Abstract
Background
Endometrial cancer is one of the most common cancers in female patients. Many studies have investigated the association between the MDM2 T309G genotype and endometrial cancer incidence, but the results have been inconclusive.
Material/Methods
We performed a systematic search in PubMed and Web of Science databases (update until October 21, 2015) for all English-language publications. The associations are indicated as pooled odds ratio (OR) and 95% confidence intervals (CI).
Results
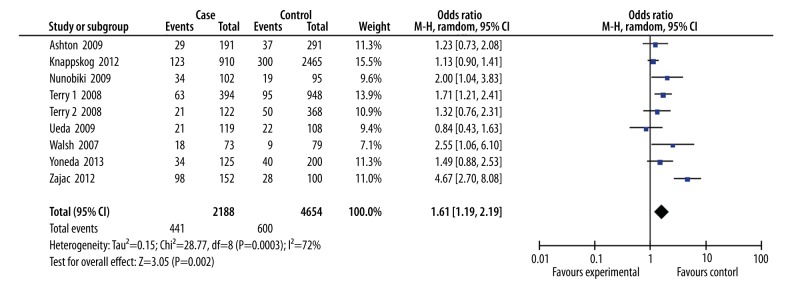
We identified 8 relevant publications (9 case-control studies), including 2188 cases and 4654 controls, that assessed the relationship between MDM2 T309G polymorphism and endometrial cancer risk. There was a significant association between MDM2 T309G polymorphism and endometrial cancer risk in the overall population in the recessive model (OR=1.61; 95% CI: 1.19–2.19; P=0.002). In the subgroup of different ethnic populations, the subgroup analysis showed MDM2 T309G polymorphism was significantly associated with increased endometrial cancer risk in Caucasians (OR=1.75; 95% CI: 1.16–2.63; P=0.007). No similar result was found in Asians.
Conclusions
Our meta-analysis provides evidence that MDM2 T309G polymorphism is associated with endometrial cancer, especially in Caucasians.
MeSH Keywords: Endometrial Neoplasms; Polymorphism, Genetic; Proto-Oncogene Proteins c-mdm2
Background
Endometrial cancer is one of the most common cancers in female patients [1]. During recent decades the incidence of endometrial cancer has been growing. The most important reasons for this growth are increased life expectancy and the global obesity epidemic [2]. Although the mechanism of endometrial cancer is known, the genetic basis of this disease is not fully understood.
Murine double minute 2 (MDM2) is one of the most important negative regulators of P53. MDM2 can inhibit the transcriptional activity of P53. This protein can function as an E3 ubiquitin ligase responsible for the ubiquitination and proteolytic degradation of p53 [3]. P53 can lead to cell cycle arrest and apoptosis, and can repair DNA damage [4]. The overexpression of MDM2 is observed in various human tumors, including endometrial cancer [5].
Many studies have investigated the association between the MDM2 T309G genotype and endometrial cancer incidence. Although a significant association was observed in some studies, a clear linkage between MDM2 T309G polymorphism and the risk of endometrial cancer has not been established [6–13]. Hence, a meta-analysis investigating MDM2 T309G polymorphism and the risk of endometrial cancer was carried out to conclusively establish the role of MDM2 T309G polymorphism in endometrial cancer.
Material and Methods
Selection of published studies
We performed a systematic search in PubMed and Web of Science databases (updated October 21, 2015) for all English-language publications using combinations of the following key words: (endometrial cancer) and („murine double minute 2” OR „MDM2”). To obtain as many eligible studies as possible, we also examined all relevant references in the selected publications. Review articles, meeting abstracts, and animal experiment studies were not considered.
Inclusion and exclusion criteria
Inclusion criteria were: (a) estimation of the association between MDM2 T309G polymorphism and the risk of endometrial cancer; (b) case-control or cohort study; and (c) sufficient original data for calculating an odds ratio (OR) with its 95% confidence interval (CIs). Studies were excluded if they did not include usable data on genotype distribution.
Data extraction
All data were carefully extracted and reviewed from each eligible study independently by 2 investigators, and any potential conflict was resolved by discussion between the 2 reviewers. The information extracted from each study included the following: the first author’s name, the publication’s year, ethnicity, the number of cases and controls, and genotype distribution.
