Abstract
Bruxism is defined as the repetitive jaw muscle activity characterized by the clenching or grinding of teeth. It can be categorized into awake and sleep bruxism (SB). Frequent SB occurs in about 13% of adults. The exact etiology of SB is still unknown and probably multifactorial in nature. Current literature suggests that SB is regulated centrally (pathophysiological and psychosocial factors) and not peripherally (morphological factors). Cited consequences of SB include temporomandibular disorders, headaches, tooth wear/fracture, implant, and other restoration failure. Chairside recognition of SB involves the use of subjective reports, clinical examinations, and trial oral splints. Definitive diagnosis of SB can only be achieved using electrophysiological tools. Pharmacological, psychological, and dental strategies had been employed to manage SB. There is at present, no effective treatment that “cures” or “stops” SB permanently. Management is usually directed toward tooth/restoration protection, reduction of bruxism activity, and pain relief.
Keywords: Consequences, diagnosis, etiology, management, sleep bruxism
INTRODUCTION
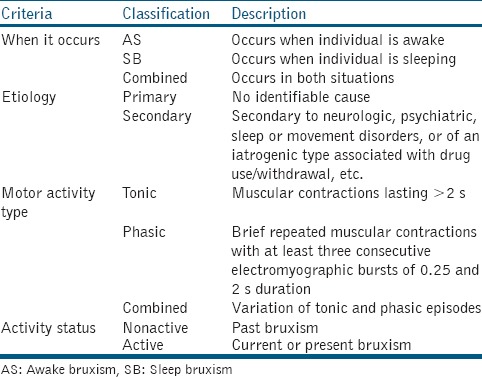
Bruxism has been defined by the American Academy of Sleep Medicine as the “repetitive jaw muscle activity characterized by the clenching or grinding of teeth and/or bracing or thrusting of the mandible.”[1] Bruxism can be classified according to when it occurs, etiology, motor activity type as activity status [Table 1].[2] It is often arduous to differentiate between the different types of bruxism. For the purpose of this article, bruxism will be categorized into awake bruxism (AB) and sleep bruxism (SB). AB is a distinct entity from SB and is characterized mainly by the clenching of teeth.[3] The prevalence of AB in adults was reported to range from 22.1% to 31% while that of “frequent” SB was more consistent at 13%.[4] The prevalence of SB in children varied from 3.5% to 40.6% according to a recent systematic review.[5] The exact prevalence of SB is hard to determine as most population studies are usually based on self-reported questionnaires due to technical/cost constraints and most bruxers (>80%) are unaware of their habit.[6] In addition, bruxism activity has been found to vary significantly over time.[7] While AB tends to be higher for women, no gender difference in SB was observed for both children and adults. Both AB and SB generally decrease with age.[3,4,5]
Table 1.
Classification of bruxism
Sleep can be divided into 3–5 cycles of non-rapid eye movement (REM) and REM periods with an REM latency ranging from 90 to 120 min. Non-REM sleep can be further broken into light sleep (stages 1 and 2) and deep sleep (stages 3 and 4). Most SB episodes occur in the light stages of non-REM sleep (i.e. stage 1 and 2 sleep) and occasionally (<10%) during REM sleep in association with sleep arousals.[8] The latter is characterized by momentary (3–15 s) cortical brain activations, increases in heart rate, and motor activity.[9,10] During REM sleep, muscles are usually relaxed to the point of paralysis, but brain activity is similar to that experienced when awake. SB during REM sleep may be a subclinical manifestation of REM sleep behavior disorder, a parasomnia where vivid dreams are acted out during sleep. Dream-enacting behaviors include talking, shouting, punching, kicking, sitting, jumping from bed, arm thrashing, and grabbing during sleep.
During sleep, rhythmic masticatory muscle activities (RMMA) are observed in up to 60% of normal subjects and 80% of patients with SB.[11] RMMA are slow (1 Hz) chewing-like movements in the absence of tooth grinding. SB is identified when RMMA are frequent or associated with tooth grinding. RMMA are three times more common and about 30% more intense in patients with SB when compared to normal subjects.[11] The physiological relationship between SB and RMMA is still undefined. RMMA may be coupled with increases in salivation to lubricate the oropharyngeal structures or to enlarge upper airway spaces. They may be associated with the central pattern generator, a complex formation situated in the trigeminal nucleus that is responsible for controlling rhythmic masticatory movements when awake.
