Abstract
The mammalian skin epidermis and its hair and sweat gland appendages provide a protective barrier that retains essential body fluids, guards against invasion by harmful microbes, and regulates body temperature through the ability to sweat. At the interface between the external environment and the body, skin is constantly subjected to physical trauma and must also be primed to repair wounds in response to injury. In adults, the skin maintains epidermal homeostasis, hair regeneration, and wound repair through the use of its stem cells. This essay focuses on when stem cells become established during skin development and where these cells reside in adult epithelial tissues of the skin. I explore how skin stem cells maintain tissue homeostasis and repair wounds and how they regulate the delicate balance between proliferation and differentiation. Finally, I tackle the relation between skin cancer and mutations that perturb the regulation of stem cells.
The mammalian skin epidermis and its hair and sweat gland appendages provide a protective barrier that retains essential body fluids, guards against invasion by harmful microbes, and regulates body temperature through the ability to sweat. At the interface between the external environment and the body, skin is constantly subjected to physical trauma and must also be primed to repair wounds in response to injury. In adults, the skin maintains epidermal homeostasis, hair regeneration, and wound repair through the use of its stem cells. For over three decades, my laboratory has been interested in the properties of skin stem cells. Where do these cells reside? When do they become established during embryogenesis, and how are they able to build tissues with such remarkably distinct architectures? How do stem cells maintain tissue homeostasis and repair wounds and how do they regulate the delicate balance between proliferation and differentiation? What is the relationship between skin cancer and mutations that perturbs the regulation of stem cells? Here, I will summarize our progress on these fronts, with emphasis on my own laboratory’s contributions to the fascinating world of skin stem cells.
1. MAMMALIAN EPIDERMIS, HOMEOSTASIS, AND STEM CELLS
The epidermis is a stratified epithelium whose innermost (basal) layer is attached to an underlying basement membrane rich in extracellular matrix and growth factors. The basal layer is the only layer with proliferative potential. Periodically, these cells will detach from the underlying basement membrane and embark upon a differentiation journey that culminates in the production of dead flattened squames that are then sloughed from the skin surface and continually replaced by inner cells moving outward.
The process of terminal differentiation can be morphologically and molecularly subdivided into four stages. The proliferative basal progenitors express high levels of integrins as well as keratins K14 and K5, which form an extensive filamentous network that provides these cells with mechanical resistance (Fuchs & Green, 1980; Jones, Harper, & Watt, 1995). Spinous suprabasal cells are transcriptionally dynamic, shutting off expression of K14 and K5, and inducing expression of K1 and K10, which form higher ordered cables that add to this mechanical infrastructure (Fuchs & Cleveland, 1998; Fuchs & Green, 1980). These cells also form elaborate desmosomes which reinforce intercellular contacts and provide the spinous-like morphology (Johnson, Najor, & Green, 2014). As spinous cells are pushed upward, they enter the granular layer, forming an elaborate array of filaggrin-rich keratohyalin granules and lamellar granules packed with lipid bilayers. Both spinous and granular cells remain transcriptionally active and make glutamine and lysine-rich proteins, including loricrin and involucrin, which are deposited beneath the plasma membrane. As terminal differentiation nears its endpoint, a calcium influx activates transglutaminases to generate γ-glutamyl-ε-lysine crosslinks that form the cornified envelope. As the organelles including the nucleus are lost, the lamellar granules extrude their lipids to form a lipid-filled sandwich of dead squames. The squames extruded from the surface are indestructible cornified sacs of keratin filaments.
Human epidermis is considerably thicker than mouse epidermis, a feature which most likely explained by the exposed status of the epidermis, and hence the need for an elaborate epidermal skin barrier, when the hair coat is thin. Consistent with this notion is the similarity of human epidermis to early neonatal mouse epidermis, i.e., before the protrusion of the hair from the skin surface. In human epidermis, which typically contains multiple spinous, granular, and stratum corneum layers, it takes ~4 weeks for cells to exit the basal layer and be shed from the skin surface. In adult mouse skin, while turnover still takes place, the thin epidermis typically consists of a barely more than four layers. An exception is the paw skin, which like human palmar and plantar skin, offers the thickest epidermis of the body.
The skin epidermis replenishes itself through a process called tissue homeostasis, in which the number of cell divisions within a tissue compensates for the number of cells lost (Xie & Spradling, 2000). Tissue homeostasis is achieved through stem cells located within the basal layer. The ability of the epidermis to turnover every few weeks places a constant demand on its stem cells. Two distinct models have been proposed to explain the behavior of basal cells. The hierarchical model suggests that the epidermis is composed of discrete proliferative units consisting of a slow-cycling stem cell that gives rise to short-lived, transit-amplifying cells which then depart the basal layer after several divisions to generate upward columnar units of differentiating cells. The stochastic model suggests that basal epidermis is composed of a single type of proliferative progenitor, whose daughter cells choose randomly to differentiate or remain as progenitors.
