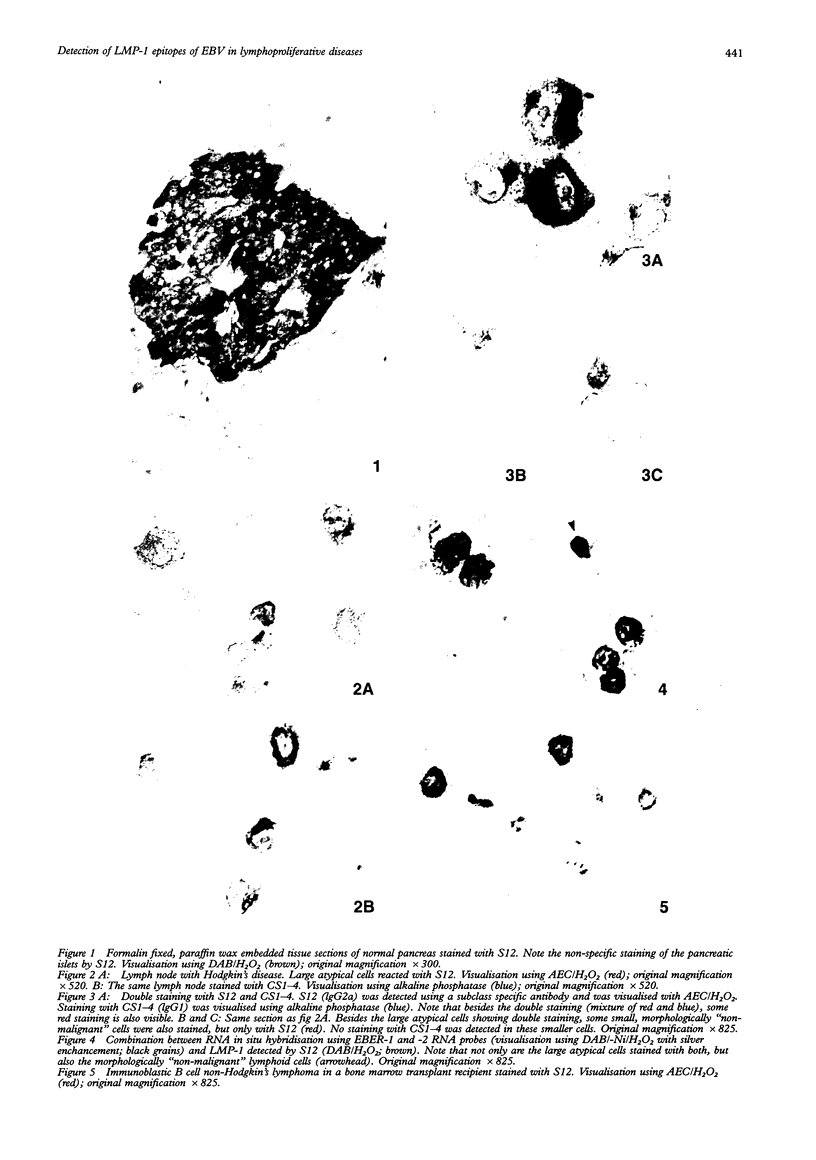
Abstract
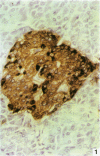
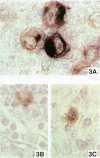
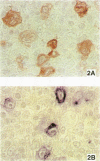
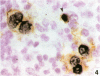
AIM--To compare the immunoreactivity of monoclonal antibodies S12 and CS1-4, which recognise different epitopes of the Epstein-Barr virus (EBV) latent membrane protein-1 (LMP-1), in EBV associated benign and malignant lymphoproliferative disorders and control tissues processed using different methods. RESULTS--Both monoclonal antibodies gave comparable results on frozen tissue sections and formalin fixed, paraffin wax embedded samples from cases with Hodgkin's disease and infectious mononucleosis. In all cases S12 stained more cells than CS1-4. For EBV associated B and T non-Hodgkin's lymphomas, frozen tissue sections yielded better LMP-1 staining results than formalin fixed material. Again, in all these cases S12 stained more cells and gave stronger results than CS1-4. For EBV negative tissues, both monoclonal antibodies showed cross-reactivity with melanocytic-like cells in the basal cell layer of the skin, synaptophysin-like staining in layers three and four of the cortex of the brain, and myelin-like staining in peripheral nerves and peripheral ganglion cells. Staining with S12 was always much stronger. Moreover, in contrast to CS1-4, S12 stained pancreatic islands in formalin fixed material but not in frozen tissue sections and sporadically stained solitary epithelial cells in the large bowel especially in formalin fixed tissue sections. CS1-4 also cross-reacted with myoepithelial cells around hair follicles and other adnexa of the skin. CONCLUSION--The results indicate that for optimal detection of LMP-1, S12 yields better results than CS1-4 and that tissue processing is very important especially when B and T non-Hodgkin's lymphomas are examined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Bruin P. C., Jiwa N. M., Van der Valk P., Van Heerde P., Gordijn R., Ossenkoppele G. J., Walboomers J. M., Meijer C. J. Detection of Epstein-Barr virus nucleic acid sequences and protein in nodal T-cell lymphomas: relation between latent membrane protein-1 positivity and clinical course. Histopathology. 1993 Dec;23(6):509–518. doi: 10.1111/j.1365-2559.1993.tb01236.x. [DOI] [PubMed] [Google Scholar]
- Fellbaum C., Hansmann M. L., Niedermeyer H., Kraus I., Alavaikko M. J., Blanco G., Aine R., Busch R., Pütz B., Fischer R. Influence of Epstein-Barr virus genomes on patient survival in Hodgkin's disease. Am J Clin Pathol. 1992 Sep;98(3):319–323. doi: 10.1093/ajcp/98.3.319. [DOI] [PubMed] [Google Scholar]
- Fåhraeus R., Rymo L., Rhim J. S., Klein G. Morphological transformation of human keratinocytes expressing the LMP gene of Epstein-Barr virus. Nature. 1990 May 31;345(6274):447–449. doi: 10.1038/345447a0. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G. Epstein-Barr virus and infectious mononucleosis. N Engl J Med. 1973 Feb 1;288(5):263–264. doi: 10.1056/NEJM197302012880512. [DOI] [PubMed] [Google Scholar]
- Hummel M., Anagnostopoulos I., Dallenbach F., Korbjuhn P., Dimmler C., Stein H. EBV infection patterns in Hodgkin's disease and normal lymphoid tissue: expression and cellular localization of EBV gene products. Br J Haematol. 1992 Dec;82(4):689–694. doi: 10.1111/j.1365-2141.1992.tb06945.x. [DOI] [PubMed] [Google Scholar]
