-ray source, an internal wheel to calibrate the bone mineral compartment, and an external lucite/aluminum phantom to calibrate the fat compartment. Standard DXA quality control and calibration measures were performed before each testing session. The subjects were instructed not to exercise within 24 h from the test. The subjects were given complete instructions on the testing procedure. Individuals were asked to remove all clothing except for undergarments including shoes, socks, and metal items before being positioned on the DXA table. Scans were performed with individuals in a supine position. The entire body was scanned beginning from the top of the head and moving in a rectilinear pattern down the body to the feet. The average measurement time was 20 min. The effective radiation dose from this procedure is about 0.01 mSv. The coefficient of variation (coefficient of variation = 100 × standard deviation/mean) intra- and inter-subjects ranged from 1% to 5%. The coefficient of variation for bone measurements is <1%; coefficient of variation on this instrument for five subjects scanned six times over a 9 month period were 2.2% for total body fat (TBFat), and 1.1% for total body lean (TBLean); total PBF was calculated as TBFat mass divided by total mass of tissues (TBFat + TBLean + TBBone) × 100.
Blood biochemical analysis
Blood samples (10 mL) were collected into sterile tubes containing ethylenediaminetetraacetic acid (evacuated tubes) through vein puncture early in the morning (07:00–09:00) after an overnight fast (12 h) and were immediately placed on ice. Serum laboratory tests included fasting glucose, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides (TG) at baseline and after 7 days. Fasting plasma glucose concentrations were measured using the glucose oxidase method with an automated glucose analyzer (COBAS INTEGRA 400, Roche Diagnostics, Indianapolis, IN, USA), serum lipid profile components were determined by standard enzymatic colorimetric techniques (Roche143 Modular P800, Roche Diagnostics, Indianapolis, IN, USA). Analyses were carried out by the accredited Clinical Chemical Laboratories of the Policlinico Casilino, Rome, Italy. Atherogenic indices were calculated as follows: total cholesterol (mg/dL)/HDL cholesterol (mg/dL) (normal value - 5 for men and 4.5 for women), LDL cholesterol (mg/dL)/HDL cholesterol (mg/dL) (normal value - 3.5 for men and 3 for women), log (TG [mg/dL]/HDL [mg/dL]) (normal value - 0.5).[11]
NLR and PLR were calculated as a simple ratio between the absolute neutrophil and the absolute lymphocyte counts, the absolute platelet, and the absolute lymphocyte counts.[12,13]
Clinical and radiological evaluation
All patients underwent the following examinations: mammography, ultrasound, and MRI before the lipofilling session and 12 months after the last lipofilling session, and ultrasound and MRI 1 month after the first lipofilling session and then 3 and 6 months after the last lipofilling session.
Platelet-rich plasma by C-Punt preparation
The authors prepared PRP from a volume of blood (55 ml) according to the method of C-punt; Biomed Device, Modena, Itlay, (http://www.biomeddevice.it). Briefly, blood was taken from a peripheral vein using sodium citrate as an anticoagulant. The current systems for preparing platelet concentrations use various centrifuges (in the C-punt system, we used 1,200 rpm for 10 minutes). PRP was prepared in all cases with approval of the transfusional service. Although the method of preparation is not selective and may include leukocytes, the final aim is to obtain a platelet pellet. Autologous PRP, not activated, obtained by the C-punt procedure after centrifugation (23 ml), was inserted in a light selector device. At the end of the procedure, 20 ml of PRP was harvested.
Surgical procedure
The donor site was chosen based on the patient’s natural fat deposition. Before AT was harvested, 200–600 ml of Klein solution was injected into the donor site using a specific cannula (Coleman Kit, Tucson, Ariz.).[14,15,16,17] The AT was then purified by centrifugation (1200 rpm for 10 min) and combined with PRP in a procedure called platelet-rich lipotransfer (PRL®). It was then re-injected aseptically with a specific microcannula, using the drop-to-drop technique in small pulses (0.2–1 ml), in a radial retrograde manner, on different planes into multiple areas of the breast. According to the patient’s needs, in each of the two session, 50–150 ml (average, 93.54 ml) was injected, for a total of 187 ml (range: 110–250 ml) per patient.
