Abstract
Introduction:
The main objective of adhesive dentistry is to create an effective, durable union between the tooth structure and restorative material. However, degradation of adhesive dentine interface remains largely responsible for the relatively short lifetime of tooth colored resin restoration.
Aim:
The aim of the study is to compare the dentin collagen stabilization property of Chlorhexidine (CHX) and Aloe barbadensis Miller using shear bond strength testing.
Materials and Methods:
Occlusal reduction was done in sixty extracted human mandibular molars to expose the mid coronal dentin and divided into three groups n = 20. Following the surface pretreatment (Group 1 = control, Group 2 = CHX, Group 3 = Aloevera), dentine bonding agent and composite resin were applied and cured. The specimens were then subjected to shear bond strength testing.
Results:
From the results analyzed, it was noted that there was statistically significant difference between the groups Control and CHX and Control and A. barbadensis Miller (P < 0.05), specifically the values of Control < CHX and Control < A. barbadensis Miller (P < 0.05). However, there was no statistically significant difference between CHX and A. barbadensis Miller (P > 0.05). Hence, the following result for the shear bond strengths to dentin was obtained: Control < CHX ≈ A. barbadensis Miller.
Conclusion:
CHX and A. barbadensis Miller, as pretreatment agents of acid demineralized dentin collagen, has no adverse effect on the immediate shear bond strength of a two-step etch and rinse adhesive to dentin.
Keywords: Collagen cross-linking agents, matrix metalloproteinases, shear bond testing
INTRODUCTION
The success of restorative procedures depend on effective removal of infected dentin prior to the placement of the restorative material.[1] Failure to mechanically remove any carious portion and not being able to achieve complete disinfection may lead to microleakage, increased pulp sensitivity, pulpal inflammation, and eventually may cause secondary caries, thereby making it necessary to replace the restoration.[2] Therefore, application of cavity disinfectant before tooth restoration is gaining acceptance.
The main objective of adhesive dentistry is to create an effective, durable union between tooth structure and the restorative material.[3] Resin-dentin bonds obtained with contemporary adhesive systems can deteriorate over time.[4] The resin to dentin adhesion occurs through the infiltration into and polymerization of hydrophilic resins within the collagen matrix exposed through acid decalcification of dentin, thus forming a hybrid layer.[5] The durability of the dentine adhesive interface is directly related to the quality of hybrid layer and the hydrophilic nature of the adhesive. In case of etch and rinse adhesives, it is pertinent that the adhesive resin monomer penetrates the acid-etched exposed dentine collagen fibrils. But studies have revealed that this goal is seldom achieved.[6]
Certain mechanisms have been proposed to improve dentin adhesion i.e., adjunctive collagen pretreatment, matrix metalloproteinases (MMP) inhibitors, etc. Different types of MMPs have been identified from human dentin, including MMPs 2, 3, 8, 9, 20.[7] Moreover, cathepsin B is identified from sound and carious dentin.[8] Both MMPs and cysteine cathepsins contribute to the degradation of denuded collagen within the hybrid layer.[9]
Chlorhexidine (CHX) has been widely used as a cavity disinfectant because of its antimicrobial property. It also has an inhibitory effect on the MMPs (against MMPs 2, 8, 9) in the dentin. This effect can be useful in preventing collagen degradation and disintegration of the bonding interface over time.[10]
In recent years, the potential for the inhibition of MMPs by substances derived from natural products has gained increasing attention. A. barbadensis Miller (Aloe vera) is a short succulent herb resembling a cactus, with green fleshy, spiny, and well marginated leaves filled with a clear viscous gel. A. vera has potent antibacterial, antifungal, and antiviral properties.[11,12] A. vera has been used to relieve thermal burn, sunburn, and promote wound healing[13] and has antimicrobial activity and can help stimulate the body's immune system.[14] The total leaf extracts contain anthraquinones.[15] Recent study has revealed A. vera exhibits MMP inhibitory effect against MMP 2 and 9.[16] Prabhakar et al. conducted a randomized clinical trial in 2015 and advocated the use of A. vera as cavity disinfectant.[17] There have been many researches to prove antimicrobial efficacy A. vera against caries causing microorganisms, i.e., Streptococcus mutans.[18] Therefore, we intended to use A. vera as a cavity disinfectant in our study.
