Abstract
Habituation is a highly conserved phenomenon that remains poorly understood at the molecular level. Invertebrate model systems, like Caenorhabditis elegans, can be a powerful tool for investigating this fundamental process. Here we established a high-throughput learning assay that used real-time computer vision software for behavioral tracking and optogenetics for stimulation of the C. elegans polymodal nociceptor, ASH. Photoactivation of ASH with ChR2 elicited backward locomotion and repetitive stimulation altered aspects of the response in a manner consistent with habituation. Recording photocurrents in ASH, we observed no evidence for light adaptation of ChR2. Furthermore, we ruled out fatigue by demonstrating that sensory input from the touch cells could dishabituate the ASH avoidance circuit. Food and dopamine signaling slowed habituation downstream from ASH excitation via D1-like dopamine receptor, DOP-4. This assay allows for large-scale genetic and drug screens investigating mechanisms of nociception modulation.
Habituation is a nonassociative form of learning characterized by a decremented response to repeated stimulation. It can be observed in organisms across phylogeny and is often considered a “cognitive building-block” (Rankin et al. 2009). Consistent with this role, deficits in habituation are associated with a variety of neuropsychiatric disorders, including autism and schizophrenia (Braff et al. 1992; Kleinhans et al. 2009). Despite its omnipresence and conservation, the cellular mechanisms underlying habituation are poorly understood. Researchers have therefore turned to a diverse array of model organisms for mechanistic insights (Kandel 2004; Bozorgmehr et al. 2013; Roberts et al. 2013; Twick et al. 2014).
C. elegans is a free-living nematode with a 302-cell nervous system of known connectivity (White et al. 1986). Its neurons are optically accessible through a transparent cuticle and genetically accessible with a sophisticated molecular biology toolkit. Despite its reproducible wiring diagram, C. elegans show a remarkable capacity for behavioral plasticity, including habituation (Rankin et al. 1990) and a variety of forms of associative learning (Ardiel and Rankin 2010). The best-characterized habituating behavior of C. elegans is the tap-withdrawal response, wherein the animal reverses following nonlocalized mechanosensory input—a tap to the side of the Petri plate (Rankin et al. 1990; Bozorgmehr et al. 2013). This behavior arises from an integration of two antagonistic reflexes: backward locomotion and forward acceleration (Wicks and Rankin 1995). In this study, we examined habituation of reversal behavior in the absence of competing forward locomotion by stimulating ASH, paired polymodal nociceptors at the worm's nose. Activation of ASH neurons promotes backward locomotion away from the stimulus using many of the same interneurons as the tap-withdrawal response (Wicks and Rankin 1995; Guo et al. 2009; Piggott et al. 2011).
ASH neurons are especially interesting in the context of habituation because of the diversity and salience of the cues they detect: toxic volatile and nonvolatile repellents, as well as osmotic pressure and physical contact (Bargmann et al. 1990; Kaplan and Horvitz 1993; Hilliard et al. 2002, 2005). Although an organism might not be expected to habituate to potentially lethal stimuli, repeated or prolonged exposure to naturally occurring ASH-sensed stimuli leads to a decreased likelihood of responding that is mostly stimulus specific (Hart et al. 1999; Ezcurra et al. 2011; Hilliard et al. 2005; Lindy et al. 2014). Monitoring calcium transients in ASH, Hilliard et al. (2005) showed that the behavioral decrement correlates with a decrease in ASH cellular responsiveness and that both are at least partially dependent on GPC-1, a G-protein γ- subunit. More recently, Lindy et al. (2014) found that reduced responding associated with prolonged exposure to hypertonicity depends on calcium flux through the TRPV-like channel OSM-9. As with tap habituation (Kindt et al. 2007), the decrement to ASH-sensed stimuli is affected by feeding status, with the presence of food promoting responding to repeated or prolonged exposure to the water-soluble repellent CuCl2 (Ezcurra et al. 2011). Exogenous dopamine mimics the presence of food and a dopamine-deficient cat-2 mutant lacking tyrosine hydroxylase behaves on food as if tested in its absence, suggesting dopamine mediates the food effect; however, the key dopamine receptor has not been identified.
Decreased cellular responsivity to specific stimuli (Hart et al. 1999; Hilliard et al. 2005) suggests that ASH may be adapting at the level of sensory transduction (e.g., by receptor internalization); however, habituation may also be occurring in parallel downstream. The molecular underpinnings of habituation are poorly understood, but a key distinction from adaptation is that habituation is readily reversible, whereas recovery from adaptation requires time away from the stimulus. To better understand the response decrement associated with ASH, we established a high-throughput training assay that used real-time computer vision software for detailed behavioral tracking and optogenetic activation of ASH for tight temporal and intensity regulation. In addition to facilitating stimulus delivery, photoactivation prevented sensory adaptation by bypassing sensory transduction, thus allowing us to probe downstream loci of plasticity. By dissecting the response into multiple metrics we identified behavioral plasticity consistent with habituation. Through mutant analysis we found that GPC-1 and OSM-9 do not play a role in habituation and that dopamine signaling modulates it via D1-like dopamine receptor, DOP-4.
Materials and Methods
Strains
Animals were maintained on nematode growth medium (NGM) seeded with Escherichia coli (OP50) as described in Brenner (1974). Two integrated Channelrhodopsin-2 (ChR2) transgenes were used for ASH photoactivation. Initial experiments were conducted using a strain with ChR2 under control of the sra-6 promoter (AQ2026 ljIs105[sra-6p::ChR2::YFP + unc-122p::GFP]), which expresses strongly in ASH and more weakly in a pair of sensory neurons (ASI) and interneurons (PVQ) (Troemel et al. 1995). To rule out a contribution of the off-target cells, we took advantage of a second strain that used intersecting promoters and FLP recombinase to specifically target ChR2 to ASH (AQ2235 lite-1(ce314) ljIs114[gpa-13p::FLPase + sra-6p::FTF::ChR2::YFP]; Ezcurra et al. 2011; Schmitt et al. 2012). AQ2026 showed robust responding at irradiances below the threshold for the C. elegans innate response to blue light (we used 70 µW/mm2); however, reduced ChR2 expression in AQ2235 necessitated increased irradiance (we used 250 µW/mm2) and therefore experiments with this strain were performed in a background lacking LITE-1, a native C. elegans short-wavelength light receptor (Edwards et al. 2008). The transgenic background used is specified in the figure captions. The ljIs105 and ljIs114 ChR2 transgenes were crossed with available mutants to generate the following:
VG53 glr-1(ky176); ljIs105
VG54 mec-4(u253); ljIs105
VG152 eat-4(ky5); ljIs105
VG154 nmr-1(ak4); ljIs105
VG155 glr-1(ky176); nmr-1(ak4); ljIs105
VG177 cat-2(e1112); ljIs105
VG224 cat-2(e1112); lite-1(ce314) ljIs114
VG236 dop-3(ok295); ljIs105
VG237 dop-1(vs101); ljIs105
VG238 dop-1(ok398); ljIs105
VG240 dop-2(vs105); lite-1(ce314) ljIs114
VG241 dop-5(ok568); lite-1(ce314) ljIs114
VG248 dop-4(ok1321); ljIs105
VG249 dop-6(ok2090); ljIs105
VG284 bas-1(ad446); cat-4(e1141); lite-1(ce314) ljIs114
VG388 trp-4(sy695); lite-1(ce314) ljIs114
VG389 trp-4(sy695); lite-1(ce314) ljIs114; xuEx584[dat-1p::trp-4-sl2-YFP + unc-122p::GFP]
VG515 osm-9(ky10); ljIs105
VG524 gpc-1(pk298); ljIs105
The dop-4 rescue plasmids (a gift from William Schafer, MRC Laboratory of Molecular Biology) (Ezcurra et al. 2011) were injected into the gonad of VG248 at 35 ng/µL with pCFJ90 (myo-2p::mCherry; 3ng/µL) (Frøkjaer-Jensen et al. 2008) for use as a visible marker and pBluescript to make the total DNA concentration 100 ng/µL (Mello et al. 1991). The following strains were generated by microinjection:
VG394 dop-4(ok1321); ljIs105; yvEx[dop-4p::dop-4 + myo-2p::mCherry]
VG384, VG385 dop-4(ok1321); ljIs105; yvEx[gpa-13p::dop-4 + myo-2p::mCherry]
VG566, VG567, VG568 dop-4(ok1321); ljIs105; yvEx[sra-6p::dop-4 + myo-2p::mCherry]
Behavioral tracking
NGM plates were spread with 50–100 µL OP50 liquid culture mixed with all-trans-retinal (ATR; or equal volume of ethanol vehicle) for a final plate concentration of 5 µM ATR. Plates were stored at room temperature in the dark for 24–48 h before use. For age-synchronized colonies, gravid adults were left 3–6 h to lay ∼20–80 eggs before being removed from the plate. Animals were reared at 20°C and tested as 3 or 4 d olds. Behavioral tracking occurred directly on the rearing plates, except for experiments evaluating the effect of food or exogenous dopamine or those utilizing strains with extra-chromosomal arrays. For food ± conditions, animals were transferred 10–15 min before testing to plates streaked at most 2 h earlier with OP50 liquid culture (food+) or just the LB (food−). For dopamine treatment, animals were transferred to freshly made 10 mM dopamine (Sigma-Aldrich) plates 10–15 min before testing. Animals with extra-chromosomal arrays were picked based on expression of a fluorescent coinjection marker to ATR-containing food plates 24 h before testing, with control strains similarly handled.
Multi-Worm Tracker software (version 1.2.0.2) was used for stimulus delivery and image acquisition (Swierczek et al. 2011). Following a 3–5 min acclimatization phase, stimuli were presented using custom-built LED rings (Luxeon Star LEDs) capable of illuminating 60 or 35 mm (diameter) Petri plates with uniform blue light (max = 70 or 250 µW/mm2, respectively). An orange filter prevented the blue light from entering the camera. For dishabituation, taps were delivered by an electromagnetic tubular push solenoid (#195205-127, Ledex, Vandalia, OH, USA). Offline behavioral quantification with Choreography software (version 1.3.0_r1035; Swierczek et al. 2011) used “—shadowless,” “--minimum-move-body 2,” and “–minimum-time 20” filters to restrict the analysis to animals that moved at least two body lengths and were tracked for at least 20 sec. The MeasureReversal plugin was used to identify reversals occurring within 3 sec (dt = 3) of the light pulse onset. Custom MatLab scripts organized and summarized Choreography output files.
Naturally sensed stimuli
The nose touch assay was conducted on rearing plates with the experimenter blind to strain (glr-1+ or −) or treatment (ATR+ or −). Nose touch sensitivity was assayed within 30 sec of the last light pulse. Positive responses were defined as reversals elicited by contact with an eyebrow hair placed perpendicular to the direction of forward motion (Kaplan and Horvitz 1993).
For octanol exposure, four 3 µL drops of 30% 1-octanol or the ethanol vehicle were placed on the Petri plate lid of rearing plates. The plates were sealed with parafilm and the Multi-Worm Tracker was used to monitor responses elicited by ChR2 activation.
Electrophysiology
Whole-cell recordings followed procedures described in Lindsay et al. (2011). Briefly, worms were immobilized with cyanoacralate glue on an agarose pad and immersed in recording saline consisting of (in mM): 5 KCl, 10 HEPES, 8 CaCl2, 143 NaCl, 30 glucose. ASH was exposed by cutting a small hole in the cuticle using a sharpened glass needle and patch clamped using an electrode filled with (in mM): 125 K-gluconate, 1 CaCl2, 18 KCl, 1 MgCl2, 10 HEPES. Neurons were voltage clamped at −75 mV and light pulses were delivered using a computer controlled LED illumination system (Rapp optoelectronics). Holding current was filtered at 2 kHz and acquired at 10 kHz. Charge transfer associated with each stimulus was calculated by integrating the holding current evoked during the stimulus epoch.
Statistics
One-way ANOVAs and Tukey's honestly significant difference criterion were used to compare responses between strains and treatments. For response probability, tests compared the mean from the proportion of worms responding on each plate (n = number of plates tested). For duration and latency metrics, data were combined across plates and collective means were compared (n = number of animals tested). For all statistical tests, α was 0.01.
Results
Population assay for repeated ASH activation
We adapted the Multi-Worm Tracker (Swierczek et al. 2011) for optogenetic experiments, allowing for simultaneous stimulation of ASH neurons with precise temporal and intensity regulation in an entire population of animals. Using a custom built LED light ring, reversal responses to blue light pulses were detected in the majority of animals with ChR2 driven by the sra-6 promoter (expresses in ASH, ASI, and PVQ; see below for data from a strain expressing ChR2 in ASH alone) and fed the essential opsin cofactor, ATR (Fig. 1A). Although the probability of an animal responding was relatively stable over a wide range of irradiance intensities and durations, the magnitude of the response was dictated by the illumination parameters, with response duration increasing with illumination irradiance and duration and response latency decreasing with increased illumination irradiance (Fig. 1B,C). This finding was similar to earlier reports using single-worm assays and is consistent with graded synaptic output of ASH (Lindsay et al. 2011; Husson et al. 2012).
Figure 1.
