Abstract
Background:
Individuals who have had an anterior cruciate ligament (ACL) tear and reconstruction continue to experience substantial knee extensor strength loss despite months of physical therapy. Identification of the alterations in muscle morphology and cellular composition are needed to understand potential mechanisms of muscle strength loss, initially as the result of the injury and subsequently from surgery and rehabilitation.
Methods:
We performed diffusion tensor imaging-magnetic resonance imaging and analyzed muscle biopsies from the vastus lateralis of both the affected and unaffected limbs before surgery and again from the reconstructed limb following the completion of rehabilitation. Immunohistochemistry was done to determine fiber type and size, Pax-7-positive (satellite) cells, and extracellular matrix (via wheat germ agglutinin straining). Using the diffusion tensor imaging data, the fiber tract length, pennation angle, and muscle volume were determined, yielding the physiological cross-sectional area (PCSA). Paired t tests were used to compare the effects of the injury between injured and uninjured limbs and the effects of surgery and rehabilitation within the injured limb.
Results:
We found significant reductions before surgery in type-IIA muscle cross-sectional area (CSA; p = 0.03), extracellular matrix (p < 0.01), satellite cells per fiber (p < 0.01), pennation angle (p = 0.03), muscle volume (p = 0.02), and PCSA (p = 0.03) in the injured limb compared with the uninjured limb. Following surgery, these alterations in the injured limb persisted and the frequency of the IIA fiber type decreased significantly (p < 0.01) and that of the IIA/X hybrid fiber type increased significantly (p < 0.01).
Conclusions:
Significant and prolonged differences in muscle quality and morphology occurred after ACL injury and persisted despite reconstruction and extensive physical therapy.
Clinical Relevance:
These results suggest the need to develop more effective early interventions following an ACL tear to prevent deleterious alterations within the quadriceps.
Every year, up to 200,000 anterior cruciate ligament (ACL) tears occur in the United States1,2. Mounting evidence suggests that substantial reductions in quadriceps strength, >20% to 40%, occur at a critical time when the individual returns to activities that involve greater muscular demands and joint loads3-14. Alterations in both neurological control and muscle physiology have been proposed as mechanisms for this protracted loss in muscle strength12,15,16. Despite the considerable attention devoted to this injury, little is still known regarding the underlying alterations in muscle physiology after an ACL tear and subsequent postoperative rehabilitation.
The results from several studies on quadriceps strength following ACL reconstruction suggest that the observed postoperative strength differences may be due to changes within the muscle13,16-18. However, the exact adaptations in muscle morphology are still not well defined. Features such as volume, physiological cross-sectional area (PCSA), muscle fiber pennation angle, and length influence muscle force generation19-21. Recently, reductions in muscle thickness but not pennation angle were reported after an ACL reconstruction22. These morphological features have been used in other populations, such as in patients with cerebral palsy and after intramedullary nailing of a unilateral femoral diaphyseal fracture, to better understand the mechanisms of strength loss23,24. Also, PCSA is a strong predictor of maximal muscle force25. Diffusion tensor imaging-magnetic resonance imaging (DTI-MRI) offers the opportunity to noninvasively evaluate these properties before and after surgery over a large area of the muscle. The use of DTI-MRI could help to improve our understanding of the muscular deficits after an ACL reconstruction.
To our knowledge, adaptations in the cellular composition of muscle after an ACL tear and reconstruction have not been fully assessed. Indirect techniques for measuring muscle composition with electromyography after surgery have led to the speculation that there is selective type-II muscle fiber atrophy6,16,26,27. Muscle stem cells (satellite cells) play a critical role in muscle repair and regeneration, and they may also play a role in muscle adaptation to injury and rehabilitation following an injury such as an ACL tear, a hypothesis that is, to our knowledge, unexplored28. In animal models, loss of satellite cells also promotes an increase in the extracellular matrix29.
The purpose of this study was to assess the underlying changes in muscle fiber tract length, pennation angle, volume, and PCSA using DTI-MRI, as well as changes in muscle fiber type and size, satellite cells, and extracellular matrix using muscle biopsies of the vastus lateralis. We evaluated both the changes occurring as the result of the injury and those observed within the injured limb after the completion of surgery and rehabilitation. We hypothesized that, following an ACL tear, there would be significant reductions in PCSA, pennation angle, fiber tract length, and satellite cells; increases in extracellular matrix; and changes in fiber type. We further hypothesized that, following surgery, these alterations would persist despite physical therapy.
