Abstract
It has long been hypothesized that conditioning mechanisms play major roles in addiction. Specifically, the associations between rewarding properties of drugs of abuse and the drug context can contribute to future use and facilitate the transition from initial drug use into drug dependency. On the other hand, the self-medication hypothesis of drug abuse suggests that negative consequences of drug withdrawal result in relapse to drug use as an attempt to alleviate the negative symptoms. In this review, we explored these hypotheses and the involvement of the hippocampus in the development and maintenance of addiction to widely abused drugs such as cocaine, amphetamine, nicotine, alcohol, opiates, and cannabis. Studies suggest that initial exposure to stimulants (i.e., cocaine, nicotine, and amphetamine) and alcohol may enhance hippocampal function and, therefore, the formation of augmented drug-context associations that contribute to the development of addiction. In line with the self-medication hypothesis, withdrawal from stimulants, ethanol, and cannabis results in hippocampus-dependent learning and memory deficits, which suggest that an attempt to alleviate these deficits may contribute to relapse to drug use and maintenance of addiction. Interestingly, opiate withdrawal leads to enhancement of hippocampus-dependent learning and memory. Given that a conditioned aversion to drug context develops during opiate withdrawal, the cognitive enhancement in this case may result in the formation of an augmented association between withdrawal-induced aversion and withdrawal context. Therefore, individuals with opiate addiction may return to opiate use to avoid aversive symptoms triggered by the withdrawal context. Overall, the systematic examination of the role of the hippocampus in drug addiction may help to formulate a better understanding of addiction and underlying neural substrates.
Addiction is a major worldwide health problem that results in maladaptive behavioral changes, some that can last a lifetime. This behavioral plasticity, often times maladaptive, must be associated changes in neural plasticity. In fact, it has been noted multiple times that there is a high degree of overlap between the neurobiology of learning and memory and the neurobiology of addiction (e.g., White 1996; Kelley 2004; Hyman et al. 2006; Volkow et al. 2014; Goodman and Packard 2016). Drugs of abuse are often linked to disrupted learning, but the relationship between drugs of abuse and learning is more complex as drug use and abuse is also associated with the development of strong but maladaptive memories that contribute to drug-seeking behavior and addiction. It is the overarching premise of this review that initial or acute use of drugs can facilitate the development of maladaptive memories between drug effects and environmental stimuli and that these associated memories can exert strong behavioral control and facilitate drug-seeking behavior and relapse. With continued use of drugs, learning deficits emerge along with cognitive inflexibility. These learning deficits and cognitive inflexibility combined with previously formed maladaptive drug-context/drug-cue associations contribute to the maintenance of addiction.
While there are multiple types of learning, this review will focus on hippocampus-mediated learning. The hippocampus is perhaps the iconic brain region associated with learning and memory. For instance, the work of Scoville and Milner (1957) with patient H.M., whose severe epilepsy was treated with complete resection of the hippocampus and surrounding medial temporal lobe tissue, demonstrated the critical importance of this brain region in the formation of new long-term declarative memories. The patient H.M. could not maintain new declarative memories. This is particularly problematic because declarative memories contribute to self-definition as they encompass memories of events and autobiographical memories. As part of an essential role in declarative memory formation, the hippocampus is especially good at binding information together to form complex representations (Sutherland and Rudy 1989; for review, see Yonelinas 2013) that are necessary for spatial and contextual memory formation (O'Keefe and Dostrovsky 1971; Kim and Fanselow 1992; Kim and Lee 2011; Loureiro et al. 2012). In addition to involvement in long-term declarative memory formation, the hippocampus is also well known as one of the brain regions that demonstrate a high-level synaptic plasticity, often assessed by changes in long-term potentiation (LTP); (Teyler and DiScenna 1987; Lynch et al. 1990). The high degree of plasticity in the hippocampus and the ability of this region to support contextual and declarative memories may facilitate drug-induced changes in hippocampal function that have a profound effect on behavior.
It is clear that the physiological effects of drugs of abuse can become associated with contextual information, contributing to future drug-seeking behavior (Bardo et al. 1984; Carr et al. 1988; Bienkowski et al. 1996; Le Foll et al. 2006; Tropea et al. 2008; Kutlu et al. 2015a). Because of the critical role of the hippocampus in learning contextual information (Smith and Mizumori 2006), drug-associated changes in hippocampal function may contribute to the development of maladaptive drug-context associations. With continued drug use, adaptations including tolerance occur and these changes could disrupt hippocampal function. Chronic drug use is often associated with cognitive deficits (Ornstein et al. 2000; Robbins et al. 2008; Stavro et al. 2013), and these deficits could contribute to addiction by interfering with acquisition of adaptive behavior that supports the cessation of drug use. Furthermore, withdrawal symptoms for multiple drugs of abuse include cognitive deficits (Solowij 1995; Jacobsen et al. 2005), which could contribute to relapse when individuals attempt to reverse these deficits (Khantzian 1985; Rukstalis et al. 2005; Patterson et al. 2010). It is beyond the scope of this review to discuss all drugs of abuse; therefore, we will focus on cocaine, amphetamine, nicotine, ethanol, opiates, and cannabis, examining for each drug the effects of acute administration, chronic administration, and withdrawal on hippocampus learning and hippocampal synaptic plasticity. In addition, because there is substantial evidence showing that self-administration and yoked-administration of drugs result in the same effects on hippocampal plasticity (Thomas and Everitt 2001; Thomas et al. 2003; Yamaguchi et al. 2004, 2005; Domínguez-Escribà et al. 2006; Noonan et al. 2008), we will review studies using both contingent and noncontingent drug administration together. Evidence from human subject studies along with laboratory animal studies will be reviewed.
Cocaine
The effects of acute administration on hippocampus-dependent learning and memory
Cocaine, a highly addictive psychostimulant derived from the leaf of Erythroxylon coca, is often characterized by compulsive use and obsessive drug seeking (Dackis and O'Brien 2001). Cocaine addiction affects roughly 2 million people in the USA, with 1.5 million of them identified as “cocaine users,” and every day there are 1700 new users (U.S. Department of Health and Human Services 2011). In addition to cocaine's negative health consequences including cardiovascular, pulmonary, and psychiatric complications (Brody et al. 1990; Haim et al. 1995; Lange and Hillis 2001), cocaine has been reported to be the most commonly used illicit drug among patients seeking emergency care (40.3%; SAMHSA 2011). These figures highlight cocaine addiction as a disease with devastating consequences; thus, understanding the processes underlying the development and maintenance of cocaine addiction is vital.
Despite its negative health consequences, when acutely administered cocaine activates the brain's reward circuitry, producing a euphoric state that serves as a reinforcer for future cocaine use (Volkow et al. 1999). In addition to the pleasurable effects, acute cocaine has also been shown to improve cognition in humans (Garavan et al. 2008) and enhance learning and memory in laboratory rodents (Wood et al. 2007). However, these procognitive effects during initial cocaine exposure may be responsible for the formation of maladaptive drug-context/-cue associations that may facilitate the development of compulsive drug-seeking behavior. In support of this hypothesis, studies have shown that laboratory rodents learn to self-administer cocaine (e.g., Richardson and Roberts 1996; España et al. 2010) and associate a specific context with cocaine reward (e.g., Spyraki et al. 1982; Vidal-Infer et al. 2012) remarkably quickly. The rewarding effects of cocaine are so powerful that a number of studies have shown that an especially addiction-prone subset of laboratory animals trained to self-administer cocaine prefer cocaine over feeding and mating and compulsively self-administer cocaine at fatal rates (Deneau et al. 1969; Lenoir et al. 2007; Kerstetter et al. 2012; Perry et al. 2013). This suggests that the coupling of cocaine's procognitive effects with overstimulation of the reward system may result in dysregulated behavioral outcomes rather than enhancement of behavioral control.
There are several brain regions within the mesolimbic circuit that are directly affected by cocaine, including reward-related regions such as the nucleus accumbens and ventral tegmental area as well as regions that control cognition such as the prefrontal cortex and hippocampus (Bardo 1998; Thomas et al. 2008). Among these regions, the hippocampus may be a critical site for both the rewarding effects of acute cocaine (Kuhar et al. 1991; Koob et al. 1994; Everitt et al. 1999; Dackis and O'Brien 2001; Anderson and Pierce 2005) as well as formation and maintenance of cocaine-context associations (Grant et al. 1996; Childress et al. 1999; Kilts et al. 2001; Wexler et al. 2001) due to its involvement in both reward and learning and memory (Aggleton et al. 1986; Burgess et al. 2002; Daumas et al. 2005; for review, see Tulving and Markowitsch 1997). For example, permanent lesions of the dorsal, but not ventral hippocampus, as well as temporary inactivation of the dorsal hippocampus by local muscimol infusions impaired cocaine conditioned place preference (CPP; Meyers et al. 2003, 2006). Similarly, studies suggest that the dorsal hippocampus controls context-induced reinstatement (Fuchs et al. 2005, 2007; Xie et al. 2010; Wells et al. 2011). In contrast to the dorsal hippocampus, the ventral hippocampus has been shown to mediate cue-induced and cocaine-primed reinstatement of cocaine self-administration (Rogers and See 2007; Ramirez et al. 2009). Moreover, Vorel et al. (2001) showed that theta burst stimulation in the ventral hippocampus resulted in relapse of extinguished cocaine self-administration. It is also possible that dorsal and ventral hippocampus may differentially contribute to the stress-induced relapse to cocaine seeking as studies have shown that stress affects synaptic plasticity in these regions in opposite ways. That is, while stress diminished synaptic plasticity in the dorsal hippocampus, ventral hippocampal synaptic plasticity was enhanced by stress (Maggio and Segal 2007, 2009; Segal et al. 2010). Therefore, ventral hippocampus may assume a greater role in reinstatement of cocaine-seeking behavior during a period of high stress such as cocaine withdrawal. But this hypothesis has not been directly examined.
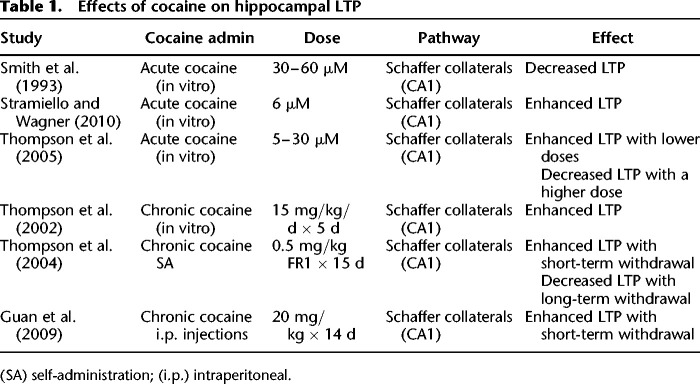
These studies establish the hippocampus as the focal region for the formation and maintenance of long-term memories that support cocaine-context and cocaine-cue associations. In support of the hippocampus as the primary target of cocaine in modulating drug-related memories, acute cocaine has also been shown to alter hippocampal LTP, a form of synaptic plasticity that may underlie learning and memory (Bliss and Collingridge 1993). However, the effects of cocaine on LTP are mixed (see Table 1 for a summary of results). For example, Smith et al. (1993) found that acute cocaine (30–60 µM) blocked induction of LTP in the CA1 region of the hippocampus without affecting NMDA receptors or already established LTP. In contrast, Stramiello and Wagner (2010) found that enhanced dopaminergic signaling associated with acute cocaine application (6 µM) increased hippocampal LTP in the CA1 subregion. Nevertheless, these contradicting results may be explained by the fact that different drug concentrations were used by these studies: 30–60 µM by Smith et al. (1993) and 6 µM by Stramiello and Wagner (2010). In support of the differential effects of low and high doses of cocaine on LTP, Thompson et al. (2005) showed that while lower cocaine concentrations (5–10 µM) enhanced hippocampal LTP, inhibition of LTP was observed with a higher concentration of cocaine (30 µM). Also in line with these results, Wood et al. (2007) found that an acute moderate dose of cocaine disrupted contextual and cued fear conditioning, whereas a low dose of acute cocaine enhanced both types of learning. Given that, in the absence of tolerance to cocaine, it is likely that most first-time cocaine users initially administer lower doses of cocaine, and therefore, they are subject to both rewarding and procognitive effects, which may underlie enhanced hippocampal plasticity that leads to maladaptive drug-associated memories.
Table 1.
Effects of cocaine on hippocampal LTP
In addition to acute cocaine-induced enhancement of hippocampal LTP, there is also evidence showing that acute cocaine positively modulates the activation of the proteins within the cell signaling cascades that support long-term memory formation. For example, phosphorylation of extracellular signal-regulated kinases -1 and -2 (ERK1/2), a protein required for both hippocampus-dependent learning and hippocampal LTP (Gooney et al. 2002), was elevated in the dorsal hippocampus following cocaine CPP (Tropea et al. 2008). Similarly, phosphorylation of hippocampal cAMP-response element binding protein (CREB), a transcription factor that plays a major role in synaptic plasticity and long-term memory formation (Abel and Lattal 2001; Trifilieff et al. 2006), was increased following cocaine CPP (Tropea et al. 2008). In addition to ERK1/2 and CREB, cAMP-dependent protein kinase (PKA) seems to be required for the formation and maintenance of cocaine-context memories. For example, Cervo et al. (1997) showed that post-training intracerebroventricular injections of a PKA inhibitor disrupted the consolidation of cocaine CPP. These results suggest that within a limited dose range, acute cocaine augments hippocampal plasticity and activity of cell signaling cascades that support hippocampal LTP, which may enhance the formation of the maladaptive drug-context memories. It is possible that augmented hippocampal function may result in drug-context-triggered cravings and drug seeking as initial cocaine use turns into cocaine addiction with chronic cocaine abuse.
