Abstract
Aim:
To evaluate the effect of diode laser and ultrasonics with and without ethylenediaminetetraacetic acid (EDTA) on the smear layer removal from root canals.
Materials and Methods:
A total of 120 mandibular premolars were decoronated to working the length of 12 mm and prepared with protaper rotary files up to size F3. Group A canals irrigated with 1 ml of 3% sodium hypochlorite (NaOCl) followed by 3 ml of 3% NaOCl. Group B canals irrigated with 1 ml of 17% EDTA followed by 3 ml of 3% NaOCl. Group C canals lased with a diode laser. Group D canals were initially irrigated with 0.8 ml of 17% EDTA the remaining 0.2 ml was used to fill the root canals, and diode laser application was done. Group E canals were irrigated with 1 ml distilled water with passive ultrasonic activation, followed by 3 ml of 3% NaOCl. Group F canals were irrigated with 1 ml EDTA with passive ultrasonic activation, followed by 3 ml of 3% NaOCl. Scanning electron microscope examination of canals was done for remaining smear layer at coronal middle and apical third levels.
Results:
Ultrasonics with EDTA had the least smear layer scores.
Conclusion:
Diode laser alone performed significantly better than ultrasonics.
Keywords: Diode laser, ethylenediaminetetraacetic acid, root canal irrigants, scanning electron microscope, ultrasonics
INTRODUCTION
One of the fundamental aims of root canal treatment is to clean the root canals as thoroughly as possible to eliminate debris and microorganisms and achieve perfect obturation without leakage. However, after preparation of the root canals, an amorphous, irregular layer is formed on the root canal walls smear layer. Various chemicals, ultrasonics, and lasers, in combination or alone, have been evaluated for the removal of the smear layer with varying results.[1,2,3] Sodium hypochlorite (NaOCl), 1–5.25% concentration as an irrigant is widely used in root canal treatment as it is bactericidal and has the ability to dissolve organic tissues but noneffective in removing the smear layer.[4,5] Decalcifying solutions used for removing the smear layer include phosphoric acid, citric acid, maleic acid, ethylenediaminetetraacetic acid (EDTA), and MTAD (a mixture of tetracycline isomer, an acid, and a detergent).[6,7] Lasers have also been used to remove the smear layer such as argon laser,[8] neodymium-doped yttrium aluminum garnet,[9] CO2 laser,[10] erbium-doped yttrium aluminum garnet,[3] and diode.[11] Currently, a final irrigation sequence with a chelating agent EDTA and NaOCl is being used to remove the inorganic and organic components of the smear layer.[12] This study evaluates the efficacy of smear layer removal from the root canals using diode laser and ultrasonics with and without EDTA during endodontic therapy.
MATERIALS AND METHODS
A total of 120 adult human noncarious mandibular premolars were taken for the study. Inclusion criteria included single-rooted teeth with straight, patent roots, and fully formed apices. Extraction for periodontal or orthodontic reasons. Standard radiographs were taken in a buccolingual and mesiodistal direction of each tooth after being held in a custom made jig to determine whether or not the sampled tooth conforms to the selection criteria adopted for the study.
Sample preparation
The teeth were stored in 10% formalin solution till they were used for the study. The root surfaces were cleaned and then decoronated using a diamond disc under water irrigation to obtain a standardized root length of 12 mm. After standardization, the working length of specimens was determined by deducting 1 mm from the length of the #10/#15 K-file after it was passively placed in the canal until the tip of the instrument visibly penetrated the apical foramen. Apices of the roots were sealed with sticky wax to simulate the clinical conditions and root canal instrumentation was initiated with ISO hand files up to #20 followed by ProTaper rotary files up to size F3. Two milliliter of 3% NaOCl was used as an irrigant after every instrument change. The irrigants were delivered with a disposable syringe and a 30-gauge Max-I-Probe needle placed 1 mm short of the working length. Finally, 3 ml of 3% NaOCl was used to flush out the debris from the root canals followed by a rinse with 3 ml of distilled water to terminate any action of the solvents remaining in the canal. A constant total volume of 15 ml of NaOCl was used as irrigant for each root canal during the study.