Platelet-rich lipotransfer preparation
Fat harvesting was performed in the same moment of the PRP preparation. We harvested fat tissue in the abdominal region using some specific cannula, with diameters of 2–3 mm and 1.5 mm, for grafting. The fat (80ml) harvested using Platelet Rich Lipotransfert system (CORIOS Soc. Coop, San Giuliano Milanese, Italy, http://www.corios.it) was subjected to automatic filtration and centrifugation cycles at 1200rpm per 10 minutes after which 40ml of the suspension was extracted from the bag. The suspension was further filtered through 120-μm filter, and 20 ml of the stromal vascular fractions cells (SVF) suspension was obtained. Subsequently, the SVF suspension was added and mixed with the centrifuged fat graft. The Blood (55 ml), according to the method of C-punt, was subjected to centrifugation cycles at 1200 rpm per 10 minutes, after which 30 ml of the suspension containing platelet poor plasma (PPP) and platelet rich Plasma (PRP) was extracted from the centrifuge and positioned in the laser selector. At the end of procedure the authors obtained 20 ml of PRP. The authors added 0.2 ml of PRP and 0.2 ml of SVF to each ml of centrifuged fat graft. This enriched-SVF fat combined with PRP was put in 10 mL syringes and aseptically reinjected using the specific microcannulas to implant it into the area to be treated. Fat tissue combined with PRP was implanted at different levels in small tunnels around the margins created earlier by forcing the cannula with precisely controlled movements. A small quantity of fat cells was laid, one or two at a time, during the exciting movement of the cannula to create a large grid to correct the vascular development around each fat cell. Layers of the aligned single cells were laid to increase the contact surface between the receiving tissue and the implant. This technique was of fundamental importance in allowing each single layer deposited to survive through the few days necessary for the growth of the blood vessels that would nourish them permanently.
RESULTS
Responder
A woman of 38-year-old affected by outcomes of breast right mastectomy [Figures 1a, c, 2a, c and 3a, b] with a BMI of 22.1 kg/m2 (normal weight according to BMI classification), waist/hip circumferences ratio of 0.68 (gynoid fat distribution), percentage of TBFat: 41.6% (obese according to the WHO and De Lorenzo classifications), normal blood biochemical parameters, and atherogenic indices but with high preoperative values of PLR (174.49) and NLR (2.65).
Figure 1.
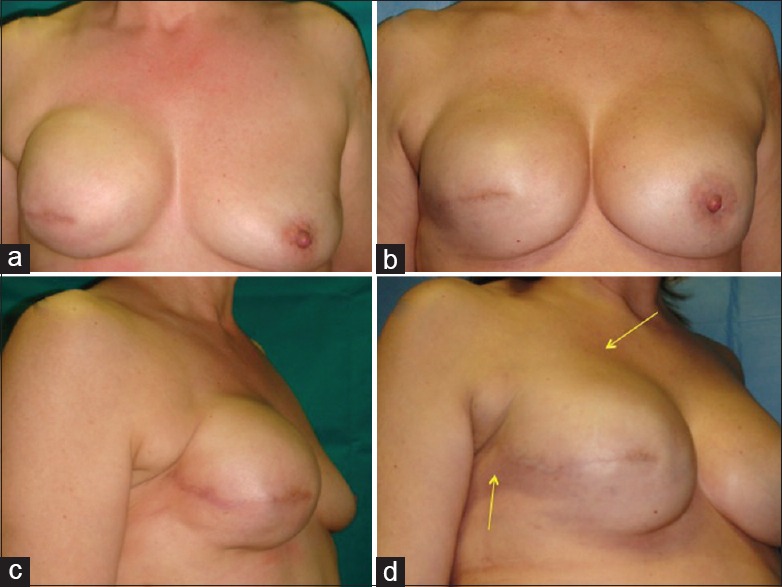
Patients “responder” with outcomes of breast reconstruction: (a) Preoperative situation in frontal projection, (b) postoperative situation in frontal projection after fat injection in the right breast and mastoplasty in the left breast, (c) preoperative situation in the three-fourth right projection with soft tissue defects in the upper pole and in axillary region, and (d) postoperative situation in the three-fourth right projection after fat injection in the right breast and mastoplasty in the left breast with increase of soft tissue volume
Figure 2.