Effects of chronic cocaine and cocaine withdrawal on hippocampal function
In contrast to acute cocaine's procognitive effects, chronic cocaine users have been repeatedly shown to exhibit a variety of neuropsychological deficits ranging from disrupted executive function, visuoperception, and psychomotor function (Bolla et al. 1999) to impairments of verbal memory and attention (Mittenberg and Motta 1993). These deficits were positively correlated with the severity of cocaine addiction (Ardila et al. 1991; for review, see Robbins et al. 2008). Accordingly, chronic cocaine administration also leads to impaired spatial learning in rodents that were exposed to cocaine during adulthood (Mendez et al. 2008) or adolescence (Santucci et al. 2004; Santucci 2008). Chronic cocaine-induced learning deficits also seem to be long-lasting as studies suggest that the impairing effects of chronic cocaine exposure persist during cocaine withdrawal (Kelley et al. 2005) as long as 3 mo (Mendez et al. 2008). These studies suggest that chronic cocaine exposure may result in a diminished ability to learn new associations. This effect of chronic cocaine use may be particularly problematic because inability to change established drug-context associations or to learn new associations that may counteract the maladaptive ones may facilitate the maintenance of cocaine addiction. For example, exposure therapy for addiction focuses on reversing learned drug-context and drug-cue associations to reduce context or cue-triggered craving, drug seeking, and drug relapse (Rosenthal and Kutlu 2014). Reduced ability to make new associations during chronic cocaine use or cocaine withdrawal may disrupt cognitive flexibility and increase the likelihood of relapse.
Importantly, reduced hippocampal function during chronic cocaine use and cocaine withdrawal may be responsible for the decreased ability to form adaptive associations to counteract context-drug memories as studies found that hippocampal function was altered with chronic administration of cocaine (London et al. 1990; Beveridge et al. 2006; Gu et al. 2010). For example, cocaine administration produced increased BOLD signal in the hippocampus of cocaine-dependent human subjects compared with saline administration (Breiter et al. 1997), while the strength of connectivity between the hippocampus and dorsomedial prefrontal cortex was decreased in chronic cocaine users (Gu et al. 2010). Also, chronic cocaine self-administration resulted in reduced glucose metabolism in the hippocampus in humans and nonhuman primates (London et al. 1990; Beveridge et al. 2006). These results suggest a central role for changes in the hippocampus in cocaine-induced cognitive deficits.
However, in contrast to reduced hippocampus-dependent learning during chronic cocaine and withdrawal, there is also evidence showing that chronic in vivo cocaine administration resulted in enhanced LTP (Thompson et al. 2002). Thompson et al. (2004) found that prior chronic cocaine self-administration resulted in enhanced hippocampal LTP in the CA1 subregion following 3 d of withdrawal but not following 30 d of withdrawal. In line with these results, other studies found enhanced LTP 3 d after chronic cocaine administration (Guan et al. 2009). Interestingly, Thompson et al. (2004) also found decreased hippocampal LTP 100 d after chronic cocaine self-administration, which suggests that chronic cocaine may have different short-term and long-term effects on hippocampal plasticity. In line with the results of Thompson et al. (2004) showing enhanced LTP during short-term withdrawal, Valzachi et al. (2013) found that chronic cocaine administration resulted in increased phosphorylated CREB levels in the hippocampus following 12 d of withdrawal. Therefore, there is a potential discrepancy between behavioral studies showing disrupted hippocampal learning and memory (Melnick et al. 2001; Santucci et al. 2004; Mendez et al. 2008; Santucci et al. 2008) and electrophysiology studies showing enhanced LTP with chronic cocaine administration (Thompson et al. 2002, 2004; Guan et al. 2009). These contradicting results might have risen because of the fact that behavioral studies cited in this review tested their subjects in long-term withdrawal between 5 wk and 4 mo. Importantly, Thompson et al. (2004) and Guan et al. (2009) showed that chronic cocaine administration enhanced LTP in short-term withdrawal (3 d), whereas disrupted LTP following long-term withdrawal (100 d). In support of this hypothesis, Del Olmo et al. (2007) found that chronic cocaine self-administration enhanced spatial learning 3 h following the last cocaine infusion. Thus, the dose of cocaine and the length of time from the last drug administration seem to be major determinants of cocaine's effects on hippocampal learning and memory and may explain some of the cognitive deficits exhibited by cocaine users. It is also important to note that increased LTP does not necessarily mean increased learning (Saucier and Cain 1995).
Overall, the studies cited clearly show that initially cocaine enhances hippocampus-dependent learning and memory resulting in strong cocaine-context associations, which may lead to drug-seeking behavior and chronic cocaine abuse. In turn, chronic cocaine exposure alters hippocampus function and results in hippocampal cognitive deficits during withdrawal, which may contribute to impaired cognitive flexibility and inability to reverse cocaine-context associations, contributing to relapse.
Amphetamine
The effects of acute amphetamine on hippocampus-dependent learning and memory
Racemic α-methylphenethylamine (amphetamine, also known as speed) was first discovered by Barger and Dale in 1910 and later synthesized and marketed under the brand name Benzedrine to treat a variety of conditions such as narcolepsy, depression, Parkinson's disease, and pulmonary dysfunction (Heal et al. 2013). However, the euphoric effects of amphetamine were quickly discovered and subsequently amphetamine has been abused for its rewarding properties such as sensations of pleasure, self-confidence, energy, and alertness (CDC 2007). Owing to these effects, millions of Benzedrine tablets, under the name of “Energy tablets,” were given to the members of the American and British military (Bett 1946) as well as Japanese soldiers during World War II, and the release of the amphetamine stockpiles after the war resulted in an amphetamine-dependency epidemic in Japan (Masaki 1956). In the USA, early studies that provided a platform for the prescription of amphetamine to reverse combat fatigue largely ignored its addictive properties (Guttmann and Sargant 1937; Tidy 1938; Bett 1946), which resulted in a widespread prescription of amphetamine-based medications and eventually amphetamine addiction peaked in mid-2000s (CDC 2007). Today amphetamine dependence is a widespread problem (CDC 2007). According to Substance Abuse and Mental Health Services Administration (SAMHSA 2005), 1.4 million Americans had used methamphetamine, an N-methylated derivative of amphetamine, in the last year. Moreover, the rate of amphetamine or methamphetamine abuse-related hospitalization more than tripled between the years 1993 and 2003 (SAMHSA 2006a). Importantly, despite its initial pleasurable effects, amphetamine has serious negative health consequences with prolonged abuse. These effects include physical symptoms such as decayed teeth, weight loss, skin lesions, stroke, and heart attack as well as mental symptoms such as paranoia, hallucinations, anxiety, irritability, social isolation, aggressiveness, and violence (CDC 2007).
Shortly after its initial release as a prescription drug in 1935, procognitive effects of amphetamine (e.g., improved intelligence, concentration, and intellectual performance) were reported in humans (Guttmann and Sargant 1937; Tidy 1938). These initial studies were later confirmed by studies showing enhanced memory consolidation (Soetens et al. 1993), memory recall (Zeeuws and Soetens 2007), attention and psychomotor performance (Johnson et al. 2000; Silber et al. 2006), information processing (Halliday et al. 1994), logical reasoning (Johnson et al. 2000), and working memory (Mattay et al. 2000) by amphetamine and its derivatives such as dextroamphetamine in humans. Because of its attention-improving properties amphetamine was used to treat ADHD but then this treatment was replaced by drugs with fewer psychoactive side effects (Wilens et al. 2008). Animal studies also showed that, similar to other stimulants, acute amphetamine and methamphetamine enhanced hippocampus-dependent learning and memory in the T-maze (Ito and Canseliet 2010), Morris water maze (Packard and McGaugh 1994; Brown et al. 2000; Cao et al. 2013), radial arm-maze (Strupp et al. 1991), and avoidance conditioning (Doty and Doty 1966). However, these procognitive effects were dose-dependent as higher doses of acute amphetamine resulted in deficits in hippocampus-dependent learning and memory (Blokland et al. 1998). Studies also showed that amphetamine and methamphetamine produced significant CPP (Carr et al. 1988; Bardo et al. 1999; Parker et al. 2004; Thorn et al. 2012, Han et al. 2014) and self-administration (Lyness et al. 1979; Piazza et al. 1989, 1990, 1991; Krasnova et al. 2010; McClung et al. 2010), which suggests that the rewarding effects of amphetamine and methamphetamine facilitated the formation of drug-context associations. In line with acute amphetamine's enhancing effects on hippocampus-dependent learning and memory, hippocampal LTP was also increased by acute amphetamine (Delanoy et al. 1983; Gold et al. 1984; Morimoto et al. 1987) and methamphetamine administration (Heysieattalab et al. 2016). Moreover, evidence suggests that acute methamphetamine-induced enhancement of hippocampus-dependent learning as well as amphetamine CPP are dependent on the upregulation of ERK1/-2 and CREB in the hippocampus (Gerdjikov et al. 2004; Cao et al. 2013). Therefore, it is possible that enhanced hippocampus-dependent learning and memory by acute amphetamine administration may drive the formation of drug/reward-context associations by enhancing hippocampal plasticity. This hypothesis is supported by the results showing that acute injections of amphetamine enhanced morphine CPP (Blaiss and Janak 2006) and conditioned approach to sucrose (Blaiss and Janak 2007). Together with studies showing that acute amphetamine produced procognitive effects as well as enhanced hippocampal plasticity, these results show that development of amphetamine-dependence may be influenced by augmentation of drug-context associations.
Effects of chronic amphetamine use on hippocampal function
In spite of its acute procognitive effects, chronic use of amphetamine and methamphetamine has devastating effects on cognition, including impaired memory, attention, cognitive flexibility, cognitive inhibition, and decision making (Ornstein et al. 2000; Simon et al. 2000, 2001; Salo et al. 2002; for review, see Nordahl et al. 2003). These cognitive deficits persist during amphetamine and methamphetamine withdrawal (Kalechstein et al. 2002; Newton et al. 2004; Johanson et al. 2006). Similar to results from human studies, animal studies also indicate cognitive deficits in hippocampus-dependent spatial learning tasks such as the spatial object recognition, T-maze and Morris water maze during amphetamine (Mandillo et al. 2003) and methamphetamine (Simões et al. 2007; North et al. 2013; Reichel et al. 2014) withdrawal. In addition, there is evidence showing that deficits in the hippocampus-dependent learning and memory paralleled reductions in hippocampal LTP induced by methamphetamine withdrawal (Swant et al. 2010; North et al. 2013). These results indicate that amphetamine withdrawal disrupts cognition, including hippocampus-dependent learning and memory and associated hippocampal plasticity. These cognitive problems may underlie relapse to amphetamine use as amphetamine administration may be seen as a way to self-medicate these deficits.
Overall, similar to other stimulants, when administered acutely amphetamine has procognitive effects including enhanced hippocampus-dependent learning and memory. Acute amphetamine's procognitive properties may result in augmentation of drug-context associations and contribute to the development of amphetamine addiction. In contrast, withdrawal of amphetamine following chronic use results in deficits in hippocampus-dependent learning and memory. In addition to the failure to reverse already established drug-context associations, relapse to amphetamine use may be motivated by attempts to self-medicated for withdrawal-induced cognitive deficits.
Nicotine
Effects of acute, chronic, and withdrawal from chronic nicotine on hippocampus-dependent learning and memory
Prolonged exposure to tobacco products, particularly cigarette smoking, continues to be the leading cause of preventable premature death claiming more than 400,000 people's lives in the USA each year (20% of all deaths; Benowitz 2010; U.S. Department of Health and Human Services 2014). Accordingly, active and passive tobacco use has been causally linked to deaths from cancer, cardiovascular disease, and pulmonary disease (Sandler et al. 1985; Carbone 1992; McBride 1992; Sherman 1992). Although nicotine, the main psychoactive component in tobacco, may play less of a role in the development of these conditions compared with other toxins in the tobacco extract, it is the main component leading to addiction. Therefore, by causing repeated use and prolonged exposure to toxins, nicotine should be considered as the indirect cause of the smoking-related diseases and deaths (Benowitz 2010).
An estimated 15%–19% of the U.S. population has been reported to use nicotine products habitually (CDC 2012). Nicotine's rewarding effects seem to play a major role in the development of nicotine dependence (Watkins et al. 2000). In humans, nicotine results in euphoria, increased energy and arousal, and suppressed anxiety (Pomerleau and Pomerleau 1985, 1992; Stolerman and Jarvis 1995; Benowitz 1996). Accordingly, in animals, acute injections of nicotine leads to CPP (Fudala et al. 1985; Risinger and Oakes 1995; Vastola et al. 2002; Grabus et al. 2006; Brielmaier et al. 2008; Kutlu et al. 2015a). Similarly, there is also evidence showing that smokers learn to associate nicotine's effects with specific contexts and cues (Dols et al. 2000, 2002; Thewissen et al. 2005), suggesting humans and animals learn to associate nicotine's rewarding effects with specific contextual cues. In addition to its rewarding effects, acute nicotine has cognitive enhancing properties in humans. Specifically, acute nicotine administration enhances attention (Parrott and Craig 1992; Bates et al. 1995; Hahn et al. 2007; Hong et al. 2011), learning and memory (Mangan and Golding 1983; Peeke and Peeke 1984; Warburton et al. 1986; Colrain et al. 1992), and information processing (Wesnes and Warburton 1983; Provost and Woodward 1991; for review, see Sherwood 1993). In agreement with human studies, there is a great body of evidence suggesting that acute nicotine augments hippocampus-dependent contextual and spatial learning and memory while not affecting hippocampus-independent subtypes of learning (e.g., cued learning) in rodents. For example, numerous studies from both our group and other laboratories showed that acute nicotine enhanced hippocampus-dependent contextual and trace fear conditioning, but not cued fear conditioning (Gould and Wehner 1999; Gould and Higgins 2003; Gould 2003a; Gould and Lommock 2003; Gould et al. 2004; Wehner et al. 2004; Davis et al. 2006, 2007; Davis and Gould 2006, 2007; Raybuck and Gould 2007; Gulick and Gould 2008; Kenney and Gould 2008; Tian et al. 2011; Portugal et al. 2012a, b), as well as spatial object recognition (Kenney et al. 2011), spatial learning and memory in Morris water maze (Abdulla et al. 1996; Sharifzadeh et al. 2005), and spatial working memory in radial arm maze tasks (Levin and Torry 1996; Levin et al. 1997, 1998). It is possible that the formation of nicotine-context associations may benefit from these procognitive effects of acute nicotine. That is, through promoting hippocampal plasticity, acute nicotine may enhance formation of drug-context associations and promote future nicotine use evoked by contextual and environmental cues. In support of this hypothesis, there is evidence showing that contextual cues associated with nicotine reward reinstate extinguished nicotine self-administration in rats (Diergaarde et al. 2008; Wing and Shoaib 2008). Therefore, these studies support the possibility that acute nicotine-induced enhancement of hippocampus-dependent learning and memory may contribute to development of nicotine-dependence.