Materials and Methods
Subjects provided written informed consent, and the protocol was approved by the university institutional review board. To qualify, subjects could not have had a previous ACL reconstruction or tear other than the current one. Subjects were excluded if they had a knee dislocation or if the ACL tear had occurred more than 2 months prior to being diagnosed; however, subjects undergoing a meniscal repair or meniscectomy were included. One of 2 orthopaedic surgeons performed the surgery. The rehabilitation protocol followed published guidelines, emphasizing early return of full knee extension and early quadriceps strength exercises30. At the time of the follow-up testing, patients were cleared by their physician to start return-to-sport drills and activities. The muscle biopsies and MRI were performed several days before surgery on both limbs, and again on the reconstructed limb at the time of resumption of sport-specific drills.
MRI
Subjects were imaged on a 3-T MAGNETOM Trio, a TIM System (Siemens) MRI system. Two packets of 11 axial slices were acquired with a slice thickness of 6 mm and no interslice gap. DTI-MRI data were acquired using a stimulated-echo sequence with a repetition time/time to echo of 4,000/36.4 ms, 3 excitations, mixing time of 173.0 ms, gradient separation of 185.8 ms, and gradient duration of 5.4 ms. The sequence captured 27 gradient directions at a diffusion weighting factor (b) of 500 s/mm2 and 4 repetitions at a b value of 0. The sequence used parallel imaging with an acceleration factor of 2, field of view of 192 × 192 mm2, and acquisition matrix of 96 × 96.
The tensor calculation, diagonalization, and fiber tracking were performed with custom MATLAB (R2013b; The MathWorks) code. The pennation angle and fiber tract length were then calculated from the tracked fibers using previously described techniques31. Lastly, muscle volume was determined by tracing the cross-sections from the anatomical images sequentially along the length of the vastus lateralis using Slicer 3D (version 4.4; www.slicer.org)32.
Muscle Biopsies
Biopsy samples were taken from the vastus lateralis under local anesthetic (1% xylocaine HCl) using a modification of the Bergstrom percutaneous biopsy technique.
Muscle Immunohistochemistry
Sections were cut in a cryostat and allowed to dry for 1 hour at room temperature. Fiber typing was performed on unfixed sections that were incubated overnight at room temperature with antibodies against myosin heavy chain (MyHC) isoforms type I (antibody BA.D5; immunoglobulin G2b [IgG2b]), type IIA (SC.71; IgG1), and type IIX (6H1; IgM) from the University of Iowa Developmental Studies Hybridoma Bank (DSHB). Fibers coexpressing type-IIA and IIX MyHC were classified as IIA/X hybrid fibers. Sections were incubated for 1 hour with Ig-specific secondary antibodies (Invitrogen): goat anti-mouse IgG2b AF647 (#A21242) for type-I fibers, goat anti-mouse IgG1 AF488 (#A21121) for type-IIA fibers, and goat anti-mouse IgM biotin (#626840) for type-IIX fibers. Sections were then incubated for 15 minutes in streptavidin-Texas Red (#SA-5006; Vector Laboratories). Sections were postfixed using methanol prior to mounting with fluorescent mounting media (#H-1000; Vector). Sections were also stained with wheat germ agglutinin (WGA) and Pax-7 according to standard immunohistochemical methods.
Image Acquisition and Analysis
Images were captured (magnification, ×10) using an upright microscope (AxioImager M1; Zeiss). The fiber type distribution and the mean cross-sectional area (CSA) of each fiber type were determined using AxioVision Rel software (version 4.8; Zeiss). Satellite cell frequency was determined by counting only the cells that were positive for both Pax-7 and DAPI (4ʹ,6-diamidino-2-phenylindole), and was expressed as Pax-7-positive cells/fiber. WGA staining was quantified using the threshold intensity feature in the AxioVision image analysis software. Assessors were not blinded to the clinical information.
Strength Assessment
Each subject’s peak isometric quadriceps strength was assessed in both limbs following the completion of rehabilitation with the knee in 90° of flexion using a Biodex 4 dynamometer. Subjects performed 1 practice trial, to familiarize themselves with the task, followed by 4 test trials. Assessors were not blinded to the side of injury.
Statistical Methods
To assess the effect of the injury on the muscle before surgery, paired t tests were used to compare muscle biopsy and DTI-MRI data between the injured limb and uninjured limb. To evaluate the effect of surgery and rehabilitation, paired t tests were used to compare muscle biopsy and DTI-MRI data between the injured limb before surgery and the same limb following surgery and the completion of rehabilitation.
