Abstract
Background:
Marine algae, also called seaweeds, are abundantly present in the coastal area of Iran, especially in Persian Gulf. These plants contain important phytochemical constituents and have potential biological activities. The present study investigated the presence of phytochemical constituents and total phenolic quantification of the seaweeds Gracilaria salicornia and Gracilaria corticata. Cytotoxicity of seaweeds was tested against HT-29, HeLa, and MCF-7 cell lines. Antioxidant potential of these two Gracilaria species was also analyzed.
Materials and Methods:
Extracts of G. salicornia and G. corticata were subjected to phytochemical and cytotoxicity tests. Phytochemical screenings were employed to identify the chemical constituents and total phenolic content. Cytotoxicity was characterized by IC50 of human cancer cell lines (MCF-7, HeLa, and HT-29) using sulforhodamine assay. Antioxidant activities were evaluated using 2,2-diphenyl-1-picrylhydrazyl.
Results:
The analysis revealed that tannins were the most abundant compounds in G. corticata while sterols and triterpenes were the most abundant ones in G. salicornia, but the total phenolic content of the two seaweeds was similar. Cytotoxic results showed that both species could inhibit cell growth effectively, especially against HT-29 cell line.
Conclusion:
Considerable phytochemicals, high antioxidant potential, and moderate cytotoxic activity of G. salicornia and G. corticata make them appropriate candidates for further studies and identification of their bioactive principles.
Keywords: Antioxidant, cytotoxic, Gracilaria corticata, Gracilaria salicornia, Persian Gulf, phytochemical, seaweed
INTRODUCTION
The unique field of marine natural products chemistry emerged in the late 1960s, developed rapidly during the 1980s, and matured in the last decade. The marine biosphere is a rich source of biologically-active principles originating from marine organisms such as seaweeds, invertebrates, coral reefs and marine bacteria. As a result of the potential for new drug discovery, marine natural products have attracted scientists from different disciplines. This interest has led to the discovery of thousands of secondary metabolites from marine organisms, many of which have shown potent biological activities.[1,2,3]
Seaweeds belong to a group of plants known as algae. Seaweeds are classified as Rhodophyta (red algae), Phaeophyta (brown algae), and Chlorophyta (green algae) depending on their nutrient and chemical composition. Like other plants, seaweeds contain various inorganic and organic substances which can benefit human health.[4] Algae contain amino acids, terpenoids, phlorotannins, steroids, phenolic compounds, halogenated ketones and alkanes and cyclic polysulphides.[5] They are rich sources of structurally novel and biologically active metabolites. The metabolites, both primary and secondary, produced by seaweeds may be potential bioactive compounds, which are used in pharmaceutical industry.[6] Marine algae have been used as medicine for a long time[7] and have been extensively studied by several researchers.[2,3,8] They are the sustainable resources in the marine ecosystem and are used as a source of food, fodder, fertilizer, industrial productions, treatment of effluents[9] and medicine. Recent studies revealed that seaweeds contain antibacterial,[10] antiviral,[11,12] antifungal,[13] cytotoxic[14] and acetylcholinesterase inhibitor[15] properties.
Iran has coastal lines about 1260 km along the Persian Gulf and the Oman Sea. More than 250 species of different alga have been identified in this area among which from Chlorophyta: Ulvaceae and Caulerpaceae;[16,17] from Phaeophyta: Dictyotaceae and Sargassaceae; and from Rhodophyta: Gracilariaceae, Gelidiaceae, and Hypneaceae are the most abundant families.[18] Despite the large extent of marine algae in this region, there are only a few studies about phytochemical and biological analysis of these seaweeds. The present study aims to identify types of active constituents of the seaweeds Gracilaria corticata and Gracilaria salicornia, antioxidant potential, and also cytotoxic activity of extracts against MCF-7, HeLa, and HT-29 cell lines.
MATERIALS AND METHODS
Authentication of plant material
The seaweeds were collected from the Persian Gulf coasts of Iran, Bushehr Province, in autumn 2014. Voucher specimens were made and deposited in the herbarium of the School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences (Code: 2666, 2667) and were identified by Agricultural and Natural Resources Research Center of Bushehr.
Preparation of the extracts
The plant samples were cut into small pieces and completely air-dried, and stored in glass containers until extraction. About 100 g of the dried plant material was extracted for 5 days with methanol. The extracts were filtered through two layers of cotton fabric and evaporated at room temperature, under reduced pressure, to a dry residue and stored in sterile vial pending phytochemical and cytotoxic screening.