ETIOLOGY OF SLEEP BRUXISM
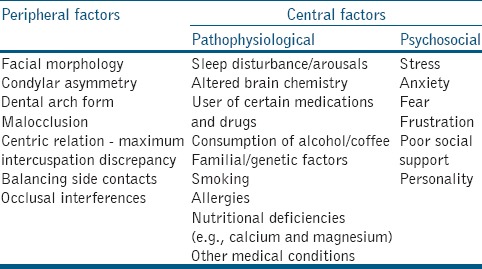
The exact etiology of SB is still not known and probably multifactorial in nature. Originally, it was attributed to peripheral (morphological) factors including malocclusion and occlusal interferences [Table 2]. The studies have, however, found similar prevalence in SB for people with or without occlusal interferences, and SB was not reduced by occlusal therapy.[12,13] In addition, there was no correlation between anatomical-structural factors and bruxism events in SB patients.[14] Current literature suggests that SB is regulated centrally and not peripherally.[15] Central issues can be categorized into pathophysiological and psychosocial factors [Table 2]. The link between SB and psychosocial factors such as emotional stress was supported by the studies reporting elevated levels of urinary catecholamine in patients with SB.[16,17] In addition, SB activity had been related to higher levels of perceived psychological stress and salivary cortisol.[18] A controlled laboratory study reported that SB patients were more competitive and felt more anxious than normal subjects.[19] Patients with both AB and SB, also showed significant differences in anxiety, depression, hostility, phobic anxiety, and paranoid ideation when compared to non-bruxers.[20]
Table 2.
Possible etiological factors for sleep bruxism
Among the many pathophysiological factors, the role of sleep-related microarousals, neurochemicals, genetics, and respiration feature prominently.[21] The link between SB and sleep microarousals had been substantiated by polysomnographic (PSG) studies. SB jaw motor activity is heralded by physiologic changes (brain activation and increased heart rate Figure 1) corroborating the central origin of SB.[9,10] Neurochemicals such as adrenalin, noradrenaline, dopamine, serotonin, and gamma-aminobutyric acid had been implicated in the genesis of SB. Due to the assumed role of noradrenaline in SB, clinical trials with propranolol and clonidine have been carried out.[22] Propranolol, a nonselective beta blocker, did not cause a reduction in SB but clonidine, a central nervous system alpha agonist, significantly reduced SB. L-dopa, a dopamine precursor, was reported to decrease SB while bromocriptine, a dopamine agonist, had no effect on SB.[23,24] Selective serotonin re-uptake inhibitors had been suggested to induce or exacerbate both AB and SB.[25] Evidence supporting the role of neurochemicals in the pathophysiology of SB is still limited and warrants additional large-scale investigations.
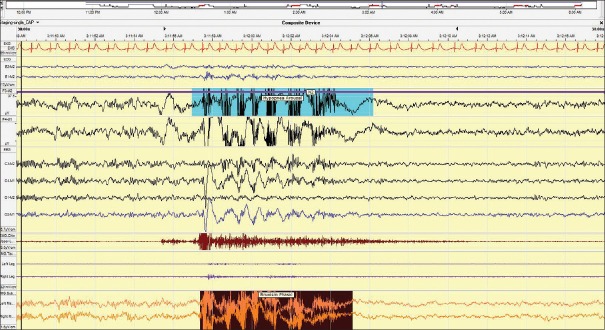
Figure 1.
A 30 s epoch showing bruxism lasting approximately 7 s with accompanying EEG arousals and transient increased heart rate. Patient was sleeping supine
Genetic factors account for half of the phenotypic variance of SB and SB was reported in up to 50% of family members of SB patients.[26,27] A recent review of the literature involving DNA analysis, family, and twin studies concluded that SB does indeed “run in families.”[28] SB appears to be a persistent trait and 35–90% of childhood SB will persists into adulthood.[29] To date, only the C allele carrier of HTR2A single nucleotide polymorphism rs6313 has been associated significantly with SB.[30] The role of respiration in SB is still not fully understood. Currently, there is no evidence supporting the association or causalty between SB and obstructive sleep apnea (OSA).[31] There, however, appears to be an association between SB and sleep position.[32] As sleep position has been found to affect both the incidence of OSA and clenching, an indirect link between SB and OSA exists.[33] Muscle activity/tone alterations are implicated in both entities. Ventilatory stimuli that activate the genioglossus during OSA also engage the masseter muscles.[34] It was hypothesized that activation of the masseter serves to stabilize the mandible and allows the genioglossus to function as a more efficient dilator of the upper airway. Mandibular advancement devices (MADs) used to improve airway patency in OSA has been shown to reduce SB motor activity.[35] SB has sometimes been reported in patients with concomitant movement and other sleep disorders. The relationship between SB and these medical conditions has not been expounded.[36]
CONSEQUENCES OF SLEEP BRUXISM
SB is characterized by the clenching and grinding of teeth.[1] Clenching of teeth is the forceful closure of the opposing dentition in a static relationship of the mandible to the maxilla in either maximum intercuspation or an eccentric position. Grinding, on the other hand, involves the forceful closure of the opposing dentition in a dynamic maxillomandibular relationship as the mandibular arch moves through various excursive positions.[37] The lateral movement of the mandibular during grinding often exceeds the edge-to-edge relation of the canines.[38] The heavy horizontal forces applied (as opposed to predominantly vertical forces during chewing and swallowing) are not well-tolerated by both masticatory and restorative systems. In addition, bite forces during SB events can exceed the amplitude of maximum voluntary bite force when awake.[39] Protective neuromuscular reflexes that are operational during waking hours appear to be suppressed during sleep.[40] The forementioned leads to significant loading of teeth (together with any restoration/prosthesis), the periodontium, temporomandibular joints (TMJ) as well as muscles of mastication.