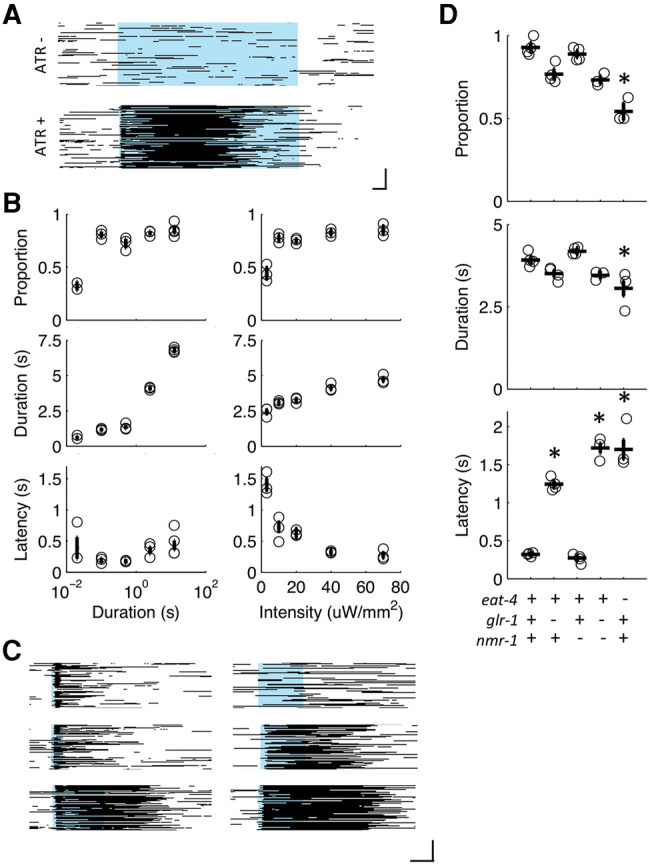
Activation of ChR2 in sra-6 expressing neurons elicits backward locomotion. (A) Raster plots of behavioral state in the presence (ATR+) or absence (ATR−) of all-trans retinal. Each line represents an animal, with black pixels depicting backward locomotion and remaining pixels (white) depicting forward locomotion or no movement. The blue bar represents whole-plate illumination with blue light at 0.07 mW/mm2. Scale bar corresponds to 1 sec (horizontal) and 30 animals (vertical). (B) Three response metrics (proportion, duration, and latency) for a 0.07 mW/mm2 light pulse of several durations (left) and a 2-sec light pulse at several intensities (right). Circles are plate means, crosses are population means ± SEM (n = 2–4 plates). (C) Representative raster plots of behavior in response to a 0.07 mW/mm light pulse of 0.5, 1.5, and 2.5 sec (left) or a 2-sec light pulse at 0.004, 0.02, and 0.07 mW/mm2 (right). (D) Proportion, duration and latency response metrics for glutamate transmission mutants stimulated with a 2-sec light pulse at 0.07 mW/mm2. Circles are plate means, crosses are population means ± SEM (n = 4 plates), and asterisks denote statistically distinguishable groups. All data collected using the ljIs105 ChR2 transgene.
Avoidance responses to the various aversive stimuli detected by ASH can be genetically dissociated both at the levels of sensory transduction and signal propagation. Although photoactivation with ChR2 bypasses native sensory transduction machinery, deficits associated with synaptic transmission should generalize to stimuli simulated by photocurrents. For example, mutants lacking the AMPA-type glutamate receptor subunit GLR-1 are unresponsive to nose touch and delayed in responding to osmotic shock (Hart et al. 1995; Maricq et al. 1995; Mellem et al. 2002). To assess which types of stimuli the photoactivation parameters used in these studies mimicked the ChR2 transgene was crossed into several glutamate transmission mutants. Loss of the vesicular glutamate transporter, EAT-4, decreased the proportion of animals reversing, as well as the duration and latency of responses elicited by a 2-sec light pulse at 70 µW/mm2 (Fig. 1D). As with EAT-4, loss of GLR-1 delayed the initiation of the reversal; however, the phenotype was less severe and neither the probability nor duration of the response was distinguishable from control (Fig. 1D), suggesting that additional glutamate receptor subunits play a role in the photoactivated response. The NMDA glutamate receptor subunit was dispensable for normal responding; however simultaneous loss of NMR-1 and GLR-1 recapitulated the extent of the eat-4 mutant's latency phenotype (Fig. 1D). This pattern of deficits was similar to those observed for osmotic shock (Mellem et al. 2002), suggesting that the 2-sec photostimulation was simulating an intense aversive stimulus and not nose touch.
To examine the plasticity of the response, the 2-sec blue light pulse was administered every 10 sec for 5 min (i.e., 30 stimuli at 0.1 Hz). Repeated stimulation had a variety of behavioral consequences that were quantified with several metrics: proportion of the population reversing, response latency (time to initiate reversal), and reversal duration (Fig. 2A). With repeated stimulation the majority of animals reversed to each stimulus; however, the duration of the reversal responses decreased, and the latency increased. The ∼1-sec increase in response latency by the end of 30 stimuli could not fully account for the ∼2.5-sec decrease in response duration, suggesting that repetitive stimulation results in both delayed initiation and earlier termination of responses.
Figure 2.

Plasticity of reversal responses elicited by repeated photoactivation of sra-6 expressing cells (ASH, ASI, and PVQ; A) or just ASH (B). Three different response metrics: proportion responding with a reversal, duration of any reversals that occurred, and latency to initiate those reversal for thirty 2-sec light pulses administered at 0.1 Hz. Gray lines correspond to the individual plates (n = 6 plates) comprising the mean (black line).
Behavioral plasticity is specific to ASH photoactivation
The experiments described above were conducted with a strain using the sra-6 promoter to drive ChR2 expression in ASH, ASI, and PVQ (Troemel et al. 1995). Neither ASI nor PVQ are thought to elicit rapid reversal responses, but chemosensory ASI neurons have been implicated in the inhibition of reversals (Gray et al. 2005; Guo et al. 2015). To rule out a potential contribution from activating these off-target cells, we tested another strain constructed with intersecting promoters and FLP recombinase to specifically target ChR2 to only ASH (Ezcurra et al. 2011). Repeated photoactivation of ASH alone with thirty 2-sec light pulses resulted in a similar pattern of plasticity to that described above (Fig. 2A), i.e., the majority of animals responded to the final stimulus, but with a decreased reversal duration and increased reversal latency compared with the first stimulus (Fig. 2B).
Photocurrents do not change with repeated stimulation
Photocurrents in ASH were measured during stimulation to determine whether the behavioral decrement associated with repeated illumination (Fig. 2) was caused by light adaptation of ChR2. As expected, a 2-sec blue light pulse evoked inward currents in ASH neurons expressing functional ChR2. Importantly, the waveform of these currents did not change noticeably over 30 stimuli administered at 0.1 Hz (Fig. 3A) and there was no decrease in the magnitude of negative charge transfer (Fig. 3B). Although the precise cellular conditions in ASH differed between intact animals and those prepared for electrophysiological recordings, these data suggest that the behavioral decrement in response to repeated photoactivation was not caused by light adaptation of ChR2, but by downstream cellular or synaptic mechanisms.
Figure 3.
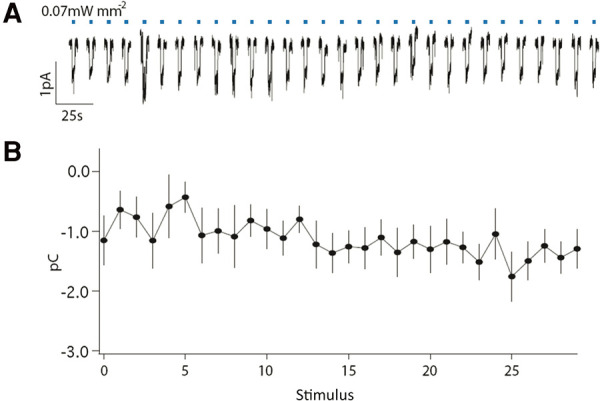
Photocurrents are unaffected by training. (A) Membrane current in ASH for thirty 2-sec light pulses administered at 0.1 Hz. (B) For each stimulus, photocurrents were quantified by calculating the total charge transfer during 2 sec of illumination. Mean ± SEM (n = 11 animals). All data collected using the ljIs105 ChR2 transgene.