In order to use these agents for extending the longevity of resin-dentin bonds, it is necessary to first evaluate whether these agents interfere with the dentin bond strength following their use to pretreat the acid-etched dentin. The aim of our study was to evaluate A. vera for resin-dentin bond stabilization. This was the first study of its kind to test A. vera for resin-dentin bond stabilization.
MATERIALS AND METHODS
Sixty freshly extracted non carious human mandibular molars were thoroughly cleaned and stored in 0.1% thymol until use. The teeth were randomly divided into three groups n = 20. They were horizontally cut using diamond disk (Markus Ink., Michigan, USA) in a high-speed hand piece under air and water spray; the long axes of the teeth were perpendicular to the surfaces cut. After the removal of enamel, the mid coronal dentin was exposed. The dentine surface was examined for the lack of enamel or pulp tissue under stereomicroscope (Olympus; zoom type, Japan). The sections of the teeth including the roots were embedded in auto polymerizing acrylic resin to form cylinders 2.5 cm in diameter and 5 cm high. Dentin surfaces were flattened with 1200 grit silicon carbide paper under running water, so that a very smooth surface and to obtain a standardized smear layer.
Acid etching of the exposed dentine was performed for 15 s with 37% phosphoric acid gel (Scotchbond Etchant, 3M ESPE, St. Paul, MN, USA). In Group 1, the specimens were not treated with any cavity disinfectant and served as control. The teeth in experimental groups were treated with one of the following cavity disinfectants i.e.
Group 2: 2% CHX solution was prepared from dilution of 20% CHX solution using distilled water (Basic Pharma, Gujrat, India).
Group 3: A. barbadensis Miller (A. vera) solution was prepared using A. vera powder of 99% purity and dissolving 20 mg of A. vera powder in 10 ml of distilled water).[16]
The acid etched dentin was pretreated with 2% CHX in Group 2 and A. barbadensis Miller solution in Group 3 for 30 s, active application with a brush applicator (Microbrush International, WI, USA) and the excess removed with cotton pellet prior to the application of bonding agent (Adper single bond 2, 3M ESPE, St. Paul, MN, USA). Adhesive tape with a 3 mm diameter hole in it was used to define the bonding agent. The dentin surfaces of the teeth were then dried with air for 10 s, resin composite was applied in 5–6 increments (Filtek Z 350, 3M ESPE, St. Paul, MN, USA) with the aid of polyethylene tubes (3 mm diameter, 2 mm height and 0.5 mm thickness) and individually light cured for 40 s using light curing unit Spectrum 800 (Dentsply, Caulk, Milford, USA) with an output of 600 mW/cm2. The tubes were then removed. The teeth were then stored in distilled water at room temperature for 24 h.
For shear bond strength testing, each tooth was secured in a specially designed attachment jig to hold the specimens to the universal testing machine (Instron, ADMET, Enkay Enterprises, New Delhi). Load was applied by the testing machine through a wire loop adjusted to the bonded interface at a cross head speed of 0.5 mm/min. Shear bond strength in MPa was calculated from the peak load at failure divided by the specimen surface area.
After testing, the fracture modes were evaluated under a stereomicroscope (Olympus, Zoom type) and classified according to the predominant mode of fracture as adhesive fracture at the resin-cement dentin interface, cohesive fracture in the resin cement, cohesive fracture in dentin, or mixed adhesive and cohesive fracture in the resin cement.
RESULTS
Data were analyzed using one-way ANOVA and post hoc Tukey test for multiple comparison between the groups. The test was done at a 0.05 level statistical significance [Tables 1 and 2].
Table 1.
Shear bond strength to dentin (mean±standard deviation)
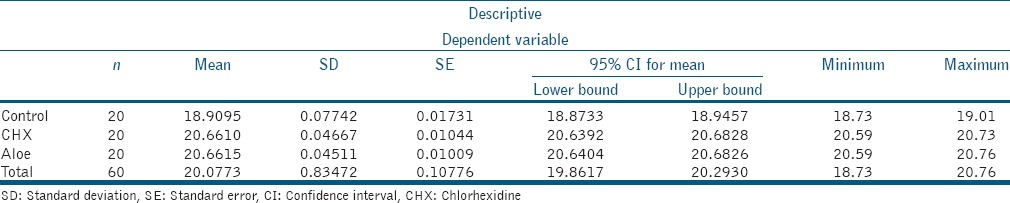
Table 2.
