Abstract
Aims:
To study the phenotypic characteristics of X-linked retinoschisis (XLRS) and report the clinical, electroretinogram (ERG), and optical coherence tomography (OCT) variables in Indian eyes.
Design:
A retrospective study.
Materials and Methods:
Medical records of 21 patients with retinoschisis who were genetically confirmed to have RS1 mutation were reviewed. The phenotype characterization included the age of onset, best-corrected visual acuity, refractive error, fundus findings, OCT, and ERG.
Statistical Analysis Used:
Data from both the eyes were used for analysis. A P < 0.05 was set as statistical significance. Data were not normally distributed (P < 0.05, Shapiro wilk); hence, nonparametric tests were used for statistical analysis.
Results:
All were males whose mean age of presentation was 9 years. Visual acuity was moderately impaired (median 0.6 logMAR, interquartile range: 0.47, 1) in these eyes with a hyperopic refractive error of median +1.75 Ds (interquartile range: +0.50 Ds, +4.25 Ds). About 54.7% of the eyes had both foveal and peripheral schisis, isolated foveal schisis was seen in 28.5% of the eyes, and schisis with retinal detachment was seen in 16.6% of the eyes. The inner nuclear layer was found to be commonly involved in the schisis, followed by outer nuclear and plexiform layers as evident on OCT. On ERG, a- and b-wave amplitudes were significantly reduced in eyes with foveal and peripheral schisis when compared to the eyes with only foveal schisis (P < 0.05).
Conclusions:
XLRS has phenotypic heterogeneity as evident on OCT, ERG, and clinical findings.
Key words: Electroretinogram, foveal schisis, optical coherence tomography, peripheral schisis, retinoschisis
X-linked retinoschisis (XLRS) is a common cause of macular dysfunction in young men due to the allelic heterogeneity at the retinoschisis (X-linked, juvenile) 1 locus (RS1), with a prevalence of 1 in 5000 to 1 in 25,000.[1] The disease retinoschisis has been described at various stages, the initial stage is characterized by spoke wheel pattern due to the formation of microcysts in the inner retinal layers, and the later stages may show increased severity of the disease with development of retinal detachment.[1] Structural and functional changes in retinoschisis have been described earlier;[2] literature in the Western population shows young patients may have moderate visual impairment and axial hypermetropia.[1,3]
Though there are a wide number of mutations giving rise to a range of phenotypes, a little genotype-phenotype correlation has been established in terms of disease severity.[4,5] Familial heterogeneity within and between families has been described in patients with XLRS.[6] Suganthalakshmi et al.[7] described two novel mutations in the Indian population besides the known genotypic features. Possible genetic heterogeneity of juvenile retinoschisis may result in variable phenotype of XLRS.[1] Hence, understanding wide spectrum phenotype characterizations in XLRS is critical, so as to provide the patient with appropriate clinical management along with the prognosis and genetic counseling.[8] There are two phenotypes described, foveal involvement alone and foveal with peripheral involvement. However, phenotypic correlation of fundus findings, optical coherence tomography (OCT) features, and electroretinogram (ERG) features of foveal versus foveal and peripheral schisis has not been described earlier.
The aim of this study was to describe phenotypic correlations in XLRS in Indian eyes.