In vitro cytotoxicity assay
The extracts were tested on MCF-7 (human breast adenocarcinoma), HeLa (cervical carcinoma), and HT-29 (human colon adenocarcinoma) cells. The cancer cell lines were purchased from the National Cell Bank of Pasteur Institute of Iran and grown in Dulbecco’s Modified Eagle Medium (D-MEM) supplemented with 10% fetal bovine serum. Cells were seeded in 96-wells (3500 cells/well) and allowed to adhere for 24 h at 37°C, with 5% CO2, in a fully humidified incubator. Then, 100 μl of serially diluted samples in medium were dispensed into the wells of the cell plates and incubated for a further 72 h. After removal of the sample medium, the cells were topped up with 200 μl D-MEM medium and incubated. After 72 h cells were fixed with cold 40% trichloroacetic acid at 4°C for 1 h and washed with tap water. These cells were determined by sulforhodamine assay.[19] The absorbance was measured at 492 nm using a microplate reader. Percentage of dead cells was calculated in comparison to control. The concentration of the extract that inhibited 50% cells growth (IC50) was determined from the graph plotted by the concentration versus percentage of dead cells.[19]
Phytochemical screening
Tests for phytochemical constituents – alkaloids, steroids and triterpenes, anthraquinones, flavonoids, saponins, cyanogenic glycosides, cardiac glycosides and tannins-followed the methods described previously.[20]
Determination of total phenolics
The powdered plant material (20 g of each sample) was weighed into a 50 ml flask, extracted with 30 ml of ethanol 40% using the sonicator for about 30 min and shaking for about 10 min. After allowing the extracts to cool down to room temperature, the flasks were filled to full volume with extraction solvent.
Preparation of standard
Twenty milligrams of gallic acid and 30 ml 40% EtOH were added into a 50 ml volumetric flask and sonicated until no solid was present in the flask. After allowing the solution to cool down to room temperature, the flask was filled with extraction solvent. The standard solution was diluted several times.
One milliliter of standard solution was transferred to 100 ml volumetric flask with 60–70 high-performance liquid chromatography (HPLC) grade water. The contents swirled to mix. Five milliliter of Folin-Ciocalteu’s phenol reagent was added and mixed again. After 1 min and before 8 min, 15 ml of sodium carbonate solution was added, the time recorded as time zero. The volume was made up to 100 ml exactly with HPLC grade water. The flask stoppered and mixed thoroughly by inverting it several times. After 2 h, the ultraviolet absorption range at 550–850 nm and maximum absorbance about 760 nm were recorded. The same solution without the extraction solution was used as blank solution.[21]
2,2-diphenyl-1-picrylhydrazyl free radical scavenging assay
The free radical scavenging activity was measured using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. Sample stock solutions (1.0 mg/ml) of the extracts were diluted to final concentrations of 243, 81, 27, 9, 3, and 1 μg/ml in ethanol. One ml of a 50 μg/ml DPPH ethanol solution was added to 2.5 ml of sample solutions of different concentrations and allowed to react at room temperature. After 30 min, the absorbance values were measured at 518 nm and converted into the percentage antioxidant activity (AA) using the following formula:

Ethanol (1.0 ml) plus plant extracts solutions (2.5 ml) were used as a blank solution.
DPPH solution (1.0 ml) plus ethanol (2.5 ml) was used as negative control. The positive controls (ascorbic acid, butylated hydroxyanisole, and butylated hydroxytoluene) were used as standard solutions. Assays were carried out in triplicate.[22]
RESULTS AND DISCUSSION
Phytochemical constituents
Phytochemical data show distinct patterns of chemical compositions in constituents of the extracts. The patterns of composition differed considerably in their quantitative values. The results of phytochemical evaluation are shown in Table 1.
Table 1.
Phytochemical constituents of Gracilaria corticata and Gracilaria salicornia
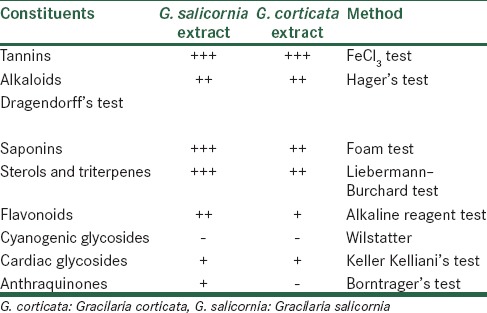
This analysis showed the most abundant compounds in G. corticata were tannins, saponins, sterols, and triterpenes followed by alkaloids, flavonoids, and cardiac glycosides. Cyanogenic glycosides were absent in this seaweed. In G. corticata, tannins were the most abundant compounds followed by saponins, alkaloids, sterols, and triterpenes. Cyanogenic glycosides and anthraquinones were not present.