Results
Two subjects did not complete the study; 1 had an ACL retear related to noncompliance with activity restriction, and the other did not return for regularly scheduled physician and physical therapy visits. A total of 8 male and 2 female subjects (mean age [and standard deviation], 23.4 ± 5.0 yr; weight, 78 ± 12.7 kg; height, 1.77 ± 0.08 m) who had sustained an ACL injury that was surgically reconstructed completed the study. Eight of the subjects had a bone-patellar tendon-bone autograft and the remaining 2 had a hamstring autograft. The mean time between injury and surgery was 82 ± 61 days. There was no relationship between time to surgery and any of the preoperative measured variables. One subject’s strength data were excluded because of equipment recording failure. Despite completing 6 months of rehabilitation, the injured limbs were significantly weaker (1.7 ± 0.53 N/kg) than the uninjured limbs (2.8 ± 0.47 N/kg, p < 0.0001).
Noninvasive Assessment of Muscle Structure and Function
MRI-DTI analyses of vastus lateralis muscle from the uninjured limb and from the injured limb before and after surgical ACL reconstruction and rehabilitation are presented in Table I. Before surgery, the injured limb had a significantly smaller pennation angle, volume, and PCSA compared with the uninjured limb, but the fiber tract length did not differ significantly. No significant improvement in any of the muscle structural properties assessed by MRI-DTI was found after surgery and rehabilitation, and muscle volume actually decreased further (Table I).
TABLE I.
Muscle Structure as Assessed by DTI-MRI
| Uninjured Limb* | Injured Limb Before Surgery* | P Value | Injured Limb After Surgery* | P Value† | |
| Pennation angle (deg) | 18.3 ± 2.7 | 16.0 ± 2.4 | 0.03 | 15.8 ± 3.0 | 0.87 |
| Fiber tract length (cm) | 4.3 ± 0.9 | 4.1 ± 1.0 | 0.57 | 4.1 ± 1.2 | 0.97 |
| Volume (cm3) | 503.6 ± 137.7 | 371.1 ± 111.0 | 0.02 | 308.7 ± 82.7 | 0.002 |
| PCSA (cm2) | 115.5 ± 40.0 | 92.0 ± 29.0 | 0.03 | 77.8 ± 24.8 | 0.25 |
The values are given as the mean and standard deviation.
Injured limb after surgery compared with before surgery.
Analysis of Muscle Morphology in Vastus Lateralis Biopsies
The results of histochemical and immunohistochemical analyses of muscle cross-sections are summarized in Table II. As shown in representative images in Figure 1, fiber size and fiber type composition were quantified using a battery of isoform-specific MyHC antibodies that recognize the slow-twitch type-I MyHC and the fast-twitch type-II MyHCs, IIA and IIX. Type-II muscle fibers were separated into 2 groups, those that expressed no type-IIX MyHC (i.e., purely type-IIA) and those that coexpressed types IIX and IIA (type-IIA/X hybrids). We found a significant reduction specifically in type-IIA fiber CSA in the muscle from the injured limb compared with the uninjured limb prior to surgery. However, we did not find any other significant changes in either frequency or CSA of any of the other muscle fiber types prior to surgery. Following surgery and rehabilitation, we found that type-IIA fibers significantly decreased in frequency and type-IIA/X hybrid fibers increased in frequency in the injured limb after surgery and rehabilitation (Fig. 1 and Table II).
TABLE II.
Muscle Morphology in Biopsies as Assessed by Immunohistochemical Analyses
| Uninjured Limb* | Injured Limb Before Surgery* | P Value | Injured Limb After Surgery* | P Value† | |
| Type-I fibers/total fibers | 0.44 ± 0.11 | 0.48 ± 0.18 | 0.53 | 0.40 ± 0.20 | 0.20 |
| Type-I fiber CSA (μm2) | 4,557 ± 1,402 | 4,344 ± 1,062 | 0.12 | 4,549 ± 1,405 | 0.35 |
| Type-IIA fibers/total fibers | 0.34 ± 0.10 | 0.35 ± 0.14 | 0.62 | 0.25 ± 0.10 | <0.01 |
| Type-IIA fiber CSA (μm2) | 5,875 ± 1,613 | 4,769 ± 1,278 | 0.03 | 5,148 ± 1,795 | 0.36 |
| Type-2IIA/X fibers/total fibers | 0.21 ± 0.07 | 0.17 ± 0.08 | 0.12 | 0.34 ± 0.16 | <0.01 |
| Type-IIA/X fiber CSA (μm2) | 5,056 ± 1,186 | 4,493 ± 1,136 | 0.33 | 4,437 ± 1,574 | 0.91 |
| Extracellular matrix/CSA | 0.10 ± 0.03 | 0.16 ± 0.05 | <0.01 | 0.15 ± 0.04 | 0.42 |
| Satellite cells/fiber | 0.17 ± 0.05 | 0.10 ± 0.04 | <0.01 | 0.11 ± 0.04 | 0.18 |
The values are given as the mean and standard deviation.