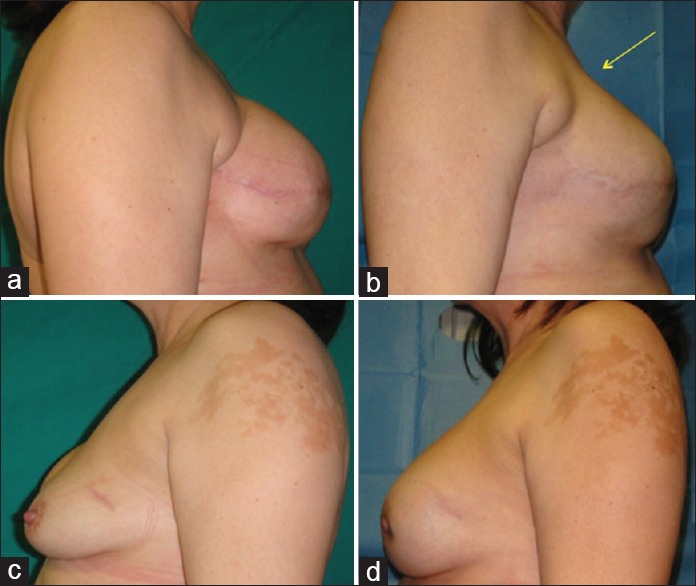
Same patients with outcomes of breast reconstruction: (a) Preoperative situation in lateral right projection with soft tissue defects in the upper pole and in axillary region, (b) postoperative situation in lateral right projection after fat injection in right breast, (c) preoperative situation in lateral left projection, and (d) postoperative situation in lateral left projection after mastoplasty with prostheses
Figure 3.
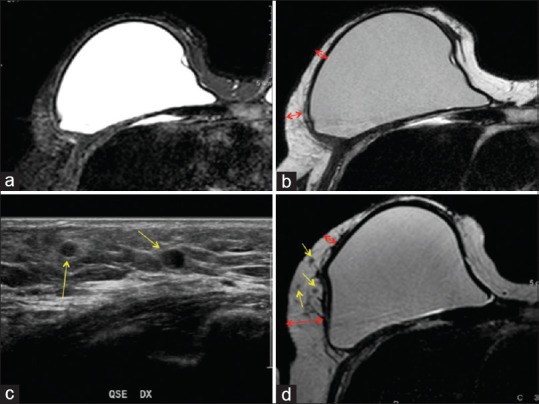
Magnetic resonance imaging of a patient “responder” treated with fat grafting. T2 spectral attenuated inversion recovery imaging (a) and T2 imaging (b) of the preoperative situation. Arrows show the critical point of the reduced thickness and the local soft tissue defect characterized by a loss of volume. Ultrasound (c) of the postoperative situation after 1 year, the yellow arrows show the oil cysts in the soft tissue after 1 year later the fat grafting. T2 imaging (d) of the postoperative situation after 1 year. Red arrows show the improvement of the critical point and the increased thickness of the tissue; the yellow arrows show the oil cysts in the soft tissue after 1 year later the fat grafting
At 6 months after the last lipofilling session, the average resorption of the injected volume was 13.88% for the right breast. At 12 months after the last lipofilling session, the average resorption was 25.85% for the right breast [Figures 1b, d and 2b, d].
In particular, the patient that the authors define “responder” showed the following parameters:
Initial left breast volume: 799.82 ml
Injected volume in left breast: 200 ml of fat grafting
Breast volume maintenance after 6th months: 972.06
Fat graft resorption after 6th month: 13.88%
Breast volume maintenance after 12 months: 948.12
Fat graft resorption after 12 months: 25.85%.
Not responder
A women of 54-year-old affected by outcomes of mastectomy with a BMI of 27.3 kg/m2 (overweight according BMI classification), waist/hip circumferences ratio: 0.89 (android fat distribution), and percentage of TBFat: 36.7% (obese according to the WHO and De Lorenzo classification) affected by metabolic syndrome according to the International Diabetes Federation definition.[13] (Waist circumference: 89 cm, diastolic blood pressure: 86 mmHg, HDL cholesterol: 47.00 mg/dL, TG: 191.00 mg/dL), high values of atherogenic indices (cholesterol total/HDL: 5.15, cholesterol LDL/HDL: 3.34, Log TGL/HDL: 0.61) but with normal preoperative NLR and PLR ratios.
At 6 months after the last lipofilling session, the average resorption of the injected volume was 17.39% for the right breast. At 12 months after the last lipofilling session, the average resorption was 31.99% for the right breast.