Generalization of simulated and naturally sensed stimuli
If the observed decrements in responding (Fig. 2) were caused by processes downstream from ChR2-mediated photocurrents, then the behavioral consequence of repeated photoactivation would be expected to affect responding to naturally occurring cues. We tested this hypothesis by delivering twenty 2-sec light pulses at 0.1 Hz prior to assessing nose touch sensitivity. Stimulated ATR-fed ChR2 transgenic animals were indeed less likely to reverse after a head-on collision with an eyelash than unstimulated controls, or stimulated animals reared in the absence of ATR (Fig. 4A). The experimental group's level of responding to nose touch was indistinguishable from the nose touch-defective glr-1 mutant. This is likely a function of the increased response latency associated with repeated photoactivation, as control animals immediately initiate backward locomotion upon contact with the eyelash.
Figure 4.

Generalization of natural and simulated stimuli. (A) Nose touch responding was affected by simulated stimuli (stim = 2-sec light pulse × 20 at 0.1 Hz), as the probability of crawling backward after a head-on collision with an eyelash was significantly reduced by light pulses if animals were reared on the essential opsin cofactor, ATR. # and & denote statistically distinguishable groups (n = 41, 56, 46, 52 animals per group). (B) For control, but not for an osm-9 mutant, preexposure to octanol (oct) decremented the duration and increased the latency of reversals elicited by ChR2 photocurrents, when compared with preexposure to the ethanol vehicle (veh). Circles are plate means, crosses are population means ± SEM (n = 4 plates), asterisks denote statistically distinguishable groups and (n.s.) no significant difference. All data collected using the ljIs105 ChR2 transgene.
Prior exposure to a stimulus naturally sensed by ASH should have a similar behavioral consequence as repeated ASH photoactivation. Indeed, we found that a 3-min exposure to the volatile repellent 1-octanol decreased the duration and increased the latency of reversal responses elicited by ChR2 photocurrents (Fig. 4B). The decrease in response duration and increase in response latency were dependent on ASH activation, as the 3-min preexposure had no effect on a mutant lacking OSM-9, the TRPV-like channel required for initiating cell excitation to most, if not all, ASH-sensed stimuli (Fig. 4B; Colbert et al. 1997; Tobin et al. 2002; Hilliard et al. 2005). These experiments demonstrate that prior exposure to simulated stimuli affect responses to naturally sensed cues and vice versa. This bidirectional generalization confirms that the behavioral decrement observed in Figure 2 was not simply light adaptation of ChR2 and supports the validity of using optogenetics to understand the nature of response decrement to repeated stimulation. Although ASH senses a variety of cues, none are easily administered discretely to a large number of animals simultaneously. Optogenetics allows for consistent repetitive stimulation, which is important for habituation assays where the intensity and timing of stimuli determine the extent of the response decrement (Rankin et al. 2009). In addition, photoactivation bypasses sensory transduction and allows for the investigation of downstream loci of plasticity independent of adaptation at the level of sensory transduction.
Dishabituation
In contrast to sensory adaptation or motor fatigue, habituation in many animals is an attentional process that can be readily reversed by presenting a novel or noxious dishabituating stimulus (Thompson and Spencer 1966). We tested whether a tap to the side of the Petri plate could dishabituate responses to repeated photoactivation of ASH. Tap was chosen because it could be discretely applied to the entire population simultaneously. Furthermore, the interneurons and motor neurons mediating the tap-withdrawal response are mostly overlapping with those required for ASH-mediated reversals (Wicks and Rankin 1995; Guo et al. 2009; Piggott et al. 2011). To test whether the response decrement associated with ASH photostimulation could be dishabituated, two groups of animals were given 2-sec light pulses at 0.1 Hz for 5 min, with the second to last stimulus (i.e., the 29th stimulus) either omitted or replaced by a tap. The results showed that tapped animals responded to the final light pulse with longer reversals (Fig. 5A) and shorter latencies than untapped controls (Fig. 5B). The untapped controls showed no evidence for spontaneous recovery over the 20 sec (Fig. 5A,B). Thus the tap dishabituated the reversal response to the light pulse. Since the behavioral decrement associated with repeated ASH photoactivation could be at least partially reversed by novel sensory input (i.e., a tap), sensory adaptation or motor fatigue could not fully account for the plasticity.
Figure 5.
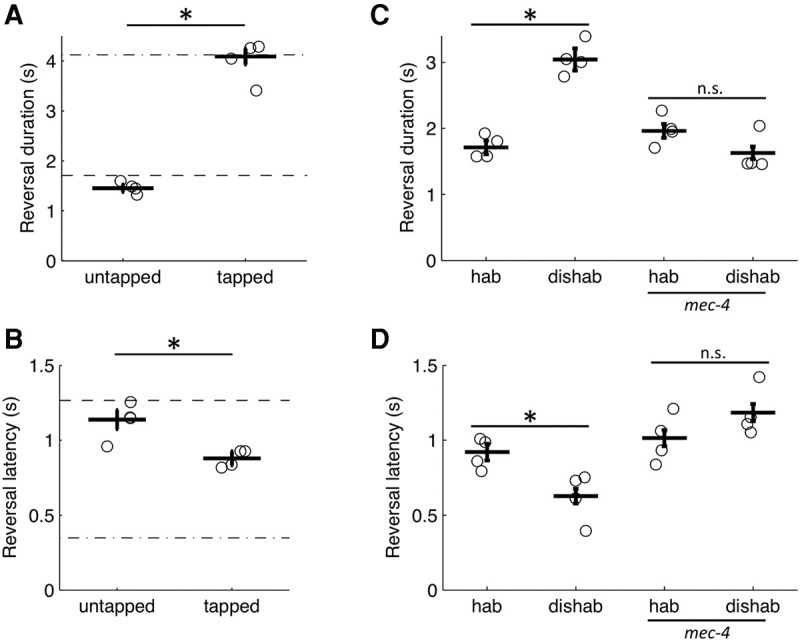
Sensory input from body touch receptors acts as a dishabituating cue. (A) Tap after training reversed the change in response (A) duration and (B) latency associated with repeated ASH activation. The dash-dot line is the initial response level and the dashed line is the habituated response level. (C,D) Habituated responding of a touch insensitive mec-4 mutant was not reversed by tap. Duration (C) and latency (D) of the reversal response elicited by the final 2-sec light pulse of training (hab) and after a dishabituating tap (dishab). Circles are plate means, crosses are population means ± SEM (n = 4 plates), asterisks denote statistically distinguishable groups, and (n.s.) no significant difference. All data collected using the ljIs105 ChR2 transgene.
The tap withdrawal response is mediated by five body touch receptor neurons located along the length of the animal (Wicks and Rankin 1995). Although ASH is not involved in the tap withdrawal response (Wicks and Rankin 1995), it is a mechanosensor and could potentially detect agar vibrations in response to tap. To test whether the body touch receptor neurons mediate dishabituation, a mec-4 loss-of-function allele was used to specifically render them insensitive to mechanical stimuli (O'Hagan et al. 2005). Although worms carrying the mec-4 mutation responded and habituated to repeated ASH photoactivations, the habituated responses were not dishabituated by tap (Fig. 5C,D), indicating that activation of the touch receptor neurons mediates dishabituation in this assay.