Statistical analysis
A chi-square test was used to estimate the Hardy-Weinberg equilibrium (HWE) among the control subjects. The risk was evaluated through the recessive model (polymorphic homozygous versus heterozygotes and homozygotes for the wild-type allele). Subgroup analysis based on different ethnic populations was also performed. Additionally, sensitivity analysis was used to examine the stability of results by omitting each study sequentially or omitting the study without HWE. The pooled OR was estimated using the fixed-effects or random-effects models according to heterogeneity. Heterogeneity among studies was calculated using the chi-square-based Q test. The effect of heterogeneity was also quantified using the I2 statistic, which ranges between 0% and 100%. When lack of heterogeneity between studies was detected, the Mantel-Haenszel method in a fixed-effects model was used. In contrast, when heterogeneity between studies was present, the DerSimonian and Laird method in a random-effects model was used. Associations were indicated as pooled OR and 95% CI. Publication bias was examined by funnel plot method, in which the standard error of log (OR) of each study was plotted against its log (OR). The asymmetry in funnel plot is detected when publication bias is present. Funnel plot asymmetry was also determined by Egger’s test. P<0.05 was considered statistically significant. Data analyses were performed using the Cochrane systematic review software Review Manager 5.2 and Stata 11.
Results
Study characteristics
After searching 44 articles meeting the search criteria, we identified 8 relevant publications (9 case-control studies), including 2188 cases and 4654 controls, to assess the relationship between MDM2 T309G polymorphism and endometrial cancer risk (Figure 1). The characteristics of the included studies are listed in Table 1. Six studies were performed in Caucasians and 3 were performed in Asians. All studies were case-control.
Figure 1.
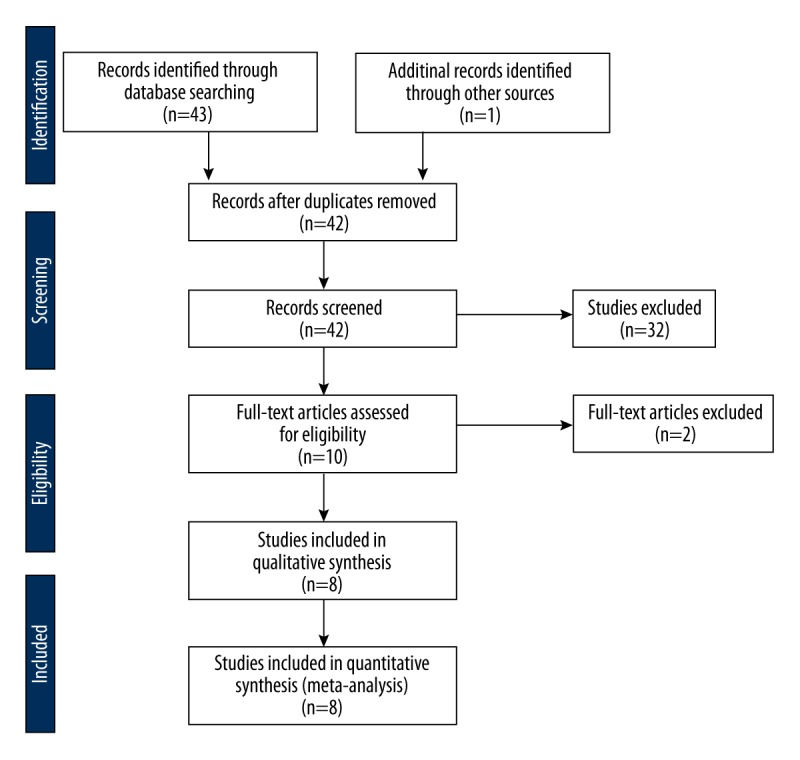
Flow chart of the literature search according to PRISMA statement.
Table 1.