Grouping of samples
After biomechanical preparation, the samples were divided into six different groups of twenty specimens each.
Group A (NaOCl) – Root canals were irrigated with a final flush of 1 ml of 3% NaOCl for 1 min followed by 3 ml of 3% NaOCl
Group B (EDTA) – Root canals were irrigated with a final flush of 1 ml of 17% EDTA for 1 min, followed by 3 ml of 3% NaOCl
Group C (diode) – For laser application, a 200-µm, 970 ± 15 nm, power max 7 watts (CW) fiberoptic tip was introduced into the root canal up to the working length; the laser was activated and gently withdrawn from the root canal to the coronal region with a helicoid movement and reintroduced to the apex for a total laser irradiation cycle of 20 s
Group D [diode + EDTA] – The root canals were initially irrigated with 0.8 ml of 17% EDTA for 40 s; the remaining 0.2 ml was used to fill the root canals, and diode laser application was done for 20 s as in Group C
Group E [ultrasonic] – The root canals were irrigated with 1 ml distilled water with passive ultrasonic activation for 1 min with a #15/21 mm ultrasonic K-file placed 1 mm short of the working length, followed by 3 ml of 3% NaOCl
Group F [ultrasonic + EDTA] The root canals were irrigated with a final flush of 1 ml EDTA with passive ultrasonic activation for 1 min with a #15/21 mm ultrasonic K-file placed 1 mm short of the working length, followed by 3 ml of 3% NaOCl.
The root canals were finally flushed with 5 ml of distilled water to terminate the action of the irrigating solutions dried and prepared for scanning electron microscope (SEM) examination.
Scanning microscope examination
The teeth were grooved along the buccal and lingual planes by using a diamond disc at low speed. The roots were then split longitudinally with a bi-beveled chisel and a mallet. One-half of each root was selected depicting the entire root canal length and prepared for SEM examination. The selected samples were progressively dehydrated using graded concentrations of aqueous ethanol (70%, 80%, 90%, and 100%) for 24 h at each concentration. After dehydration, samples were placed in a vacuum chamber and sputter coated with a 30 nm gold layer. The dentinal wall of the root canals was examined at coronal, middle, and apical thirds at a magnification of ×1000 for the presence or absence of smear layer and patency of dentinal tubules. Photomicrographs of the root canals were taken at coronal, middle, and apical level for scoring individually in a calibrated single-blind manner according to the rating system developed by Gutmann et al.[13]
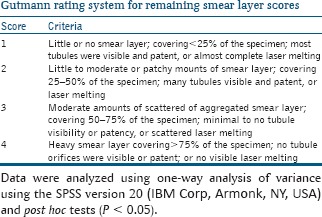
Data were analyzed using one-way analysis of variance using the SPSS version 20 (IBM Corp, Armonk, NY, USA) and post hoc tests (P < 0.05).
RESULTS
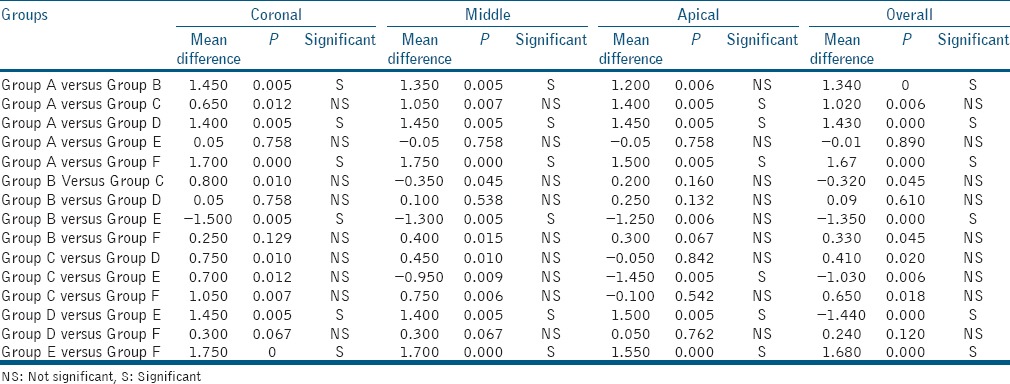
At the coronal third level: Group F followed by Group B had the least smear layer scores with no significant difference between them. This was followed by Group D and Group C with a significant difference between Group C and Groups B, D, and F, respectively. At the middle third level: Group F followed by Group D had the least smear layer scores with no significant difference between them. This was followed by Group B and Group C with a significant difference between them. The highest smear layer scores were for Group A and Group E, respectively [Table 1]. At the apical third level: the lowest smear layer scores were for Group F followed by Group D, Group C, and Group B, respectively, with a significant difference between Group F and Group B [Table 2].