Lineage tracing involves the genetic marking of one or a group of cells in their normal physiological context in a way that their subsequent progeny retain marker expression. This method has proven to be powerful in evaluating the contribution of stem cells to tissue homeostasis (Xie & Spradling, 2000). When individually labeled basal cells in tail, ear, or hindpaw epidermis were marked by lineage tracing and their progeny were then monitored long term, the clonal fate data were compatible with the stochastic model (Clayton et al., 2007; Doupe, Klein, Simons, & Jones, 2010; Lim et al., 2013). However, a crucial issue still unresolved is whether the basal cells in these various labeling strategies were marked randomly or selectively. In contrast, a recent study on tailskin employed two inducible Cre-lineage tracers: one was driven by a Krt14-promoter, active in all basal cells, and the other by an Involucrin-promoter, active only in a discrete subset of K14+ basal cells that precociously express this differentiation-specific gene. In this study, purportedly slow-cycling, basal cells marked by Krt14-CreER but not Involucrin-CreER behaved like long-lived stem cells, which gave rise to the Involucrin-CreER subset that displayed features of more committed basal progenitors (Mascre et al., 2012). This study supports a hierarchical model, and yet shows that progenitors within the basal layer exist and can behave in a stochastic manner. While it is tempting to speculate that the differences in these collective studies arise from the tools used to mark the basal cells, it is still possible that variation in the epidermis at different body sites accounts for the differences. An additional confounding problem for analyzing epidermal homeostasis and lineage tracing in the laboratory mouse is their behavioral traits of scratching and grooming, which can inadvertently affect the outcome. At first glance, it may seem that the elegant live-imaging studies recently performed on mouse ear skin (Pineda et al., 2015; Rompolas, Mesa, & Greco, 2013) will offer a better avenue for tracing clonal behavior. Here, however is the issue of compressing the ear between glass plates in order to capture the image. These mechanical stresses may also affect the outcome of clonal analyses. Single-cell analyses may provide the best current route to a more definitive answer in the future.
While the exact number and characteristics of the epidermal stem cell still remain elusive, their existence and reliance upon underlying extracellular matrix have long been known. Indeed, the first functional demonstration of an epidermal stem cell was made when methods were identified to culture human keratinocytes under conditions where they could be maintained and propagated for hundreds of generations without losing stemness (Rheinwald & Green, 1975, 1977). When grown from an unaffected region of a burn patient, expanded epidermal cultures could be stably engrafted onto the damaged skin (Green, 1991). Some 30 years later, the engrafted epidermis had not developed cancer or other abnormalities, which indicated that, under the right conditions—in this case, coculture with irradiated dermal fibroblasts—in vitro stem cell expansion and differentiation can be achieved without deleterious consequence.
The requirement of dermal neighbors for successful culturing of epidermal stem cells highlights the reliance of stem cells upon crosstalk with their niche microenvironment. Indeed, by elucidating key heterologous niche components and/or the crosstalk involved, stem cells from many different epithelia have since been cultured. The key to long-term self-renewal of tissue stem cells in vitro is predicated on unraveling the complexities of signaling circuitry governing stem cell behavior. While efforts on the epidermal front are ever improving, we still cannot make an epidermal appendage in a culture dish. Interestingly, the epidermis of the engrafted cultured keratinocytes also never grew hair nor produced sweat. There is still much research to do!
2. MAMMALIAN EPIDERMAL APPENDAGES: HAIR FOLLICLES AND SWEAT GLANDS
Most mammals have a thick hair coat and devote much of their time to taking care of it for warmth and protection. They do so by undergoing cyclical bouts of hair growth (anagen), followed by destruction (catagen) in which the hair stops growing and the lower two thirds of the follicle degenerates, and then rest (telogen) in which not much happens. Such cycles can operate sequentially from a single signaling (dermal papilla), which can activate a single stem cell niche (the bulge) that overlies it (Fig. 1). Each time a hair follicle cycles, a new hair is born and a new stem cell niche is produced at the end of the cycle (Hsu, Pasolli, & Fuchs, 2011). If the old hair is released at the end of each hair cycle, a process known as exogen, only one hair will protrude from each orifice. If, however, the old hair is tightly anchored to its niche, then by the time the hair cycle is launched, the hair coat becomes thicker than it was as a juvenile.
Figure 1.
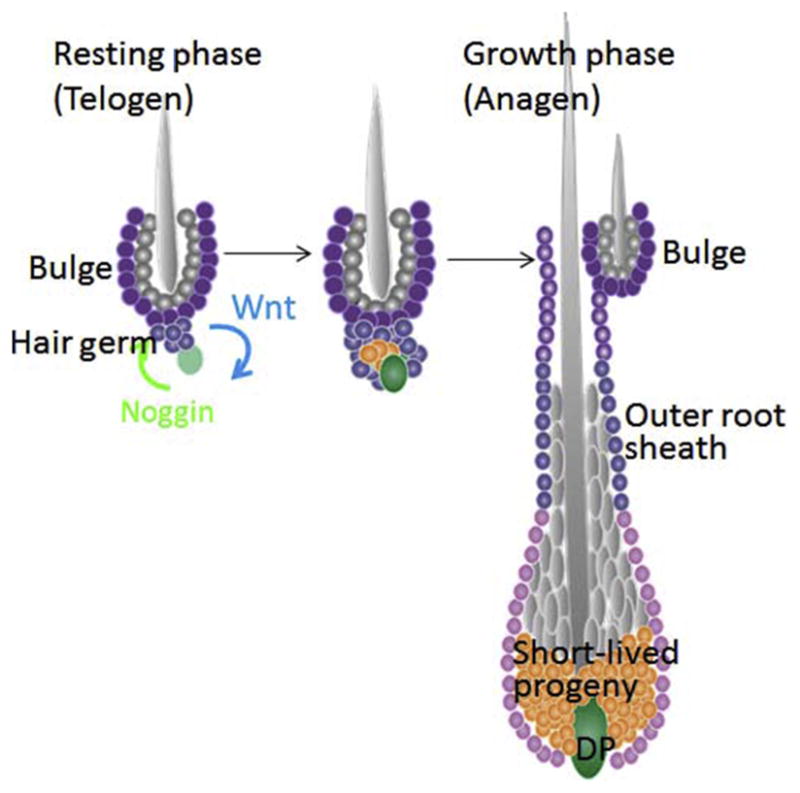
Schematic of the resting and growth phases of the hair follicle. The stem cells (purple) reside in the outer layer of the bulge niche, which anchors the hair from the previous hair cycle. The bulge cells at the base are referred to as the hair germ, which during telogen, is abutted next to the dermal papilla (DP), the mesenchymal stimulus of the hair follicle. Following a buildup of BMP inhibitors (Noggin) in the DP and WNTs in the hair germ, the stem cells at the base are activated and generate the short-lived progeny (orange), which ultimately will differentiate into the hair and its channel. This figure is patterned after Figure S5 in Hsu, Li, and Fuchs (2014b). Elsevier Press.