Characteristics of the included studies in this meta-analysis.
| First author | Year | Ethnicity | Case number | Control number | Case distribution | Control distribution | HWE |
|---|---|---|---|---|---|---|---|
| Walsh | 2007 | Caucasian | 73 | 79 | 28/27/18 | 32/38/9 | Yes |
| Terry 1 | 2008 | Caucasian | 394 | 948 | 169/162/63 | 433/429/95 | Yes |
| Terry 2 | 2008 | Caucasian | 122 | 368 | 47/54/21 | 163/155/50 | Yes |
| Ashton | 2009 | Caucasian | 191 | 291 | 78/84/29 | 128/126/37 | Yes |
| Nunobiki | 2009 | Asian | 102 | 95 | 24/44/34 | 17/59/19 | No |
| Ueda | 2009 | Asian | 119 | 108 | 26/54/21 | 20/66/22 | No |
| Zajac | 2012 | Caucasian | 152 | 100 | 24/30/98 | 24/48/28 | Yes |
| Knappskog | 2012 | Caucasian | 910 | 2465 | 361/426/123 | 1072/1093/300 | Yes |
| Yoneda | 2013 | Asian | 125 | 200 | 30/61/34 | 62/98/40 | Yes |
HWE – Hardy-Weinberg equilibrium.
Quantitative data synthesis
As shown in Figure 2, there was a significant association between MDM2 T309G polymorphism and endometrial cancer risk in the overall population in the recessive model (OR=1.61; 95% CI: 1.19–2.19; P=0.002). However, there was significant between-study heterogeneity (I2=72%); therefore, we performed sensitive analysis. When the studies without HWE were excluded, the result was still significant (OR=1.70; 95% CI: 1.20–2.42; P=0.003; I2=76%). When the study was excluded once, we found that the study by Zajac might be the main resource of heterogeneity. When this study was omitted, the heterogeneity decreased (I2=29%). In the subgroup of different ethnic populations, the subgroup analysis showed MDM2 T309G polymorphism was significantly associated with increased endometrial cancer risk in Caucasians (OR=1.75; 95% CI: 1.16–2.63; P=0.007; I2=80%), but not in Asians.
Figure 2.
Combined meta-analysis of the association between MDM2 T309G polymorphism and endometrial cancer risk.
We used funnel plots and Egger’s test to assess the publication bias. The funnel plots appeared to be symmetrical (Figure 3) and Egger’s test did not reveal any evidence of publication bias (P>0.05).
Figure 3.
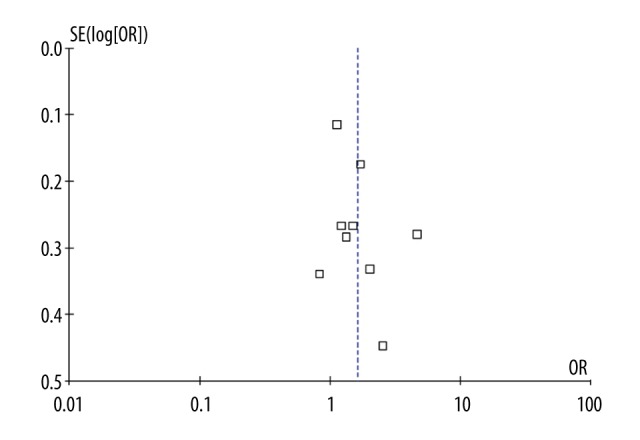
Funnel plot of association between MDM2 T309G polymorphism and endometrial cancer risk.
Discussion
The present meta-analysis consists of an evaluation of MDM2 T309G polymorphism and endometrial cancer risk. Our results show a significant association between MDM2 T309G polymorphism and endometrial cancer risk. Additionally, after the population was stratified by ethnicity, an increased risk for endometrial cancer was observed in Caucasians.
MDM2 was discovered in a locus amplified on double minute chromosomes in a tumorigenic mouse cell line [14]. MDM2 can block the transcriptional activity of p53. In addition, MDM2 can promote p53 protein degradation. Thus, the main function of MDM2 is to inhibit p53 activity. A previous study suggested that high levels of MDM2 could decrease p53 protein levels. This progress can attenuate p53 function; therefore, it can increase cancer risk [15]. Additionally, MDM2 might play a critical role in cancer progression [16]. In tumor cells, overexpression of MDM2 can induce cell proliferation and inhibit cell apoptosis [17,18].
Several potential limitations of the present study should be mentioned. Firstly, our results were based on unadjusted single-factor estimates, but if detailed individual information on age, sex, family history, and environmental factors were available, more precise analyses could have been conducted. Secondly, because we only searched for articles with sufficient original data and published in English, some inevitable bias might occur in our results, although the funnel plot and Egger’s test showed no obvious publication bias. Thirdly, this meta-analysis contained a relatively small sample size, and deficient control populations, and the limitation of clinicopathological data may have especially affected our final conclusion.