Recapitulating the cat-2 mutant phenotype
In previous research, the G-protein γ-subunit GPC-1, the TRPV-like channel OSM-9, and dopamine signaling have all been implicated in the behavioral decrement associated with repeated or prolonged exposure to stimuli naturally detected by ASH (Hilliard et al. 2005; Ezcurra et al. 2011; Lindy et al. 2014). We investigated whether mutations in genes encoding GPC-1, OSM-9, or CAT-2 (tyrosine hydroxylase, the rate limiting enzyme in dopamine biosynthesis) would similarly alter the decrement in response to repeated ASH photoactivation. We found no evidence for involvement of OSM-9 or GPC-1, as the corresponding loss of function mutants were indistinguishable from control in terms of the probability (Fig. 6A), duration (Fig. 6B), and latency (Fig. 6C) of the first and final responses of training. The lack of a habituation phenotype in the optogenetic assay may be explained by stimulus-specific roles for GPC-1 and OSM-9 in ASH; however, we hypothesize that both of these molecules are functioning upstream of cell excitation to mediate adaptation at the level of sensory transduction and are therefore not relevant when ASH is directly depolarized with ChR2 photocurrents. In contrast, the dopamine deficient cat-2 mutant showed habituation deficits. Of the three response metrics, loss of cat-2 had the clearest effect on the proportion of worms responding, fewer mutants than control animals reversed after just a few trials and this continued to decline with repeated stimulation (Fig. 6A). This rapid decrement in proportion of animals reversing recapitulates the cat-2 mutant deficit associated with repeated or prolonged exposure to a naturally sensed stimulus, CuCl2 (Ezcurra et al. 2011). For the cat-2 mutant it is difficult to interpret the duration and latency metrics because there were so few reversals at the end of the 30 stimuli; however restricting our analysis to the first few responses, we observed more rapid increase in reversal latency for the cat-2 mutant than for control (Fig. 6C), but no obvious difference for reversal duration after the initial stimulus (Fig. 6B). Because of the paucity of responses from the cat-2 mutant toward the end of the assay, the magnitude metrics (i.e., duration and latency) were not used in subsequent analyses.
Figure 6.
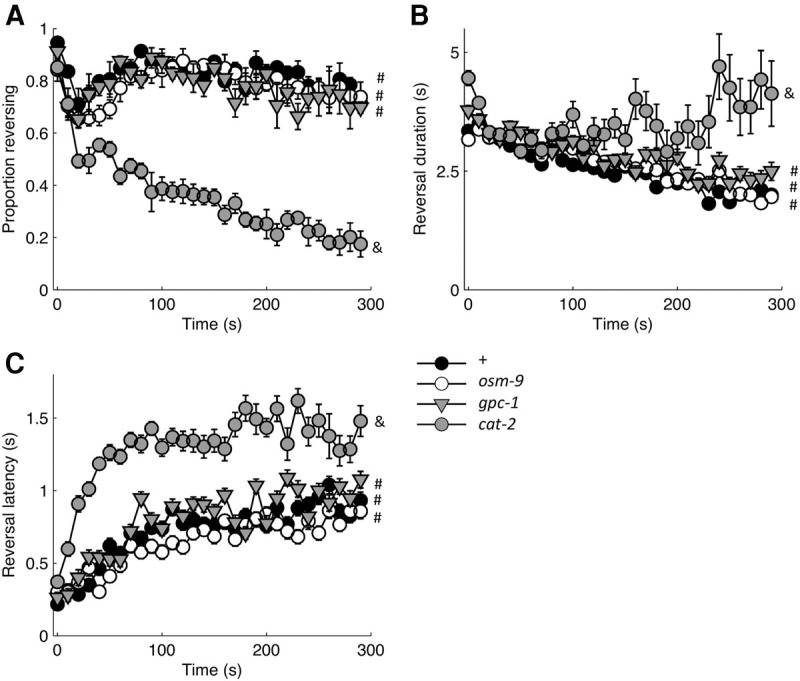
Analysis of mutations in genes previously implicated in repeated responding to naturally sensed ASH stimuli. Response probability (A), duration (B), and latency (C) for reversals elicited by thirty 2-sec light pulses administered at 0.1 Hz (n = 4 plates). # and & denote statistically distinguishable groups based on the response to the final stimulus. All data collected using the ljIs105 ChR2 transgene.
Food texture slows habituation via dopamine
Because of its specificity to ASH, the ljIs114 ChR2 transgene was preferentially used for subsequent mutant analysis, except when the gene of interest was located on chromosome X, where the ljIs114 ChR2 transgene was integrated. As with the ljIs105 ChR2 transgene, we found that disrupting dopamine biosynthesis in the ljIs114 background resulted in a decreased likelihood of responding at the end of the habituation assay (Fig. 7A), thereby ruling out a necessary contribution from ASI and PVQ. This was true for both the monoamine deficient cat-4;bas-1 double mutant, as well as the dopamine deficient cat-2 mutant. C. elegans has eight dopaminergic neurons in three classes (CEP, ADE, and PDE). These cells are thought to directly detect the texture of the bacterial lawn (Sawin et al. 2000). To evaluate the role of food, we transferred animals to unseeded or freshly seeded plates immediately before testing. Recapitulating the observations of Ezcurra et al. (2011) for a naturally sensed cue (CuCl2), we found that populations tested in the absence of food habituated faster in response to repeated ASH activation than populations tested on a bacterial lawn (Fig. 7B).
Figure 7.
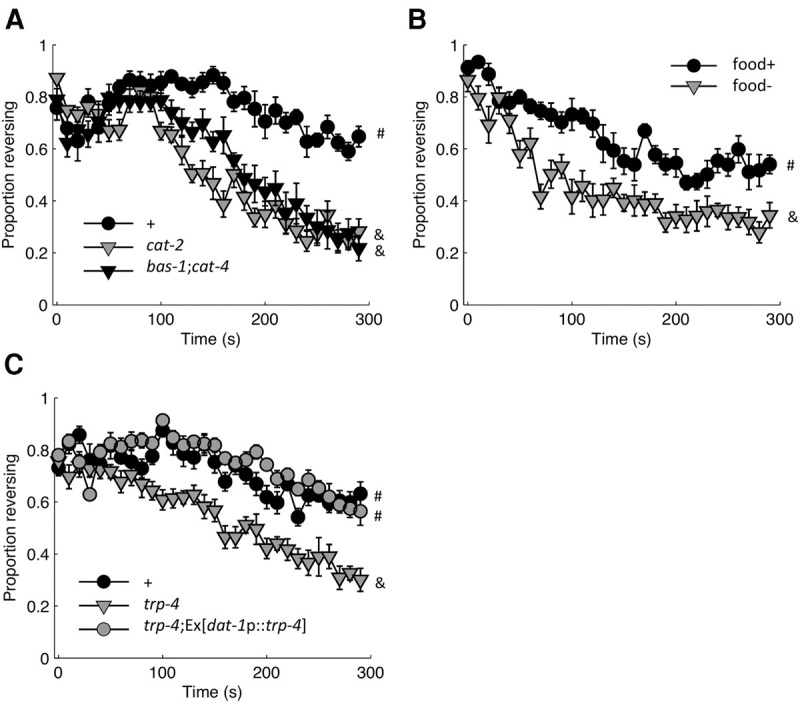
Dopamine signaling promotes responding during habituation training. (A) Loss of monoamine biosynthetic enzymes (CAT-2, BAS-1, CAT-4) altered the probability of responding relative to control (+; n = 6 plates). (B) Comparing populations off of food (food−) with those tested on a bacterial lawn (food+; n = 6 plates). Although all other experiments were conducted in the presence of a bacterial lawn, the food+ condition for Figure 7B refers to a very thin bacterial lawn that was spread with liquid culture at most 2 h before testing (compared with the usual 24 h or more before testing), thus the more rapid decline in responding than was observed for other trials with food (n = 6 plates). (C) Loss of trp-4 recapitulated the dopamine deficient cat-2 mutant phenotype. Loss of trp-4 could be compensated for by restoring trp-4 expression to dopaminergic neurons (n = 8 plates). Mean ± SEM. # and & denote statistically distinguishable groups based on the likelihood of responding to the final stimulus. All data collected using the ljIs114 ChR2 transgene.