The amount of total phenol was determined with the Folin–Ciocalteu reagent. Gallic acid was used as a standard compound, and the total phenols were expressed as mg/g gallic acid equivalent using the standard curve equation:
y = 1.771x – 0.0252, R2 = 0.9958.
Where y is absorbance at 760 nm and x is total phenolic content in the extracts of different alga expressed in mg/L [Figure 1]. Phenolic compounds are a class of antioxidant agents which act as free radical terminators.[13] Table 2 shows the contents of total phenols that were measured by Folin–Ciocalteu reagent in terms of gallic acid equivalent. The total phenol in selected seaweeds was 0.012–0.013 mg in 1 g dry extract in G. salicornia and G. corticata, respectively.
Figure 1.
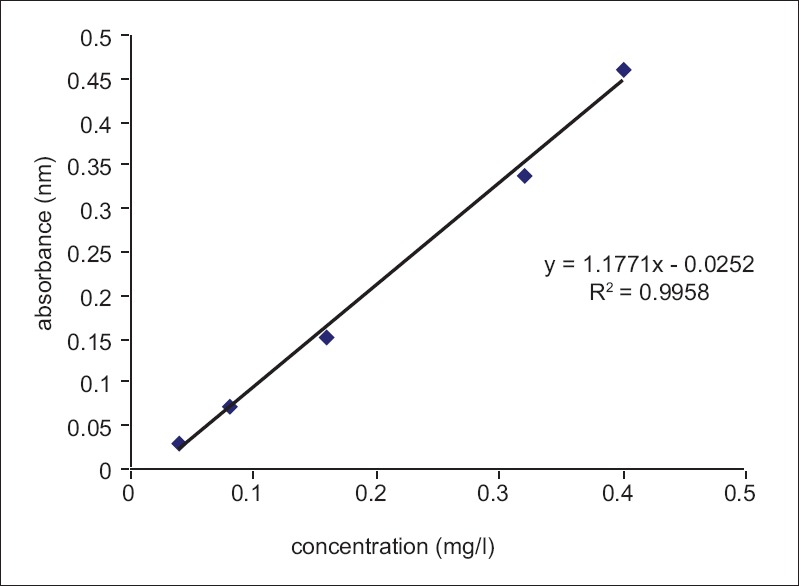
Standard curve of gallic acid
Table 2.
Total phenolic content of seaweeds

Cytotoxic assay
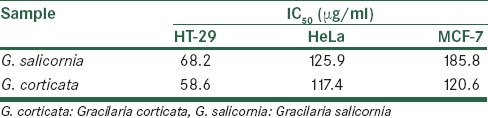
The criteria used to categorize the activity of extracts against human breast, cervix, colon (MCF-7, HeLa, and HT-29) cell lines based on IC50 values were modified from those of NCI and Geran et al.[20] as follows: IC50 ≤20 μg/ml = highly active, IC50 21–200 μg/ml = moderately active, IC50 201–500 μg/ml = weakly active, and IC50 >501 μg/ml = inactive.
The cytotoxicity data for the extracts against MCF-7 (human breast adenocarcinoma), HeLa (cervical carcinoma), and HT-29 (human colon adenocarcinoma) cells are displayed in Table 3.
Table 3.
Cytotoxic activity (IC50) of the seaweed Gracilaria corticata and Gracilaria salicornia
Antioxidant activity
The AA of the seaweed extracts was measured on the basis of the scavenging activity of the stable DPPH free radical. IC50 value is inversely related to AA. The extracts of the two algae were found to have different levels of AA. Table 4 shows that higher AA was found in G. salicornia extract.
Table 4.