The patient that the authors define “not responder” showed the following parameters:
Initial right breast volume: 713.18 ml
Injected volume in the right breast: 180 ml of fat grafting
Right breast volume maintenance after 6th months: 861.487
Fat graft resorption after 6th month in the right breast: 17.39%
Breast volume maintenance after 12 months in the right breast: 835.59
Fat graft resorption after 12 months in the right breast: 31.99%.
The authors considered as the cutoff of fat resorption 16.8% at 6 months and 30.8% at 12 months.
Volumetric assessment at 6 and 12 months after the last lipofilling session
At 6 months after the last lipofilling session, the average resorption of the injected volume was 15.36 ± 1.76% (range: 9.95–17.55%) for all the breasts and 14.40 ± 0.68% (range: 13.92–14.88%) for only the two breasts with oily cyst resorption and 15.94 ± 1.31% (range: 13.88–17.55%) for only the breasts without oily cyst resorption. At 12 months after the last lipofilling session, the average resorption was 28.23 ± 1.55% (range: 25.47–31.99%) for all the breasts, 27.84 ± 1.15% (range: 26.72–29.88%) for the six breasts with oily cyst resorption (Figure 3c, d), and 28.23 ± 1.96% (range: 25.47–31.99%) for the breasts without oily cyst resorption.
DISCUSSION
One of the main reasons why the fat grafting was questioned is that there may be lipofilling resorption. Therefore, the results are unpredictable. In the literature, the resorption rate reported over the 1st year is highly variable (20–90%), most evidently between the 4th and 6th months.[18,19,20,21,22,23]
However, so far, in many studies, the evidence of breast lipofilling survival was based on patient satisfaction and plastic surgeons' evaluations. In a study where mammary volumes were calculated by computed tomography using a three-dimensional program, a resorption rate of 47.5% at 9 months was reported.[24]
However, computed tomography is not indicated for longitudinal studies because it can cause tumors induced by radiation. Instead, MRI allows for a good volume estimate and does not pose this risk.
We found a total average resorption percentage of 15.36% at 6 months after the last lipofilling session and of 28.23% at 12 months after the last lipofilling session. To prevent, or rather minimize, resorption, it is crucial to perform each step of the procedure carefully, paying close attention to the technical details. We injected 200–600 ml average of Klein solution into the donor site before liposuction. In our experience, the lipoaspirate must then be purified in various ways: filtered and centrifuged. Its injection causes an inflammatory response that can be reduced by injecting only adipocytes.[25,26]
In particular, in our patients, the lipoaspirate was purified by centrifugation and combined with PRP. PRP has no impact on the diagnostic images but improves lipofilling results and reduces the resorption rate, increasing fat graft survival.[27] Then, the lipoaspirate was injected using the drop-to-drop technique and in multiple sessions to maximize the contact surface between the lipoaspirate and the host’s capillaries.[17] Diffusion of nutrients from neighboring capillaries is essential for adipocyte survival and favors their integration with the surrounding tissue.[24,28] In addition, PRP added in a concentration of 0.4 mL (40%) per each mL of fat tissue favors an optimal adipose-derived stem cells (ASCs) proliferation with correct architectural adipocytes distribution,[27] better cell-to-cell interaction, AT growth, and differentiation from ASCs; this offers early protection from surrounding inflammatory events. Second, PRP-induced early development of neoangiogenetic microcapillary network favors the delivering of proper nutrient and oxygen levels to grafted cells.[27]
Obese individuals vary in their body fat distribution, their metabolic profile, and the degree of associated cardiovascular and metabolic risks. There is substantial evidence providing that fat distribution is a better predictor of cardiovascular disease than the degree of obesity.[29,30]
An excess of abdominally located fat, even without manifestations of obesity, is associated with metabolic disturbances that indicate an increased risk of atherogenesis and of higher morbidity and mortality, possible due to inherent characteristics of abdominal adipocytes.[31] Thus, regional fat distribution rather than overall fat volume has been considered to be more important in understanding the link between obesity and metabolic disorders.