Once nicotine dependence is established, it is especially difficult to reverse habitual use of nicotine. There is evidence showing that even though 80% of smokers express willingness to quit (CDC 2002) and 40% of them attempt to quit (CDC 2005), only 3% of them successfully quit (Hughes et al. 2004). One of the main contributors to this low rate of successful quitting is the negative symptoms experienced during nicotine withdrawal. Negative symptoms include irritability and restlessness, anxiety, social problems, increased food consumption, constipation, and craving for nicotine (Benowitz 2008). In addition to these general symptoms, cognitive deficits such as difficulty concentrating (Pomerleau et al. 2000), disrupted working memory (Jacobsen et al. 2005; Mendrek et al. 2006), verbal memory problems (Jacobsen et al. 2005), increased response time (Snyder et al. 1989; Bell et al. 1999), and problems in paired-associate learning (Kleinman et al. 1973) were observed during nicotine withdrawal. In line with these reports, animal studies also showed that while chronic nicotine did not have any effect on hippocampus-dependent contextual and trace fear conditioning, nicotine withdrawal impaired hippocampal learning and memory (Davis et al. 2005; Davis and Gould 2009; Raybuck and Gould 2009; Gould et al. 2012, 2014a; Portugal et al. 2012a, b; Wilkinson and Gould 2013). Overall, human and animal studies demonstrate hippocampus-dependent learning and memory enhancement during initial nicotine exposure and cognitive deficits during nicotine withdrawal. These results may suggest that initial nicotine exposure results in strong nicotine-context associations that support drug-seeking behavior, which may facilitate the transition into chronic nicotine use. Chronic use leads to neuronal adaptations that produce tolerance to the enhancing effects of nicotine and withdrawal deficits in hippocampus learning (Wilkinson et al. 2013; Gould et al. 2014a). Importantly, the nicotine withdrawal deficits in learning may increase the chance of relapse to avoid these negative symptoms. In line with this interpretation is the self-medication hypothesis of addiction, which states that one of the major contributors to addiction is the drive to reduce the negative symptoms that arise during drug withdrawal (Khantzian 1985). In support, nicotine has been shown to alleviate cognitive deficits in various mental disorders such as schizophrenia (Adler et al. 1993; for review, see Parikh et al. 2016) and attention deficit/hyperactivity disorder (ADHD, Potter and Newhouse 2008; Evans and Drobes 2009; for review, see Kutlu et al. 2015b), two populations that show higher rates of smoking (38%–42% of ADHD population, Pomerleau et al. 1995; 80%–90% in schizophrenia, George and Krystal 2000; de Leon and Diaz 2005). Also in support of the self-medication hypothesis, there is evidence showing that cognitive deficits during nicotine withdrawal predict future relapse to nicotine use (Rukstalis et al. 2005; Patterson et al. 2010). Thus, increased relapse to nicotine use during withdrawal may be a mechanism to self-medicate for withdrawal-induced cognitive deficits akin to what occurs with the cognitive impairments seen in mental disorders.
Modulation of hippocampal plasticity by nicotine
As the previous section explained, while acute nicotine enhances hippocampus-dependent learning and memory, these processes are disrupted during nicotine withdrawal. Evidence from electrophysiology studies support these conclusions and suggest that altered hippocampal plasticity by acute nicotine and withdrawal from chronic nicotine is largely responsible for these effects. For example, Alkondon et al. (2003) found that nicotinic acetylcholine receptor (nAChR) agonists lead to AMPA and NMDA receptor-mediated excitatory postsynaptic currents (EPSCs) in CA1 subregion of the hippocampus. Moreover, several studies showed that hippocampal nAChR activation by nicotine and other nAChR agonists resulted in enhanced hippocampal LTP (Fujii and Sumikawa 2000; Welsby et al. 2006, 2007; Jia et al. 2010); that is, strengthening of weak stimulation-induced short-term LTP (Fujii et al. 1999; Matsuyama et al. 2000; Matsuyama and Matsumoto 2003) as well as direct induction of LTP (He et al. 2000; Matsuyama et al. 2000; Matsuyama and Matsumoto 2003). Moreover, antagonism of nAChRs blocked learning-induced CA1 LTP (Mitsushima et al. 2012). In addition to the effects of acute administration of nicotine and other direct nAChR agonists, hippocampal plasticity has been shown to be altered during nicotine withdrawal. For example, Yamazaki et al. (2006) found that the lowered threshold for hippocampal LTP induction during nicotine administration was reversed during withdrawal. Therefore, the alterations of hippocampal LTP are in parallel with behavioral effects of acute nicotine and nicotine withdrawal on hippocampus-dependent learning. Thus, altered LTP may underlie the development of nicotine addiction through formation of drug-context associations during early use and the development of cognitive deficits during chronic use and withdrawal.
In further support of nicotine modulating synaptic plasticity, nicotine can alter key cell signaling kinases and transcription factors known to modulate hippocampal learning and plasticity such as PKA, ERK1/2, and CREB (for review, see Kutlu and Gould 2016). For example, Gould et al. (2014b) showed that infusions of a subthreshold dose of the PKA inhibitor PKI 14–22 amide into the dorsal hippocampus reversed enhancement of contextual fear conditioning by acute nicotine. Similarly, the same study also showed a temporal shift in learning-related dorsal hippocampal PKA peak activation as a result of systemic acute nicotine administration. There is also evidence showing that acute nicotine administration increased ERK1/-2 phosphorylation (Nakayama et al. 2001) and this effect was reversed by PKA inhibitors (Dajas-Bailador et al. 2002), suggesting that acute nicotine-induced alterations of PKA translates into changes in downstream MAPK signaling. Finally, nicotine also modulates the activation of the transcription factor CREB in the hippocampus. For example, acute nicotine enhanced CREB activation (Nakayama et al. 2001; Hu et al. 2002). CREB activity was also enhanced during nicotine CPP (Pascual et al. 2009) and acute nicotine-induced enhancement of contextual fear conditioning (Kenney et al. 2012), suggesting that CREB may be critical for the formation of drug-context memories. Overall, the above-mentioned studies provide strong evidence indicating that nicotine's control over hippocampal cell signaling cascades and consequently hippocampal plasticity may be an underlying factor for enhancement of drug-context memories by acute nicotine and cognitive deficits observed during nicotine withdrawal.
In summary, the results of the cited studies suggest that initially nicotine enhances drug-context associations, which leads to the development of sustained nicotine use and consequently nicotine dependence. While chronic nicotine does not seem to alter hippocampus-dependent learning and memory, these processes are impaired during nicotine withdrawal. This effect also seems to contribute to maintenance of nicotine dependence as smokers may self-medicate for withdrawal-induced cognitive deficits by returning to nicotine use.
Alcohol
The effects of acute ethanol on hippocampus-dependent learning and memory
Alcoholism is a complex behavioral and physiological phenomenon in which the abuser progressively loses control over alcohol consumption despite its negative health consequences (Koob et al. 1998). Indeed, severe health problems have been associated with alcohol overconsumption, including medical conditions ranging from cardiovascular and psychiatric diseases to certain cancers (e.g., mouth, liver, and esophageal cancers), and liver cirrhosis (Rehm et al. 2003). In its 2014 “Global status report on alcohol and health” the World Health Organization (WHO) stated that alcohol abuse is linked to more than 200 health problems and responsible for ∼3.3 million deaths world-wide every year (5.9% of all deaths), which establishes alcohol as one of the most harmful drugs of abuse to human health.
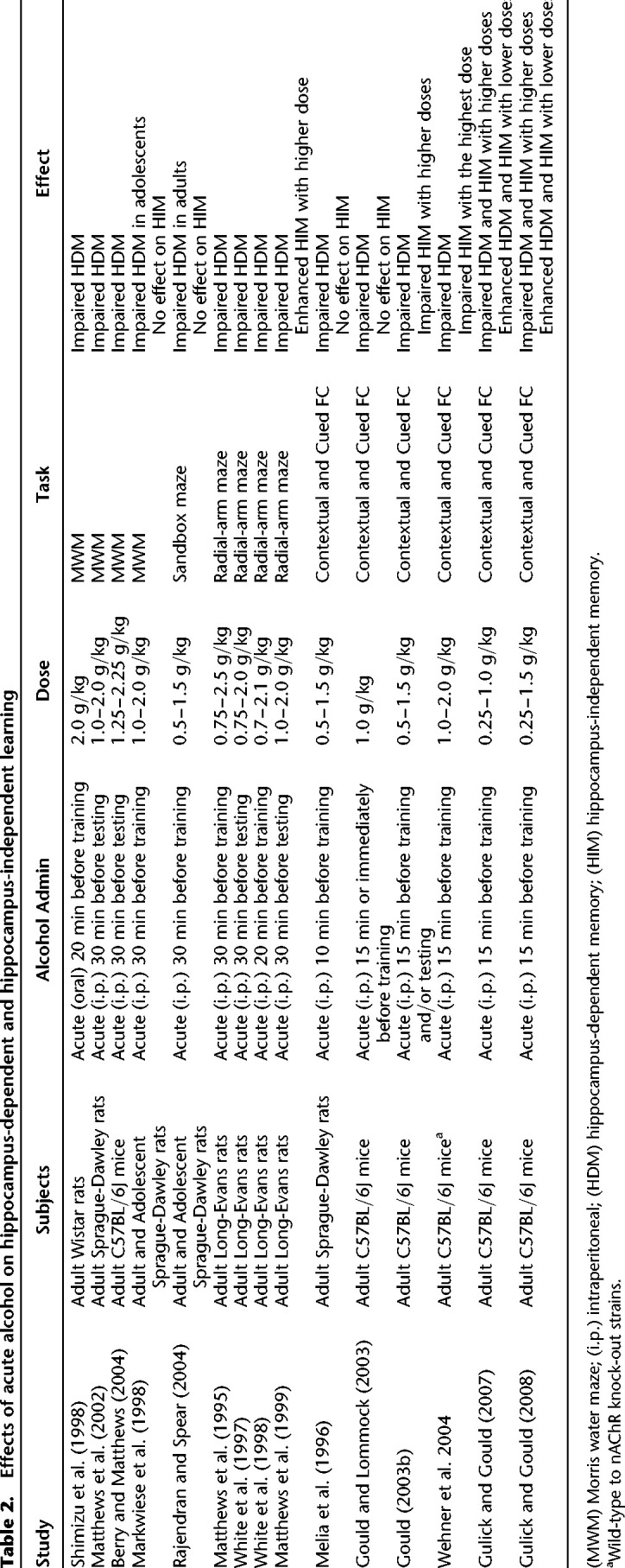
In addition to the overall health problems caused by alcohol abuse, alcoholism is also known to cause cognitive impairments (Ryan and Butters 1983; see Stavro et al. 2013 for a meta-analysis) with over 50% of alcoholics reporting memory and cognition problems (Vetreno et al. 2011). The results from laboratory animal studies also demonstrate alcohol's impairing effects on cognition. Specifically, acute ethanol administration impaired hippocampus-dependent learning and memory in the Morris Water Maze (Markwiese et al. 1998; Shimizu et al. 1998; Matthews et al. 2002; Berry and Matthews 2004), the radial arm maze (Matthews et al. 1995, 1999; Vandergriff et al. 1995; White et al. 1997, 1998), the sandbox maze (Rajendran and Spear 2004), and contextual fear conditioning (see Table 2 for the summary of acute ethanol's effects on hippocampus-dependent learning and memory) (Melia et al. 1996; Gould 2003b; Gould and Lommock 2003; Wehner et al. 2004; Gulick and Gould 2007, 2008).
Table 2.
Effects of acute alcohol on hippocampus-dependent and hippocampus-independent learning
In contrast, there is evidence showing that light to moderate alcohol intake (1–2 drinks per day) may decrease the risk of cardiovascular mortality, coronary heart disease, and stroke in humans (Fagrell et al. 1999; O'Keefe et al. 2014), which suggests that lower doses of ethanol may have some beneficial effects on human health. In addition, there is evidence showing that unlike higher doses of ethanol, lower doses are required for ethanol-induced dopaminergic reward signaling (Gessa et al. 1985), successful ethanol self-administration (Sinden and Le Magnen 1982) and ethanol CPP (Bienkowski et al. 1996; Cunningham and Henderson 2000). In fact, higher doses resulted in decreased ethanol self-administration and conditioned place aversion (CPA; Cunningham and Henderson 2000). Furthermore, Sinden and Le Magnen (1982) showed that low-dose ethanol self-administration linearly increased during 5 d of training whereas self-administration of a high-dose ethanol decreased significantly below saline levels. In line with these observations, Gulick and Gould (2007) found that while higher doses of acute ethanol resulted in deficits in both hippocampus-dependent contextual and hippocampus-independent cued fear conditioning, lower doses of ethanol enhanced both types of learning. This report is critical in terms of understanding how alcohol-context and alcohol-cue associations are formed following initial alcohol consumption. That is, Gulick and Gould's (2007) results suggest that lower doses of ethanol may enhance the formation of ethanol-context/cue associations initially, which may facilitate future ethanol intake. The data showing sustained self-administration and CPP with lower doses and decreased self-administration and CPA with higher doses of ethanol are also in support of this hypothesis. Therefore, these results suggest that a lower dose of ethanol facilitates maladaptive ethanol-context associations by enhancing reward processes and contextual and cued learning necessary for these maladaptive associations to occur.