Antioxidant activity (IC50) of the seaweed Gracilaria corticata and Gracilaria salicornia

So far more than 2400 marine natural products have been isolated from seaweeds of subtropical and tropical populations.[23] Certain algae have long been used in traditional Chinese herbal medicine in the treatment of cancer.[24] Many studies have been developed to determine the bioactive compounds produced by marine algae. Antioxidant and cytotoxic activities are one of the most important specificities of marine algae. Some metabolites such as bromophenols, carotene, and steroids were isolated and purified from some algae, and their activity against some cancer cell lines were demonstrated.[25]
In the case of red seaweeds, five major edible genera have been introduced, including Porphyra, Palmaria, Gracilaria, Gelidium, and Kappaphycus (Eucheuma). In particular, numerous red seaweeds of the genus Gracilaria are utilized as fresh food in many parts of the world.[26,27] In this research, an effort was made to study the similarity and differences between the two species on the basis of phytochemical analysis and bioevaluation. Phytochemical tests revealed lots of similarities than differences. The only difference between the two plants was about the detection of anthraquinones which were not present in G. corticata. The amount of tannins, sterols, triterpenes, saponins, and flavonoids were also different, but the phenolic content of these seaweeds were similar. Regarding cytotoxic activity, both species showed better results against HT-29 cell lines as compared to MCF-7 and HeLa cell lines, but G. salicornia had better results. In a study about cytotoxic activities often different algae from the Persian Gulf and the Oman Sea, G. salicornia was tested against MDA-MB-231, MCF-7, and T-47D cell lines but IC50 of this alga was reported higher than 400 μg/ml.[28] The difference between our data and this study may be because of different solvents used for extraction, season and time of collection, and also different methods. Saeidnia et al. also reported that ethyl acetate extract of G. salicornia showed a potent cytotoxic effect against Artemia salina nauplii.[29] Phytochemical analysis showed that sterols and triterpenes are the main compounds in the ethyl acetate fraction and may be responsible for the cytotoxic activity of this seaweed. Antioxidant capacity is widely used as a parameter for medicinal bioactive components. Natural antioxidants are known to exhibit a wide range of biological effects including antibacterial, antiviral, anti-inflammatory, antiallergic, antithrombotic, and vasodilatory activities.[30] The present study infers that G. salicornia and G. corticata extracts exhibit effective antitumor activity and seem to have potent secondary metabolites. They are cost-effective, easy to produce and purify. In future, they can be recommended to patients as an effective therapeutic tool in the form of a food or drug. Further research needs to be explored to study the bioactive compounds of these two seaweeds and for the successful implication of them as a potent therapeutic tool against cancer.
CONCLUSION
The extracts of two seaweeds from Persian Gulf, Iran were screened for their antioxidant, cytotoxic and phytochemical analysis. Phytochemical and cytotoxic results were similar with a few differences about all species. These seaweed extracts and their active components could emerge as natural and alternative antioxidants or serve as starting points for synthesizing more effective cytotoxic drugs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Smit AJ. Medicinal and pharmaceutical uses of seaweed natural products: A review. J Appl Phycol. 2004;16:245–62. [Google Scholar]
- 2.Yegdaneh A, Putchakarn S, Yuenyongsawad S, Ghannadi A, Plubrukarn A. 3-oxoabolene and 1-oxocurcuphenol, aromatic bisabolanes from the sponge Myrmekioderma sp. Nat Prod Commun. 2013;8:1355–7. [PubMed] [Google Scholar]
- 3.Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2007;24:31–86. doi: 10.1039/b603047p. [DOI] [PubMed] [Google Scholar]
- 4.Kuda T, Taniguchi E, Nishizawa M, Araki Y. Fate of water-soluble polysaccharides in dried Chorda filum a brown alga during water washing. J Food Compost Anal. 2002;15:3–9. [Google Scholar]
- 5.Mtolera MS, Semesi AK. Uppsala, Sweden: Gotab AB; 2000. Gotab AB: Uppsala Sweden; 2000. pp. 211–7. [Google Scholar]
- 6.Kusumoto IT, Nakabayashi T, Kida H, Miyashrio T, Hattori M, Namba T. Shimotohno Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficiency virus-I (HIV-I) protease. Phytother Res. 1995;9:180. [Google Scholar]