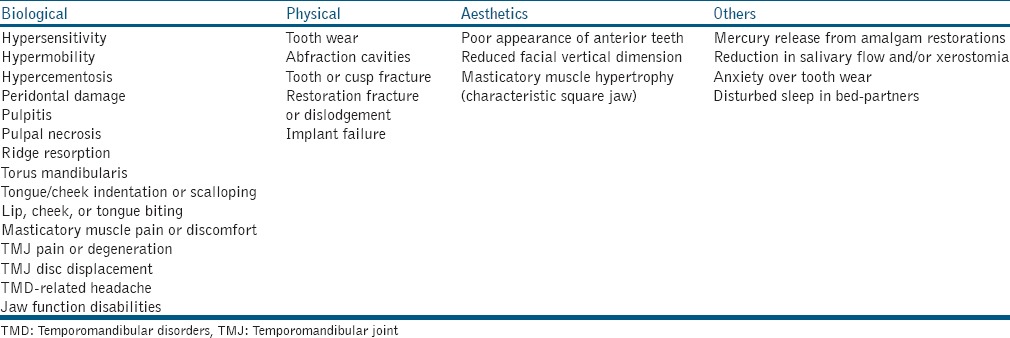
The possible consequences of SB are listed in Table 3. The clinical features cited are, however, not specific to SB and may be related to other oral habits and conditions. Of interest to restorative dentists is the effect of SB on prosthodontic rehabilitation. Review of the current literature suggests that bruxism is associated with increased mechanical and/or technical complications of tooth and implant-supported prostheses.[41] It is, however, an unlikely risk factor for biological complications of dental implants.[42] When prosthodontic interventions are indicated in SB patients, efforts must be made to reduce occlusal loading on all prosthetic components, especially during sleep. Failure to do so may result in early restorative failure.
Table 3.
Possible consequences of sleep bruxism
DIAGNOSIS OF SLEEP BRUXISM
Chairside recognition of SB includes the use of subjective reports, clinical examinations, and trial oral splints. Diagnostic criteria for SB based on the International Classification of Sleep Disorders [1] was as follows: The presence of (a) regular or frequent tooth grinding sounds during sleep and (b) one or more of the following clinical signs (i) abnormal tooth wear, (ii) transient morning jaw muscle pain or fatigue, and/or temporal headache, and/or jaw locking on awakening consistent with reports of tooth grinding during sleep. Chairside diagnosis has many limitations and may not be reliable as patients may sleep alone (or is edentulous) and suffer from comorbid TMD or medical conditions.[3]
Definitive diagnosis of SB can only be achieved using electrophysiological tools. Laboratory-based PSG allows for the detection of SB as well as other sleep disorders including sleep apnea, periodic limb movements, and parasomnias. Objective PSG recordings include brain activity (electroencephalogram), eye movements (electrooculogram), jaw/leg movements (electromyogram), heart rate/rhythm (electrocardiogram), thoracoabdominal movements, oronasal airflow and oxygen saturation. As the cost of PSG is relatively high and patients are substantially inconvenienced, it is not viable for identifying SB in the dental setting. In addition, recordings are also performed in an unfamiliar environment.
Ambulatory systems can record similar information as PSG and are less expensive to conduct. They, however, have fewer channels and do not allow for sleep staging as well as audio-video footage, unlike PSG. Ambulatory tests can be used at home but assess overall masticatory muscle activity rather that SB specifically.[43] Portable devices for diagnosis SB (e.g. Bitestrip and Bruxoff) are also available. The validity of such devices with respect to PSG recordings is still scarce and requires more research.[44]
MANAGEMENT OF SLEEP BRUXISM
At present, there is no effective treatment that “cures” or “stops” SB permanently.[45] Management is usually directed toward tooth/restoration protection, reduction of bruxism activity, and pain relief. Pharmacological, psychological, and dental strategies had been employed to manage SB. Although the use of a variety of drugs had been reported, only clonidine, L-dopa, and clonazepam had been shown to reduce SB in controlled clinical trials.[22,23,46] When compared with placebo, clonazepam (a benzodiazepine) significantly decreased SB index by 42 ± 15%. It also improved sleep efficiency, maintenance, latency, awakenings and nocturnal wake time, arousal index as well as subjective sleep and awakening quality.[46] The risk of dependency and other psychological side-effects, however, limits its long-term use. Locally, administered botulinum toxin (BTX type A) has also been used to manage SB. BTX-A is a peripheral cholinergic synapse-blocking agent that produces motor weakness to the point of paralysis. It may be considered for patients with severe bruxism, particularly those with movement disorders, refractory to conventional therapy.[47] BTX-A was found to reduce the number of bruxism events and intensity in clinical studies.[48,49] A single BTX-A injection was found to be effective for controlling SB for at least a month.[49] Possible local side effects of BTX-A masseter injections include masticatory difficulty, speech disturbance, muscle ache, prominent zygoma, and facial asymmetry secondary to muscle size reduction arising from masseter atrophy.[50]
Psychological approaches to managing SB include biofeedback, hypnotherapy, cognitive therapy, behavioral therapy, stress, and relaxation management. The efficacies of these methods have not been established despite the documented associations between SB and psychosocial factors. When cognitive-behavioral therapy (comprising of muscle relaxation, nocturnal biofeedback as well as training of recreation and enjoyment) was compared to the use of occlusal splints, no difference in SB activity reduction, self-assessment of SB activity and associated symptoms, psychological impairment as well as increased positive stress-coping strategies was found between the two treatment groups.[51] Dental treatment for SB usually takes the form of occlusal therapy (i.e., occlusal adjustment and/or rehabilitation) and occlusal splints. As there is no scientific evidence for the role of occlusion in the etiology of SB,[15] extensive irreversible occlusal therapy is not advocated unless the dentition is markedly worn and requires reconstruction. Occlusal splints are removable appliances made of hard acrylic or soft vinyl that fits between the maxillary and mandibular teeth. The purpose of occlusal splints is to protect teeth and restorations from attrition and adverse traumatic loading. Depending of their designs, occlusal splints can also unload, stabilize, and improve functions of the TMJ as well as reduce abnormal muscle activity, muscle pain, and improve functions of the masticatory motor system.