Detection of the texture of the bacterial lawn requires the mechanosensitive TRPN channel, TRP-4 in the dopamine neurons (Li et al. 2006; Kindt et al. 2007). To confirm the link between food and dopamine signaling, we evaluated the loss-of-function phenotype for trp-4. Consistent with an inability to detect the bacterial lawn, the trp-4 mutant expressing ChR2 in ASH displayed a more rapid decrement in responding than control (Fig. 7C). In addition to the dopaminergic neurons, TRP-4 is expressed in at least two classes of interneurons, including DVA, where it functions as a stretch receptor for proprioceptive feedback (Li et al. 2006). To localize the TRP-4 site of action we rescued it in dopaminergic neurons by expressing TRP-4 cDNA with a dat-1 promoter. The behavior of the trp-4 rescue strain was indistinguishable from control, with rescued animals being more responsive at the end of the assay than the trp-4 mutant (Fig. 7C). This indicates TRP-4 function in dopaminergic neurons is sufficient for food dependent suppression of habituation. Collectively, these results suggest a model where the texture of the bacterial food source stimulates dopamine release, which promotes persistent responses to aversive stimuli (i.e., slows habituation of response probability) by modulating the avoidance circuit downstream from ASH excitation.
DOP-4 slows habituation
To identify the receptor by which food and dopamine slowed habituation, we evaluated loss-of-function phenotypes for confirmed (DOP-1, DOP-2, DOP-3, and DOP-4) and potential (DOP-5 and DOP-6) dopamine GPCRs. The initial response probability of all mutant strains was indistinguishable from control animals (Fig. 8A). However, when evaluated at the final stimulus presentation, loss of DOP-4—an invertebrate-specific D1-like receptor (Sugiura et al. 2005) recapitulated the rapid habituation phenotype of the dopamine-deficient cat-2 mutant (Fig. 8B). To confirm the dop-4 mutation as the causative allele, we reintroduced dop-4 genomic DNA with its promoter. This fully rescued the deficit of the dop-4 mutant (Fig. 8C). Ezcurra et al. (2011) demonstrated that DOP-4 signaling increases ASH excitability in the presence of food. However, our data suggested that for repetitive stimulation, dopamine signaling modulated responses downstream from ASH excitation. In contrast to the naïve response described by Ezcurra et al. (2011), we found that DOP-4 expression in ASH did not rescue the habituation deficit of the dop-4 mutant—as determined by five independent lines each expressing one of two rescue constructs used by Ezcurra et al. (2011) (gpa-13p::dop-4 or sra-6p::dop-4).
Figure 8.
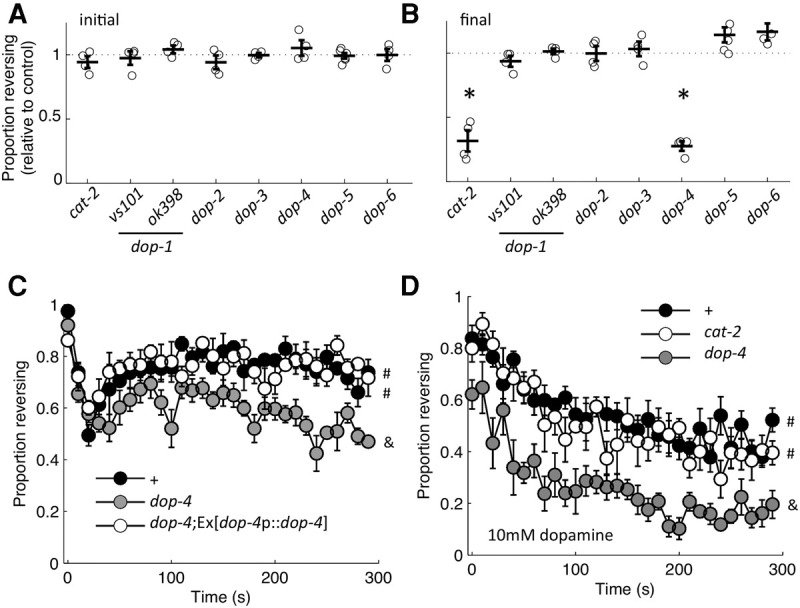
Loss of dop-4 recapitulates the phenotype of a dopamine deficient cat-2 mutant. Proportion of the population reversing relative to control for the initial (A) and 30th (B) 2-sec light pulse delivered at 0.1 Hz. Circles are plate means, crosses are population means± SEM (n = 4 plates), and the asterisks denote statistically distinguishable groups. (C) Proportion of animals responding to each stimulus of habituation training, with reintroduction of dop-4 (dop-4p::dop-4) rescuing the rapid response decrement of the dop-4 mutant (n = 4 plates). Mean ± SEM. # and & denote statistically distinguishable groups based on the likelihood of responding to the final stimulus. (D) Proportion of animals responding on 10 mM exogenous dopamine (n = 6 plates). Mean± SEM. # and & denote statistically distinguishable groups based on the likelihood of responding to the final stimulus. All data collected using the ljIs105 ChR2 transgene, except for dop-2 and dop-5 mutants, which had the ljIs114 ChR2 transgene.
To confirm DOP-4 as the receptor by which dopamine signaling promotes responding to repetitive ASH activation, we exposed animals to exogenous dopamine during habituation training. This treatment compensated for loss of tyrosine hydroxylase, but not loss of DOP-4, as the cat-2 mutant was indistinguishable from control, while the dop-4 mutant was less responsive than both strains (Fig. 8D). Thus, the D1-like receptor DOP-4 is essential for dopamine to exert its influence on habituation of the ASH avoidance circuit.
Discussion
Population assay for habituation of ASH-mediated reversals
We have established a new C. elegans learning assay, combining optogenetics with real-time computer vision software (the Multi-Worm Tracker) (Swierczek et al. 2011) to enable both high-throughput and highly detailed behavioral analysis of responses elicited by the polymodal nociceptor, ASH. Although population assays can be used to obtain avoidance indices for naturally sensed stimuli, probing the dynamics of the reversal response itself requires discrete stimulus presentation. This has traditionally been accomplished with single worm assays (Kaplan and Horvitz 1993; Hilliard et al. 2002; Chao et al. 2004), although recently developed microfluidic devices could be used for stimulating multiple animals with water-soluble repellents (Larsch et al. 2013). In addition to increasing throughput, optogenetics allowed us to specifically target ASH and bypass sensory transduction to investigate downstream loci of plasticity.