Materials and Methods
This study was a retrospective review of patients who visited our Tertiary Eye Care Centre between 2010 and 2013, who were recruited in an ocular genetic study and were confirmed to have RS1 mutation. The project was approved by the Institutional Review Board of Vision Research Foundation, and the tenets of the Declaration of Helsinki were followed. Medical records of 21 patients who belonged to different parts of North and South India with a diagnosis of retinoschisis were reviewed. Basic demographic information was collected from the electronic medical records including age, gender, duration of complaints, and age of onset of disease. Information about the best-corrected visual acuity and refraction were also noted. The fundus findings were obtained from the fundus charts for both central and peripheral retina; however, central retinal findings were confirmed from the color fundus photographs (FF450IR Carl Zeiss), if available. Fifteen patients underwent cirrus high definition-OCT (Carl Zeiss Meditec, Germany) using macular cube (A-scans 512, B-scans 128) and 5-line raster scan (4096 A-scans) protocols; macular thickness measurements were obtained from Early Treatment Diabetic Retinopathy Study macular cube protocol. Qualitative OCT characteristics were also assessed by a trained retinal specialist using the high definition raster scans. Full-field ERG using Ganzfeld simulator was performed following International Society for Clinical Electrophysiology of Vision guidelines. Burian-allen contact lens electrodes were used to record the measurements which included dark-adapted 0.01 ERG (rod response), dark-adapted 3.0 ERG (combined rod-cone response), dark-adapted 3.0 oscillatory potentials, light-adapted 3.0 ERG (cone response), and light-adapted 3.0 flicker (30 Hz flicker).
Statistical analysis
Statistical analysis was performed using statistical software (SPSS for Windows, version 17.0 SPSS Science, Chicago, IL, USA). A P < 0.05 was set as statistical significance. Data from both the eyes were used for analysis, assuming independent nature of the variables (intraclass correlation between the eyes was < 0.8). Normality tests were performed using Shapiro wilk, which showed that the data was not normally distributed (P < 0.05); hence, nonparametric tests were used for statistical analysis. Mann-Whitney U-test was used to compare the significance in difference between two independent groups.
Results
Data from 21 males (42 eyes) who were diagnosed to have XLRS were taken for analysis. Of 21, six patients were related and had positive family history of the disease. Mean age of presentation was 9 years (range, 6-21 years), and all patients had an age of onset in the first decade of their life. There was no history of night blindness, color vision difficulties, and history of field loss in these patients; the chief complaints were related to board work difficulties. At the time of presentation, they had moderate visual impairment – median 0.6 logMAR (interquartile range, 0.47-1 logMAR) and showed a hyperopic refractive error of median + 1.75 Ds (interquartile range, 0.50 Ds until + 4.25 Ds). Anterior segment in these patients was normal except for four eyes which had an early cataract, one eye had a total cataract, one was aphakic, and one eye was pseudophakic.
Fundus evaluation was documented in all 42 eyes. The posterior margin of the peripheral retina was identified as a line joining the points at which the vortex veins pass from the choroid to the sclera. This rather constant landmark is 3 mm (nearly 2 disc diameters) posterior to the equator of the eye. Presence of schisis in this area was referred to as peripheral schisis. Based on location of the schisis, disease was categorized as foveal + peripheral schisis which was seen in maximum 54.7% (n = 23) of the eyes followed by foveal schisis seen in 28.5% (n = 12) of the eyes, and schisis with retinal detachment was seen in 16.6% (n = 7) of the eyes [Fig. 1]. Tapetal reflex was present in eight (19%) eyes [Fig. 2], and vitreous veils were present in five (11.9%) eyes. Vitrectomy was performed in four eyes for retinal detachment.
Figure 1.
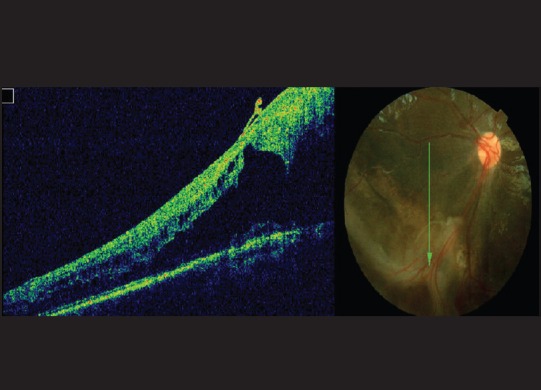
Optical coherence tomography and fundus picture showing schisis in the peripheral retina
Figure 2.