Injured limb after surgery compared with before surgery.
Fig. 1.
Representative immunohistochemical images showing myosin heavy chain (MyHC) fiber type identification in the injured limb (scale bar = 50 μm). Type-IIA fibers are green, type-IIX fibers are red, type-IIA/X fibers are yellow/green, and type-I fibers are light pink. The distribution of fiber types IIA and IIA/X after surgery differed from that before surgery. Fig. 1-A Composite image (showing all stains) before surgery. Fig. 1-B Type-IIX fibers (red) in the preceding image. Fig. 1-C Composite image after surgery and rehabilitation. Fig. 1-D Type-IIX fibers in the preceding image.
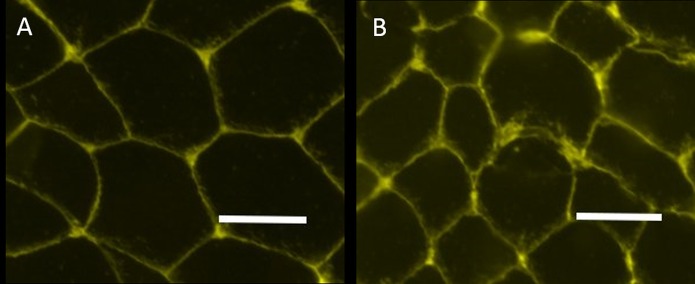
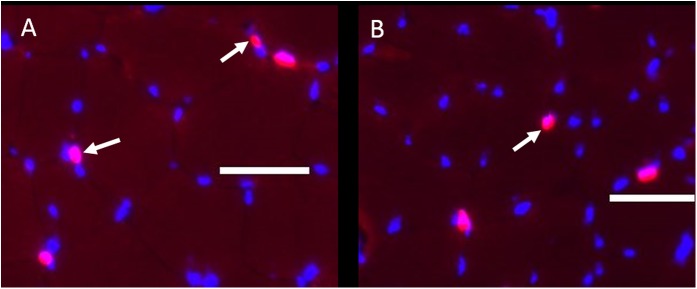
Extracellular matrix (representative images shown in Figure 2) was higher in the muscle from the injured limb (Fig. 2-B) compared with the uninjured limb before surgery (Fig. 2-A) (p < 0.01). Surgical reconstruction and rehabilitation did not significantly reduce extracellular matrix (Table II). Satellite cells (Fig. 3) were significantly reduced in injured (Fig. 3-B) compared with uninjured muscle prior to surgery (Fig. 3-A) and did not increase after surgery and rehabilitation (Table II).
Fig. 2.
Representative immunohistochemical images showing WGA staining (yellow). There is greater extracellular matrix in the injured limb before surgery (Fig. 2-B) compared with the uninjured limb (Fig. 2-A) (scale bar = 50 μm).
Fig. 3.
Representative immunohistochemical composite images showing Pax-7 (pink) and DAPI (blue) staining in the uninjured limb (Fig. 3-A) and the injured limb before surgery (Fig. 3-B) (scale bar = 50 μm). Arrows indicate representative positive staining for Pax-7 (which indicates satellite cells).
Discussion
We performed a comprehensive assessment of muscle alterations as a result of ACL injury and rehabilitation through a combined approach involving muscle structural quantification using DTI-MRI and muscle morphology analysis using biopsied tissue. Alterations occurred in the vastus lateralis muscle of the injured limb compared with the control limb, and the alterations did not improve following subsequent surgery and rehabilitation. These results indicate that there are several pathophysiological responses of the muscle to an ACL tear as well as other alterations that become more apparent following reconstruction and rehabilitation of the ACL.