Table 1.
Mean remaining smear layer scores among various groups
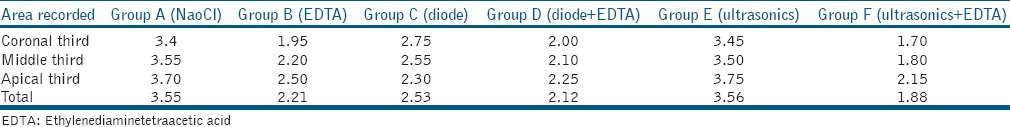
Table 2.
Intragroup comparison of remaining smear layer scores at various levels
DISCUSSION
In the present study, Group A showed the presence of heavy smear layer throughout the length of the canals which is similar to previous studies by Baumgartner and Mader,[1] Torabinejad et al.,[14] and Goldman et al.[15] that show NaOCl to be ineffective in removing the smear layer. In Group B, EDTA showed effective smear layer removal from coronal and middle third as compared to the apical third which was statistically significant and in agreement with the results of various studies.[1,14] A larger canal diameter in the coronal and middle third exposes the dentin to a higher volume of irrigants, allowing a better flow of the solution and hence, improving the efficacy of smear layer removal.[16] The addition of ultrasonics to EDTA increased the smear layer removing efficacy of EDTA by enhancing its penetration into the narrow apical regions of the root canals. In Group C, the choice for the power 1.5 watts in CW parameter settings used in this study was based on the results of study by Alfredo et al.,[17] who demonstrated that these parameters yielded a temperature rise approximately 10°C, which does not exceed the limit supported by the periapical tissues.[18] Twenty seconds time application was used according to the study by Marchesan et al.[19] The diode laser was able to remove the smear layer from the root canal surfaces; however, most of the dentinal tubules were obliterated with smear plugs. The apical third had lesser smear layer scores than the middle and coronal third, with a statistically significant difference between coronal and apical third. This can be attributed to the narrower diameter of the canal in the apical region resulting in a closer approximation of the laser tip to the root canal walls and thus melting and evaporating the smear layer easily. Localized melting, fusion, and constriction of the dentinal tubule openings were found in many samples as opposed to findings of Wang et al.[11] He found clean root canal walls with open dentinal tubules. This difference may be due to different laser setting and irrigants used in the preparation of the root canals before laser application in the two studies. In Group D, the smear layer was removed from the root canals; the dentinal tubules were obliterated mostly at the middle and apical level. The results were similar to the study of Faria et al.[20] who found absence of smear layer and partially obliterated dentinal tubules after application of 980 nm diode laser on root canals irrigated with 1% NaOCl plus 17% EDTA. In Group E, the entire root canal surface was covered with thick layers of smear layer. These results are in accordance with studies done by Cameron[2] and Huque et al.[21] who reported that passive ultrasonic irrigation with water as irrigant is unable to remove the smear layer. In Group F, the root canal surfaces were clean and free of smear layer in the coronal and middle third, whereas the apical third showed scattered areas with smear layer. No significant difference in smear layer scores was recorded at the coronal and middle levels; however, higher smear layer scores were recorded at the apical level which were significant in comparison to coronal third. The results were statistically significant in comparison to Group B and Group C and nonsignificant in comparison to Group D. On comparing Group B and Group F, the results are in accordance to Kuah et al.[22] who recorded a significant difference in the smear layer and debris removal at the 2 mm and 6 mm level from the apical foramen in groups with both ultrasonic and EDTA as compared to groups with EDTA alone. The addition of ultrasonics to EDTA increased the smear layer removing efficacy of EDTA by enhancing its penetration into the narrow apical regions of the root canals.