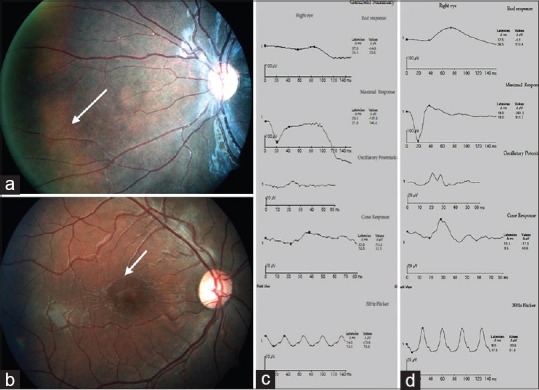
(a) Fundus picture depicting tapetal reflex. (b) Foveal schisis seen on fundus photography. (c) Full field electroretinogram showing reduced waveforms on all recordings. Combined response shows a negative waveform in a case of foveal + peripheral schisis. (d) Full field electroretinogram in a case of foveal schisis shows better waveforms compared to the peripheral schisis scenario
Median visual acuity in the foveal schisis group was 0.3 logMAR and in the foveal + peripheral schisis group was 1 logMAR. ERG and OCT measurements were not documented in the group schisis with Retinal Detachment (RD) (n = 7) due to poor vision (counting fingers, perception of light).
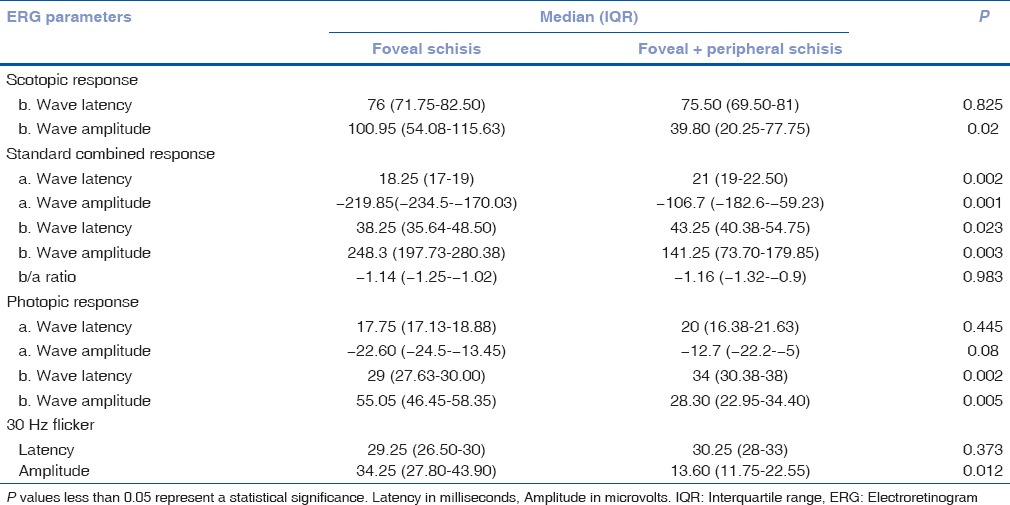
Of the 42 eyes, OCT and ERG were performed in 30 eyes. Seven out of 30 eyes had a negative waveform on standard combined response [Fig. 2]. Foveal + peripheral schisis showed statistically significant reduction of amplitudes when compared to the eyes with foveal schisis on all dark-adapted 0.01 ERG (rod response), dark-adapted 3.0 ERG (combined rod-cone response), light-adapted 3.0 ERG (cone response), and light-adapted 3.0 flicker responses [Table 1], [Fig. 2]. Latencies of standard combined response (both a- and b-wave) along with that of photopic b-wave were significantly delayed in eyes with foveal + peripheral schisis when compared to the foveal schisis [Table 1].
Table 1.