There were several notable alterations in the vastus lateralis muscle prior to surgery. One of the most important early alterations was the significant reduction in the PCSA as a result of ACL injury. Similar findings have been reported in research assessing the effects of disuse33. We examined the 3 individual components of the PCSA—volume, pennation angle, and fiber length—to determine which were driving this change (Table I). The fiber tract length did not change, which we speculate was because subjects did not maintain the limb in a shortened position. Previous work has shown that when subjects have undergone casting, fiber length decreases only if the limb is immobilized in a shortened position34. In contrast, we found that the pennation angle was significantly different. Although the pennation angle is not a major determinant of the PCSA, a reduction in this angle will limit the number of fibers within a given area and subsequently result in less force development. The observed reduction in the PCSA is most likely primarily attributable to a reduction in the volume of the vastus lateralis. The reduction in muscle volume is consistent with other studies, although the magnitude of the reduction in the present study is larger than in those studies35,36. Potential differences in subject characteristics and in the methods used to determine volume may explain some of the differences between studies.
There were several significant cellular alterations that occurred following the injury but prior to surgery. Although we did not find significant changes in the distribution of muscle fiber types before surgery, there was a significant reduction in the CSA of type-IIA muscle fibers. The selective atrophy of the type-IIA fibers confirms the speculations of previous authors6,16,26,27. Potentially, the selective reduction of type-IIA fiber CSA is due to a lack of input from the gamma motor neuron loop, which affects high-threshold motor neurons activating type-IIA fibers37,38. Additional work is needed to more directly test the link between sensory alterations due to an ACL tear and adaptations within the muscle.
Other cellular adaptations of the vastus lateralis muscle due to the ACL injury included greater extracellular matrix and fewer satellite cells prior to surgery. Little research has defined fibrotic changes in muscle as a result of an orthopaedic injury. For example, imaging studies in subjects who have had a hamstring strain have shown an increase in nonactive contractile components39,40. The end result of greater muscle extracellular matrix is less area occupied by active contractile components, effectively reducing force generation. Additionally, the reduction in satellite cell content in the injured muscle before surgery may impair the muscle’s ability to respond to subsequent rehabilitation. Satellite cell content is strongly correlated with strength gains in response to resistance training in the elderly, with the satellite cells providing myonuclei by fusing to the growing fibers41-43.
Muscle morphology did not improve significantly after surgery and rehabilitation. For example, the PCSA did not improve following reconstruction of the ACL and rehabilitation. Previous reports have found a reduction in muscle volume following surgery and rehabilitation but have not reported on PCSA35,36. We also found little change in muscle volume or pennation angle. Our results are in contrast to another report that used ultrasonography and found no difference in pennation angle at 2 years of follow-up22. Differences in the time to follow-up and in the technique used to assess pennation angle make a direct comparison between these studies difficult. However, these data provide further evidence of the need for rehabilitation strategies focused on earlier intervention, such as eccentric exercises, which are known to improve PCSA and the components contributing to it44-48.
We also report several other adaptations that occur after surgery and rehabilitation. There was a selective reduction in type-IIA muscle fiber frequency and increased abundance of type-IIA/X hybrid fibers. In addition, we found no significant improvement in the CSA of specific muscle fiber types. In the only previous study of fiber atrophy after ACL surgery that we are aware of, type-II muscle fiber CSA was reduced up to 1 year after surgery relative to that in the nonoperative limb15. However, that study focused only on fiber CSA, did not consider type-IIA/X hybrid fibers, and used outdated surgical and rehabilitation techniques15. The shift from type-IIA to type-IIA/X hybrid fibers has been shown in other populations to be indicative of a detrained state49-51. By contrast, subjects in the present study underwent a rehabilitation period, and at the time of the second biopsy they had begun to return to their previous activity levels. These results suggest that deficits in type-IIA fiber CSA and deleterious alterations in fiber type composition continue during current rehabilitation protocols.
The persistence of the increase in extracellular matrix and decrease in satellite cell content despite surgical reconstruction and rehabilitation demonstrate the need to intervene early following an ACL tear to prevent changes at the cellular level that may ultimately limit muscle adaptation during rehabilitation. Additionally, whether thickening of the muscle extracellular matrix can be reversed in humans through rehabilitation has yet to be determined. If irreversible, these alterations may necessitate the need to develop strategies to optimize the remaining muscle function. Emerging evidence suggests that satellite cells may play an important role in the regulation of extracellular matrix in muscle29. The lack of restoration of satellite cells following surgery may contribute to the protracted weakness, greater extracellular matrix, and reduced ability of muscle fibers to hypertrophy.
The between-limb differences in quadriceps strength were larger than in some previous reports but similar to the differences in others3,12,52-54. Differences in the mode of testing (isometric versus isokinetic), type of graft, timing of the testing, and age are all factors that may potentially explain these differences. Future studies in larger cohorts comparing the morphological features of those with the least and greatest between-limb differences are warranted given the observed differences.