The results do not agree with those of Abbott et al.[23] who reported that intermittent activation of EDTA followed by NaOCl was not effective in the removal of smear layer. A power setting of 1, #20 K-file, and the irrigant was delivered with a 25-gauge needle. Lower power setting and larger size of needle and file might have been responsible for the different results in that study.
In this study, the apical third of the canals was least influenced by the ultrasonic irrigation. The oscillation of the tips of ultrasonic instruments is decreased by constraining it in the root canal. Because the amplitude of the oscillation is largest at the instrument's tip, any attenuation affects the apical part most significantly where the diameter of the canal is smallest.[24]
The combined use of ultrasonic and EDTA followed by a combination of diode and EDTA performed better than EDTA, diode, and ultrasonics used alone in removing smear layer suggesting that the incorporation of ultrasonics and diode laser with EDTA might prove beneficial in increasing the ability of EDTA to remove the smear layer by enhancing its interaction with the root canal walls particularly in the apical regions. The diode laser used alone proved to be effective than EDTA in the apical regions in removing the smear layer; however, the difference was not significant.
CONCLUSION
Within the limitations of the current study, all the tested groups were able to remove the smear layer from the prepared root canals to different degrees except NaOCl and ultrasonics. When used alone diode laser performed significantly better than ultrasonic. Diode laser could be a good addition to the armamentarium used for smear layer removal and along with its bactericidal effects on the root canal microbes could increase the success rate of endodontic therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Baumgartner JC, Mader CL. A scanning electron microscopic evaluation of four root canal irrigation regimens. J Endod. 1987;13:147–57. doi: 10.1016/s0099-2399(87)80132-2. [DOI] [PubMed] [Google Scholar]
- 2.Cameron JA. The use of ultrasonics in the removal of the smear layer: a scanning electron microscope study. J Endod. 1983;9:289–92. doi: 10.1016/S0099-2399(83)80119-8. [DOI] [PubMed] [Google Scholar]
- 3.Takeda FH, Harashima T, Kimura Y, Matsumoto K. Efficacy of Er:YAG laser irradiation in removing debris and smear layer on root canal walls. J Endod. 1998;24:548–51. doi: 10.1016/S0099-2399(98)80075-7. [DOI] [PubMed] [Google Scholar]
- 4.Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: Part 3. J Endod. 1983;9:137–42. doi: 10.1016/S0099-2399(83)80032-6. [DOI] [PubMed] [Google Scholar]
- 5.Prati C, Selighini M, Ferrieri P, Mongiorgi R. Scanning electron microscopic evaluation of different endodontic procedures on dentin morphology of human teeth. J Endod. 1994;20:174–9. doi: 10.1016/S0099-2399(06)80330-4. [DOI] [PubMed] [Google Scholar]
- 6.Wayman BE, Kopp WM, Pinero GJ, Lazzari EP. Citric and lactic acids as root canal irrigants in vitro. J Endod. 1979;5:258–65. doi: 10.1016/S0099-2399(79)80171-5. [DOI] [PubMed] [Google Scholar]
- 7.Aktener BO, Bilkay U. Smear layer removal with different concentrations of EDTA-ethylenediamine mixtures. J Endod. 1993;19:228–31. doi: 10.1016/S0099-2399(06)81296-3. [DOI] [PubMed] [Google Scholar]
- 8.Harashima T, Takeda FH, Zhang C, Kimura Y, Matsumoto K. Effect of argon laser irradiation on instrumented root canal walls. Endod Dent Traumatol. 1998;14:26–30. doi: 10.1111/j.1600-9657.1998.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 9.Dederich D, Zakariasen K, Tulip J. Scanning electron microscopic analysis of root canal wall dentin following neodymium-yttrium aluminium-garnet laser. J Endod. 1984;10:428–31. doi: 10.1016/S0099-2399(84)80264-2. [DOI] [PubMed] [Google Scholar]