One of the major questions still unanswered is what controls hair anchorage. There are plenty of us in the world who would like to unlock this mystery, not just for scientific purposes! One clue we might be able to glean about this process is hormonal and/or metabolic influence. A musk ox must have a very thick protective coat when it is out on the tundra, but it sheds this coat in the summer. To date, studies of hair shedding en masse have been limited largely to sheep and larger mammals, where genetics and biochemistry are largely impractical. That said, the process of exogen occurs in mice and would be worth exploring in the future.
When the skin epithelium uses only hair follicles to contribute to thermal regulation, the process is inefficient and most mammals are confined to small climate variations. Horses and camels are among the best examples of animals able to withstand greater temperature extremes. They are able to secrete an oily like fluid through apocrine sweat glands which are an appendage of the hair follicle. This sweating function is critical for their survival and performance, through the ability of apocrine secretions to dissipate heat (McEwan Jenkinson, Elder, & Bovell, 2006; Schmidt-Nielsen, Schmidt-Nielsen, Houpt, & Jarnum, 1957; Schmidt-Nielsen, Schmidt-Nielsen, Jarnum, & Houpt, 1957).
By contrast, higher primates, including humans, have elaborated upon a new type of sweat gland, the eccrine gland, which protrudes directly onto the skin surface rather than from the hair follicle orifice, and secrete a salt–water mixture as opposed to oily sweat. As a result of having a high density of sweat glands over our body, we are able to secrete as much as 3 l of sweat during a marathon, and can regulate body temperature far better than other animals. In humans, as many as 700/cm2 sweat glands can be found in the skin from the palms and soles.
As the most commonly used laboratory mammal, mouse has eccrine sweat glands exclusively present in the pads of their paws, and its trunk skin lacks sweat glands altogether. Although animals such as this are sensitive to extremes in climate, the sweat glands they do have provide a segue to dissecting the genetics underlying glandular development. In particular, mutant mice lacking either ligand EDA (Tabby) or receptor EDAR (Downless) exhibit a loss of sweat glands as well as several other curious features, including tooth loss and a loss of the coarsest type of hair, the guard hair, on the mouse’s coat (Srivastava et al., 2001). Excessive activation of signaling leads to supernumerary sweat glands and guard hairs (Mustonen et al., 2003). Intriguingly, a natural EDAR variant (V370A) has been found within an East Asian population of humans who have especially coarse hair and an increase in sweat glands (Cluzeau et al., 2012; Fujimoto, Kimura, et al., 2008; Fujimoto, Ohashi, et al., 2008; Kimura et al., 2009; Sabeti et al., 2007). This variant arose more than 30,000 years ago and persists in an ever-expanding population (Kamberov et al., 2013). Thus, the increased sweat gland density confers a selective advantage in the hot and humid climate of this region (Lu & Fuchs, 2014).
Can we simply activate a V370A EDAR variant and humanize the mouse skin? While it would clearly make it easier to unlock the molecular mysteries of sweat glands as well as making a better laboratory model for investigating the biology of skin disorders, the goal is not so simple. Thus, the EDA/EDAR pathway controls the numbers of appendages, but their regional positioning remains largely unaffected by overactivating the pathway. Similarly, many of the distinctive characteristics of different appendages/glands are preserved even under excessive signaling. One exception may be in branching morphology, which occurs in mammary and salivary glands but not in sweat glands or hair follicles. It has been observed that both mammary and salivary glands display defective branching in Tabby mice (Blecher, Debertin, & Murphy, 1983; Voutilainen et al., 2012), and that ductal branching appears to be enhanced on excessive EDA/EDAR signaling (Chang, Jobling, Brennan, & Headon, 2009; Mustonen et al., 2003). It will be interesting in the future to determine whether the “nonbranched” morphologies of sweat glands and hair follicles are achieved by modulating EDA/EDAR signaling during development. Most likely, a complex interplay of signaling pathways, involving EDA/EDAR but not exclusive of them, will be needed before the perfect model in all its elegant regalia will emerge.