Occlusal splints can be classified according to material types, occlusal contacts, and condylar position. Soft polyvinyl splints do not allow for occlusal prescriptions and are less durable than hard acrylic splints. They have been found to increase masseter and temporalis muscle activity [52,53] and may lead to increased muscle pain/discomfort in some patients. Hard acrylic splints for SB includes stabilization and anterior splints. Repositioning (protrusive) and posterior (distraction or pivot) splints are usually not employed for managing SB. Stabilization splints are the most widely used and researched splints. They are full coverage flat plane appliances designed with balanced contacts with all opposing teeth in centric relation. Canine ramps are often included as they had been reported to reduce elevator muscle activity.[54] The effect of stabilization and palatal (with no occlusal coverage) splints on SB was compared using a cross-over design and portable electromyographic recording system.[55] Both splint types reduced SB immediately after insertion but effects were transient, and no reduction was observed at 2, 4, and 6 weeks. The results corroborated an earlier study using the same two splints. Neither occlusal nor palatal splints were found to influence SB outcome variables.[56] Results of a recent exploratory trial, however, indicate that intermittent use of stabilization splints (every other week) may reduce SB activity for a longer period (up to 4 weeks) when compared to continuous use.[57] Anterior splints that only make contact with anterior teeth in the opposing dentition have gained some popularity in recent years. They are based on the ability of anterior bite stops to reduce both temporalis and masseter activity during clenching and grinding.[58] Both anterior and stabilization splints were found to reduce SB on the first night and at 1 week but had no effect on sleep microarousals. Anterior splints were, however, reported to be more effective than stabilization splints.[59] Anterior and other segmental splints must be used with caution as they may allow for unwanted tooth and condylar movements if worn continuously over a period. Although there is insufficient evidence to support the effectiveness of occlusal splints for treating SB, they are still recommended in patients with SB to protect against dental wear and restoration damage.[60]
Although occlusal splints remain the most popular dental strategy for managing SB, MADs have shown good results and are a promising alternative treatment for SB. MAD are intraoral appliances used to treat snoring and OSA. They retain the mandible in an anterior position relative to the centric relation by means of dental anchorage and aim to reduce soft tissue vibration, decrease upper airway collapsibility, and enlarge oropharyngeal airway space.[61] A recent systematic review reported that MAD brought the soft palate, tongue, and hyoid bone forward and activated the masseter and submental muscles, preventing airway closure. All these effects reduced apnea-hypopnea index, increased oxygen saturation and improved the symptoms of OSA.[62] Although continuous positive airway pressure (CPAP) treatment is more effective, MAD is an appropriate treatment for patients who are intolerant of CPAP and may be comparable to CPAP in mild OSA cases.[63] Several sleep laboratory studies have confirmed the greater efficacy of MAD in reducing SB episodes when compared to occlusal splints.[35,64,65] Significantly improved sleep quality was also reported with MAD.[65] The findings of these studies were supported by other PSG studies that reported reduction of SB activity, SB signs, and symptoms, occlusal forces as well as headaches with MAD.[66,67,68] The exact mechanism of SB reduction remains to be explained but may be related to a concomitant reduction in RMMA.[68] The role of mandibular protrusion with MAD on SB reduction is equivocal. While some studies have found no difference between MAD positions, others had reported differences in SB activity between differing degrees of mandibular protrusion.[35,64,67,68] Despite its efficacy in reducing SB, MAD treatment had to be stopped in a considerable number of patients due to complications.[35,66] The latter included pain of the teeth, gums, masticatory muscles, and TMJ. Muscular and TMJ discomfort usually resolves after a month of MAD wear.[69] In addition to pain, clinically significant progressive changes in occlusion had also been observed with MAD use.[70] The efficacy and safety of MAD for managing SB require further studies with larger sample sizes and longer time periods.