- 10.Onal B, Ertl T, Siebert G, Müller G. Preliminary report on the application of pulsed CO2 laser radiation on root canals with AgCl fibers: a scanning and transmission electron microscopic study. J Endod. 1993;19:272–6. doi: 10.1016/s0099-2399(06)80455-3. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Sun Y, Kimura Y, Kinoshita J, Ishizaki NT, Matsumoto K. Effects of diode laser irradiation on smear layer removal from root canal walls and apical leakage after obturation. Photomed Laser Surg. 2005;23:575–81. doi: 10.1089/pho.2005.23.575. [DOI] [PubMed] [Google Scholar]
- 12.Hülsmann M, Heckendorff M, Lennon A. Chelating agents in root canal treatment: mode of action and indications for their use. Int Endod J. 2003;36:810–30. doi: 10.1111/j.1365-2591.2003.00754.x. [DOI] [PubMed] [Google Scholar]
- 13.Gutmann JL, Saunders WP, Nguyen L, Guo IY, Saunders EM. Ultrasonic root-end preparation. Part 1. SEM analysis. Int Endod J. 1994;27:318–24. doi: 10.1111/j.1365-2591.1994.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 14.Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, et al. A new solution for the removal of the smear layer. J Endod. 2003;29:170–5. doi: 10.1097/00004770-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Goldman LB, Goldman M, Kronman JH, Lin PS. The efficacy of several irrigating solutions for endodontics: a scanning electron microscopic study. Oral Surg Oral Med Oral Pathol. 1981;52:197–204. doi: 10.1016/0030-4220(81)90319-4. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira CS, Felippe MC, Felippe WT. The effect of application time of EDTA and NaOCl on intracanal smear layer removal: an SEM analysis. Int Endod J. 2005;38:285–90. doi: 10.1111/j.1365-2591.2005.00930.x. [DOI] [PubMed] [Google Scholar]
- 17.Alfredo E, Silva SR, Ozório JE, Sousa-Neto MD, Brugnera-Júnior A, Silva-Sousa YT. Bond strength of AH plus and epiphany sealers on root dentine irradiated with 980 nm diode laser. Int Endod J. 2008;41:733–40. doi: 10.1111/j.1365-2591.2008.01418.x. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson AR, Albrektsson T. Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent. 1983;50:101–7. doi: 10.1016/0022-3913(83)90174-9. [DOI] [PubMed] [Google Scholar]
- 19.Marchesan MA, Junior AB, Gabriel AE, Silva SR, Neto MD. Ultrastructural analysis of root canal dentine irradiated with 980-nm diode laser energy at different parameters. Photomed Laser Surg. 2008;26:235–40. doi: 10.1089/pho.2007.2136. [DOI] [PubMed] [Google Scholar]
- 20.Faria MI, Souza-Gabriel AE, Alfredo E, Messias DC, Silva-Sousa YT. Apical microleakage and SEM analysis of dentin surface after 980 nm diode laser irradiation. Braz Dent J. 2011;22:382–7. doi: 10.1590/s0103-64402011000500006. [DOI] [PubMed] [Google Scholar]
- 21.Huque J, Kota K, Yamaga M, Iwaku M, Hoshino E. Bacterial eradication from root dentine by ultrasonic irrigation with sodium hypochlorite. Int Endod J. 1998;31:242–50. doi: 10.1046/j.1365-2591.1998.00156.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuah HG, Lui JN, Tseng PS, Chen NN. The effect of EDTA with and without ultrasonics on removal of the smear layer. J Endod. 2009;35:393–6. doi: 10.1016/j.joen.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Abbott PV, Heijkoop PS, Cardaci SC, Hume WR, Heithersay GS. An SEM study of the effects of different irrigation sequences and ultrasonics. Int Endod J. 1991;24:308–16. doi: 10.1111/j.1365-2591.1991.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 24.Walmsley AD, Williams AR. Effects of constraint on the oscillatory pattern of endosonic files. J Endod. 1989;15:189–94. doi: 10.1016/S0099-2399(89)80233-X. [DOI] [PubMed] [Google Scholar]