Effects of prolonged alcohol exposure on hippocampal function
As described earlier, both rewarding and procognitive effects of initial alcohol exposure may predict increased rates of subsequent alcohol intake through enhanced drug-context learning. In addition to possible enhancement of hippocampus-dependent learning and memory with low doses of ethanol, studies also showed that prolonged ethanol consumption uniformly resulted in hippocampus-dependent spatial learning deficits (Bond and Di Giusto 1976; Beatty et al. 1984; Ehrlich and Humpel 2012), which were shown to be irreversible in some cases (Cippitelli et al. 2010). Binge ethanol exposure also results in necrotic cell death and neurodegeneration in the hippocampus (Obernier et al. 2002; Hamelink et al. 2005). Moreover, evidence from studies examining the effects of ethanol on hippocampal plasticity suggests that chronic ethanol inhibits hippocampal LTP (Durand and Carlen 1984; Tremwel and Hunter 1994; Roberto et al. 2002, 2003), which may underlie chronic ethanol-induced deficits in hippocampus-dependent learning and memory. Ethanol inhibition of LTP may result from decreased excitability of hippocampal neurons due to blockage of NMDA receptors, specifically NR1/NR2A or NR1/NR2B subtypes (Hoffman et al. 1989; Lovinger et al. 1989; Schummers and Browning 2001; Izumi et al. 2005; for review, see Allgaier 2002) as well as activation of inhibitory GABAA receptors (Allan and Harris 1986; Aguayo 1990; Reynolds et al. 1992; Schummers and Browning 2001). In addition to alterations of NMDA and GABA receptor function in the hippocampus, ethanol-induced suppression of hippocampal LTP has been associated with the inhibition of MAPK signaling (Sanna et al. 2002; Roberto et al. 2003; Chandler and Sutton 2005; Wang et al. 2012). Roberto et al. (2003) showed that following chronic intermittent ethanol treatment, both LTP and ERK1/2 activation were reduced in the hippocampus. Similarly, multiple studies found that ERK1/-2 phosphorylation was reduced in the hippocampus after chronic ethanol administration, whereas this effect was reversed during ethanol withdrawal and occurred again during ethanol reexposure (Sanna et al. 2002; Chandler and Sutton 2005; Wang et al. 2012). Moreover, CREB phosphorylation in the hippocampus was also inhibited following chronic ethanol exposure while ethanol withdrawal enhanced hippocampal CREB (Bison and Crews 2003). The ethanol-induced changes in MAPK-CREB pathway are crucial to understanding the molecular mechanisms underlying the hippocampal deficits during chronic ethanol exposure because numerous studies have shown that activation of this cell signaling pathway is necessary for long-term memory formation (e.g., Atkins et al. 1998; Vianna et al. 2000) and LTP (e.g., Winder et al. 1999; Kelleher et al. 2004; for review, see Kutlu and Gould 2016). Overall, these studies show that chronic ethanol can inhibit cell signaling cascades involved in learning through activation of GABAergic receptors and blockage of NMDA receptors in the hippocampus.
Evidence from human studies seems to be in agreement with animal studies showing chronic ethanol-induced cognitive impairments and disrupted hippocampal function. For example, impairments in learning and memory (Ryan and Butters 1983; Vetreno et al. 2011), attention and executive function (Loeber et al. 2009), and abstraction (Klisz and Parsons 1977; for review, see Bates et al. 2002) were reported after chronic alcohol use. These cognitive deficits also persisted during abstinence (Fein et al. 1990; Stavro et al. 2013). These learning and memory problems may contribute to relapse to alcohol use and maintenance of alcohol addiction as individuals with withdrawal-induced cognitive deficits attempt to self-medicate.
In line with chronic ethanol's impairing effects on cognition is a condition known as Wernicke–Korsakoff syndrome (WKS), which results from a prolonged alcohol exposure-induced thiamine deficiency. WKS is characterized by gradual impairments in memory function that spread into other cognitive domains as chronic alcohol use is maintained (Ryan et al. 1980; Cermak et al. 1988; for review, see Isenberg-Grzeda et al. 2012). Thus, WKS is associated with myriad of cognitive deficits such as impairment in verbal processing (Cermak et al. 1973, 1974; Oscar-Berman et al. 2004), discrimination learning (Jones et al. 1975), decision making (Brand et al. 2005), and spatial working memory (Joyce and Robbins 1991). WKS patients exhibited extensive damage to the hippocampus that was linked to the memory deficits (Sullivan and Marsh 2003; Caulo et al. 2005; Sullivan and Pfefferbaum 2009). Specifically, hippocampal volume in WKS patients was greatly reduced to a level comparable to patients with Alzheimer's disease (Sullivan and Marsh 2003). Moreover, an fMRI study showed that the hippocampal activity detected in controls during memory encoding and recognition was absent in the patients with WKS (Caulo et al. 2005). Results from WKS patients clearly indicate that prolonged alcohol exposure has deleterious effects on hippocampus function and morphology, which may be the source of hippocampus-dependent learning and memory deficits. Therefore, ethanol-induced neural damage and hippocampus-dependent learning deficits may contribute to the negative symptoms of alcoholism.
In conclusion, lower doses of acute ethanol enhance both hippocampus-dependent contextual and hippocampus-independent cued learning while higher doses of ethanol disrupt both types of learning. Nevertheless, hippocampus-dependent learning may be especially sensitive to the effects of acute ethanol because numerous studies suggest that acute ethanol alters hippocampal function. In contrast, chronic ethanol's detrimental effects on hippocampus-dependent learning and hippocampal function are relatively ubiquitous and one-sided. That is, chronic ethanol exposure alters hippocampal anatomy, disrupts hippocampal plasticity, and changes hippocampal cell signaling leading to impairments in hippocampus-dependent learning. These effects may contribute to the cognitive deficits that characterize ethanol-related syndromes such as WKS. Overall, given that acute ethanol enhances and chronic ethanol disrupts hippocampus-dependent learning, it is possible that initially learned drug-context memories are enhanced by acute ethanol and remain relatively unaltered during chronic ethanol use due to an inability to reverse established ethanol-context memories. This hindered ability to update drug-context association with new information may contribute to the persistence of and relapse to ethanol addiction.
Opiates
The effects of acute and chronic opiate exposure on hippocampus-dependent learning and memory
Opiates are a class of psychoactive compounds naturally derived from the opium poppy or synthetically produced that include morphine, diacetylmorphine (diamorphine or heroin), codeine, oxycodone, and methadone. Opiates such as oxycodone are legally prescribed to patients for pain management. Importantly, legally prescribed opiates lose their analgesic effects as tolerance develops, which, in some cases, results in drug dependence as patients try to achieve analgesic potency or avoid withdrawal symptoms. Consequently, although the prevalence of opiate addiction in the U.S. population is ∼8% (SAMHSA 2006b), opiate prescription use in chronic pain patients can be as high as 90% (Chabal et al. 1997; Manchikanti et al. 2004) and up to 16% of these patients have been documented to abuse prescribed opiates (Manchikanti et al. 2006). This suggests that opiate addiction is a potential risk to widespread segments of the population.
In addition to its analgesic effects, opiate-induced euphoria is the leading cause for opiate-seeking in nontherapeutic addicts but not in therapeutic addicts who abuse opiates for its analgesic potency (McAuliffe et al. 1985). However, as Koob et al. (1989) hypothesized, opiates’ positive effects are counterbalanced by severe negative consequences of opiate withdrawal such as aches and pain, agitation, anxiety, muscle cramps, nausea, and sleep disturbances (Alexander and Hadaway 1982; Jaffe 1990; West and Gossop 1994). Accordingly, while laboratory rodents learn CPP for morphine (Bardo et al. 1984, 1995; Tzschentke and Schmidt 1995; Milekic et al. 2006), heroin (Bozarth 1987; Bardo et al. 1995), methadone (Steinpreis et al. 1996), and oxycodone (Niikura et al. 2013), they also show CPA to the contexts associated with spontaneous withdrawal (Myers and Carlezon 2010) and precipitated withdrawal induced by opiate receptor antagonists such as naloxone (Jin et al. 2005; Stinus et al. 2005; Li et al. 2007; Manwell et al. 2009). It is important to note that development of CPA to withdrawal symptoms is in line with the fact that opiate withdrawal symptoms are usually considered more severe than symptoms associated with stimulant withdrawal (e.g., amphetamine and cocaine). Thus, it is possible that avoidance of withdrawal plays an especially important role in opiate dependence.
Similar to cocaine and ethanol, opiate abuse also correlates with long-lasting cognitive deficits in humans (Rogers et al. 1999; Darke et al. 2000; Ornstein et al. 2000; Curran et al. 2001; Davis et al. 2002). Abusers of morphine, heroin, and methadone show impairments in episodic memory (Curran et al. 2001), visual memory, verbal memory, information processing, problem solving (Darke et al. 2000), word fluency and attention (Davis et al. 2002), and spatial tactile and verbal memory (Hill and Mikhael 1979; Ornstein et al. 2000). In addition to the effects of chronic abuse of opiates, Curran et al. (2001) showed that a single dose of methadone resulted in impaired episodic memory in a population tolerant to opiates. Although several studies have suggested that opiate abuse-related cognitive decline may be linked to compromised frontal lobe function (Robinson and Kolb 1999; Ornstein et al. 2000), studies examining the effects of opiate exposure on hippocampus morphology and hippocampus-dependent learning and memory suggested that some of the cognitive deficits observed in opiate abusers may be related to altered hippocampal function. For example, opiates have been shown to inhibit adult neurogenesis in the hippocampus (Eisch et al. 2000) and alter proteins associated with hippocampal synaptic density such as clathrin (Morón et al. 2007). Moreover, acute morphine has also been shown to disrupt spatial memory retention in Morris water maze (Farahmandfar et al. 2010) and Y-maze (Ma et al. 2007), another hippocampus-dependent task (Retailleau et al. 2013). There is also evidence showing that chronic heroin and morphine impair hippocampus-dependent spatial learning in Morris water maze (Means et al. 1996; Tramullas et al. 2008), radial arm maze and Y-maze (Spain and Newsom 1991). Moreover, the impairing effects of chronic administration of opiates on spatial memory have been linked to an increase in proteins associated with apoptosis such as Fas, FasL, and Bad in the cortex and hippocampus (Tramullas et al. 2008), which suggests that chronic opiates may interfere with hippocampus-dependent learning through increased hippocampal neurotoxicity and cell-death. Finally, in addition to their effects on hippocampus-dependent learning and hippocampal function, both acute (Ito et al. 2001) and chronic opiate exposure (Ito et al. 2001; Salmanzadeh et al. 2003) have been shown to disrupt hippocampal plasticity in the form of decreased hippocampal LTP. These results show that both acute and chronic opiate administration result in deficits in hippocampus-dependent learning and memory and, therefore, it is difficult to explain sustained opiate use by enhanced drug-context memories in this case as there is no evidence suggesting that acute administration of opiates may enhance hippocampus-dependent learning and memory. Nevertheless, studies showing that laboratory rodents successfully learn CPP to opiates suggest that normal drug-context learning occurs through the reinforcing effects of the opiates.
Opiate withdrawal-induced enhancement of drug-context associations
Opiates may be different from the other drugs of abuse reviewed here as low doses of opiates do not enhance memory; instead, opiate withdrawal may alter hippocampal function contributing to formation of memories linking the aversive effects of withdrawal and withdrawal context. As mentioned, opiate withdrawal is often more severe than stimulant withdrawal (Alexander and Hadaway 1982; Jaffe 1990; West and Gossop 1994). Earlier models of opiate addiction, such as Wikler's (1948), proposed that avoidance of withdrawal symptoms is a major motivator for continued drug use. Also, latter models followed Wikler's hypothesis by describing opiate addiction as a balance between positive (reward) and negative (withdrawal) reinforcers (Koob et al. 1989). In line with these models, rodents learn to avoid contexts paired with spontaneous (Myers and Carlezon 2010) or opioid antagonist precipitated opiate withdrawal (Jin et al. 2005; Stinus et al. 2005; Li et al. 2007; Manwell et al. 2009). Human studies also report conditioned withdrawal symptoms to specific contexts (O'Brien et al. 1977; Childress et al. 1986; McLellan et al. 1986). For example, O'Brien et al. (1977) precipitated unconditioned withdrawal symptoms such as tearing, yawning, decreased skin temperature, increased heart rate, and rhinorrhea by using naloxone injections in a methadone-dependent group. These withdrawal symptoms occurred in a sound-attenuated room in the presence of a specific background music and odor. Conditioned withdrawal symptoms were tested following saline injections. The results of this study showed that participants exhibited strong conditioned withdrawal symptoms to the withdrawal context in the absence of naloxone. Moreover, there is qualitative evidence suggesting that contexts associated with withdrawal increased drug craving in opiate addicts (Wikler 1973). These results suggest that human opiate addicts also learn conditioned place aversion to the withdrawal context, which may contribute to future use of opiates in attempts to self-medicate to reduce negative symptoms associated with a withdrawal context.
Interestingly, opiate withdrawal is linked to normalization and enhancement of hippocampus-dependent learning and memory. For example, studies showed that while short-term withdrawal disrupted performance in the Y-maze (Ma et al. 2007) and Morris water maze (Dougherty et al. 1996), long-term withdrawal and naloxone-precipitated withdrawal reversed chronic opiate-induced impairments in these tasks (Dougherty et al. 1996; Li et al. 2001; Ma et al. 2007). Furthermore, there are data showing that spontaneous withdrawal from chronic morphine enhanced the acquisition of cocaine-self administration (He and Grasing 2004), suggesting that drug-context learning may be enhanced during opiate withdrawal. More direct evidence for enhancement of future drug seeking as a result of associations between withdrawal symptoms and withdrawal context came from Kenny et al. (2006) who showed that associations between contextual cues and naloxone-induced precipitated withdrawal symptoms enhanced heroin self-administration in heroin-dependent rats. These studies indicate that hippocampus-dependent drug withdrawal-context associations may reinforce future drug use to avoid negative withdrawal symptoms.
Studies examining hippocampal plasticity are in agreement with the view of enhanced opiate withdrawal-related memories. For example, Mansouri et al. (1997) found that hippocampal LTP was increased during withdrawal following 20 d of chronic morphine treatment via drinking water. Similarly, Ito et al. (2001) showed that hippocampal LTP was attenuated following acute (1 h) intracerebroventricular morphine administration via osmotic minipumps whereas it was enhanced when tested in withdrawal following 72 h of chronic morphine administration via the same route. This effect was reversed when the slices were treated with morphine, suggesting reexposure to morphine following withdrawal reversed the withdrawal-induced enhancement of hippocampal plasticity. Similarly, Salmanzadeh et al. (2003) showed that while LTP was reduced in hippocampal slices treated with morphine following chronic morphine administration via drinking water, treatment with artificial cerebrospinal fluid (ACSF, spontaneous withdrawal) or naloxone (precipitated withdrawal) reversed the chronic morphine-induced impairments in hippocampal LTP. There was also another study showing that morphine withdrawal following repeated injections of morphine reduced hippocampal LTP (Pu et al. 2002). However, discrepancies between chronic morphine administration regimens (continuous vs. intermittent) employed by these studies make it difficult to compare results. Still, it is possible that continuous administration of opiates via osmotic mini-pumps or drinking water may mimic prolonged opiate abuse better than short-term intermittent injections. Finally, there is evidence showing that hippocampal ERK1/-2 phosphorylation is enhanced during heroin withdrawal (Edwards et al. 2009), which indicates upregulated MAPK signaling may underlie opiate-induced enhancement of hippocampal plasticity. In summary, in contrast to acute and chronic opiate administration, converging evidence from multiple studies suggests that hippocampal plasticity may be enhanced during opiate withdrawal.