CONCLUSIONS
Current knowledge on the prevalence, etiology, consequences, and diagnosis of SB was reviewed. Contemporary management of SB involving pharmacological, psychological, and dental strategies was appraised. At present, there is no effective treatment that “cures” or “stops” SB permanently. A combination of different strategies may be warranted to protect teeth/restorations, reduce bruxism activity, and relieve pain. More high quality randomized controlled trials on the efficacy and safety of promising treatments for SB including BTX-A and MAD are needed. The association between SB and OSA also warrants further exploration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.International Classification of Sleep Disorders. 3rd ed. Westchester, Darien, Illinois: American Academy of Sleep Medicine; 2014. American Academy of Sleep Medicine. Sleep related bruxism. [Google Scholar]
- 2.de la Hoz-Aizpurua JL, Díaz-Alonso E, LaTouche-Arbizu R, Mesa-Jiménez J. Sleep bruxism. Conceptual review and update. Med Oral Patol Oral Cir Bucal. 2011;16:e231–8. doi: 10.4317/medoral.16.e231. [DOI] [PubMed] [Google Scholar]
- 3.Kato T, Dal-Fabbro C, Lavigne GJ. Current knowledge on awake and sleep bruxism: Overview. Alpha Omegan. 2003;96:24–32. [PubMed] [Google Scholar]
- 4.Manfredini D, Winocur E, Guarda-Nardini L, Paesani D, Lobbezoo F. Epidemiology of bruxism in adults: A systematic review of the literature. J Orofac Pain. 2013;27:99–110. doi: 10.11607/jop.921. [DOI] [PubMed] [Google Scholar]
- 5.Manfredini D, Restrepo C, Diaz-Serrano K, Winocur E, Lobbezoo F. Prevalence of sleep bruxism in children: A systematic review of the literature. J Oral Rehabil. 2013;40:631–42. doi: 10.1111/joor.12069. [DOI] [PubMed] [Google Scholar]
- 6.Thompson BA, Blount BW, Krumholz TS. Treatment approaches to bruxism. Am Fam Physician. 1994;49:1617–22. [PubMed] [Google Scholar]
- 7.Lavigne GJ, Guitard F, Rompré PH, Montplaisir JY. Variability in sleep bruxism activity over time. J Sleep Res. 2001;10:237–44. doi: 10.1046/j.1365-2869.2001.00261.x. [DOI] [PubMed] [Google Scholar]
- 8.Lavigne GJ, Kato T, Kolta A, Sessle BJ. Neurobiological mechanisms involved in sleep bruxism. Crit Rev Oral Biol Med. 2003;14:30–46. doi: 10.1177/154411130301400104. [DOI] [PubMed] [Google Scholar]
- 9.Kato T, Rompré P, Montplaisir JY, Sessle BJ, Lavigne GJ. Sleep bruxism: An oromotor activity secondary to micro-arousal. J Dent Res. 2001;80:1940–4. doi: 10.1177/00220345010800101501. [DOI] [PubMed] [Google Scholar]
- 10.Macaluso GM, Guerra P, Di Giovanni G, Boselli M, Parrino L, Terzano MG. Sleep bruxism is a disorder related to periodic arousals during sleep. J Dent Res. 1998;77:565–73. doi: 10.1177/00220345980770040901. [DOI] [PubMed] [Google Scholar]
- 11.Lavigne GJ, Rompré PH, Poirier G, Huard H, Kato T, Montplaisir JY. Rhythmic masticatory muscle activity during sleep in humans. J Dent Res. 2001;80:443–8. doi: 10.1177/00220345010800020801. [DOI] [PubMed] [Google Scholar]
- 12.Kato T, Thie NM, Huynh N, Miyawaki S, Lavigne GJ. Topical review: Sleep bruxism and the role of peripheral sensory influences. J Orofac Pain. 2003;17:191–213. [PubMed] [Google Scholar]
- 13.Clark GT, Adler RC. A critical evaluation of occlusal therapy: Occlusal adjustment procedures. J Am Dent Assoc. 1985;110:743–50. doi: 10.14219/jada.archive.1985.0430. [DOI] [PubMed] [Google Scholar]
- 14.Lobbezoo F, Rompré PH, Soucy JP, Iafrancesco C, Turkewicz J, Montplaisir JY, et al. Lack of associations between occlusal and cephalometric measures, side imbalance in striatal D2 receptor binding, and sleep-related oromotor activities. J Orofac Pain. 2001;15:64–71. [PubMed] [Google Scholar]
- 15.Lobbezoo F, Naeije M. Bruxism is mainly regulated centrally, not peripherally. J Oral Rehabil. 2001;28:1085–91. doi: 10.1046/j.1365-2842.2001.00839.x. [DOI] [PubMed] [Google Scholar]
- 16.Clark GT, Rugh JD, Handelman SL. Nocturnal masseter muscle activity and urinary catecholamine levels in bruxers. J Dent Res. 1980;59:1571–6. doi: 10.1177/00220345800590100301. [DOI] [PubMed] [Google Scholar]