Comparison of immediate mean shear bond strength values (MPa) of all groups
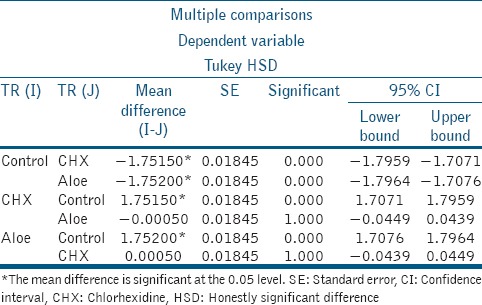
From the results analyzed, it was noted that there was statistically significant difference between the groups Control and CHX and Control and A. vera (P < 0.05), specifically values of Control < CHX and Control < A. vera (P < 0.05). However, there was no statistically significant difference between CHX and A. vera (P > 0.05). Hence, the following result for the shear bond strengths to dentin was obtained: Control < CHX ≈ A. vera.
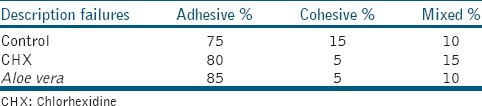
Fracture analysis indicated that most specimens showed adhesive fracture after 24 h [Table 3].
Table 3.
Failure modes of all test groups
DISCUSSION
Acid etching creates a low pH which activates the dentin MMPs in the presence of zinc and calcium ions.[19] It has been advocated that to improve the durability of restorations, pretreatment of acid-etched dentin with MMP inhibitors should be carried out.
CHX increased the immediate shear bond strength in this study, which was in support of the study by Brackett et al., 2009.[20] In total etch adhesive resins, the use of CHX after etching prevents collagen fiber degradation and preserves the hybrid layer due to its inhibitory effect on MMPs, thereby increasing the bond strength. Yet few studies have showed that applying CHX before acid etching did not significantly affect the bond strength.[21] This increase in the immediate bond strength can be attributed to the MMP inhibitory action of CHX.
The present study was conducted from agents derived from natural products that has been recently reported to possess anti-MMP potential, especially against MMP-2 and MMP-9.[16] Aloe contains alloins and barbadoins as main chemical constituents. Its bactericidal activity is because of anthraquinone.[22,23] The results of this study revealed that the application of A. barbadensis Miller to acid-etched dentine improves the longevity of resin dentin bonds by inhibiting MMPs.
The purpose of this study was to introduce new herbal product with possible potential for the inhibition of MMPs in order to maintain the dentin adhesive interface. The parameters compared were the control group (with no pretreatment) and CHX. The shear bond testing was performed only after 24 h of storage as this was the first study of its kind and it aimed to determine whether the tested concentration of the herbal product would be really effective and thereafter conduct longitudinal studies. As if immediate bond strength was negatively affected by the use of A. vera, there would have been no point for conducting long duration studies.
Although the use of A. barbadensis Miller on acid-etched dentin prevents the degradation of collagen and improves the longevity of composite restorations, it is imperative that they do not adversely affect adhesive bonding to dentin. As pretreatment with the herbal used was able to maintain immediate dentin bond strength, there is not enough evidence to reject the null hypothesis. The ability of this agent to improve the durability of resin-dentin should be evaluated in future studies.