3. THE HAIR FOLLICLE AND ITS STEM CELLS
Given the abundance of hair follicles in the laboratory mouse, they have emerged as one of the best systems to explore the key facets of homeostasis and stem cells within an adult tissue. The wonderful aspect about mouse hair follicles is their ability to undergo synchronized cyclical bouts of regeneration and rest. This process is fueled by stem cells located in a region known as the bulge, and in a cluster of cells at the bulge base known as the hair germ. Melanocyte stem cells are intermingled with hair follicle stem cells in the bulge and the hair germ (Chang et al., 2013; Nishimura et al., 2002; Rabbani et al., 2011). When a new hair cycle begins, melanocyte and hair follicle stem cells must synchronize their differentiation programs so that the mature melanocytes that produce melanin will do so at the right time to transfer their melanin pigment to the differentiating hair cells so that they have their distinctive coloring.
The synchronization of the first three hair cycles in the mouse hair coat provided a unique means of studying hair follicle stem cells in their resting, quiescent phase, and comparing them to their anagen phase when they are actively fueling hair follicle regeneration and hair growth. Cells with proliferative potential that spend extended periods in quiescence can thus be marked and monitored by nucleotide analog pulse-chase experiments, which were useful before the advent of lineage tracing with fluorescent markers. Such label-retaining cells reside at the base of the resting telogen follicle, within the outermost layer of the bulge and its associated hair germ (Cotsarelis, Sun, & Lavker, 1990). Label-retaining cells are stem cells, as later demonstrated by using a regulatable fluorescent histone to label the resting bulge cells and then monitor their cell divisions, during normal hair regeneration and following wounding to the skin surface (Tumbar et al., 2004). Similar studies with classical lineage-tracing markers bolstered these findings (Morris et al., 2004).
Both bulge and hair germ share many molecular features of stemness, including expression of Lgr5 and Sox9 (Greco et al., 2009; Sennett et al., 2015). However, hair germ cells are always the first to be activated at the start of each new hair cycle, and they undergo more divisions than bulge cells (Greco et al., 2009; Rompolas et al., 2013). Their close proximity to the underlying mesenchymal signaling center, the dermal papillae (DP), impacts the early activation process through activating WNT signals from the germ and inhibitory BMP signals from the DP.
Activated hair germ cells do not maintain stemness in vitro (Chen et al., 2012) and in, they generate the short-lived progeny that produce the vivo hair and its channel (Greco et al., 2009; Hsu, Li, & Fuchs, 2014a, 2014b). By contrast, once the new hair cycle initiates, some bulge cells leave their niche and form an inverse proliferative gradient along the emerging outer root sheath of the follicle. Early in the hair growth phase, a subset of the short-lived progeny produce a Sonic Hedgehog signal that stimulates the remaining bulge cells to proliferate and replenish the niche (Hsu et al., 2014a, 2014b). SHH also undergoes crosstalk with the DP that fuels the proliferation of the bulb cells (matrix) that will form the seven concentric cones of differentiating cells that will form the central hair shaft, its inner root sheath, and the companion layer that separates it from the outer root sheath.
As the hair follicle grows downward, the SHH-expressing signaling cells and the DP move too far away from the niche and the bulge cells return to quiescence. The outer root sheath cells that are in closest proximity to the bulge soon follow suit, returning to quiescence as well. Soon thereafter, these cells form a new bulge and hair germ for the next cycle (Hsu et al., 2011). Many of these features, first established by staggered pulse-chase and lineage-tracing experiments (Hsu et al., 2011), have been more recently corroborated by elegant live-imaging studies (Rompolas et al., 2013). Moreover, even though bulge normally gives rise to the hair germ, the hair germ can conversely replenish an emptied bulge niche, as shown by laser ablation and live imaging (Rompolas et al., 2013). This not only underscores the close relation between the bulge and hair germ but also the plasticity of the system. Intriguingly, when the DP is ablated the stem cells cannot function. Taken together, these findings suggest a greater importance for the stem cell niche than its residents.
These roots of stem cell plasticity appear to be governed at the level of transcriptional landscaping as my laboratory recently showed (Adam et al., 2015). Several years ago, we showed that hair follicle lineage progression is governed in part by dynamic regulation of Polycomb (PcG)-mediated repression/derepression of chromatin as typified by a trimethylation mark on lysine 27 of histone H3 (H3K27me3) (Ezhkova et al., 2009, 2011). However, hair follicle stem cell identity and function are mainly independent of PcG-regulated genes, indicating that additional epigenetic mechanisms underlie the governance of critical cell identity genes.
Recent studies on cultured embryonic stem cells showed that genes controlling unique cellular identities are driven by so-called super-enhancers, which are marked by acetylation of the H3K27 site over large chromatin regulatory domains bountiful in the pluripotency transcription factor-binding motifs (Whyte et al., 2013). This mechanism enables the factors to bind cooperatively, and since the genes encoding these factors are themselves regulated by super-enhancers, this provides a feed-forward loop that sustains stemness.
The availability of large quantities of hair follicle stem cells allowed us to test this paradigm for the first time in an adult tissue stem cell in its native niche. By conducting super-enhancer analyses, we learned that SOX9, LHX2, TCF3/4, and NFATc1, all bind to small regions (epicenters) within these open chromatin domains of hair follicle bulge stem cells (Adam et al., 2015). All of these factors are genetically essential for maintaining aspects of the bulge stem cells. Some are required to maintain stemness, while others are important in controlling quiescence (Horsley, Aliprantis, Polak, Glimcher, & Fuchs, 2008; Lien & Fuchs, 2014; Lien et al., 2014; Nguyen et al., 2009; Nowak, Polak, Pasolli, & Fuchs, 2008; Rhee, Polak, & Fuchs, 2006; Vidal et al., 2005). These epicenters harbor all of the information necessary for targeting a gene to the stem cell niche, as evidenced by using GFP as a reporter (Fig. 2).