- 17.Seraidarian P, Seraidarian PI, das Neves Cavalcanti B, Marchini L, Claro Neves AC. Urinary levels of catecholamines among individuals with and without sleep bruxism. Sleep Breath. 2009;13:85–8. doi: 10.1007/s11325-008-0193-7. [DOI] [PubMed] [Google Scholar]
- 18.Karakoulaki S, Tortopidis D, Andreadis D, Koidis P. Relationship between sleep bruxism and stress determined by saliva biomarkers. Int J Prosthodont. 2015;28:467–74. doi: 10.11607/ijp.4296. [DOI] [PubMed] [Google Scholar]
- 19.Major M, Rompré PH, Guitard F, Tenbokum L, O’Connor K, Nielsen T, et al. Acontrolled daytime challenge of motor performance and vigilance in sleep bruxers. J Dent Res. 1999;78:1754–62. doi: 10.1177/00220345990780111301. [DOI] [PubMed] [Google Scholar]
- 20.Bayar GR, Tutuncu R, Acikel C. Psychopathological profile of patients with different forms of bruxism. Clin Oral Investig. 2012;16:305–11. doi: 10.1007/s00784-010-0492-9. [DOI] [PubMed] [Google Scholar]
- 21.Lavigne GJ, Khoury S, Abe S, Yamaguchi T, Raphael K. Bruxism physiology and pathology: An overview for clinicians. J Oral Rehabil. 2008;35:476–94. doi: 10.1111/j.1365-2842.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 22.Huynh N, Lavigne GJ, Lanfranchi PA, Montplaisir JY, de Champlain J. The effect of 2 sympatholytic medications – Propranolol and clonidine – On sleep bruxism: Experimental randomized controlled studies. Sleep. 2006;29:307–16. doi: 10.1093/sleep/29.3.307. [DOI] [PubMed] [Google Scholar]
- 23.Lobbezoo F, Lavigne GJ, Tanguay R, Montplaisir JY. The effect of catecholamine precursor L-dopa on sleep bruxism: A controlled clinical trial. Mov Disord. 1997;12:73–8. doi: 10.1002/mds.870120113. [DOI] [PubMed] [Google Scholar]
- 24.Lavigne GJ, Soucy JP, Lobbezoo F, Manzini C, Blanchet PJ, Montplaisir JY. Double-blind, crossover, placebo-controlled trial of bromocriptine in patients with sleep bruxism. Clin Neuropharmacol. 2001;24:145–9. doi: 10.1097/00002826-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Ellison JM, Stanziani P. SSRI-associated nocturnal bruxism in four patients. J Clin Psychiatry. 1993;54:432–4. [PubMed] [Google Scholar]
- 26.Rintakoski K, Hublin C, Lobbezoo F, Rose RJ, Kaprio J. Genetic factors account for half of the phenotypic variance in liability to sleep-related bruxism in young adults: A nationwide Finnish twin cohort study. Twin Res Hum Genet. 2012;15:714–9. doi: 10.1017/thg.2012.54. [DOI] [PubMed] [Google Scholar]
- 27.Glaros AG. Incidence of diurnal and nocturnal bruxism. J Prosthet Dent. 1981;45:545–9. doi: 10.1016/0022-3913(81)90044-5. [DOI] [PubMed] [Google Scholar]
- 28.Lobbezoo F, Visscher CM, Ahlberg J, Manfredini D. Bruxism and genetics: A review of the literature. J Oral Rehabil. 2014;41:709–14. doi: 10.1111/joor.12177. [DOI] [PubMed] [Google Scholar]
- 29.Hublin C, Kaprio J, Partinen M, Koskenvuo M. Sleep bruxism based on self-report in a nationwide twin cohort. J Sleep Res. 1998;7:61–7. doi: 10.1046/j.1365-2869.1998.00091.x. [DOI] [PubMed] [Google Scholar]
- 30.Abe Y, Suganuma T, Ishii M, Yamamoto G, Gunji T, Clark GT, et al. Association of genetic, psychological and behavioral factors with sleep bruxism in a Japanese population. J Sleep Res. 2012;21:289–96. doi: 10.1111/j.1365-2869.2011.00961.x. [DOI] [PubMed] [Google Scholar]
- 31.Balasubramaniam R, Kiasser GD, Cistulli PA, Lavigne GJ. The link between sleep bruxism, sleep disordered breathing and temporomandibular disorders: An eviden-based review. J Dent Sleep Med. 2014;1:27–37. [Google Scholar]
- 32.Miyawaki S, Lavigne GJ, Pierre M, Guitard F, Montplaisir JY, Kato T. Association between sleep bruxism, swallowing-related laryngeal movement, and sleep positions. Sleep. 2003;26:461–5. [PubMed] [Google Scholar]
- 33.Phillips BA, Okeson J, Paesani D, Gilmore R. Effect of sleep position on sleep apnea and parafunctional activity. Chest. 1986;90:424–9. doi: 10.1378/chest.90.3.424. [DOI] [PubMed] [Google Scholar]
- 34.Hollowell DE, Bhandary PR, Funsten AW, Suratt PM. Respiratory-related recruitment of the masseter: Response to hypercapnia and loading. J Appl Physiol. 1991;70:2508–13. doi: 10.1152/jappl.1991.70.6.2508. [DOI] [PubMed] [Google Scholar]