Overall, the studies reviewed here suggest a potential contributing factor for sustained opiate abuse. That is, strong memories are formed about the rewarding effects of opiates, as reflected by strong CPP, whereas the association between aversive withdrawal symptoms and the context is augmented by enhanced hippocampus-dependent learning and plasticity during withdrawal from chronic opiate administration. Thus, the withdrawal-associated context may produce a strong drive for self-medication in an attempt to ameliorate severe context-evoked withdrawal symptoms, which could result in relapse to opiate use and continued addiction. This effect may be dependent on the enhancement of hippocampal LTP and phosphorylation of cell signaling kinases critical for hippocampal plasticity such as MAPKs.
Cannabis
The effects of cannabis on hippocampus-dependent learning and memory
Cannabis is a flowering plant that has long been used for the production of hemp-based goods (e.g., hemp oil and hemp fiber) and medicinal purposes (Hillig 2005). Some subspecies of cannabis that are selectively bred for a high yield of Δ9-tetrahydrocannabinol (THC), the main psychoactive constituent in the cannabis, are used in recreational drugs such as hashish and marijuana. Cannabis use currently receives increasing amount of attention in the USA as both medicinal and recreational marijuana are legalized or decriminalized in more than 20 states. In addition to the discussions on legalization of cannabis products, with 40% of the population having used marijuana at least once in their lifetime and ∼23% current users, the prevalence of marijuana use is well-above cocaine, heroin, methamphetamine, inhalant abuse; matching cigarette smoking and alcohol use (SAMHSA 2014). Although usually perceived as a milder form of drug, like other drugs of abuse, short-term and long-term marijuana use has been linked to adverse health effects such as anxiety, psychosis, pulmonary problems as well as cognitive problems with prolonged use (Hall and Degenhardt 2009; Volkow et al. 2014). However, marijuana users also self-report positive effects of cannabis, including relaxation, analgesia, happiness, creativity, social benefits, and improved sleep (Goode 1970; Berke and Hernton 1974; Green et al. 2003). In line with the positive emotional effects of cannabis, THC-induced CPP (Valjent and Maldonado 2000; Braida et al. 2004; Ji et al. 2006; Le Foll et al. 2006) and THC self-administration (Takahashi and Singer 1979; Justinova et al. 2003; Braida et al. 2004) have been shown in laboratory animals. In these regards, cannabis exhibits similar properties with other drugs of abuse, namely rewarding effects with initial use. However, like opiates and unlike ethanol, cocaine, amphetamine, and nicotine, acute administration of THC and cannabidiol have been shown to disrupt hippocampus-dependent spatial learning in the Morris water maze and radial arm maze (Lichtman et al. 1995; Lichtman and Martin 1996; Da Silva and Takahashi 2002; Cha et al. 2007; Niyuhire et al. 2007) as well as contextual fear conditioning (Lemos et al. 2010; Stern et al. 2012). In line with the animal studies, human studies also suggest that acute cannabinoids result in impaired memory (Tinklenberg et al. 1970; Ferraro 1980; for review, see Ranganathan and D'Souza 2006). Therefore, in the case of cannabis, drug-context associations are formed in spite of hippocampal learning difficulties associated with cannabis use.
Cannabis dependence also results in a withdrawal syndrome during abstinence, which is characterized by heightened anxiety, irritability, negative mood, restlessness, shakiness, sleeping difficulty, stomach pain, strange dreams, sweating, and weight loss (Kouri and Pope 2000; Budney et al. 2003; for review, see Budney and Hughes 2006). These symptoms have been shown to be reversed by reexposure to THC (Budney et al. 2007). Comparative studies found that nicotine and cannabis withdrawal syndromes share similar negative symptoms (Vandrey et al. 2005, 2008; Budney et al. 2008). Moreover, as shown by self-report studies, the majority of cannabis users indicated that negative withdrawal symptoms were the major reason for their inability to quit and they attempted to self-medicate these symptoms, which usually resulted in relapse to cannabis use (Budney et al. 1998, 1999; Crowley et al. 1998; Copeland et al. 2001; Coffey et al. 2002; Stephens et al. 2002; Vandrey et al. 2005; Copersino et al. 2006). Also similar to nicotine withdrawal, marijuana users show cognitive deficits during abstinence from cannabis such as impaired mathematical skills, disrupted verbal expression, altered encoding and retrieval of verbal memories, attentional problems, and executive function deficits (Block and Ghoneim 1993; Solowij 1995; Pope and Yurgelun-Todd 1996). There is evidence showing that rats undergoing THC withdrawal show deficits in hippocampus-dependent spatial learning (Wise et al. 2011). Overall, these studies suggest that both acute administration and abstinence from cannabis result in difficulties in cognition. Therefore, in line with self-medication hypothesis, it is possible that while hippocampus-dependent memory enhancement seen with nicotine is not apparent for cannabis, relapse to cannabis use due to withdrawal-induced cognitive and emotional deficits is a possible contributing factor for the maintenance of cannabis addiction.
Alterations in hippocampal plasticity by cannabinoids
Disruption of hippocampus-dependent learning as a result of acute cannabis use may result from deficits in hippocampal plasticity. For example, acute THC reduced the amplitude of both spontaneous and conditioned stimulus evoked potentials in the hippocampus (Campbell et al. 1986a,b). Moreover, THC eliminated the firing of the neurons in the CA1 subregion of the hippocampus induced by delayed match-to-sample task performance (Heyser et al. 1993). Furthermore, like THC, acute cannabinoid receptor agonists (e.g., WIN-55,212-2 and CP 55,940) also disrupted spatial memory in rats (Lichtman et al. 1995) and reduced hippocampal LTP (Nowicky et al. 1987; Collins et al. 1994, 1995; Terranova et al. 1995; Puighermanal et al. 2009; for review, see Sullivan 2000). For example, Puighermanal et al. (2009) showed that cannabinoid receptor CB1 activation impaired hippocampal LTP and this effect was associated with amnesic effects of acute THC in a hippocampus-dependent context recognition task. Moreover, enhanced hippocampal plasticity has been documented in mice lacking CB1 receptors (Bohme et al. 1999). In line with a modulatory role of CB1 receptors in hippocampal plasticity, CB1 activation has been shown to inhibit glutamatergic synapses in the hippocampus (Takahashi and Castillo 2006), indicating a negative regulation of NMDAR-dependent long-term memory formation by CB1 receptors. Although a majority of studies examining the effects of acute cannabinoids on hippocampal plasticity reported impaired hippocampal LTP, there is also evidence for disruption of LTP during withdrawal following chronic THC administration (Hoffman et al. 2007). Specifically, Hoffman et al. (2007) found that withdrawal following 3 or 7 d of chronic THC administration, but not following 1 d of administration, resulted in decreased hippocampal LTP 24 h after the last injection. Similarly, Fan et al. (2010) showed that following 7 d of repeated administration of THC, hippocampal LTP as well as phosphorylation of hippocampal CREB were attenuated during withdrawal and these effects were reversed by inhibition or deletion of hippocampal CB1 receptors. However, human chronic marijuana smokers have been shown to exhibit downregulated CB1 receptors in a variety of brain regions including the hippocampus (Hirvonen et al. 2012). This result seems to be conflicting with studies showing improved hippocampal plasticity with deletion or inhibition of CB1 receptors. The downregulation does not speak to the functional state of the receptors and thus, it is possible that these receptors may be more responsive to endocannabinoids, which may result in increased efficiency of the endocannabinoid system compared with systems where CB1 receptors are inhibited or completely absent. Thus, these studies suggest that both acute and withdrawal from chronic THC disrupted hippocampal LTP and these effects were associated with CB1 receptor activation. Given the apparent parallels between impairments in hippocampus-dependent learning and memory and disrupted hippocampal LTP, it is likely that disrupted hippocampal plasticity may underlie the hippocampus-dependent learning and memory deficits observed following acute cannabinoid agonism and withdrawal from chronic cannabinoid administration.
Overall, the studies cited here clearly show that both acute administration of THC and THC withdrawal lead to cognitive deficits and these effects are mediated by CB1 receptor-mediated disruption of hippocampal plasticity. Given that removal of cognitive deficits exhibited by chronic marijuana smokers is one of the major motivators for relapse to smoke marijuana (Budney et al. 1998, 1999; Crowley et al. 1998; Copeland et al. 2001; Coffey et al. 2002; Stephens et al. 2002; Vandrey et al. 2005; Copersino et al. 2006), it is possible that hippocampal cognitive deficits trigger a self-medication response and contributes to maintenance of cannabis addiction.
Conclusion
In summary, the studies reviewed here provide evidence for the involvement of hippocampus-dependent learning and memory as well as hippocampal plasticity in development and maintenance of addiction. Specifically, acute administration of stimulants such as cocaine, nicotine, and amphetamine, as well as alcohol enhances hippocampus-dependent learning and memory. It is possible that this augments drug-context associations and contributes to future drug use. On the other hand, opiates and cannabis seem to disrupt hippocampus-dependent learning and memory following acute administration. Nevertheless, both opiates and cannabis produce strong CPP, suggesting that successful drug-context associations are formed regardless. In addition to the enhancement of drug-context associations during acute administration of these drugs, all drugs of abuse reviewed here except opiates produce strong deficits in hippocampus-dependent learning and memory and attenuated hippocampal plasticity during withdrawal, which may motivate attempts to self-medicate resulting in relapse and maintenance of drug use. In the case of opiates, unlike other drugs of abuse, withdrawal leads to enhanced hippocampus-dependent learning and memory, which may facilitate the development of context-evoked withdrawal symptoms that could facilitate relapse. Overall, human and laboratory animal studies suggest a significant role of drug-induced alterations of hippocampus-dependent learning and memory in development and maintenance of drug addiction.
Competing interest statement
We declare no potential conflict of interest.
Acknowledgments
T.J.G. was funded with grant support from the National Institute on Drug Abuse (DA017949).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.042192.116.