Comparison of ERG parameters between the groups
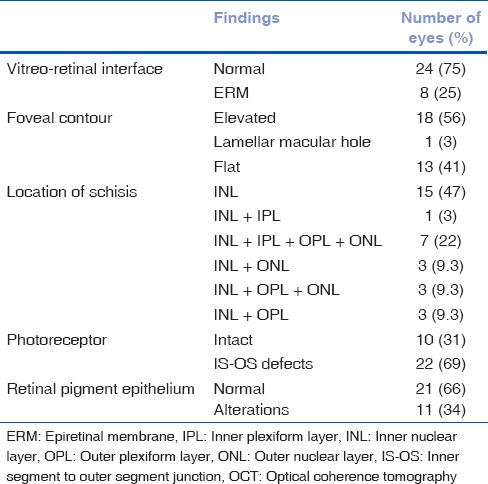
Quantitative and qualitative OCT analyses were performed. Foveal contour was elevated in 56% of the eyes, inner nuclear layer had schisis in 47% of the eyes, schitic cavities were also seen in the ganglion cell layer in peripheral retina (n = 3) in cases with foveal and peripheral involvement, 69% of the eyes had inner segment/outer segment (IS-OS) junction defects of the photoreceptor layer, and alterations and thinning of retinal pigment epithelium (RPE) were also noted. Table 2 shows the profile of qualitative features on OCT in these eyes.
Table 2.
OCT characteristics in patients with retinoschisis (n=32 eyes)
[Figs. 3–5] show delineation of defects on OCT seen in different layers of the retina in eyes with retinoschisis. Quantitative thickness parameters such as central subfield foveal thickness, inner 3 mm ring. and outer 6 mm ring did not differ significantly between the groups [Table 3].
Figure 3.
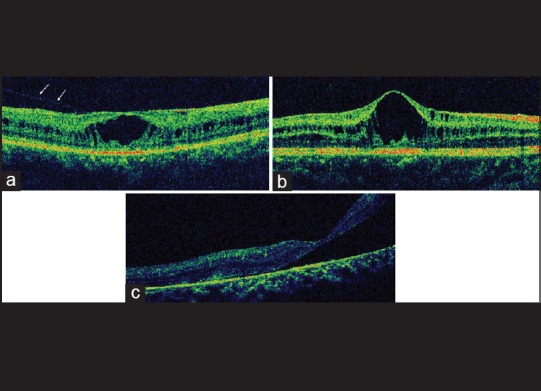
(a) Raster optical coherence tomography scan showing the presence of an epiretinal membrane. (b) Normal vitreoretinal interface with elevated foveal contour. (c) Optical coherence tomography of an eye representing a large schisis and thinning of retinal pigment epithelium
Figure 5.
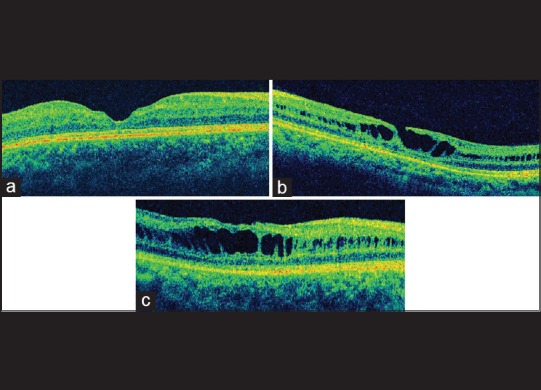
(a) Raster optical coherence tomography scan shows a foveal thinning with intact photoreceptor layer. (b) Lamellar macular hole with inner segment/outer segment defects of the photoreceptors. (c) Optical coherence tomography image showing an abnormal and flat foveal contour along with retinal pigment epithelium alterations
Table 3.
Comparison of OCT parameters between the groups
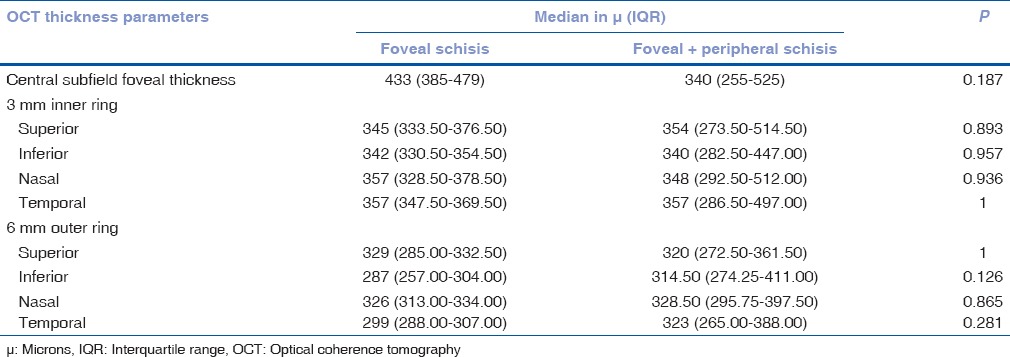
Figure 4.