- 35.Landry ML, Rompré PH, Manzini C, Guitard F, de Grandmont P, Lavigne GJ. Reduction of sleep bruxism using a mandibular advancement device: An experimental controlled study. Int J Prosthodont. 2006;19:549–56. [PubMed] [Google Scholar]
- 36.Kato T, Thie NM, Montplaisir JY, Lavigne GJ. Bruxism and orofacial movements during sleep. Dent Clin North Am. 2001;45:657–84. [PubMed] [Google Scholar]
- 37.De Laat A, Macaluso GM. Sleep bruxism as a motor disorder. Mov Disord. 2002;17(Suppl 2):S67–9. doi: 10.1002/mds.10064. [DOI] [PubMed] [Google Scholar]
- 38.Yap AU. Effects of stabilization appliances on nocturnal parafunctional activities in patients with and without signs of temporomandibular disorders. J Oral Rehabil. 1998;25:64–8. doi: 10.1046/j.1365-2842.1998.00194.x. [DOI] [PubMed] [Google Scholar]
- 39.Nishigawa K, Bando E, Nakano M. Quantitative study of bite force during sleep associated bruxism. J Oral Rehabil. 2001;28:485–91. doi: 10.1046/j.1365-2842.2001.00692.x. [DOI] [PubMed] [Google Scholar]
- 40.Reding GR, Rubright WC, Zimmerman SO. Incidence of bruxism. J Dent Res. 1966;45:1198–204. doi: 10.1177/00220345660450042701. [DOI] [PubMed] [Google Scholar]
- 41.Johansson A, Omar R, Carlsson GE. Bruxism and prosthetic treatment: A critical review. J Prosthodont Res. 2011;55:127–36. doi: 10.1016/j.jpor.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Manfredini D, Poggio CE, Lobbezoo F. Is bruxism a risk factor for dental implants? A systematic review of the literature. Clin Implant Dent Relat Res. 2014;16:460–9. doi: 10.1111/cid.12015. [DOI] [PubMed] [Google Scholar]
- 43.Kato T. Sleep bruxism and its relation to obstructive sleep apnea-hyponea syndrome. Sleep Biol Rhythms. 2004;2:1–15. [Google Scholar]
- 44.Manfredini D, Ahlberg J, Castroflorio T, Poggio CE, Guarda-Nardini L, Lobbezoo F. Diagnostic accuracy of portable instrumental devices to measure sleep bruxism: A systematic literature review of polysomnographic studies. J Oral Rehabil. 2014;41:836–42. doi: 10.1111/joor.12207. [DOI] [PubMed] [Google Scholar]
- 45.Huynh N, Manzini C, Rompré PH, Lavigne GJ. Weighing the potential effectiveness of various treatments for sleep bruxism. J Can Dent Assoc. 2007;73:727–30. [PubMed] [Google Scholar]
- 46.Saletu A, Parapatics S, Anderer P, Matejka M, Saletu B. Controlled clinical, polysomnographic and psychometric studies on differences between sleep bruxers and controls and acute effects of clonazepam as compared with placebo. Eur Arch Psychiatry Clin Neurosci. 2010;260:163–74. doi: 10.1007/s00406-009-0034-0. [DOI] [PubMed] [Google Scholar]
- 47.Tan EK, Jankovic J. Treating severe bruxism with botulinum toxin. J Am Dent Assoc. 2000;131:211–6. doi: 10.14219/jada.archive.2000.0149. [DOI] [PubMed] [Google Scholar]
- 48.Lee SJ, McCall WD, Jr, Kim YK, Chung SC, Chung JW. Effect of botulinum toxin injection on nocturnal bruxism: A randomized controlled trial. Am J Phys Med Rehabil. 2010;89:16–23. doi: 10.1097/PHM.0b013e3181bc0c78. [DOI] [PubMed] [Google Scholar]
- 49.Shim YJ, Lee MK, Kato T, Park HU, Heo K, Kim ST. Effects of botulinum toxin on jaw motor events during sleep in sleep bruxism patients: A polysomnographic evaluation. J Clin Sleep Med. 2014;10:291–8. doi: 10.5664/jcsm.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park MY, Ahn KY, Jung DS. Botulinum toxin type A treatment for contouring of the lower face. Dermatol Surg. 2003;29:477–83. doi: 10.1046/j.1524-4725.2003.29116.x. [DOI] [PubMed] [Google Scholar]
- 51.Ommerborn MA, Schneider C, Giraki M, Schäfer R, Handschel J, Franz M, et al. Effects of an occlusal splint compared with cognitive-behavioral treatment on sleep bruxism activity. Eur J Oral Sci. 2007;115:7–14. doi: 10.1111/j.1600-0722.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 52.al-Quran FA, Lyons MF. The immediate effect of hard and soft splints on the EMG activity of the masseter and temporalis muscles. J Oral Rehabil. 1999;26:559–63. doi: 10.1046/j.1365-2842.1999.00421.x. [DOI] [PubMed] [Google Scholar]
- 53.Okeson JP. The effects of hard and soft occlusal splints on nocturnal bruxism. J Am Dent Assoc. 1987;114:788–91. doi: 10.14219/jada.archive.1987.0165. [DOI] [PubMed] [Google Scholar]