References
- Abdulla FA, Gray JA, Sinden JD, Bradbury E, Calaminici MR, Lippiello PM, Wonnacott S. 1996. Relationship between up-regulation of nicotine binding sites in rat brain and delayed cognitive enhancement observed after chronic or acute nicotinic receptor stimulation. Psychopharmacology 124: 323–331. [DOI] [PubMed] [Google Scholar]
- Abel T, Lattal KM. 2001. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol 11: 180–187. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LD, Wiser A, Freedman R. 1993. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 150: 1856–1861. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Rawlins JNP. 1986. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res 19: 133–146. [DOI] [PubMed] [Google Scholar]
- Aguayo LG. 1990. Demonstration that ethanol potentiates the GABAA-activated Cl− current in central mammalian neurons. Alcohol Alcohol Suppl 1: 187–190. [PubMed] [Google Scholar]
- Alexander BK, Hadaway PF. 1982. Opiate addiction: the case for an adaptive orientation. Psychol Bull 92: 367. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. 2003. NMDA and AMPA receptors contribute to the nicotinic cholinergic excitation of CA1 interneurons in the rat hippocampus. J Neurophysiol 90: 1613–1625. [DOI] [PubMed] [Google Scholar]
- Allan AM, Harris RA. 1986. Gamma-aminobutyric acid and alcohol actions: neurochemical studies of long sleep and short sleep mice. Life Sci 39: 2005–2015. [DOI] [PubMed] [Google Scholar]
- Allgaier C. 2002. Ethanol sensitivity of NMDA receptors. Neurochem Int 41: 377–382. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Pierce RC. 2005. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther 106: 389–403. [DOI] [PubMed] [Google Scholar]
- Ardila A, Rosselli M, Strumwasser S. 1991. Neuropsychological deficits in chronic cocaine abusers. Int J Neurosci 57: 73–79. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. 1998. The MAPK cascade is required for mammalian associative learning. Nat Neurosci 1: 602–609. [DOI] [PubMed] [Google Scholar]
- Bardo MT. 1998. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol 12: 37–67. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Miller JS, Neisewander JL. 1984. Conditioned place preference with morphine: the effect of extinction training on the reinforcing CR. Pharmacol Biochem Behav 21: 545–549. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. 1995. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev 19: 39–51. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Valone JM, Bevins RA. 1999. Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology 143: 39–46. [DOI] [PubMed] [Google Scholar]
- Bates T, Mangan G, Stough C, Corballis P. 1995. Smoking, processing speed and attention in a choice reaction time task. Psychopharmacology 120: 209–212. [DOI] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, Barry D. 2002. Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Exp Clin Psychopharmacol 10: 193. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Bengtson KR, Lunn RJ, Staton RD, Brumback RA. 1984. Comparative effects of long-term ethanol consumption and forebrain lesions on maze learning and active avoidance behavior in rats. Alcohol 1: 465–470. [DOI] [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. 1999. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine Tob Res 1: 45–52. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. 1996. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol 36: 597–613. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. 2008. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther 83: 531–541. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. 2010. Nicotine addiction. N Engl J Med 362: 2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JH, Hernton CC. 1974. The cannabis experience: an interpretative study of the effects of marijuana and hashish. Peter Owen Publishers. [Google Scholar]
- Berry RB, Matthews DB. 2004. Acute ethanol administration selectively impairs spatial memory in C57BL/6J mice. Alcohol 32: 9–18. [DOI] [PubMed] [Google Scholar]
- Bett WR. 1946. Benzedrine sulphate in clinical medicine: a survey of the literature. Postgrad Med J 22: 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. 2006. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci 23: 3109–3118. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kuca P, Piasecki J, Kostowski W. 1996. Low dose of ethanol induces conditioned place preference in rats after repeated exposures to ethanol or saline injections. Alcohol Alcohol 31: 547–553. [DOI] [PubMed] [Google Scholar]
- Bison S, Crews F. 2003. Alcohol withdrawal increases neuropeptide Y immunoreactivity in rat brain. Alcohol Clin Exp Res 27: 1173–1183. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Janak PH. 2006. Post-training and post-reactivation administration of amphetamine enhances morphine conditioned place preference. Behav Brain Res 171: 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaiss CA, Janak PH. 2007. Post-training, but not post-reactivation, administration of amphetamine and anisomycin modulates Pavlovian conditioned approach. Neurobiol Learn Mem 87: 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. 1993. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39. [DOI] [PubMed] [Google Scholar]
- Block RI, Ghoneim MM. 1993. Effects of chronic marijuana use on human cognition. Psychopharmacology 110: 219–228. [DOI] [PubMed] [Google Scholar]
- Blokland A, Honig W, Prickaerts J. 1998. Effects of haloperidol and d-amphetamine on working and reference memory performance in a spatial cone field task. Behav Pharmacol 9: 429–436. [DOI] [PubMed] [Google Scholar]
- Bohme GA, Laville M, Ledent C, Parmentier M, Imperato A. 1999. Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience 95: 5–7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Rothman R, Cadet JL. 1999. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci 11: 361–369. [DOI] [PubMed] [Google Scholar]
- Bond NW, Di Giusto EL. 1976. Impairment of Hebb-Williams maze performance following prolonged alcohol consumption in rats. Pharmacol Biochem Behav 5: 85–86. [DOI] [PubMed] [Google Scholar]
- Bozarth MA. 1987. Conditioned place preference: a parametric analysis using systemic heroin injections. In Methods of assessing the reinforcing properties of abused drugs (pp. 241–273). Springer, New York. [Google Scholar]
- Braida D, Iosuè S, Pegorini S, Sala M. 2004. Δ9-Tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol 506: 63–69. [DOI] [PubMed] [Google Scholar]
- Brand M, Fujiwara E, Borsutzky S, Kalbe E, Kessler J, Markowitsch HJ. 2005. Decision-making deficits of korsakoff patients in a new gambling task with explicit rules: associations with executive functions. Neuropsychology 19: 267–277. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, et al. 1997. Acute effects of cocaine on human brain activity and emotion. Neuron 19: 591–611. [DOI] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. 2008. Nicotine place preference in a biased conditioned place preference design. Pharmacol Biochem Behav 89: 94–100. [DOI] [PubMed] [Google Scholar]
- Brody SL, Slovis CM, Wrenn KD. 1990. Cocaine-related medical problems: consecutive series of 233 patients. Am J Med 88: 325–331. [DOI] [PubMed] [Google Scholar]
- Brown RW, Bardo MT, Mace DD, Phillips SB, Kraemer PJ. 2000. D-amphetamine facilitation of Morris water task performance is blocked by eticlopride and correlated with increased dopamine synthesis in the prefrontal cortex. Behav Brain Res 114: 135–143. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR. 2006. The cannabis withdrawal syndrome. Curr Opin Psychiatry 19: 233–238. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Radonovich KJ, Higgins ST, Wong CJ. 1998. Adults seeking treatment for marijuana dependence: a comparison with cocaine-dependent treatment seekers. Exp Clin Psychopharmacol 6: 419. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. 1999. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction 94: 1311–1322. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, Hughes JR. 2003. The time course and significance of cannabis withdrawal. J Abnorm Psychol 112: 393–402. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. 2007. Oral Δ-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend 86: 22–29. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. 2008. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat 35: 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. 2002. The human hippocampus and spatial and episodic memory. Neuron 35: 625–641. [DOI] [PubMed] [Google Scholar]
- Campbell KA, Foster TC, Hampson RE, Deadwyler SA. 1986a. Δ9-Tetrahydrocannabinol differentially affects sensory-evoked potentials in the rat dentate gyrus. J Pharmacol Exp Ther 239: 936–940. [PubMed] [Google Scholar]
- Campbell KA, Foster TC, Hampson RE, Deadwyler SA. 1986b. Effects of Δ9-tetrahydrocannabinol on sensory-evoked discharges of granule cells in the dentate gyrus of behaving rats. J Pharmacol Exp Ther 239: 941–945. [PubMed] [Google Scholar]
- Cao G, Zhu J, Zhong Q, Shi C, Dang Y, Han W, Liu X, Xu M, Chen T. 2013. Distinct roles of methamphetamine in modulating spatial memory consolidation, retrieval, reconsolidation and the accompanying changes of ERK and CREB activation in hippocampus and prefrontal cortex. Neuropharmacology 67: 144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone D. 1992. Smoking and cancer. Am J Med 93: S13–S17. [DOI] [PubMed] [Google Scholar]
- Carr GD, Phillips AG, Fibiger HC. 1988. Independence of amphetamine reward from locomotor stimulation demonstrated by conditioned place preference. Psychopharmacology 94: 221–226. [DOI] [PubMed] [Google Scholar]
- Caulo M, Van Hecke J, Toma L, Ferretti A, Tartaro A, Colosimo C, Romani GL, Uncini A. 2005. Functional MRI study of diencephalic amnesia in Wernicke–Korsakoff syndrome. Brain 128: 1584–1594. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2002. Cigarette smoking among adults—United States, 2000. MMWR Morb Mortal Wkly Rep 51: 642–645. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2005. Cigarette smoking among adults—United States, 2004. MMWR Morb Mortal Wkly Rep 54: 1121–1124. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2007. CDC HIV/AIDS fact sheet: methamphetamine use and risk for HIV/AIDS. Author, Atlanta, GA. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2012. Current cigarette smoking among adults—United States, 2011. MMWR Morb Mortal Wkly Rep 61: 889–894. [PubMed] [Google Scholar]
- Cermak LS, Butters N, Gerrein J. 1973. The extent of the verbal encoding ability of Korsakoff patients. Neuropsychologia 11: 85–94. [DOI] [PubMed] [Google Scholar]
- Cermak LS, Butters N, Moreines J. 1974. Some analyses of the verbal encoding deficit of alcoholic Korsakoff patients. Brain Lang 1: 141–150. [Google Scholar]
- Cermak LS, Bleich RP, Blackford SP. 1988. Deficits in the implicit retention of new associations by alcoholic Korsakoff patients. Brain Cogn 7: 312–323. [DOI] [PubMed] [Google Scholar]
- Cervo L, Mukherjee S, Bertaglia A, Samanin R. 1997. Protein kinases A and C are involved in the mechanisms underlying consolidation of cocaine place conditioning. Brain Res 775: 30–36. [DOI] [PubMed] [Google Scholar]
- Cha YM, Jones KH, Kuhn CM, Wilson WA, Swartzwelder HS. 2007. Sex differences in the effects of Δ9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav Pharmacol 18: 563–569. [DOI] [PubMed] [Google Scholar]
- Chabal C, Erjavec MK, Jacobson L, Mariano A, Chaney E. 1997. Prescription opiate abuse in chronic pain patients: clinical criteria, incidence, and predictors. Clin J Pain 13: 150–155. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Sutton G. 2005. Acute ethanol inhibits extracellular signal-regulated kinase, protein kinase B, and adenosine 3′: 5′-cyclic monophosphate response element binding protein activity in an age- and brain region-specific manner. Alcohol Clin Exp Res 29: 672–682. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O'Brien CP. 1986. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict 81: 655–660. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. 1999. Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Zook M, Bell L, Damadzic R, Eskay RL, Schwandt M, Heilig M. 2010. Reversibility of object recognition but not spatial memory impairment following binge-like alcohol exposure in rats. Neurobiol Learn Mem 94: 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey C, Carlin JB, Degenhardt L, Lynskey M, Sanci L, Patton GC. 2002. Cannabis dependence in young adults: an Australian population study. Addiction 97: 187–194. [DOI] [PubMed] [Google Scholar]
- Collins DR, Pertwee RG, Davies SN. 1994. The action of synthetic cannabinoids on the induction of long-term potentiation in the rat hippocampal slice. Eur J Pharmacol 259: R7–R8. [DOI] [PubMed] [Google Scholar]
- Collins DR, Pertwee RG, Davies SN. 1995. Prevention by the cannabinoid antagonist, SR141716A, of cannabinoid-mediated blockade of long-term potentiation in the rat hippocampal slice. Br J Pharmacol 115: 869–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Mangan GL, Pellett OL, Bates TC. 1992. Effects of post-learning smoking on memory consolidation. Psychopharmacology 108: 448–451. [DOI] [PubMed] [Google Scholar]
- Copeland J, Swift W, Rees V. 2001. Clinical profile of participants in a brief intervention program for cannabis use disorder. J Subst Abuse Treat 20: 45–52. [DOI] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. 2006. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict 15: 8–14. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Macdonald MJ, Whitmore EA, Mikulich SK. 1998. Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct symptoms and substance use disorders. Drug Alcohol Depend 50: 27–37. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Henderson CM. 2000. Ethanol-induced conditioned place aversion in mice. Behav Pharmacol 11: 591–602. [DOI] [PubMed] [Google Scholar]
- Curran HV, Kleckham J, Bearn J, Strang J, Wanigaratne S. 2001. Effects of methadone on cognition, mood and craving in detoxifying opiate addicts: a dose-response study. Psychopharmacology 154: 153–160. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O'Brien CP. 2001. Cocaine dependence: a disease of the brain's reward centers. J Subst Abuse Treat 21: 111–117. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Soliakov L, Wonnacott S. 2002. Nicotine activates the extracellular signal-regulated kinase 1/2 via the α7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J Neurochem 80: 520–530. [DOI] [PubMed] [Google Scholar]
- Darke S, Sims J, McDonald S, Wickes W. 2000. Cognitive impairment among methadone maintenance patients. Addiction 95: 687–695. [DOI] [PubMed] [Google Scholar]
- Da Silva GE, Takahashi RN. 2002. SR 141716A prevents Δ9-tetrahydrocannabinol-induced spatial learning deficit in a Morris-type water maze in mice. Prog Neuropsychopharmacol Biol Psychiatry 26: 321–325. [DOI] [PubMed] [Google Scholar]
- Daumas S, Halley H, Francés B, Lassalle JM. 2005. Encoding, consolidation, and retrieval of contextual memory: differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learn Mem 12: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. 2006. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology 184: 345–352. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. 2007. β2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology 190: 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. 2009. Hippocampal nAChRs mediate nicotine withdrawal-related learning deficits. Eur Neuropsychopharmacol 19: 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PE, Liddiard H, McMillan TM. 2002. Neuropsychological deficits and opiate abuse. Drug Alcohol Depend 67: 105–108. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. 2005. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci 25: 8708–8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Porter J, Gould TJ. 2006. Nicotine enhances both foreground and background contextual fear conditioning. Neurosci Lett 394: 202–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. 2007. Hippocampal α4β2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci 27: 10870–10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanoy RL, Tucci DL, Gold PE. 1983. Amphetamine effects on long term potentiation in dentate granule cells. Pharmacol Biochem Behav 18: 137–139. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. 2005. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res 76: 135–157. [DOI] [PubMed] [Google Scholar]
- Del Olmo N, Higuera-Matas A, Miguéns M, García-Lecumberri C, Ambrosio E. 2007. Cocaine self-administration improves performance in a highly demanding water maze task. Psychopharmacology 195: 19–25. [DOI] [PubMed] [Google Scholar]
- Deneau G, Yanagita T, Seevers MH. 1969. Self-administration of psychoactive substances by the monkey. Psychopharmacologia 16: 30–48. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, De Vries W, Raasø H, Schoffelmeer ANM, De Vries TJ. 2008. Contextual renewal of nicotine seeking in rats and its suppression by the cannabinoid-1 receptor antagonist Rimonabant (SR141716A). Neuropharmacology 55: 712–716. [DOI] [PubMed] [Google Scholar]
- Dols M, Willems B, van den Hout M, Bittoun R. 2000. Smokers can learn to influence their urge to smoke. Addict Behav 25: 103–108. [DOI] [PubMed] [Google Scholar]
- Dols M, van den Hout M, Kindt M, Willems B. 2002. The urge to smoke depends on the expectation of smoking. Addiction 97: 87–93. [DOI] [PubMed] [Google Scholar]
- Domínguez-Escribà L, Hernández-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, García-Verdugo JM, Canales JJ. 2006. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur J Neurosci 24: 586–594. [DOI] [PubMed] [Google Scholar]