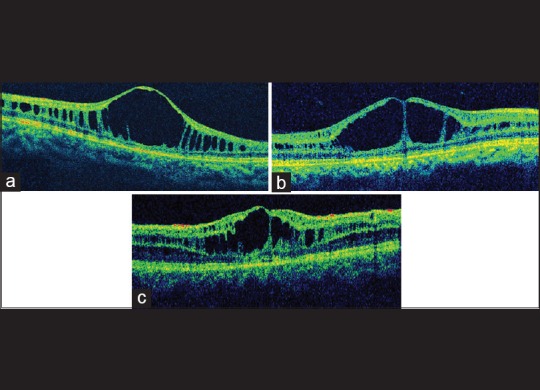
(a) Optical coherence tomography image showing schisis involving inner nuclear layer. (b) Schisis involving inner nuclear and inner plexiform layer. (c) Schisis involving both inner and outer (nuclear, plexiform) layers
Discussion
The disease typically occurs in the early stage of life; eventually, results in compromised vision by school age, few of them may develop retinal detachment at an older age. The disease is characterized by spoke wheel like foveal schisis; splitting of the retinal layers seen is believed to be caused due to the intracellular retention of the protein, retinoschisin.[9] Though, features seems to have been well established in this condition, variability in the disease expression is seen across literature emphasizing the need for detailed studies on retinoschisis phenotypes. As far as our knowledge, limited studies exist in India describing the detailed features of retinoschisis, though, unique structural and functional case scenarios have been reported.[2,10,11,12]
Mizuo-Nakamura phenomenon usually associated with oguchi disease and fundus albipunctatus is rarely seen in retinoschisis. This was first reported in four unrelated males by Jong et al.[13] Tapetal reflex was found to be seen in the eight eyes (four patients) in our study. Etiology of tapetal reflex has been well-described by the mechanism of increased concentrations of potassium or potassium chloride giving rise to yellowish fundus reflex in eyes with retinoschisis.[13,14]
Cell to cell adhesion is lost in eyes with retinoschisis resulting in a break in the retinal layers,[15] this mostly involves foveal schisis although peripheral schisis is not uncommon. In our study, we have seen that maximum eyes had schisis both in the foveal and peripheral retina and few eyes had retinal detachment as well. Evidence of inner retinal layers splitting on OCT, and electronegative confirmation on ERG helps in diagnosis.
Various studies have used OCT to delineate the schisis location precisely, in which inner nuclear layer was found to be the most common site for schisis.[16,17,18] Yu et al.[19] showed that inner nuclear layer schisis along with the involvement of outer plexiform and the nuclear layer was seen in XLRS. In our sample, we found that inner nuclear layer had schisis, in most of the cases; few eyes had schisis extending into the inner plexiform layer along with the inner nuclear layer. Though we could not find isolated outer retinal schisis, outer nuclear and outer plexiform layers were found to be having schisis cavities along with the inner nuclear layer in the fovea. Based on our OCT features, as we did not see any isolated outer layer schisis we postulate that it is probably the inner layers which are involved early, followed by outer layers. Along with the schisis, macular hole was reported to be seen in eyes with retinoschisis,[2,10] one eye in our study had a lamellar macular hole. Structural alterations of photoreceptors like thinning, IS-OS defects along with the RPE alterations, and atrophy were also noted on OCT.