Among fat depots, fat accumulation in the abdominal area has a greater risk of developing diabetes and future cardiovascular events than the peripheral area. There are differences between AT present in subcutaneous areas and in the abdominal cavity. These include anatomical, cellular, molecular, physiological, clinical, and prognostic differences.[32]
In obese subjects, there are an increased number of macrophages in the AT, which produce several cytokines that contribute to local AT inflammation and to systemic low-grade inflammation.[33,34]
It is believed that adipocyte hypertrophy and local hypoxia due to adipocyte expansion are two important contributing factors to the increased accumulation of macrophages in AT in the obese state.[35,36]
In recent years, increasing evidence have shown that chronic low-grade inflammation is closely related to ectopic fat deposition and metabolic diseases, for example, elevated inflammatory factors are often observed in patients with ectopic fat deposition, such as fatty liver and fatty pancreas.[37,38] Usually, this inflammatory condition is linked with over-nutrition; however, a recent study reported elevated C-reactive protein in nonobese or overweight subjects with the nonalcoholic fatty liver disease, revealing that inflammation may play a critical and direct role, independent of excessive lipid from the diet, in the development of ectopic fat accumulation.[39]
Finally, in light of what we have observed and since the inflammation plays an important role in the ectopic fat distribution, we assume that the problem of fat resorption may be resolved by analysis of body composition and by examining the predictive role of preoperative markers of low-grade inflammation.
CONCLUSION
The authors observed patients with normal weight, gynoid fat distribution, normal blood biochemical parameters, and atherogenic indices but with high preoperative values of PLR and NLR showed an increase of breast fat graft maintenance at 6 and 12 months after the last lipofilling session.
“Compliance with ethical standards.”
The study design was clearly written in layperson language and provided to each study subject. A written informed consent was obtained from each patient, and the study protocol conforms to the ethical guidelines of the Declaration of Helsinki.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hu G, Bouchard C, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, et al. Trunk versus extremity adiposity and cardiometabolic risk factors in white and African American adults. Diabetes Care. 2011;34:1415–8. doi: 10.2337/dc10-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The nurses' health study. Am J Epidemiol. 1997;145:614–9. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 3.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–60. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 4.Snijder MB, Flyvbjerg A, Stehouwer CD, Frystyk J, Henry RM, Seidell JC, et al. Relationship of adiposity with arterial stiffness as mediated by adiponectin in older men and women: The Hoorn study. Eur J Endocrinol. 2009;160:387–95. doi: 10.1530/EJE-08-0817. [DOI] [PubMed] [Google Scholar]
- 5.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: The Hoorn study. Diabetes Care. 2004;27:372–7. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 6.Herishanu Y, Rogowski O, Polliack A, Marilus R. Leukocytosis in obese individuals: Possible link in patients with unexplained persistent neutrophilia. Eur J Haematol. 2006;76:516–20. doi: 10.1111/j.1600-0609.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 7.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Renzo L, Del Gobbo V, Bigioni M, Premrov MG, Cianci R, De Lorenzo A. Body composition analyses in normal weight obese women. Eur Rev Med Pharmacol Sci. 2006;10:191–6. [PubMed] [Google Scholar]
- 9.De Lorenzo A, Bianchi A, Maroni P, Iannarelli A, Di Daniele N, Iacopino L, et al. Adiposity rather than BMI determines metabolic risk. Int J Cardiol. 2013;166:111–7. doi: 10.1016/j.ijcard.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Thornton JC, Bari S, Williamson B, Gallagher D, Heymsfield SB, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutr. 2003;77:379–84. doi: 10.1093/ajcn/77.2.379. [DOI] [PubMed] [Google Scholar]
- 11.Millán J, Pintó X, Muñoz A, Zúñiga M, Rubiés-Prat J, Pallardo LF, et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–65. [PMC free article] [PubMed] [Google Scholar]
- 12.Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. 2008;395:27–31. doi: 10.1016/j.cca.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome – A new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 14.Coleman SR. Structural fat grafting: More than a permanent filler. Plast Reconstr Surg. 2006;118(3 Suppl):108S–20S. doi: 10.1097/01.prs.0000234610.81672.e7. [DOI] [PubMed] [Google Scholar]