- Doty BA, Doty LA. 1966. Facilitative effects of amphetamine on avoidance conditioning in relation to age and problem difficulty. Psychopharmacologia 9: 234–241. [DOI] [PubMed] [Google Scholar]
- Dougherty KD, Walsh TJ, Bailey S, Schlussman S, Grasing K. 1996. Acquisition of a Morris water maze task is impaired during early but not late withdrawal from morphine. Pharmacol Biochem Behav 55: 227–235. [DOI] [PubMed] [Google Scholar]
- Durand D, Carlen PL. 1984. Impairment of long-term potentiation in rat hippocampus following chronic ethanol treatment. Brain Res 308: 325–332. [DOI] [PubMed] [Google Scholar]
- Edwards S, Graham DL, Whisler KN, Self DW. 2009. Phosphorylation of GluR1, ERK, and CREB during spontaneous withdrawal from chronic heroin self-administration. Synapse 63: 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich D, Humpel C. 2012. Chronic vascular risk factors (cholesterol, homocysteine, ethanol) impair spatial memory, decline cholinergic neurons and induce blood–brain barrier leakage in rats in vivo. J Neurol Sci 322: 92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. 2000. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci 97: 7579–7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts D, Jones SR. 2010. The hypocretin–orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci 31: 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Drobes DJ. 2009. Nicotine self-medication of cognitive-attentional processing. Addict Biol 14: 32–42. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. 1999. Associative processes in addiction and reward the role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci 877: 412–438. [DOI] [PubMed] [Google Scholar]
- Fagrell B, De Faire U, Bondy S, Criqui M, Gaziano M, Gronbaek M, Jackson R, Klatsky A, Salonen J, Shaper AG. 1999. The effects of light to moderate drinking on cardiovascular diseases. J Intern Med 246: 331–340. [DOI] [PubMed] [Google Scholar]
- Fan N, Yang H, Zhang J, Chen C. 2010. Reduced expression of glutamate receptors and phosphorylation of CREB are responsible for in vivo Δ9-THC exposure-impaired hippocampal synaptic plasticity. J Neurochem 112: 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahmandfar M, Karimian SM, Naghdi N, Zarrindast MR, Kadivar M. 2010. Morphine-induced impairment of spatial memory acquisition reversed by morphine sensitization in rats. Behav Brain Res 211: 156–163. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. 1990. Cognitive impairments in abstinent alcoholics. West J Med 152: 531–537. [PMC free article] [PubMed] [Google Scholar]
- Ferraro DP. 1980. Acute effects of marijuana on human memory and cognition. NIDA Res Monogr 31: 98–119. [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. 2005. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30: 296–309. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. 2007. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci 26: 487–498. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Teoh KW, Iwamoto ET. 1985. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav 22: 237–241. [DOI] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Morita N, Sumikawa K. 1999. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res 846: 137–143. [DOI] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Sumikawa K. 2000. Inactivation of α7 ACh receptors and activation of non-α7 ACh receptors both contribute to long term potentiation induction in the hippocampal CA1 region. Neurosci Lett 286: 134–138. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R. 2008. Acute effects of cocaine on the neurobiology of cognitive control. Philos Trans R Soc Lond B Biol Sci 363: 3267–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Krystal JH. 2000. Comorbidity of psychiatric and substance abuse disorders. Curr Opin Psychiatry 13: 327–331. [Google Scholar]
- Gerdjikov TV, Ross GM, Beninger RJ. 2004. Place preference induced by nucleus accumbens amphetamine is impaired by antagonists of ERK or p38 MAP kinases in rats. Behav Neurosci 118: 740. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. 1985. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res 348: 201–203. [DOI] [PubMed] [Google Scholar]
- Gold PE, Delanoy RL, Merrin J. 1984. Modulation of long-term potentiation by peripherally administered amphetamine and epinephrine. Brain Res 305: 103–107. [DOI] [PubMed] [Google Scholar]
- Goode E. 1970. The marijuana smokers (pp. p201–202). Basic Books, New York. [Google Scholar]
- Goodman J, Packard MG. 2016. Memory systems and the addicted brain. Front Psychiatry 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooney M, Shaw K, Kelly A, O'Mara SM, Lynch MA. 2002. Long-term potentiation and spatial learning are associated with increased phosphorylation of TrkB and extracellular signal-regulated kinase (ERK) in the dentate gyrus: evidence for a role for brain-derived neurotrophic factor. Behav Neurosci 116: 455. [DOI] [PubMed] [Google Scholar]
- Gould TJ. 2003a. Nicotine produces a within-subject enhancement of contextual fear conditioning in C57BL/6 mice independent of sex. Integr Physiol Behav Sci 38: 124–132. [DOI] [PubMed] [Google Scholar]
- Gould TJ. 2003b. Ethanol disrupts fear conditioning in C57BL/6 mice. J Psychopharmacol 17: 77–81. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. 2003. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem 80: 147–157. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lommock JA. 2003. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci 117: 1276. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. 1999. Nicotine enhancement of contextual fear conditioning. Behav Brain Res 102: 31–39. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Feiro O, Moore D. 2004. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behav Brain Res 155: 167–173. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Portugal GS, André JM, Tadman MP, Marks MJ, Kenney JW, Yildirim E, Adoff M. 2012. The duration of nicotine withdrawal-associated deficits in contextual fear conditioning parallels changes in hippocampal high affinity nicotinic acetylcholine receptor upregulation. Neuropharmacology 62: 2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Wilkinson DS, Yildirim E, Blendy JA, Adoff MD. 2014a. Dissociation of tolerance and nicotine withdrawal-associated deficits in contextual fear. Brain Res 1559: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Wilkinson DS, Yildirim E, Poole RL, Leach PT, Simmons SJ. 2014b. Nicotine shifts the temporal activation of hippocampal protein kinase A and extracellular signal-regulated kinase 1/2 to enhance long-term, but not short-term, hippocampus-dependent memory. Neurobiol Learn Mem 109: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. 2006. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology 184: 456–463. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. 1996. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci 93: 12040–12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BOB, Kavanagh D, Young R. 2003. Being stoned: a review of self-reported cannabis effects. Drug Alcohol Rev 22: 453–460. [DOI] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. 2010. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage 53: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Zhang R, Xu Y, Li S. 2009. Cocaine withdrawal enhances long-term potentiation in rat hippocampus via changing the activity of corticotropin-releasing factor receptor subtype 2. Neuroscience 161: 665–670. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. 2007. Acute ethanol has biphasic effects on short- and long-term memory in both foreground and background contextual fear conditioning in C57BL/6 mice. Alcohol Clin Exp Res 31: 1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. 2008. Interactive effects of ethanol and nicotine on learning in C57BL/6J mice depend on both dose and duration of treatment. Psychopharmacology 196: 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann E, Sargant W. 1937. Observations on benzedrine. Br Med J 1: 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. 2007. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci 27: 3477–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim DY, Lippmann ML, Goldberg SK, Walkenstein MD. 1995. The pulmonary complications of crack cocaine. A comprehensive review. Chest 107: 233–240. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. 2009. Adverse health effects of non-medical cannabis use. Lancet 374: 1383–1391. [DOI] [PubMed] [Google Scholar]
- Halliday R, Naylor H, Brandeis D, Callaway E, Yano L, Herzig K. 1994. The effect of D-amphetamine, clonidine, and yohimbine on human information processing. Psychophysiology 31: 331–337. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. 2005. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Ther 314: 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han WY, Du P, Fu SY, Wang F, Song M, Wu CF, Yang JY. 2014. Oxytocin via its receptor affects restraint stress-induced methamphetamine CPP reinstatement in mice: involvement of the medial prefrontal cortex and dorsal hippocampus glutamatergic system. Pharmacol Biochem Behav 119: 80–87. [DOI] [PubMed] [Google Scholar]
- He S, Grasing K. 2004. Chronic opiate treatment enhances both cocaine-reinforced and cocaine-seeking behaviors following opiate withdrawal. Drug Alcohol Depend 75: 215–221. [DOI] [PubMed] [Google Scholar]
- He J, Deng CY, Chen RZ, Zhu XN, Yu JP. 2000. Long-term potentiation induced by nicotine in CA1 region hippocampal slice is Ca2+-dependent. Acta Pharmacol Sin 21: 429–432. [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Gosden J, Nutt DJ. 2013. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol 27: 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA. 1993. Effects of Δ-9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther 264: 294–307. [PubMed] [Google Scholar]
- Heysieattalab S, Naghdi N, Hosseinmardi N, Zarrindast MR, Haghparast A, Khoshbouei H. 2016. Methamphetamine-induced enhancement of hippocampal long-term potentiation is modulated by NMDA and GABA receptors in the shell–accumbens. Synapse 70: 325–335. [DOI] [PubMed] [Google Scholar]
- Hill SY, Mikhael MA. 1979. Computerized transaxial tomographic and neuropsychological evaluations in chronic alcoholics and heroin abusers. Am J Psychiatry 136: 598–602. [PubMed] [Google Scholar]
- Hillig KW. 2005. Genetic evidence for speciation in Cannabis (Cannabaceae). Genet Resour Crop Evol 52: 161–180. [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. 2012. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 17: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PL, Rabe CS, Moses F, Tabakoff B. 1989. N-methyl-d-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J Neurochem 52: 1937–1940. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Yang R, Lichtman AH, Lupica CR. 2007. Opposing actions of chronic Δ9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn Mem 14: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Schroeder M, Ross TJ, Buchholz B, Salmeron BJ, Wonodi I, Thaker GK, Stein EA. 2011. Nicotine enhances but does not normalize visual sustained attention and the associated brain network in schizophrenia. Schizophr Bull 37: 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Liu QS, Chang KT, Berg DK. 2002. Nicotinic regulation of CREB activation in hippocampal neurons by glutamatergic and nonglutamatergic pathways. Mol Cell Neurosci 21: 616–625. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. 2004. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 99: 29–38. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. 2006. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29: 565–598. [DOI] [PubMed] [Google Scholar]
- Isenberg-Grzeda E, Kutner HE, Nicolson SE. 2012. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics 53: 507–516. [DOI] [PubMed] [Google Scholar]
- Ito R, Canseliet M. 2010. Amphetamine exposure selectively enhances hippocampus-dependent spatial learning and attenuates amygdala-dependent cue learning. Neuropsychopharmacology 35: 1440–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Tabata K, Makimura M, Fukuda H. 2001. Acute and chronic intracerebroventricular morphine infusions affect long-term potentiation differently in the lateral perforant path. Pharmacol Biochem Behav 70: 353–358. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Nagashima K, Murayama K, Zorumski CF. 2005. Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience 136: 509–517. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. 2005. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry 57: 56–66. [DOI] [PubMed] [Google Scholar]
- Jaffe JH. 1990. Drug addiction and drug abuse. In The pharmacological basis of therapeutics, 8th ed., pp. 522–573. [Google Scholar]
- Ji SP, Zhang Y, Van Cleemput J, Jiang W, Liao M, Li L, Wan Q, Backstrom JR, Zhang X. 2006. Disruption of PTEN coupling with 5-HT2C receptors suppresses behavioral responses induced by drugs of abuse. Nat Med 12: 324–329. [DOI] [PubMed] [Google Scholar]
- Jia Y, Yamazaki Y, Nakauchi S, Ito KI, Sumikawa K. 2010. Nicotine facilitates long-term potentiation induction in oriens-lacunosum moleculare cells via Ca2+ entry through non-α7 nicotinic acetylcholine receptors. Eur J Neurosci 31: 463–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Araki H, Nagata M, Shimosaka R, Shibata K, Suemaru K, Kawasaki H, Gomita Y. 2005. Expression of c-Fos in the rat central amygdala accompanies the acquisition but not expression of conditioned place aversion induced by withdrawal from acute morphine dependence. Behav Brain Res 161: 107–112. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Wells LT. 2000. Effects of isradipine, a dihydropyridine-class calcium channel antagonist, on D-methamphetamine-induced cognitive and physiological changes in humans. Neuropsychopharmacology 22: 504–512. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, et al. 2006. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology 185: 327–338. [DOI] [PubMed] [Google Scholar]
- Jones BP, Moskowitz HR, Butters N. 1975. Olfactory discrimination in alcoholic Korsakoff patients. Neuropsychologia 13: 173–179. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Robbins TW. 1991. Frontal lobe function in Korsakoff and non-Korsakoff alcoholics: planning and spatial working memory. Neuropsychologia 29: 709–723. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. 2003. Self-administration of Δ9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology 169: 135–140. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Green M. 2002. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J Neuropsychiatry Clin Neurosci 15: 215–220. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, Govindarajan A, Jung HY, Kang H, Tonegawa S. 2004. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 116: 467–479. [DOI] [PubMed] [Google Scholar]
- Kelley AE. 2004. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44: 161–179. [DOI] [PubMed] [Google Scholar]
- Kelley BJ, Yeager KR, Pepper TH, Beversdorf DQ. 2005. Cognitive impairment in acute cocaine withdrawal. Cogn Behav Neurol 18: 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. 2008. Nicotine enhances context learning but not context-shock associative learning. Behav Neurosci 122: 1158–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Adoff MD, Wilkinson DS, Gould TJ. 2011. The effects of acute, chronic, and withdrawal from chronic nicotine on novel and spatial object recognition in male C57BL/6J mice. Psychopharmacology 217: 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Poole RL, Adoff MD, Logue SF, Gould TJ. 2012. Learning and nicotine interact to increase CREB phosphorylation at the jnk1 promoter in the hippocampus. PLoS One 7: e39939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. 2006. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci 26: 5894–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. 2012. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology 37: 2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. 1985. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry 142: 1259–1264. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. 2001. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 58: 334–341. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. 1992. Modality-specific retrograde amnesia of fear. Science 256: 675–677. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee I. 2011. Hippocampus is necessary for spatial discrimination using distal cue-configuration. Hippocampus 21: 609–621. [DOI] [PubMed] [Google Scholar]
- Kleinman KM, Vaughn RL, Christ TS. 1973. Effects of cigarette smoking and smoking deprivation on paired-associate learning of high and low meaningful nonsense syllables. Psychol Rep 32: 963–966. [DOI] [PubMed] [Google Scholar]
- Klisz DK, Parsons OA. 1977. Hypothesis testing in younger and older alcoholics. J Stud Alcohol 38: 1718–1729. [DOI] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M, Bloom FE. 1989. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev 13: 135–140. [DOI] [PubMed] [Google Scholar]
- Koob GF, Caine B, Markou A, Pulvirenti L, Weiss F. 1994. Role for the mesocortical dopamine system in the motivating effects of cocaine. NIDA Res Monogr 145: 1–1. [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytiä P, Merlo-Pich E, Weiss F. 1998. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res 22: 3–9. [PubMed] [Google Scholar]