Figure 2.
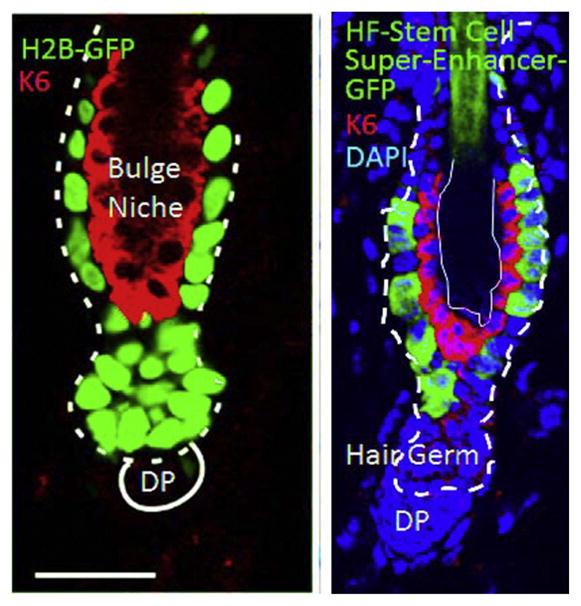
Immunofluorescence images showing a resting phase hair follicle, replete with bulge stem cells and keratin 6+ inner bulge nonstem cells. At left, bulge and hair germ cells are label-retaining and marked by H2B-GFP used in a pulse-chase labeling experiment. At right, bulge is labeled with GFP driven by a HF stem cell-specific super-enhancer epicenter element. Left panel is Figure 5 Frame C of Hsu et al. (2011). Elsevier Press. Right panel is Figure 2e of Adam et al. (2015). Nature Press.
Interestingly, when hair follicle stem cells are removed from their native niche and placed into culture under conditions where engraftment could restore their multilineage capacity (Blanpain, Lowry, Geoghegan, Polak, & Fuchs, 2004), the chromatin landscape changes dramatically, as most stemness transcription factor genes are silenced, and many super-enhancers are decommissioned (Adam et al., 2015). Intriguingly, new super-enhancers form and regulate a new set of transcription factors that allow these cells to survive in a new environment rich for serum and growth factors. Perhaps not surprisingly, the genes and regulatory factors induced in vitro are also similar to those acquired when stem cells exit the bulge and participate in wound repair. Together, these findings further underscore the overpowering importance of the stem cell niche.
4. ADDITIONAL LESSONS LEARNED FROM STUDYING SKIN STEM CELLS: SWEAT GLAND STEM CELLS
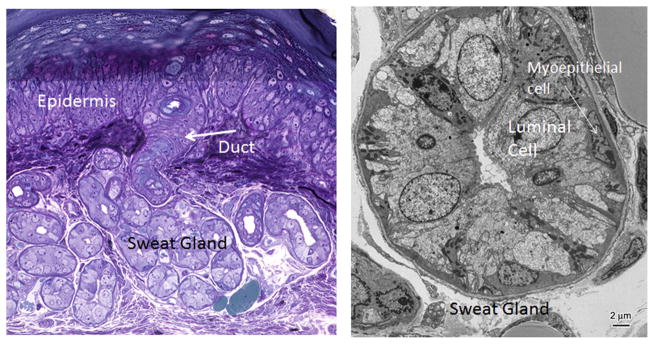
Like mammary glands, eccrine sweat glands consist of an outer myoepithelial layer of cells, attached to an underlying basement membrane and an inner layer of luminal cells (Lu & Fuchs, 2014). The sweat gland duct is a single tubular structure that protrudes through the skin surface to secrete sweat and cool the body surface (Fig. 3). A major difference between mammary glands and sweat glands is their activity: whereas mammary glands undergo enormous branching and leafing morphogenesis following lactation, sweat glands remain relatively inert in size and simply crank up sweat output to match the demands on the gland. This has led to speculation as to whether sweat glands have stem cells.
Figure 3.
Semi-thin section and ultrastructure of a sweat gland from the mouse paw pad. The gland is a bilayered epithelium consisting of myoepithelial and luminal epithelial cells. The duct opens out onto the surface of the epidermis. This figure is courtesy of H.A. Pasolli.
Several years ago, we set out to address this question. Using lineage tracing, we first established the existence of multipotent and unipotent progenitors in the sweat glands (Lu et al., 2012). At the initiation of sweat bud downgrowth during development, labeling of K14+ or K5+ basal cells in the overlying epidermis generated first the duct and then the sweat glands. These data confirm that the sweat glands are derived from multipotent epidermal basal progenitors. Labeling of emerging K15+/K18+ luminal cells followed by lineage tracing into mature glands showed that they clonally expand nearly exclusively as luminal cells, indicating that once they form, luminal progenitors are unipotent. Similar lineage tracing of emerging myoepithelial cells showed their unipotency. These findings revealed a switch from multipotency to unipotency in the progenitor properties during sweat gland morphogenesis (Lu et al., 2012).