- Jiwa N. M., Kanavaros P., De Bruin P. C., van der Valk P., Horstman A., Vos W., Mullink H., Walboomers J. M., Meijer C. J. Presence of Epstein-Barr virus harbouring small and intermediate-sized cells in Hodgkin's disease. Is there a relationship with Reed-Sternberg cells? J Pathol. 1993 Jun;170(2):129–136. doi: 10.1002/path.1711700206. [DOI] [PubMed] [Google Scholar]
- Jiwa N. M., Van der Valk P., Mullink H., Vos W., Horstman A., Maurice M. M., Olde-Weghuis D. E., Walboomers J. M., Meijer C. J. Epstein-Barr virus DNA in Reed-Sternberg cells of Hodgkin's disease is frequently associated with CR2 (EBV receptor) expression. Histopathology. 1992 Jul;21(1):51–57. doi: 10.1111/j.1365-2559.1992.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Jones J. F., Shurin S., Abramowsky C., Tubbs R. R., Sciotto C. G., Wahl R., Sands J., Gottman D., Katz B. Z., Sklar J. T-cell lymphomas containing Epstein-Barr viral DNA in patients with chronic Epstein-Barr virus infections. N Engl J Med. 1988 Mar 24;318(12):733–741. doi: 10.1056/NEJM198803243181203. [DOI] [PubMed] [Google Scholar]
- Kanavaros P., Jiwa N. M., de Bruin P. C., van der Valk P., Noorduyn L. A., van Heerde P., Gordijn R., Horstman A., Mullink R., Willemze R. High incidence of EBV genome in CD30-positive non-Hodgkin's lymphomas. J Pathol. 1992 Nov;168(3):307–315. doi: 10.1002/path.1711680311. [DOI] [PubMed] [Google Scholar]
- Liebowitz D., Kopan R., Fuchs E., Sample J., Kieff E. An Epstein-Barr virus transforming protein associates with vimentin in lymphocytes. Mol Cell Biol. 1987 Jul;7(7):2299–2308. doi: 10.1128/mcb.7.7.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. P., Staunton D., Thorley-Lawson D. A. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J Virol. 1985 Sep;55(3):710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy R. K., Thorley-Lawson D. A. Biochemical, genetic, and functional analyses of the phosphorylation sites on the Epstein-Barr virus-encoded oncogenic latent membrane protein LMP-1. J Virol. 1993 May;67(5):2637–2645. doi: 10.1128/jvi.67.5.2637-2645.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedobitek G., Fahraeus R., Herbst H., Latza U., Ferszt A., Klein G., Stein H. The Epstein-Barr virus encoded membrane protein (LMP) induces phenotypic changes in epithelial cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62(1):55–59. doi: 10.1007/BF02899665. [DOI] [PubMed] [Google Scholar]
- Rowe M., Evans H. S., Young L. S., Hennessy K., Kieff E., Rickinson A. B. Monoclonal antibodies to the latent membrane protein of Epstein-Barr virus reveal heterogeneity of the protein and inducible expression in virus-transformed cells. J Gen Virol. 1987 Jun;68(Pt 6):1575–1586. doi: 10.1099/0022-1317-68-6-1575. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Movahed L. A., Warnke R. A., Sklar J. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin's disease. N Engl J Med. 1989 Feb 23;320(8):502–506. doi: 10.1056/NEJM198902233200806. [DOI] [PubMed] [Google Scholar]
- Young L., Alfieri C., Hennessy K., Evans H., O'Hara C., Anderson K. C., Ritz J., Shapiro R. S., Rickinson A., Kieff E. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989 Oct 19;321(16):1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Schulte-Holthausen H., Klein G., Henle W., Henle G., Clifford P., Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970 Dec 12;228(5276):1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]