- 15.Coleman SR. Long-term survival of fat transplants: Controlled demonstrations. Aesthetic Plast Surg. 1995;19:421–5. doi: 10.1007/BF00453875. [DOI] [PubMed] [Google Scholar]
- 16.Coleman SR. Structural fat grafts: The ideal filler? Clin Plast Surg. 2001;28:111–9. [PubMed] [Google Scholar]
- 17.Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: Safety and efficacy. Plast Reconstr Surg. 2007;119:775–85. doi: 10.1097/01.prs.0000252001.59162.c9. [DOI] [PubMed] [Google Scholar]
- 18.Niechajev I, Sevcuk O. Long-term results of fat transplantation: Clinical and histologic studies. Plast Reconstr Surg. 1994;94:496–506. doi: 10.1097/00006534-199409000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Hörl HW, Feller AM, Biemer E. Technique for liposuction fat reimplantation and long-term volume evaluation by magnetic resonance imaging. Ann Plast Surg. 1991;26:248–58. doi: 10.1097/00000637-199103000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Chajchir A. Fat injection: Long-term follow-up. Aesthetic Plast Surg. 1996;20:291–6. doi: 10.1007/BF00228458. [DOI] [PubMed] [Google Scholar]
- 21.Ersek RA. Transplantation of purified autologous fat: A 3-year follow-up is disappointing. Plast Reconstr Surg. 1991;87:219–27. [PubMed] [Google Scholar]
- 22.Lewis CM. The current status of autologous fat grafting. Aesthetic Plast Surg. 1993;17:109–12. doi: 10.1007/BF02274730. [DOI] [PubMed] [Google Scholar]
- 23.Chan CW, McCulley SJ, Macmillan RD. Autologous fat transfer – A review of the literature with a focus on breast cancer surgery. J Plast Reconstr Aesthet Surg. 2008;61:1438–48. doi: 10.1016/j.bjps.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Amar O, Bruant-Rodier C, Lehmann S, Bollecker V, Wilk A. Fat tissue transplant: Restoration of the mammary volume after conservative treatment of breast cancers, clinical and radiological considerations. Ann Chir Plast Esthet. 2008;53:169–77. doi: 10.1016/j.anplas.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Goodpasture JC, Bunkis J. Quantitative analysis of blood and fat in suction lipectomy aspirates. Plast Reconstr Surg. 1986;78:765–72. doi: 10.1097/00006534-198678060-00009. [DOI] [PubMed] [Google Scholar]
- 26.Carpaneda CA. Study of aspirated adipose tissue. Aesthetic Plast Surg. 1996;20:399–402. doi: 10.1007/BF02390314. [DOI] [PubMed] [Google Scholar]
- 27.Gentile P, Di Pasquali C, Bocchini I, Floris M, Eleonora T, Fiaschetti V, et al. Breast reconstruction with autologous fat graft mixed with platelet-rich plasma. Surg Innov. 2013;20:370–6. doi: 10.1177/1553350612458544. [DOI] [PubMed] [Google Scholar]
- 28.Zheng DN, Li QF, Lei H, Zheng SW, Xie YZ, Xu QH, et al. Autologous fat grafting to the breast for cosmetic enhancement: Experience in 66 patients with long-term follow up. J Plast Reconstr Aesthet Surg. 2008;61:792–8. doi: 10.1016/j.bjps.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 29.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 30.Kang SM, Yoon JW, Ahn HY, Kim SY, Lee KH, Shin H, et al. Android fat depot is more closely associated with metabolic syndrome than abdominal visceral fat in elderly people. PLoS One. 2011;6:e27694. doi: 10.1371/journal.pone.0027694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oka R, Miura K, Sakurai M, Nakamura K, Yagi K, Miyamoto S, et al. Impacts of visceral adipose tissue and subcutaneous adipose tissue on metabolic risk factors in middle-aged Japanese. Obesity (Silver Spring) 2010;18:153–60. doi: 10.1038/oby.2009.180. [DOI] [PubMed] [Google Scholar]
- 32.Després JP. Cardiovascular disease under the influence of excess visceral fat. Crit Pathw Cardiol. 2007;6:51–9. doi: 10.1097/HPC.0b013e318057d4c9. [DOI] [PubMed] [Google Scholar]
- 33.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–86. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 35.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 36.Dalmas E, Clément K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32:307–14. doi: 10.1016/j.it.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167–74. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol. 2011;8:169–77. doi: 10.1038/nrgastro.2011.4. [DOI] [PubMed] [Google Scholar]
- 39.Park SH, Kim BI, Yun JW, Kim JW, Park DI, Cho YK, et al. Insulin resistance and C-reactive protein as independent risk factors for non-alcoholic fatty liver disease in non-obese Asian men. J Gastroenterol Hepatol. 2004;19:694–8. doi: 10.1111/j.1440-1746.2004.03362.x. [DOI] [PubMed] [Google Scholar]