The present study had several limitations. First, we were unable to control the time window between injury and surgery, although subjects had surgery within 2 months of the injury. Second, the sample size is small and includes subjects with either bone-patellar tendon-bone or hamstring grafts, but the level of complexity of the study and time commitments from subjects make large sample sizes for this work a challenge. Lastly, we were not able to image or take a biopsy from the uninjured limb following surgery, limiting our ability to identify contralateral changes in the examined variables.
In conclusion, we have shown that there are pathophysiological responses within muscle following an ACL injury, with reductions in muscle fiber volume and pennation angle resulting in reduced PCSA as well as greater extracellular matrix and reduced satellite cell frequency. These measures do not improve following surgery and rehabilitation. There was also a significant shift to a greater frequency of type-IIA/X muscle fibers following surgery and rehabilitation. Future strategies to address these clinical issues must be developed.
Footnotes
Investigation performed at the University of Kentucky, Lexington, Kentucky
A commentary by Robert A. Magnussen, MD, is linked to the online version of this article at jbjs.org.
Disclosure: This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH R01 AR050101 and K23 AR062069). On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author received payment or services from a third party (government, commercial, private foundation, etc.) for an aspect of the submitted work.
Disclaimer: The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, Demaio M, Dick RW, Engebretsen L, Garrett WE Jr, Hannafin JA, Hewett TE, Huston LJ, Ireland ML, Johnson RJ, Lephart S, Mandelbaum BR, Mann BJ, Marks PH, Marshall SW, Myklebust G, Noyes FR, Powers C, Shields C Jr, Shultz SJ, Silvers H, Slauterbeck J, Taylor DC, Teitz CC, Wojtys EM, Yu B. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006. September;34(9):1512-32. [DOI] [PubMed] [Google Scholar]
- 2.McLean SG, Beaulieu ML. Complex integrative morphological and mechanical contributions to ACL injury risk. Exerc Sport Sci Rev. 2010. October;38(4):192-200. [DOI] [PubMed] [Google Scholar]
- 3.Hiemstra LA, Webber S, MacDonald PB, Kriellaars DJ. Knee strength deficits after hamstring tendon and patellar tendon anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 2000. August;32(8):1472-9. [DOI] [PubMed] [Google Scholar]
- 4.DeVita P, Hortobagyi T, Barrier J. Gait biomechanics are not normal after anterior cruciate ligament reconstruction and accelerated rehabilitation. Med Sci Sports Exerc. 1998. October;30(10):1481-8. [DOI] [PubMed] [Google Scholar]
- 5.Paterno MV, Ford KR, Myer GD, Heyl R, Hewett TE. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin J Sport Med. 2007. July;17(4):258-62. [DOI] [PubMed] [Google Scholar]
- 6.Bryant AL, Kelly J, Hohmann E. Neuromuscular adaptations and correlates of knee functionality following ACL reconstruction. J Orthop Res. 2008. January;26(1):126-35. [DOI] [PubMed] [Google Scholar]
- 7.Webster KE, Feller JA. Alterations in joint kinematics during walking following hamstring and patellar tendon anterior cruciate ligament reconstruction surgery. Clin Biomech (Bristol, Avon). 2011. February;26(2):175-80. Epub 2010 Oct 15. [DOI] [PubMed] [Google Scholar]
- 8.Feller JA, Webster KE. A randomized comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction. Am J Sports Med. 2003. Jul-Aug;31(4):564-73. [DOI] [PubMed] [Google Scholar]
- 9.Jansson KA, Linko E, Sandelin J, Harilainen A. A prospective randomized study of patellar versus hamstring tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2003. Jan-Feb;31(1):12-8. [DOI] [PubMed] [Google Scholar]
- 10.Maletis GB, Cameron SL, Tengan JJ, Burchette RJ. A prospective randomized study of anterior cruciate ligament reconstruction: a comparison of patellar tendon and quadruple-strand semitendinosus/gracilis tendons fixed with bioabsorbable interference screws. Am J Sports Med. 2007. March;35(3):384-94. Epub 2007 Jan 11. [DOI] [PubMed] [Google Scholar]
- 11.Natri A, Järvinen M, Latvala K, Kannus P. Isokinetic muscle performance after anterior cruciate ligament surgery. Long-term results and outcome predicting factors after primary surgery and late-phase reconstruction. Int J Sports Med. 1996. April;17(3):223-8. [DOI] [PubMed] [Google Scholar]