CONCLUSION
Within the limitations of this in vitro study, it may be stated that the use of CHX or A. barbadensis Miller, as pretreatment agents of acid demineralized dentin collagen, has no adverse effect on the shear immediate bond strength of a two-step etch and rinse adhesive to dentin. Further, in vitro and in vivo studies are still warranted to evaluate the effect of A. barbadensis Miller for cavity disinfection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sharma V, Rampal P, Kumar S. Shear bond strength of composite resin to dentin after application of cavity disinfectants – SEM study. Contemp Clin Dent. 2011;2:155–9. doi: 10.4103/0976-237X.86438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brännström M. The cause of postrestorative sensitivity and its prevention. J Endod. 1986;12:475–81. doi: 10.1016/S0099-2399(86)80202-3. [DOI] [PubMed] [Google Scholar]
- 3.Fonseca BM, Pleffken PR, Balducci I, Pucci CR, Tay FR, Araujo MA. New trends in dentin bonding: Treatment with chlorhexidine, hyaluronic acid, Vitamin C and green tea. Braz Dent Sci. 2013;16:56–62. [Google Scholar]
- 4.Burrow MF, Satoh M, Tagami J. Dentin bond durability after three years using a dentin bonding agent with and without priming. Dent Mater. 1996;12:302–7. doi: 10.1016/s0109-5641(96)80038-8. [DOI] [PubMed] [Google Scholar]
- 5.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16:265–73. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 6.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 7.Bourd-Boittin K, Fridman R, Fanchon S, Septier D, Goldberg M, Menashi S. Matrix metalloproteinase inhibition impairs the processing, formation and mineralization of dental tissues during mouse molar development. Exp Cell Res. 2005;304:493–505. doi: 10.1016/j.yexcr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Nascimento FD, Minciotti CL, Geraldeli S, Carrilho MR, Pashley DH, Tay FR, et al. Cysteine cathepsins in human carious dentin. J Dent Res. 2011;90:506–11. doi: 10.1177/0022034510391906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90:953–68. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. A critical review of the durability of adhesion to tooth tissue: Methods and results. J Dent Res. 2005;84:118–32. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 11.Ramasubrimanyan TS, Sivakumar VT, Thirumalai AV. Antimicrobial activity of Aloe vera (L) Burm. f. against pathogenic microorgaisms. J Biosci Res. 2010;4:251–8. [Google Scholar]
- 12.Arunkumar S, Muthuselvam M. Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J Agric Sci. 2009;5:572–6. [Google Scholar]
- 13.Foster S. Aloe vera: The succulent with skin soothing cell protecting properties. Herbs for health magazine: Health World Online. 1999 [Google Scholar]
- 14.Davis HR. Aloe vera: A Scientific Approach. New York: Vantage Press; 1997. pp. 3–5. [Google Scholar]
- 15.Newali CA, Anderson LA, Phillipson JD. Herbal Medicines: A Guide for Healthcare Professionals. London: The Pharmaceutical Press; 1996. pp. 46–7. [Google Scholar]
- 16.Kudalkar MD, Nayak A, Bhat KS, Nayak RN. Effect of Azadirachta indica (Neem) and Aloe vera as compared to subantimicrobial dose doxycycline on matrix metalloproteinases (MMP)-2 and MMP-9: An in-vitro study. Ayu. 2014;35:85–9. doi: 10.4103/0974-8520.141947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prabhakar AR, Karuna YM, Yavagal C, Deepak BM. Cavity disinfection in minimally invasive dentistry – Comparative evaluation of Aloe vera and propolis: A randomized clinical trial. Contemp Clin Dent. 2015;6(Suppl 1):S24–31. doi: 10.4103/0976-237X.152933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertolini PF, Biondi Filho O, Pomilio A, Pinheiro SL, Carvalho MS. Antimicrobial capacity of Aloe vera and propolis dentifrice against Streptococcus mutans strains in toothbrushes: An in vitro study. J Appl Oral Sci. 2012;20:32–7. doi: 10.1590/S1678-77572012000100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osorio R, Yamauti M, Osorio E, Ruiz-Requena ME, Pashley D, Tay F, et al. Effect of dentin etching and chlorhexidine application on metalloproteinase-mediated collagen degradation. Eur J Oral Sci. 2011;119:79–85. doi: 10.1111/j.1600-0722.2010.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brackett MG, Tay FR, Brackett WW, Dib A, Dipp FA, Mai S, et al. In vivo chlorhexidine stabilization of hybrid layers of an acetone-based dentin adhesive. Oper Dent. 2009;34:379–83. doi: 10.2341/08-103. [DOI] [PubMed] [Google Scholar]
- 21.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 22.Wynn RL. Aloe vera gel: Update for dentistry. Gen Dent. 2005;53:6–9. [PubMed] [Google Scholar]
- 23.Sinha DJ, Sinha AA. Natural medicaments in dentistry. Ayu. 2014;35:113–8. doi: 10.4103/0974-8520.146198. [DOI] [PMC free article] [PubMed] [Google Scholar]