Repeated photoactivation of ASH induced a decrement in responding consistent with the characteristics of habituation, as electrophysiological and behavioral data ruled out sensory adaptation or motor fatigue. Most notably, mechanosensory input from the touch receptor neurons could reverse the habituated response (Fig. 5). The behavioral plasticity was observed using two different ChR2 transgenes and various stimulation protocols. Photoactivation with ChR2 bypassed sensory transduction, suggesting that at least one locus of plasticity lies downstream from cell excitation. Earlier work using naturally sensed cues implicated the G-protein γ- subunit GPC-1, the TRPV-like channel OSM-9, and dopamine signaling in behavioral decrement associated with repeated or prolonged ASH activation (Hilliard et al. 2005; Ezcurra et al. 2011; Lindy et al. 2014). In our optogenetic-based assay, dopamine deficient animals showed altered habituation; however loss of GPC-1 or OSM-9 had no effect on the ASH-mediated response or plasticity (Fig. 6). We propose that sensory adaptation at the level of stimulus detection is mediated by GPC-1 and OSM-9 and occurs in parallel with more downstream mechanisms of habituation. Consistent with this model, previous assays (Hilliard et al. 2005; Ezcurra et al. 2011) found more rapid decrements in the probability of responding than those observed here.
Independence of response measures
A number of results demonstrate that different aspects of a response (e.g., probability, duration, and latency) can be independently controlled. For example, loss of the vesicular glutamate transporter EAT-4 decreased the likelihood of responding to ASH activation and delayed and shortened the responses that did occur, but loss of GLR-1 affected only response latency, a deficit exacerbated by simultaneous loss of NMR-1. These data show that multiple glutamate receptors shape the response elicited by ASH activation. Furthermore, both the response duration and latency habituated, but the ∼1 sec increase in response latency by the end of 30 stimuli could not fully account for the ∼2.5 sec decrease in response duration, suggesting they are independent metrics. Finally, mutations in genes altering dopamine transmission had the largest effect on habituation of response probability, but also altered response latency early in habituation training. In contrast, the dopamine deficit had little effect on habituation of response duration. Taken together, these data support the hypothesis that the proportion of worms reversing and the duration and latency of the response are mediated by different mechanisms.
Dopamine modulates habituation
Dopamine is a neuromodulator frequently implicated in neural and behavioral plasticity in a wide range of organisms. A role for dopamine signaling has also been proposed for habituation in mammals (Lloyd et al. 2014), for which it is well-positioned based on responding of mesolimbocortical and nigrostriatal dopaminergic neurons to unconditioned salient and arousing sensory input (Horvitz 2000). In C. elegans, dopamine influences a variety of behaviors, including locomotion (Sawin et al. 2000; Hills et al. 2004; Vidal-Gadea et al. 2011) and learning (Bettinger and McIntire 2004; Sanyal et al. 2004; Kindt et al. 2007; Hukema et al. 2008; Voglis and Tavernarakis 2008; Kimura et al. 2010). Previous work has demonstrated that dopamine signaling modulates naïve responding to ASH-sensed cues, increasing or decreasing sensitivity depending on the stimulus (Ferkey et al. 2007; Wragg et al. 2007; Ezcurra et al. 2011). However, unlike habituation, this modulation is thought to occur upstream of cell excitation. Here we showed that loss of dopamine signaling led to a rapid decrease in the proportion of worms responding to repeated ASH photoactivation and identified the dopamine receptor, DOP-4, as a key mediator. Using CuCl2 as a stimulus, Ezcurra et al. (2011) also found that loss of dopamine signaling resulted in a more rapid decrement in responding; however, in their assay the dop-4 mutant did not have a phenotype. It is possible that DOP-4 signaling modulates habituation to some, but not all ASH-sensed stimuli. Alternatively, a role for DOP-4 may have been masked by sensory adaptation to CuCl2. Our optogenetic approach removed the contribution of adaptation to specifically evaluate habituation, which may better reveal the dop-4 mutant phenotype.
For habituation to nonlocalized mechanosensory input (taps), the D1-like DOP-1 dopamine receptor functions in the body touch receptor neurons to promote cell excitability (Kindt et al. 2007). Although DOP-1 and DOP-4 are both D1-like receptors, DOP-4 belongs to an invertebrate specific subfamily that includes the Drosophila receptor, DAMB, and the Apis mellifera receptor, DOP-2 (Sugiura et al. 2005). The deficits associated with each dopamine receptor appear to be assay-specific, as loss of DOP-4 did not decrease the probability of response in the tap habituation assay (data not shown) and loss of DOP-1 did not affect habituation of ASH-mediated reversals (Fig. 8A). Thus habituation of these avoidance circuits is similarly affected at the behavioral level by the same neuromodulator, but via distinct receptors. It remains to be determined whether the cellular signals are also divergent: one difference appears to be their site of action, with DOP-1 functioning in the sensory cells to promote excitability and DOP-4 signaling in some as-yet-unidentified downstream cell or cells. Further comparisons between habituation to ASH stimulation and habituation to tap will be useful for evaluating the generalizability of findings between circuits.
As mechanistic insights emerge, it is apparent that multiple cellular processes can underlie habituation, for example the depression of excitatory synapses described for the gill and siphon withdrawal reflex of Aplysia (Kandel et al. 2004) and the recurrent inhibition motif of the Drosophila olfactory system (Twick et al. 2014). The best prospect for a complete characterization of habituation is the continued use of model systems with tractable nervous systems. Combining optogenetics with the Multi-Worm Tracker has removed the bottleneck of data collection and analysis, making it feasible to conduct large parametric studies or drug or genetic screens targeting habituation of the ASH avoidance circuit.
Acknowledgments
Strains were provided by the CGC (funding from NIH Office of Research Infrastructure Programs P40 OD010440), as well as the laboratories of William Schafer (MRC Laboratory of Molecular Biology) and Shawn Xu (University of Michigan). Andrew Lee, Myron Huen, and Alex Liang helped cross strains and run preliminary experiments. Research was funded with a NSERC CGSD3 to E.L.A. and a NSERC Discovery grant (NSERC RGPIN 1222216-13) to C.H.R.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.041830.116.