Interestingly, when we looked for stem cell activity in the adult sweat gland, we did not see signs of proliferation, even though this was readily detected in the basal layer of the overlying epidermis as well as in the basal layer of the ductal epithelium. When we introduced superficial epidermal wounds, the basal progenitors repaired the epidermis, while the ductal epithelial progenitors repaired the orifice of the duct. The gland remained quiescent. Deeper wounds mobilized the gland progenitors: myoepithelial progenitors repaired the myoepithelium while luminal progenitors repaired the damaged lumen (Lu et al., 2012). When myoepithelial progenitors were purified and then engrafted into a mammary gland fat pad, however, now these progenitors could regenerate not only a functional sweat gland but also the duct. These progenitors could also repair the epidermis.
These studies illustrate an important paradigm that we have since seen in many epithelial stem cell populations and which again illuminate our knowledge of plasticity. As long as resident tissue stem cells are present, they function to replenish dying cells and repair local injuries. When the resident tissue stem cells are lost, niches further distant from the wound site are mobilized. With massive damage, a 911 call is placed—any stem cell with the capacity to make and repair the tissue. Alas, for the skin epithelium, the limits appear to be epidermis, sebaceous and sweat glands and hair follicles. However, with a boost of a transcription factor or two, new choices can be coaxed, as illustrated by the recent studies on corneal stem cells (Ouyang et al., 2014).
4.1 Development
During embryogenesis, the single layer of K14+ epidermal basal progenitors undergoes a spindle orientation shift from >90% lateral to ~70% perpendicular cell divisions, which leaves one daughter in the basal layer and one suprabasal differentiating daughter cell (Lechler & Fuchs, 2005) These divisions result in activated Notch signaling in the differentiating daughter cells (Williams, Beronja, Pasolli, & Fuchs, 2011), and involve a mechanism with ancient roots in Drosophila neuroblast divisions (Lechler & Fuchs, 2005; Williams, Ratliff, Postiglione, Knoblich, & Fuchs, 2014).
Notably, although all of the epidermal appendages are distinct, they all begin development in a similar way (Mikkola, 2007), and knowledge of this process has been gained by studying the embryonic skin at a stage at which it exists as a single-layered epithelium (Ahtiainen et al., 2014). Before hair follicle morphogenesis, a uniform layer of epithelial cells overlies a disperse population of dermal cells. At about E14.5 of mouse development, mesenchymal–epithelial interactions result in the formation of the first wave of hair placodes that appear as small epidermal invaginations into the dermis (DasGupta & Fuchs, 1999; Hardy, 1992).
Once specified, signals from the epithelium cause dermal cells to aggregate and form a dermal condensate under each placode. This specification process occurs in four overlapping waves, with rare primary guard hairs being specified at E14.5 and the remainder of follicles being specified from E15.5 to P0. Once placodes have formed, they become highly proliferative and grow downward into the dermis, forming hair germs and then hair pegs. Maturation is completed toward the end of the first postnatal week, when follicle downgrowth stops and the upward-moving, terminally differentiated hairs break through the skin surface (Schmidt-Ullrich & Paus, 2005).
The developmental decision to form a hair follicle is the result of mesenchymal–epithelial cross talk that integrates multiple instructive signals necessary to initiate hair follicle morphogenesis. Activation of Wnt/β-catenin signaling in epithelial cells is a key initial step in placode formation. The bipartite transcription factor complex composed of the DNA-binding protein LEF1 and its activation partner (stabilized β-catenin) can be readily detected in the nuclei of developing placode cells, and over a decade ago, the “Wnt reporter” gene TOPGAL, containing an enhancer element composed of multimerized LEF1-binding sites, confirmed the specific transcriptional activity of these complexes in the placode (DasGupta & Fuchs, 1999). Notably, when the Wnt inhibitor Dickoff 1 (Dkk1) is expressed ectopically or when β-catenin is conditionally targeted for ablation in epithelial cells, hair follicle morphogenesis is blocked altogether, whereas mice lacking LEF1 or β-catenin display fewer or no follicles (Andl, Reddy, Gaddapara, & Millar, 2002; Huelsken, Vogel, Erdmann, Cotsarelis, & Birchmeier, 2001; van Genderen et al., 1994). Strong evidence for Wnt signaling as a sufficient, instructive cue for placode formation comes from experiments expressing excessive stabilized β-catenin in the epithelium, which result in super-furry mice exhibiting ectopic hair follicles within their interfollicular epidermis (Gat, DasGupta, Degenstein, & Fuchs, 1998; Jamora, DasGupta, Kocieniewski, & Fuchs, 2003). Many studies since then have corroborated these findings in various ways, including chromatin landscaping, lineage-tracing analyses, and genetics (see, for example, Deschene et al., 2014; Genander et al., 2014; Lien & Fuchs, 2014; Lien et al., 2014). That said, surprisingly, despite all of these years of investigation, we still do not fully understand how WNT signaling is involved and how it exerts its instructive powers.
Another major question in hair follicle biology is where and when the adult stem cells become established. Several years ago, my laboratory addressed this question for the bulge niche by exploiting the relatively slow-cycling behavior of adult follicle stem cells to trace their developmental origins (Nowak et al., 2008). Our embryonic pulse-chase studies revealed that, surprisingly, label-retaining cells are specified early in skin development and later become adult bulge stem cells. These early label-retaining cells express SOX9, TCF3/4, and LHX2, which have emerged as key transcriptional regulators of bulge stem cell niche identity. However, our recent studies that show that bulge stem cells can retain stemness outside their niche by mobilizing a new transcriptional network to adapt to a new microenvironment (Adam et al., 2015) now raises an interesting question. Are stem cells formed before their niche or are they formed only once the niche is established? Given the importance of the niche, it is tempting to speculate that the niche must form first. However, it is also formally possible that a microenvironment where stem cells can be born occurs much earlier in skin development. This fascinating question awaits future investigation.