Retinoschisis, as described not only involves fovea but may also involve a periphery. We found that the peripheral schisis involved the ganglion cell layer, whereas the foveal schisis had more of the inner nuclear and outer retinal layers involved, similar to the findings reported by Gregori et al.[20] It was found in our study that schisis involved only inner retina in few eyes and outer retina in other eyes. This restates that schisis can involve different layers of retina, iterating a phenotypical variability. This variability can be witnessed as reduction in amplitudes of either a or b wave or both under scotopic or photopic conditions in different eyes. Depressed b-wave amplitudes may imply disruption of the inner retinal morphology. We found that both a and b wave amplitudes cum latencies were reduced and delayed under scotopic conditions. This may be attributed to the dysfunction of photoreceptor, muller/bipolar cells, respectively. Abnormal oscillatory potentials seen indicates possible role of amacrine cells in the pathogenesis of retinoschisis. Although electronegative configuration on ERG is a diagnostic sign for retinoschisis, there are few exceptions in which all the affected eyes may not show a negative ERG.[21] Only seven eyes in our sample had an electronegative waveform.
Fundus findings in our study showed that 50% of the eyes had peripheral involvement and about one in five developed retinal detachments. The possible assumption is that, in advanced stages, the peripheral schisis can result in atrophic outer layer holes leading to retinal detachment. ERG amplitudes were statistically reduced, and latencies were delayed in cases with peripheral schisis when compared to the eyes with foveal schisis alone. This emphasizes the clinical importance of critically examining the peripheral fundus (even if the foveal findings may or may not be obvious) in outpatient department and in screenings, especially pediatric population as all these patients had their age of onset > 10 years.
The limitation of this study is the retrospective design. However, this study describes the phenotypic features (clinical, ERG and OCT) in XLRS. A differentiation of foveal and foveal + peripheral involvement is important as the structural and functional damage as assessed by the tools in the study are more severe in the later.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.George ND, Yates JR, Moore AT. X linked retinoschisis. Br J Ophthalmol. 1995;79:697–702. doi: 10.1136/bjo.79.7.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nittala MG, Laxmi G, Raman R, Rani PK, Bhargava A, Pal SS, et al. Spectral-domain OCT and microperimeter characterization of morphological and functional changes in X-linked retinoschisis. Ophthalmic Surg Lasers Imaging. 2009;40:71–4. doi: 10.3928/15428877-20090101-16. [DOI] [PubMed] [Google Scholar]
- 3.Kato K, Miyake Y, Kachi S, Suzuki T, Terasaki H, Kawase Y, et al. Axial length and refractive error in X-linked retinoschisis. Am J Ophthalmol. 2001;131:812–4. doi: 10.1016/s0002-9394(00)00923-5. [DOI] [PubMed] [Google Scholar]
- 4.Vincent A, Robson AG, Neveu MM, Wright GA, Moore AT, Webster AR, et al. A phenotype-genotype correlation study of X-linked retinoschisis. Ophthalmology. 2013;120:1454–64. doi: 10.1016/j.ophtha.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Bowles K, Cukras C, Turriff A, Sergeev Y, Vitale S, Bush RA, et al. X-linked retinoschisis: RS1 mutation severity and age affect the ERG phenotype in a cohort of 68 affected male subjects. Invest Ophthalmol Vis Sci. 2011;52:9250–6. doi: 10.1167/iovs.11-8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eksandh LC, Ponjavic V, Ayyagari R, Bingham EL, Hiriyanna KT, Andréasson S, et al. Phenotypic expression of juvenile X-linked retinoschisis in Swedish families with different mutations in the XLRS1 gene. 2000;118:1098–104. doi: 10.1001/archopht.118.8.1098. [DOI] [PubMed] [Google Scholar]