References
- Ardiel EL, Rankin CH. 2010. An elegant mind: learning and memory in Caenorhabditis elegans. Learn Mem 17: 191–201. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Thomas JH, Horvitz HR. 1990. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol 55: 529–538. [DOI] [PubMed] [Google Scholar]
- Bettinger JC, McIntire SL. 2004. State-dependency in C. elegans. Genes Brain Behav 3: 266–272. [DOI] [PubMed] [Google Scholar]
- Bozorgmehr T, Ardiel EL, McEwan AH, Rankin CH. 2013. Mechanisms of plasticity in a Caenorhabditis elegans mechanosensory circuit. Front Physiol 4: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. 1992. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry 49: 206–215. [DOI] [PubMed] [Google Scholar]
- Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MY, Komatsu H, Fukuto HS, Dionne HM, Hart AC. 2004. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci 101: 15512–15517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI. 1997. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci 17: 8259–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SL, Charlie NK, Milfort MC, Brown BS, Gravlin CN, Knecht JE, Miller KG. 2008. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol 6(8): e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezcurra M, Tanizawa Y, Swoboda P, Schafer WR. 2011. Food sensitizes C. elegans avoidance behaviours through acute dopamine signalling. EMBO J 30: 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkey DM, Hyde R, Haspel G, Dionne HM, Hess HA, Suzuki H, Schafer WR, Koelle MR, Hart AC. 2007. C. elegans G protein regulator RGS-3 controls sensitivity to sensory stimuli. Neuron 53: 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, Jorgensen EM. 2008. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet 40: 1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, Bargmann CI. 2005. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci 102: 3184–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZV, Hart AC, Ramanathan S. 2009. Optical interrogation of neural circuits in Caenorhabditis elegans. Nat Methods 6: 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Wu TH, Song YX, Ge MH, Su CM, Niu WP, Li L-L, Xu Z-J, Ge C-L, Al-Mhanawi MTH, et al. 2015. Reciprocal inhibition between sensory ASH and ASI neurons modulates nociception and avoidance in Caenorhabditis elegans. Nat Commun 6: 5655. [DOI] [PubMed] [Google Scholar]
- Hart AC, Sims S, Kaplan JM. 1995. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature 378: 82–85. [DOI] [PubMed] [Google Scholar]
- Hart AC, Kass J, Shapiro JE, Kaplan JM. 1999. Distinct signaling pathways mediate touch and osmosensory responses in a polymodal sensory neuron. J Neurosci 19: 1952–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard MA, Bargmann CI, Bazzicalupo P. 2002. C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr Biol 12: 730–734. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR. 2005. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J 24: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills T, Brockie PJ, Maricq AV. 2004. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci 24: 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. 2000. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience 96: 651–656. [DOI] [PubMed] [Google Scholar]
- Hukema RK, Rademakers S, Jansen G. 2008. Gustatory plasticity in C. elegans involves integration of negative cues and NaCl taste mediated by serotonin, dopamine, and glutamate. Learn Mem 15: 829–836. [DOI] [PubMed] [Google Scholar]
- Husson SJ, Liewald JF, Schultheis C, Stirman JN, Lu H, Gottschalk A. 2012. Microbial light-activatable proton pumps as neuronal inhibitors to functionally dissect neuronal networks in C. elegans. PLoS One 7: e40937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. 2004. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep 24: 475–522. [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Horvitz HR. 1993. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc Natl Acad Sci 90: 2227–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura KD, Fujita K, Katsura I. 2010. Enhancement of odor avoidance regulated by dopamine signaling in Caenorhabditis elegans. J Neurosci 30: 16365–16375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR. 2007. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron 55: 662–676. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Johnson LC, Richards T, Mahurin R, Greenson J, Dawson G, Aylward E. 2009. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry 166: 467–475. [DOI] [PubMed] [Google Scholar]
- Larsch J, Ventimiglia D, Bargmann CI, Albrecht DR. 2013. High-throughput imaging of neuronal activity in Caenorhabditis elegans. Proc Natl Acad Sci 110: E4266–E4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Feng Z, Sternberg PW, Xu XZ. 2006. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 440: 684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay TH, Thiele TR, Lockery SR. 2011. Optogenetic analysis of synaptic transmission in the central nervous system of the nematode Caenorhabditis elegans. Nat Commun 2: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindy AS, Parekh PK, Zhu R, Kanju P, Chintapalli SV, Tsvilovskyy V, et al. 2014. TRPV channel-mediated calcium transients in nociceptor neurons are dispensable for avoidance behaviour. Nat Commun 5: 4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DR, Medina DJ, Hawk LW, Fosco WD, Richards JB. 2014. Habituation of reinforcer effectiveness. Front Integr Neurosci 7: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq AV, Peckol E, Driscoll M, Bargmann CI. 1995. Mechanosensory signalling in C. elegans mediated by the GLR-1 glutamate receptor. Nature 378: 78–81. [DOI] [PubMed] [Google Scholar]
- Mellem JE, Brockie PJ, Zheng Y, Madsen DM, Maricq AV. 2002. Decoding of polymodal sensory stimuli by postsynaptic glutamate receptors in C. elegans. Neuron 36: 933–944. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. 1991. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hagan R, Chalfie M, Goodman MB. 2005. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci 8: 43–50. [DOI] [PubMed] [Google Scholar]
- Piggott BJ, Liu J, Feng Z, Wescott SA, Xu XZ. 2011. The neural circuits and synaptic mechanisms underlying motor initiation in C. elegans. Cell 147: 922–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH, Beck CD, Chiba CM. 1990. Caenorhabditis elegans: a new model system for the study of learning and memory. Behav Brain Res 37: 89–92. [DOI] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, et al. 2009. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem 92: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Bill BR, Glanzman DL. 2013. Learning and memory in zebrafish larvae. Front Neural Circuits 7: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Wintle RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, et al. 2004. Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J 23: 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. 2000. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631. [DOI] [PubMed] [Google Scholar]
- Schmitt C, Schultheis C, Husson SJ, Liewald JF, Gottschalk A. 2012. Specific expression of channelrhodopsin-2 in single neurons of Caenorhabditis elegans. PLoS One 7: e43164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M, Fuke S, Suo S, Sasagawa N, Van Tol HH, Ishiura S. 2005. Characterization of a novel D2-like dopamine receptor with a truncated splice variant and a D1-like dopamine receptor unique to invertebrates from Caenorhabditis elegans. J Neurochem 94: 1146–1157. [DOI] [PubMed] [Google Scholar]
- Swierczek NA, Giles AC, Rankin CH, Kerr RA. 2011. High-throughput behavioral analysis in C. elegans. Nat Methods 8: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. 1966. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 73: 16–43. [DOI] [PubMed] [Google Scholar]
- Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, et al. 2002. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35: 307–318. [DOI] [PubMed] [Google Scholar]
- Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI. 1995. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83: 207–218. [DOI] [PubMed] [Google Scholar]
- Twick I, Lee JA, Ramaswami M. 2014. Olfactory habituation in Drosophila-odor encoding and its plasticity in the antennal lobe. Prog Brain Res 208: 3–38. [DOI] [PubMed] [Google Scholar]
- Vidal-Gadea A, Topper S, Young L, Crisp A, Kressin L, Elbel E, Maples T, Brauner M, Erbguth K, Axelrod A, et al. 2011. Caenorhabditis elegans selects distinct crawling and swimming gaits via dopamine and serotonin. Proc Natl Acad Sci 108: 17504–17509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglis G, Tavernarakis N. 2008. A synaptic DEG/ENaC ion channel mediates learning in C. elegans by facilitating dopamine signalling. EMBO J 27: 3288–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 314: 1–340. [DOI] [PubMed] [Google Scholar]
- Wicks SR, Rankin CH. 1995. Integration of mechanosensory stimuli in Caenorhabditis elegans. J Neurosci 15: 2434–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wragg RT, Hapiak V, Miller SB, Harris GP, Gray J, Komuniecki PR, Komuniecki RW. 2007. Tyramine and octopamine independently inhibit serotonin-stimulated aversive behaviors in Caenorhabditis elegans through two novel amine receptors. J Neurosci 27: 13402–13412. [DOI] [PMC free article] [PubMed] [Google Scholar]