- 54.Fitins D, Sheikholeslam A. Effect of canine guidance of maxillary occlusal splint on level of activation of masticatory muscles. Swed Dent J. 1993;17:235–41. [PubMed] [Google Scholar]
- 55.Harada T, Ichiki R, Tsukiyama Y, Koyano K. The effect of oral splint devices on sleep bruxism: A 6-week observation with an ambulatory electromyographic recording device. J Oral Rehabil. 2006;33:482–8. doi: 10.1111/j.1365-2842.2005.01576.x. [DOI] [PubMed] [Google Scholar]
- 56.van der Zaag J, Lobbezoo F, Wicks DJ, Visscher CM, Hamburger HL, Naeije M. Controlled assessment of the efficacy of occlusal stabilization splints on sleep bruxism. J Orofac Pain. 2005;19:151–8. [PubMed] [Google Scholar]
- 57.Matsumoto H, Tsukiyama Y, Kuwatsuru R, Koyano K. The effect of intermittent use of occlusal splint devices on sleep bruxism: A 4-week observation with a portable electromyographic recording device. J Oral Rehabil. 2015;42:251–8. doi: 10.1111/joor.12251. [DOI] [PubMed] [Google Scholar]
- 58.Roark AL, Glaros AG, O’Mahony AM. Effects of interocclusal appliances on EMG activity during parafunctional tooth contact. J Oral Rehabil. 2003;30:573–7. doi: 10.1046/j.1365-2842.2003.01139.x. [DOI] [PubMed] [Google Scholar]
- 59.Liu W, Wang H, Li Q. Investigation of nociceptive trigeminal inhibitory tension suppression system and occlusal stabilization splint on bruxism patients by using polysomnography. Hua Xi Kou Qiang Yi Xue Za Zhi. 2012;30:54–6, 60. [PubMed] [Google Scholar]
- 60.Macedo CR, Silva AB, Machado MA, Saconato H, Prado GF. Occlusal splints for treating sleep bruxism (tooth grinding) Cochrane Database Syst Rev. 2007;17:CD005514. doi: 10.1002/14651858.CD005514.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: A review. Sleep. 2006;29:244–62. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 62.Serra-Torres S, Bellot-Arcís C, Montiel-Company JM, Marco-Algarra J, Almerich-Silla JM. Effectiveness of mandibular advancement appliances in treating obstructive sleep apnea syndrome: A systematic review. Laryngoscope. 2016;126:507–14. doi: 10.1002/lary.25505. [DOI] [PubMed] [Google Scholar]
- 63.Sharples LD, Clutterbuck-James AL, Glover MJ, Bennett MS, Chadwick R, Pittman MA, et al. Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea. Sleep Med Rev. 2016;27:108–24. doi: 10.1016/j.smrv.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Landry-Schönbeck A, de Grandmont P, Rompré PH, Lavigne GJ. Effect of an adjustable mandibular advancement appliance on sleep bruxism: A crossover sleep laboratory study. Int J Prosthodont. 2009;22:251–9. [PubMed] [Google Scholar]
- 65.Singh PK, Alvi HA, Singh BP, Singh RD, Kant S, Jurel S, et al. Evaluation of various treatment modalities in sleep bruxism. J Prosthet Dent. 2015;114:426–31. doi: 10.1016/j.prosdent.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 66.Mainieri VC, Saueressig AC, Fagondes SC, Teixeira ER, Rehm DD, Grossi ML. Analysis of the effects of a mandibular advancement device on sleep bruxism using polysomnography, the BiteStrip, the sleep assessment questionnaire, and occlusal force. Int J Prosthodont. 2014;27:119–26. doi: 10.11607/ijp.3675. [DOI] [PubMed] [Google Scholar]
- 67.Carra MC, Huynh NT, El-Khatib H, Remise C, Lavigne GJ. Sleep bruxism, snoring, and headaches in adolescents: Short-term effects of a mandibular advancement appliance. Sleep Med. 2013;14:656–61. doi: 10.1016/j.sleep.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Franco L, Rompre PH, de Grandmont P, Abe S, Lavigne GJ. A mandibular advancement appliance reduces pain and rhythmic masticatory muscle activity in patients with morning headache. J Orofac Pain. 2011;25:240–9. [PubMed] [Google Scholar]
- 69.Smith AM, Battagel JM. Non-apneic snoring and the orthodontist: The effectiveness of mandibular advancement splints. J Orthod. 2004;31:115–23. doi: 10.1179/146531204225020409. [DOI] [PubMed] [Google Scholar]
- 70.Pliska BT, Nam H, Chen H, Lowe AA, Almeida FR. Obstructive sleep apnea and mandibular advancement splints: Occlusal effects and progression of changes associated with a decade of treatment. J Clin Sleep Med. 2014;10:1285–91. doi: 10.5664/jcsm.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]