- 12.Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008. July;27(3):405-24, vii-ix. [DOI] [PubMed] [Google Scholar]
- 13.Snyder-Mackler L, Delitto A, Bailey SL, Stralka SW. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Joint Surg Am. 1995. August;77(8):1166-73. [DOI] [PubMed] [Google Scholar]
- 14.Witvrouw E, Bellemans J, Verdonk R, Cambier D, Coorevits P, Almqvist F. Patellar tendon vs. doubled semitendinosus and gracilis tendon for anterior cruciate ligament reconstruction. Int Orthop. 2001;25(5):308-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopresti C, Kirkendall DT, Street GM, Dudley AW. Quadriceps insufficiency following repair of the anterior cruciate ligament*. J Orthop Sports Phys Ther. 1988;9(7):245-9. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res. 2011. May;29(5):633-40. Epub 2011 Jan 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder-Mackler L, De Luca PF, Williams PR, Eastlack ME, Bartolozzi AR 3rd. Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1994. April;76(4):555-60. [DOI] [PubMed] [Google Scholar]
- 18.Chmielewski TL, Wilk KE, Snyder-Mackler L. Changes in weight-bearing following injury or surgical reconstruction of the ACL: relationship to quadriceps strength and function. Gait Posture. 2002. August;16(1):87-95. [DOI] [PubMed] [Google Scholar]
- 19.Lieber RL, Fridén J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000. November;23(11):1647-66. [DOI] [PubMed] [Google Scholar]
- 20.Lieber RL, Ward SR. Skeletal muscle design to meet functional demands. Philos Trans R Soc Lond B Biol Sci. 2011. May 27;366(1570):1466-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward SR, Eng CM, Smallwood LH, Lieber RL. Are current measurements of lower extremity muscle architecture accurate? Clin Orthop Relat Res. 2009. April;467(4):1074-82. Epub 2008 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longo UG, Rizzello G, Frnaceschi F, Campi S, Maffulli N, Denaro V. The architecture of the ipsilateral quadriceps two years after successful anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft. Knee. 2014. June;21(3):721-5. Epub 2014 Feb 12. [DOI] [PubMed] [Google Scholar]
- 23.Moreau NG, Simpson KN, Teefey SA, Damiano DL. Muscle architecture predicts maximum strength and is related to activity levels in cerebral palsy. Phys Ther. 2010. November;90(11):1619-30. Epub 2010 Sep 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleakney R, Maffulli N. Ultrasound changes to intramuscular architecture of the quadriceps following intramedullary nailing. J Sports Med Phys Fitness. 2002. March;42(1):120-5. [PubMed] [Google Scholar]
- 25.Powell PL, Roy RR, Kanim P, Bello MA, Edgerton VR. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol Respir Environ Exerc Physiol. 1984. December;57(6):1715-21. [DOI] [PubMed] [Google Scholar]
- 26.Hart JM, Ko JW, Konold T, Pietrosimone B. Sagittal plane knee joint moments following anterior cruciate ligament injury and reconstruction: a systematic review. Clin Biomech (Bristol, Avon). 2010. May;25(4):277-83. Epub 2010 Jan 25. [DOI] [PubMed] [Google Scholar]
- 27.Bisciotti GN, Combi F, Forloni F, Iodice P, Petrone N. Increased force endurance and change of muscle type following anterior cruciate ligament reconstruction. Eur J Sports Traumatol Relat Res. 2001;23(4):159-63. [Google Scholar]
- 28.Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012. August;139(16):2845-56. [DOI] [PubMed] [Google Scholar]
- 29.Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med. 2015. January;21(1):76-80. Epub 2014 Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright RW, Haas AK, Anderson J, Calabrese G, Cavanaugh J, Hewett TE, Lorring D, McKenzie C, Preston E, Williams G; MOON Group. Anterior cruciate ligament reconstruction rehabilitation: MOON guidelines. Sports Health. 2015. May;7(3):239-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noehren B, Andersen A, Feiweier T, Damon B, Hardy P. Comparison of twice refocused spin echo versus stimulated echo diffusion tensor imaging for tracking muscle fibers. J Magn Reson Imaging. 2015. March;41(3):624-32. Epub 2014 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012. November;30(9):1323-41. Epub 2012 Jul 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol. 2007. November 15;585(Pt 1):241-51. Epub 2007 Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blazevich AJ. Effects of physical training and detraining, immobilisation, growth and aging on human fascicle geometry. Sports Med. 2006;36(12):1003-17. [DOI] [PubMed] [Google Scholar]