- 7.Fitton JH. In: Antiviral properties of marine algae. World Seaweed Resources. Critchley AT, Ohno M, Largo DB, editors. Wokingham, UK: Windows and Macintosch, ETI Information Services; 2006. p. 7. [Google Scholar]
- 8.González del Val A, Platas G, Basilio A, Cabello A, Gorrochategui J, Suay I, et al. Screening of antimicrobial activities in red, green and brown macroalgae from Gran Canaria (Canary Islands, Spain) Int Microbiol. 2001;4:35–40. doi: 10.1007/s101230100006. [DOI] [PubMed] [Google Scholar]
- 9.Sharmila S, Rebecca LJ. A comparative study on the degradation of leather industry effluent by marine algae. Int J Pharm Sci Rev Res. 2014;25:46–50. [Google Scholar]
- 10.Tuney I, Cadirci BH, Unal D, Sukatar A. Antimicrobial activities of the extracts of marine algae from the coast of Urla (Izmir, Turkey) Turk J Biol. 2006;30:171–5. [Google Scholar]
- 11.Garg HS, Sharma T, Bhakuni DS, Pramanik BN, Bose AK. An antiviral sphingosine derivative from green alga Ulva fasiata. Tetrahedron Lett. 1992;33:1641–4. [Google Scholar]
- 12.Tang HF, Yang-Hua Y, Yao XS, Xu QZ, Zhang SY, Lin HW. Bioactive steroids from the brown alga Sargassum carpophyllum. J Asian Nat Prod Res. 2002;4:95–101. doi: 10.1080/10286020290027362. [DOI] [PubMed] [Google Scholar]
- 13.Aliya R, Shameel M. Phycochemical evaluation of four coenocytic green seaweeds from the coast of Karachi. Pak J Mar Biol. 1999;5:65–76. [Google Scholar]
- 14.Thangam TS, Kathiresan K. Mosquito larvicidal activity of marine plant extracts with synthetic insecticides. Bot Mar. 1991;34:537–9. [Google Scholar]
- 15.Ghannadi A, Plubrukarn A, Zandi K, Sartavi K, Yegdaneh A. Screening for antimalarial and acetylcholinesterase inhibitory activities of some Iranian seaweeds. Res Pharm Sci. 2013;8:113–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Sohrabipour J, Rabiei R. The checklist of green algae of the Iranian coastal lines of the Persian Gulf and Gulf of Oman. Iran J Bot. 2007;13:146–9. [Google Scholar]
- 17.Sohrabipour J, Nejadsatari T, Assadi M, Rebei R. The marine algae of the Southern coast of Iran, Persian Gulf, Lengeh area. Iran J Bot. 2004;10:83–93. [Google Scholar]
- 18.Safa O, Soltanipoor MA, Rastegar S, Kazemi M, Nourbakhsh Dehkordi K, Ghannadi A. An ethnobotanical survey on hormozgan province, Iran. Avicenna J Phytomed. 2013;3:64–81. [PMC free article] [PubMed] [Google Scholar]
- 19.Kamba AS, Hassan LG. Phytochemical screening and antimicrobial activities of Euphorbia balsamifera leaves, stems and root against some pathogenic microorganisms. Afr J Pharm Pharmacol. 2010;4:645–52. [Google Scholar]
- 20.Geran RI, Greenberg NH, Macdonald MM, Shumacher AM, Abbott BJ. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chem Rep. 1972;3:1–103. [Google Scholar]
- 21.Pourmorad F, Hosseinimehr SJ, Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotechnol. 2006;5:1142–5. [Google Scholar]
- 22.Braca A, De Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia tarapotensis. J Nat Prod. 2001;64:892–5. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- 23.Manilal A, Sujith S, Kiran GS, Selvin J, Shakir C, Gandhimathi R, et al. Biopotentials of seaweeds collected from South West Coast of India. J Mar Sci Technol. 2009;17:67–73. [Google Scholar]
- 24.Yamamoto I, Takahashi M, Tamura E, Maruyama H, Mori H. Antitumor activity of edible marine algae: Effect of crude fucoidan fractions prepared from edible brown seaweeds against L-1210 leukemia. Hydrobiologia. 1984;116:145–8. [Google Scholar]
- 25.Xu N, Fan X, Yan X, Tseng C. Screening marine algae from China for their antitumor activities. J Appl Phycol. 2004;16:451–6. [Google Scholar]
- 26.McDermid KJ, Stuercke B. Nutritional composition of edible Hawaiian seaweeds. J Appl Phycol. 2003;15:513–24. [Google Scholar]
- 27.Norziah MH, Ching CY. Nutritional composition of edible seaweed Gracilaria changgi. Food Chem. 2006;68:69–76. [Google Scholar]
- 28.Erfani N, Nazemosadat Z, Moein M. Cytotoxic activity of ten algae from the Persian Gulf and Oman Sea on human breast cancer cell lines; MDA-MB-231, MCF-7, and T-47D. Pharmacognosy Res. 2015;7:133–7. doi: 10.4103/0974-8490.150539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saeidnia S, Gohari AR, Shahverdi AR, Permeh P, Nasiri M, Mollazadeh K. In vitro antitumor activity of Gracilaria corticata (a red alga) against jurkat and molt-4 human cancer cell lines. Afr J Biotechnol. 2010;9:6787–90. [Google Scholar]
- 30.Cook NC, Samman S. Flavonoids: Chemistry, metabolism, cardioprotective effects and dietary sources. J Nutr Biochem. 1996;7:66–76. [Google Scholar]