- 7.Suganthalakshmi B, Shukla D, Rajendran A, Kim R, Nallathambi J, Sundaresan P. Genetic variations in the hotspot region of RS1 gene in Indian patients with juvenile X-linked retinoschisis. Mol Vis. 2007;13:611–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Pimenides D, George ND, Yates JR, Bradshaw K, Roberts SA, Moore AT, et al. X-linked retinoschisis: Clinical phenotype and RS1 genotype in 86 UK patients. J Med Genet. 2005;42:e35. doi: 10.1136/jmg.2004.029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang T, Waters CT, Rothman AM, Jakins TJ, Römisch K, Trump D. Intracellular retention of mutant retinoschisin is the pathological mechanism underlying X-linked retinoschisis. Hum Mol Genet. 2002;11:3097–105. doi: 10.1093/hmg/11.24.3097. [DOI] [PubMed] [Google Scholar]
- 10.Gautam M, Muralidhar NS, Murthy H. Bilateral macular holes in X-linked retinoschisis: Now the spectrum is wider. Indian J Ophthalmol. 2011;59:507–9. doi: 10.4103/0301-4738.86326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tandon M, Shukla D. Macular drusenoid deposits in X-linked retinoschisis. Indian J Ophthalmol. 2013;61:366–7. doi: 10.4103/0301-4738.115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla D, Rajendran A, Gibbs D, Suganthalakshmi B, Zhang K, Sundaresan P. Unusual manifestations of x-linked retinoschisis: Clinical profile and diagnostic evaluation. Am J Ophthalmol. 2007;144:419–423. doi: 10.1016/j.ajo.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 13.De Jong PT, Zrenner E, van Meel GJ, Keunen JE, van Norren D. Mizuo phenomenon in X-linked retinoschisis. Pathogenesis of the Mizuo phenomenon. Arch Ophthalmol. 1991;109:1104–8. doi: 10.1001/archopht.1991.01080080064029. [DOI] [PubMed] [Google Scholar]
- 14.Vincent A, Shetty R, Yadav NK, Shetty BK. Foveal schisis with Mizuo phenomenon: Etio-pathogenesis of tapetal reflex in X-linked retinoschisis. Eye (Lond) 2009;23:1240–1. doi: 10.1038/eye.2008.170. [DOI] [PubMed] [Google Scholar]
- 15.Mooy CM, Van Den Born LI, Baarsma S, Paridaens DA, Kraaijenbrink T, Bergen A, et al. Hereditary X-linked juvenile retinoschisis:A review of the role of Müller cells. Arch Ophthalmol. 2002;120:979–84. [PubMed] [Google Scholar]
- 16.Ozdemir H, Karacorlu S, Karacorlu M. Optical coherence tomography findings in familial foveal retinoschisis. Am J Ophthalmol. 2004;137:179–81. doi: 10.1016/s0002-9394(03)00736-0. [DOI] [PubMed] [Google Scholar]
- 17.Brucker AJ, Spaide RF, Gross N, Klancnik J, Noble K. Optical coherence tomography of X-linked retinoschisis. Retina. 2004;24:151–2. doi: 10.1097/00006982-200402000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Apushkin MA, Fishman GA, Janowicz MJ. Correlation of optical coherence tomography findings with visual acuity and macular lesions in patients with X-linked retinoschisis. Ophthalmology. 2005;112:495–501. doi: 10.1016/j.ophtha.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Ni Y, Keane PA, Jiang C, Wang W, Xu G. Foveomacular schisis in juvenile X-linked retinoschisis: An optical coherence tomography study. Am J Ophthalmol. 2010;149:973–8.e2. doi: 10.1016/j.ajo.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Gregori NZ, Berrocal AM, Gregori G, Murray TG, Knighton RW, Flynn HW, Jr, et al. Macular spectral-domain optical coherence tomography in patients with X linked retinoschisis. Br J Ophthalmol. 2009;93:373–8. doi: 10.1136/bjo.2007.136127. [DOI] [PubMed] [Google Scholar]
- 21.Renner AB, Kellner U, Fiebig B, Cropp E, Foerster MH, Weber BH. ERG variability in X-linked congenital retinoschisis patients with mutations in the RS1 gene and the diagnostic importance of fundus autofluorescence and OCT. Doc Ophthalmol. 2008;116:97–109. doi: 10.1007/s10633-007-9094-5. [DOI] [PubMed] [Google Scholar]