Conclusions
Our meta-analysis provides evidence that MDM2 T309G polymorphism is associated with endometrial cancer, especially in Caucasians.
Footnotes
Disclosure of conflict of interest
None.
Source of support: Departmental sources
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Odagiri T, Watari H, Hosaka M, et al. Multivariate survival analysis of the patients with recurrent endometrial cancer. J Gynecol Oncol. 2011;22:3–8. doi: 10.3802/jgo.2011.22.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–99. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 4.Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013;13:83–96. doi: 10.1038/nrc3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundgren K, Montes de Oca Luna R, McNeill YB, et al. Targeted expression of MDM2 uncouples S phase from mitosis and inhibits mammary gland development independent of p53. Genes Dev. 1997;11:714–25. doi: 10.1101/gad.11.6.714. [DOI] [PubMed] [Google Scholar]
- 6.Ashton KA, Proietto A, Otton G, et al. Polymorphisms in TP53 and MDM2 combined are associated with high grade endometrial cancer. Gynecol Oncol. 2009;113:109–14. doi: 10.1016/j.ygyno.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 7.Knappskog S, Trovik J, Marcickiewicz J, et al. MoMaTEC study group. SNP285C modulates oestrogen receptor/Sp1 binding to the MDM2 promoter and reduces the risk of endometrial but not prostatic cancer. Eur J Cancer. 2012;48:1988–96. doi: 10.1016/j.ejca.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Nunobiki O, Ueda M, Yamamoto M, et al. Polymorphisms of p53 codon 72 and MDM2 promoter 309 and the risk of endometrial cancer. Hum Cell. 2009;22:101–6. doi: 10.1111/j.1749-0774.2009.00075.x. [DOI] [PubMed] [Google Scholar]
- 9.Terry K, McGrath M, Lee IM, et al. MDM2 SNP309 is associated with endometrial cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:983–86. doi: 10.1158/1055-9965.EPI-07-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueda M, Yamamoto M, Nunobiki O, et al. Murine double-minute 2 homolog single nucleotide polymorphism 309 and the risk of gynecologic cancer. Hum Cell. 2009;22:49–54. doi: 10.1111/j.1749-0774.2009.00068.x. [DOI] [PubMed] [Google Scholar]
- 11.Walsh CS, Miller CW, Karlan BY, Koeffler HP. Association between a functional single nucleotide polymorphism in the MDM2 gene and sporadic endometrial cancer risk. Gynecol Oncol. 2007;104:660–64. doi: 10.1016/j.ygyno.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Yoneda T, Kuboyama A, Kato K, et al. Association of MDM2 SNP309 and TP53 Arg72Pro polymorphisms with risk of endometrial cancer. Oncol Rep. 2013;30:25–34. doi: 10.3892/or.2013.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zając A, Stachowiak G, Pertyński T, et al. Association betweenMDM2 SNP309 polymorphismand endometrial cancer risk in Polish women. Pol J Pathol. 2012;63:278–83. doi: 10.5114/pjp.2012.32776. [DOI] [PubMed] [Google Scholar]
- 14.Pichiorri F, Suh SS, Rocci A, et al. Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell. 2010;18:367–81. doi: 10.1016/j.ccr.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Nag S, Qin J, Srivenugopal KS, et al. The MDM2-p53 pathway revisited. J Biomed Res. 2013;27:254–71. doi: 10.7555/JBR.27.20130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Günther T, Schneider-Stock R, Häckel C, et al. Mdm2 gene amplification in gastric cancer correlation with expression of Mdm2 protein and p53 alterations. Mod Pathol. 2000;13:621–26. doi: 10.1038/modpathol.3880107. [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Lozano G. Molecular pathways: targeting Mdm2 and Mdm4 in cancer therapy. Clin Cancer Res. 2013;19:34–41. doi: 10.1158/1078-0432.CCR-12-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong P, Meng LR. Effect of mTOR inhibitors in nude mice with endometrial carcinoma and variable PTEN expression status. Med Sci Monit Basic Res. 2014;20:146–52. doi: 10.12659/MSMBR.892514. [DOI] [PMC free article] [PubMed] [Google Scholar]