- 35.Williams GN, Snyder-Mackler L, Barrance PJ, Buchanan TS. Quadriceps femoris muscle morphology and function after ACL injury: a differential response in copers versus non-copers. J Biomech. 2005. April;38(4):685-93. [DOI] [PubMed] [Google Scholar]
- 36.Macleod TD, Snyder-Mackler L, Buchanan TS. Differences in neuromuscular control and quadriceps morphology between potential copers and noncopers following anterior cruciate ligament injury. J Orthop Sports Phys Ther. 2014. February;44(2):76-84. Epub 2013 Nov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konishi Y, Aihara Y, Sakai M, Ogawa G, Fukubayashi T. Gamma loop dysfunction in the quadriceps femoris of patients who underwent anterior cruciate ligament reconstruction remains bilaterally. Scand J Med Sci Sports. 2007. August;17(4):393-9. Epub 2006 Jun 28. [DOI] [PubMed] [Google Scholar]
- 38.Konishi Y, Fukubayashi T, Takeshita D. Mechanism of quadriceps femoris muscle weakness in patients with anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2002. December;12(6):371-5. [DOI] [PubMed] [Google Scholar]
- 39.Reurink G, Almusa E, Goudswaard GJ, Tol JL, Hamilton B, Moen MH, Weir A, Verhaar JA, Maas M. No association between fibrosis on magnetic resonance imaging at return to play and hamstring reinjury risk. Am J Sports Med. 2015. May;43(5):1228-34. Epub 2015 Mar 6. [DOI] [PubMed] [Google Scholar]
- 40.Silder A, Reeder SB, Thelen DG. The influence of prior hamstring injury on lengthening muscle tissue mechanics. J Biomech. 2010. August 26;43(12):2254-60. Epub 2010 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verney J, Kadi F, Charifi N, Féasson L, Saafi MA, Castells J, Piehl-Aulin K, Denis C. Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle Nerve. 2008. September;38(3):1147-54. [DOI] [PubMed] [Google Scholar]
- 42.Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr). 2014. April;36(2):545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985). 2008. June;104(6):1736-42. Epub 2008 Apr 24. [DOI] [PubMed] [Google Scholar]
- 44.Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, Halkjaer-Kristensen J, Simonsen EB. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001. July 15;534(Pt. 2):613-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blazevich AJ, Gill ND, Bronks R, Newton RU. Training-specific muscle architecture adaptation after 5-wk training in athletes. Med Sci Sports Exerc. 2003. December;35(12):2013-22. [DOI] [PubMed] [Google Scholar]
- 46.Blazevich AJ, Cannavan D, Coleman DR, Horne S. Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J Appl Physiol (1985). 2007. November;103(5):1565-75. Epub 2007 Aug 23. [DOI] [PubMed] [Google Scholar]
- 47.Brockett CL, Morgan DL, Proske U. Human hamstring muscles adapt to eccentric exercise by changing optimum length. Med Sci Sports Exerc. 2001. May;33(5):783-90. [DOI] [PubMed] [Google Scholar]
- 48.Seynnes OR, de Boer M, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol (1985). 2007. January;102(1):368-73. Epub 2006 Oct 19. [DOI] [PubMed] [Google Scholar]
- 49.Andersen JL, Aagaard P. Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle Nerve. 2000. July;23(7):1095-104. [DOI] [PubMed] [Google Scholar]
- 50.Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013. October;45(10):2191-9. Epub 2013 May 21. [DOI] [PubMed] [Google Scholar]
- 51.Andersen JL, Gruschy-Knudsen T, Sandri C, Larsson L, Schiaffino S. Bed rest increases the amount of mismatched fibers in human skeletal muscle. J Appl Physiol (1985). 1999. February;86(2):455-60. [DOI] [PubMed] [Google Scholar]
- 52.Schmitt LC, Paterno MV, Hewett TE. The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2012. September;42(9):750-9. Epub 2012 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lepley LK. Deficits in quadriceps strength and patient-oriented outcomes at return to activity after ACL reconstruction: a review of the current literature. Sports Health. 2015. May;7(3):231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmieri-Smith RM, Lepley LK. Quadriceps strength asymmetry after anterior cruciate ligament reconstruction alters knee joint biomechanics and functional performance at time of return to activity. Am J Sports Med. 2015. July;43(7):1662-9. Epub 2015 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]