5. SUMMARY
In this synopsis, I have highlighted the findings from my group that I think will be particularly interesting to developmental biologists. I have also touched upon the boundaries of our knowledge, and where pushing these is likely to yield exciting new areas for future exploration. In the three decades of skin epithelial research, we have learned a lot about skin development, homeostasis, and wound repair. However, many of the complex interactions that occur between stem cells and their microenvironment remain unexplored. Our new lentiviral delivery technology that allows us to knockdown expression of any desired gene specifically in the skin epidermis and its appendages (Adam et al., 2015; Beronja, Livshits, Williams, & Fuchs, 2010; Williams et al., 2011) expedites our ability to carry out complex genetics and interrogate the physiological relevance of genes in the skin in a matter of days a great improvement over conventional conditional knockout approaches. We will need these and other new advances in order to address the ever-escalating questions that emerge from exploring this mesmerizing system in all of its dimensions.
I have had the good fortune to mentor a number of outstanding students and postdoctoral fellows, many of whom have continued in this field and have made exceptional contributions to it. I have also had the pleasure to interact with a cohort of wonderful skin biologists and dermatologists, and even though my concentration has been on our own work, their research contributions are enormous and highly insightful. All this aside, however, the frontiers are still vast for the field of skin biology, and the questions will keep us all and those who follow, busy for the decades to come.
References
- Adam RC, Yang H, Rockowitz S, Larsen SB, Nikolova M, Oristian DS, et al. Pioneer factors govern super-enhancer dynamics in stem cell plasticity and lineage choice. Nature. 2015;521:366–370. doi: 10.1038/nature14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahtiainen L, Lefebvre S, Lindfors PH, Renvoise E, Shirokova V, Vartiainen MK, et al. Directional cell migration, but not proliferation, drives hair placode morphogenesis. Developmental Cell. 2014;28:588–602. doi: 10.1016/j.devcel.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Developmental Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Beronja S, Livshits G, Williams S, Fuchs E. Rapid functional dissection of genetic networks via tissue-specific transduction and RNAi in mouse embryos. Nature Medicine. 2010;16:821–827. doi: 10.1038/nm.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Blecher SR, Debertin M, Murphy JS. Pleiotropic effect of Tabby gene on epidermal growth factor-containing cells of mouse submandibular gland. The Anatomical Record. 1983;207:25–29. doi: 10.1002/ar.1092070104. [DOI] [PubMed] [Google Scholar]
- Chang SH, Jobling S, Brennan K, Headon DJ. Enhanced Edar signalling has pleiotropic effects on craniofacial and cutaneous glands. PLoS One. 2009;4:e7591. doi: 10.1371/journal.pone.0007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CY, Pasolli HA, Giannopoulou EG, Guasch G, Gronostajski RM, Elemento O, et al. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature. 2013;495:98–102. doi: 10.1038/nature11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Heller E, Beronja S, Oshimori N, Stokes N, Fuchs E. An RNA interference screen uncovers a new molecule in stem cell self-renewal and long-term regeneration. Nature. 2012;485:104–108. doi: 10.1038/nature10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- Cluzeau C, Hadj-Rabia S, Bal E, Clauss F, Munnich A, Bodemer C, et al. The EDAR370A allele attenuates the severity of hypohidrotic ectodermal dysplasia caused by EDA gene mutation. The British Journal of Dermatology. 2012;166:678–681. doi: 10.1111/j.1365-2133.2011.10620.x. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Deschene ER, Myung P, Rompolas P, Zito G, Sun TY, Taketo MM, et al. β-Catenin activation regulates tissue growth non-cell autonomously in the hair stem cell niche. Science. 2014;343:1353–1356. doi: 10.1126/science.1248373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe DP, Klein AM, Simons BD, Jones PH. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Developmental Cell. 2010;18:317–323. doi: 10.1016/j.devcel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Ezhkova E, Lien WH, Stokes N, Pasolli HA, Silva JM, Fuchs E. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes & Development. 2011;25:485–498. doi: 10.1101/gad.2019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, et al. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell. 2009;136:1122–1135. doi: 10.1016/j.cell.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–519. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Fujimoto A, Kimura R, Ohashi J, Omi K, Yuliwulandari R, Batubara L, et al. A scan for genetic determinants of human hair morphology: EDAR is associated with Asian hair thickness. Human Molecular Genetics. 2008;17:835–843. doi: 10.1093/hmg/ddm355. [DOI] [PubMed] [Google Scholar]
- Fujimoto A, Ohashi J, Nishida N, Miyagawa T, Morishita Y, Tsunoda T, et al. A replication study confirmed the EDAR gene to be a major contributor to population differentiation regarding head hair thickness in Asia. Human Genetics. 2008;124:179–185. doi: 10.1007/s00439-008-0537-1. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Genander M, Cook PJ, Ramskold D, Keyes BE, Mertz AF, Sandberg R, et al. BMP signaling and its pSMAD1/5 target genes differentially regulate hair follicle stem cell lineages. Cell Stem Cell. 2014;15:619–633. doi: 10.1016/j.stem.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. Cultured cells for the treatment of disease. Scientific American. 1991;265:96–102. doi: 10.1038/scientificamerican1191-96. [DOI] [PubMed] [Google Scholar]
- Hardy MH. The secret life of the hair follicle. Trends in Genetics. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nature Medicine. 2014a;20:847–856. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell. 2014b;157:935–949. doi: 10.1016/j.cell.2014.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Najor NA, Green KJ. Desmosomes: Regulators of cellular signaling and adhesion in epidermal health and disease. Cold Spring Harbor Perspectives in Medicine. 2014;4:a015297. doi: 10.1101/cshperspect.a015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PH, Harper S, Watt FM. Stem cell patterning and fate in human epidermis. Cell. 1995;80:83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- Kamberov YG, Wang S, Tan J, Gerbault P, Wark A, Tan L, et al. Modeling recent human evolution in mice by expression of a selected EDAR variant. Cell. 2013;152:691–702. doi: 10.1016/j.cell.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura R, Yamaguchi T, Takeda M, Kondo O, Toma T, Haneji K, et al. A common variation in EDAR is a genetic determinant of shovel-shaped incisors. American Journal of Human Genetics. 2009;85:528–535. doi: 10.1016/j.ajhg.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Fuchs E. Wnt some lose some: Transcriptional governance of stem cells by Wnt/beta-catenin signaling. Genes & Development. 2014;28:1517–1532. doi: 10.1101/gad.244772.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Polak L, Lin M, Lay K, Zheng D, Fuchs E. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nature Cell Biology. 2014;16:179–190. doi: 10.1038/ncb2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, et al. Inter-follicular epidermal stem cells self-renew via autocrine Wnt signaling. Science. 2013;342:1226–1230. doi: 10.1126/science.1239730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Fuchs E. Sweat gland progenitors in development, homeostasis, and wound repair. Cold Spring Harbor Perspectives in Medicine. 2014;4:1–17. doi: 10.1101/cshperspect.a015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CP, Polak L, Rocha AS, Pasolli HA, Chen SC, Sharma N, et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- McEwan Jenkinson D, Elder HY, Bovell DL. Equine sweating and anhidrosis Part 1—Equine sweating. Veterinary Dermatology. 2006;17:361–392. doi: 10.1111/j.1365-3164.2006.00545.x. [DOI] [PubMed] [Google Scholar]
- Mikkola ML. p63 in skin appendage development. Cell Cycle. 2007;6:285–290. doi: 10.4161/cc.6.3.3798. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, et al. Capturing and profiling adult hair follicle stem cells. Nature Biotechnology. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Mustonen T, Pispa J, Mikkola ML, Pummila M, Kangas AT, Pakkasjarvi L, et al. Stimulation of ectodermal organ development by Ectodysplasin-A1. Developmental Biology. 2003;259:123–136. doi: 10.1016/s0012-1606(03)00157-x. [DOI] [PubMed] [Google Scholar]
- Nguyen H, Merrill BJ, Polak L, Nikolova M, Rendl M, Shaver TM, et al. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nature Genetics. 2009;41:1068–1075. doi: 10.1038/ng.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang H, Xue Y, Lin Y, Zhang X, Xi L, Patel S, et al. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature. 2014;511:358–361. doi: 10.1038/nature13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda CM, Park S, Mesa KR, Wolfel M, Gonzalez DG, Haberman AM, et al. Intravital imaging of hair follicle regeneration in the mouse. Nature Protocols. 2015;10:1116–1130. doi: 10.1038/nprot.2015.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science. 2006;312:1946–1949. doi: 10.1126/science.1128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977;265:421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti PC, Varilly P, Fry B, Lohmueller J, Hostetter E, Cotsapas C, et al. Genome-wide detection and characterization of positive selection in human populations. Nature. 2007;449:913–918. doi: 10.1038/nature06250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Nielsen B, Schmidt-Nielsen K, Houpt TR, Jarnum SA. Urea excretion in the camel. The American Journal of Physiology. 1957a;188:477–484. doi: 10.1152/ajplegacy.1957.188.3.477. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K, Schmidt-Nielsen B, Jarnum SA, Houpt TR. Body temperature of the camel and its relation to water economy. The American Journal of Physiology. 1957b;188:103–112. doi: 10.1152/ajplegacy.1956.188.1.103. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- Sennett R, Wang Z, Rezza A, Grisanti L, Roitershtein N, Sicchio C, et al. An integrated transcriptome atlas of embryonic hair follicle progenitors, their niche, and the developing skin. Developmental Cell. 2015;34:S1534–S5807. doi: 10.1016/j.devcel.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AK, Durmowicz MC, Hartung AJ, Hudson J, Ouzts LV, Donovan DM, et al. Ectodysplasin-A1 is sufficient to rescue both hair growth and sweat glands in Tabby mice. Human Molecular Genetics. 2001;10:2973–2981. doi: 10.1093/hmg/10.26.2973. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, et al. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes & Development. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, Lutzkendorf S, Cotsarelis G, Mill P, Hui CC, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Current Biology. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Voutilainen M, Lindfors PH, Lefebvre S, Ahtiainen L, Fliniaux I, Rysti E, et al. Ectodysplasin regulates hormone-independent mammary ductal morphogenesis via NF-kappaB. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5744–5749. doi: 10.1073/pnas.1110627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Beronja S, Pasolli HA, Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Ratliff LA, Postiglione MP, Knoblich JA, Fuchs E. Par3-mInsc and Gαi3 cooperate to promote oriented epidermal cell divisions through LGN. Nature Cell Biology. 2014;16:758–769. doi: 10.1038/ncb3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]