- Kouri EM, Pope HG Jr. 2000. Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol 8: 483. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL. 2010. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One 5: e8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. 1991. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 14: 299–302. [DOI] [PubMed] [Google Scholar]
- Kutlu MG, Gould TJ. 2016. Nicotinic modulation of hippocampal cell signaling and associated effects on learning and memory. Physiol Behav 155: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Ortega LA, Gould TJ. 2015a. Strain-dependent performance in nicotine-induced conditioned place preference. Behav Neurosci 129: 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Parikh V, Gould TJ. 2015b. Chapter seven—nicotine addiction and psychiatric disorders. Int Rev Neurobiol 124: 171–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange RA, Hillis LD. 2001. Cardiovascular complications of cocaine use. N Engl J Med 345: 351–358. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wiggins M, Goldberg SR. 2006. Nicotine pre-exposure does not potentiate the locomotor or rewarding effects of Δ-9-tetrahydrocannabinol in rats. Behav Pharmacol 17: 195–199. [DOI] [PubMed] [Google Scholar]
- Lemos JI, Resstel LB, Guimarães FS. 2010. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav Brain Res 207: 105–111. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. 2007. Intense sweetness surpasses cocaine reward. PLoS One 2: e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Torry D. 1996. Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology 123: 88–97. [DOI] [PubMed] [Google Scholar]
- Levin ED, Kaplan S, Boardman A. 1997. Acute nicotine interactions with nicotinic and muscarinic antagonists: working and reference memory effects in the 16-arm radial maze. Behav Pharmacol 8: 236–242. [PubMed] [Google Scholar]
- Levin ED, Bettegowda C, Weaver T, Christopher NC. 1998. Nicotine-dizocilpine interactions and working and reference memory performance of rats in the radial-arm maze. Pharmacol Biochem Behav 61: 335–340. [DOI] [PubMed] [Google Scholar]
- Li Z, Wu CF, Pei G, Xu NJ. 2001. Reversal of morphine-induced memory impairment in mice by withdrawal in Morris water maze: possible involvement of cholinergic system. Pharmacol Biochem Behav 68: 507–513. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu X, Chen H, Deng H, Xiang X, Chen H, Hao W. 2007. Development, extinction and reinstatement of morphine withdrawal-induced conditioned place aversion in rats. Addict Biol 12: 470–477. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. 1996. Δ9-Tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology 126: 125–131. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. 1995. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology 119: 282–290. [DOI] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, Mann K. 2009. Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol 44: 372–381. [DOI] [PubMed] [Google Scholar]
- London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, Herning R, Grayson R, Jaffe JH, Wagner HN Jr. 1990. Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry 47: 567–574. [DOI] [PubMed] [Google Scholar]
- Loureiro M, Lecourtier L, Engeln M, Lopez J, Cosquer B, Geiger K, Kelche C, Cassel JC, Pereira de Vasconcelos A. 2012. The ventral hippocampus is necessary for expressing a spatial memory. Brain Struct Funct 217: 93–106. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. 1989. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243: 1721–1724. [DOI] [PubMed] [Google Scholar]
- Lynch G, Kessler M, Arai A, Larson J. 1990. The nature and causes of hippocampal long-term potentiation. Prog Brain Res 83: 233–250. [DOI] [PubMed] [Google Scholar]
- Lyness WH, Friedle NM, Moore KE. 1979. Destruction of dopaminergic nerve terminals in nucleus accumbens: effect on d-amphetamine self-administration. Pharmacol Biochem Behav 11: 553–556. [DOI] [PubMed] [Google Scholar]
- Ma MX, Chen YM, He J, Zeng T, Wang JH. 2007. Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience 147: 1059–1065. [DOI] [PubMed] [Google Scholar]
- Maggio N, Segal M. 2007. Striking variations in corticosteroid modulation of long-term potentiation along the septotemporal axis of the hippocampus. J Neurosci 27: 5757–5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio N, Segal M. 2009. Differential modulation of long-term depression by acute stress in the rat dorsal and ventral hippocampus. J Neurosci 29: 8633–8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Damron KS, McManus CD, Barnhill RC. 2004. Patterns of illicit drug use and opioid abuse in patients with chronic pain at initial evaluation: a prospective, observational study. Pain Physician 7: 431–437. [PubMed] [Google Scholar]
- Manchikanti L, Manchukonda R, Pampati V, Damron KS, Brandon DE, Cash KA, McManus CD. 2006. Does random urine drug testing reduce illicit drug use in chronic pain patients receiving opioids? Pain Physician 9: 123–129. [PubMed] [Google Scholar]
- Mandillo S, Rinaldi A, Oliverio A, Mele A. 2003. Repeated administration of phencyclidine, amphetamine and MK-801 selectively impairs spatial learning in mice: a possible model of psychotomimetic drug-induced cognitive deficits. Behav Pharmacol 14: 533–544. [DOI] [PubMed] [Google Scholar]
- Mangan GL, Golding JF. 1983. The effects of smoking on memory consolidation. J Psychol 115: 65–77. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Motamedi F, Fathollahi Y, Atapour N, Semnanian S. 1997. Augmentation of LTP induced by Primed–Bursts tetanic stimulation in hippocampal CA1 area of morphine dependent rats. Brain Res 769: 119–124. [DOI] [PubMed] [Google Scholar]
- Manwell LA, Satvat E, Lang ST, Allen CP, Leri F, Parker LA. 2009. FAAH inhibitor, URB-597, promotes extinction and CB(1) antagonist, SR141716, inhibits extinction of conditioned aversion produced by naloxone-precipitated morphine withdrawal, but not extinction of conditioned preference produced by morphine in rats. Pharmacol Biochem Behav 94: 154–162. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. 1998. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res 22: 416–421. [PubMed] [Google Scholar]
- Masaki T. 1956. The amphetamine problem in Japan. WHO Tech. Rep. Ser 102: 14–21. [Google Scholar]
- Matsuyama S, Matsumoto A. 2003. Epibatidine induces long-term potentiation (LTP) via activation of α4β2 nicotinic acetylcholine receptors (nAChRs) in vivo in the intact mouse dentate gyrus: both α7 and α4β2 nAChRs essential to nicotinic LTP. J Pharmacol Sci 93: 180–187. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Matsumoto A, Enomoto T, Nishizaki T. 2000. Activation of nicotinic acetylcholine receptors induces long-term potentiation in vivo in the intact mouse dentate gyrus. Eur J Neurosci 12: 3741–3747. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R, Berman KF, Goldberg TE, Weinberger DR. 2000. Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage 12: 268–275. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Simson PE, Best PJ. 1995. Acute ethanol impairs spatial memory but not stimulus/response memory in the rat. Alcohol Clin Exp Res 19: 902–909. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Ilgen M, White AM, Best PJ. 1999. Acute ethanol administration impairs spatial performance while facilitating nonspatial performance in rats. Neurobiol Learn Mem 72: 169–179. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Morrow AL, Tokunaga S, McDaniel JR. 2002. Acute ethanol administration and acute allopregnanolone administration impair spatial memory in the Morris water task. Alcohol Clin Exp Res 26: 1747–1751. [DOI] [PubMed] [Google Scholar]
- McAuliffe WE, Rohman M, Feldman B, Launer EK. 1985. The role of euphoric effects in the opiate addictions of heroin addicts, medical patients and impaired health professionals. J Drug Issues 15: 203–224. [Google Scholar]
- McBride PE. 1992. The health consequences of smoking. Cardiovascular diseases. Med Clin North Am 76: 333–353. [DOI] [PubMed] [Google Scholar]
- McClung J, Fantegrossi W, Howell LL. 2010. Reinstatement of extinguished amphetamine self-administration by 3,4-methylenedioxymethamphetamine (MDMA) and its enantiomers in rhesus monkeys. Psychopharmacology 210: 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Childress AR, Ehrman R, O'Brien CP, Pashko S. 1986. Extinguishing conditioned responses during opiate dependence treatment turning laboratory findings into clinical procedures. J Subst Abuse Treat 3: 33–40. [DOI] [PubMed] [Google Scholar]
- Means LW, Holsten RD, Long M, High KM. 1996. Scopolamine- and morphine-induced deficits in water maze alternation: failure to attenuate with glucose. Neurobiol Learn Mem 66: 167–175. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Corodimas KP, Wilson MC, Ledoux JE. 1996. Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience 74: 313–322. [DOI] [PubMed] [Google Scholar]
- Melnick SM, Kubie JL, Laungani R, Dow-Edwards DL. 2001. Impairment of spatial learning following preweaning cocaine exposure in the adult rat. Neurotoxicol Teratol 23: 445–451. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Montgomery KS, LaSarge CL, Simon NW, Bizon JL, Setlow B. 2008. Long-term effects of prior cocaine exposure on Morris water maze performance. Neurobiol Learn Mem 89: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. 2006. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav 31: 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Neisewander JL. 2003. Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport 14: 2127–2131. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Speer CM, Neisewander JL. 2006. Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci 120: 401–412. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. 2006. Persistent disruption of an established morphine conditioned place preference. J Neurosci 26: 3010–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsushima D, Sano A, Takahashi T. 2012. A cholinergic trigger drives learning-induced plasticity at hippocampal synapses. Nat Commun 4: 2760–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittenberg W, Motta S. 1993. Effects of chronic cocaine abuse on memory and learning. Arch Clin Neuropsychol 8: 477–483. [PubMed] [Google Scholar]
- Morimoto K, Otani S, Goddard GV. 1987. Effects of acute and long-term treatment with amphetamine on evoked responses and long-term potentiation in the dentate gyrus of anesthetized rats. Brain Res 407: 137–143. [DOI] [PubMed] [Google Scholar]
- Morón JA, Abul-Husn NS, Rozenfeld R, Dolios G, Wang R, Devi LA. 2007. Morphine administration alters the profile of hippocampal postsynaptic density-associated proteins: a proteomics study focusing on endocytic proteins. Mol Cell Proteomics 6: 29–42. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA. 2010. D-cycloserine facilitates extinction of naloxone-induced conditioned place aversion in morphine-dependent rats. Biol Psychiatry 67: 85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Numakawa T, Ikeuchi T, Hatanaka H. 2001. Nicotine-induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells. J Neurochem 79: 489–498. [DOI] [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. 2004. Methamphetamine abstinence syndrome: preliminary findings. Am J Addict 13: 248–255. [DOI] [PubMed] [Google Scholar]
- Niikura K, Ho A, Kreek MJ, Zhang Y. 2013. Oxycodone-induced conditioned place preference and sensitization of locomotor activity in adolescent and adult mice. Pharmacol Biochem Behav 110: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyuhire F, Varvel SA, Martin BR, Lichtman AH. 2007. Exposure to marijuana smoke impairs memory retrieval in mice. J Pharmacol Exp Ther 322: 1067–1075. [DOI] [PubMed] [Google Scholar]
- Noonan MA, Choi KH, Self DW, Eisch AJ. 2008. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci 28: 2516–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Leamon M. 2003. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci 15: 317–325. [DOI] [PubMed] [Google Scholar]
- North A, Swant J, Salvatore MF, Gamble-George J, Prins P, Butler B, Mittal MK, Heltsley R, Clark JT, Khoshbouei H. 2013. Chronic methamphetamine exposure produces a delayed, long-lasting memory deficit. Synapse 67: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicky AV, Teyler TJ, Vardaris RM. 1987. The modulation of long-term potentiation by Δ-9-tetrahydrocannabinol in the rat hippocampus, in vitro. Brain Res Bull 19: 663–672. [DOI] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. 2002. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res 26: 547–557. [PubMed] [Google Scholar]
- O'Brien CP, Testa T, O'Brien TJ, Brady JP, Wells B. 1977. Conditioned narcotic withdrawal in humans. Science 195: 1000–1002. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. 1971. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34: 171–175. [DOI] [PubMed] [Google Scholar]
- O'Keefe JH, Bhatti SK, Bajwa A, DiNicolantonio JJ, Lavie CJ. 2014. Alcohol and cardiovascular health: the dose makes the poison… or the remedy. In Mayo Clinic Proceedings (Vol. 89, No. 3, pp. 382–393). Elsevier. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. 2000. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology 23: 113–126. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Kirkley SM, Gansler DA, Couture A. 2004. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcohol Clin Exp Res 28: 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. 1994. Quinpirole and d-amphetamine administration posttraining enhances memory on spatial and cued discriminations in a water maze. Psychobiology 22: 54–60. [Google Scholar]
- Parikh V, Kutlu MG, Gould TJ. 2016. nAChR dysfunction as a common substrate for schizophrenia and comorbid nicotine addiction: current trends and perspectives. Schizophr Res 171: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Burton P, Sorge RE, Yakiwchuk C, Mechoulam R. 2004. Effect of low doses of Δ9-tetrahydrocannabinol and cannabidiol on the extinction of cocaine-induced and amphetamine-induced conditioned place preference learning in rats. Psychopharmacology 175: 360–366. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Craig D. 1992. Cigarette smoking and nicotine gum (0, 2 and 4 mg): effects upon four visual attention tasks. Neuropsychobiology 25: 34–43. [DOI] [PubMed] [Google Scholar]
- Pascual MM, Pastor V, Bernabeu RO. 2009. Nicotine-conditioned place preference induced CREB phosphorylation and Fos expression in the adult rat brain. Psychopharmacology 207: 57–71. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. 2010. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend 106: 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeke SC, Peeke HV. 1984. Attention, memory, and cigarette smoking. Psychopharmacology 84: 205–216. [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. 2013. The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS One 8: e79465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Le Moal M, Simon H. 1989. Factors that predict individual vulnerability to amphetamine self-administration. Science 245: 1511–1513. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, le Moal M, Simon H. 1990. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res 514: 22–26. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. 1991. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci 88: 2088–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS. 1985. Neuroregulators and the reinforcement of smoking: towards a biobehavioral explanation. Neurosci Biobehav Rev 8: 503–513. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF. 1992. Euphoriant effects of nicotine in smokers. Psychopharmacology 108: 460–465. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. 1995. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse 7: 373–378. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Marks JL, Pomerleau OF. 2000. Who gets what symptom? Effects of psychiatric cofactors and nicotine dependence on patterns of smoking withdrawal symptomatology. Nicotine Tob Res 2: 275–280. [DOI] [PubMed] [Google Scholar]
- Pope HG, Yurgelun-Todd D. 1996. The residual cognitive effects of heavy marijuana use in college students. JAMA 275: 521–527. [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Turner JR, Blendy JA, Gould TJ. 2012a. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol Learn Mem 97: 482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Kenney JW, Sullivan C, Gould TJ. 2012b. Strain-dependent effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Behav Genet 42: 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. 2008. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol Biochem Behav 88: 407–417. [DOI] [PubMed] [Google Scholar]
- Provost SC, Woodward R. 1991. Effects of nicotine gum on repeated administration of the Stroop test. Psychopharmacology 104: 536–540. [DOI] [PubMed] [Google Scholar]
- Pu L, Bao GB, Xu NJ, Ma L, Pei G. 2002. Hippocampal long-term potentiation is reduced by chronic opiate treatment and can be restored by re-exposure to opiates. J Neurosci 22: 1914–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. 2009. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci 12: 1152–1158. [DOI] [PubMed] [Google Scholar]
- Rajendran P, Spear LP. 2004. The effects of ethanol on spatial and nonspatial memory in adolescent and adult rats studied using an appetitive paradigm. Ann N Y Acad Sci 1021: 441–444. [DOI] [PubMed] [Google Scholar]
- Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA, Fuchs RA. 2009. Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur J Neurosci 30: 901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, D'Souza DC. 2006. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 188: 425–444. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. 2007. Extracellular signal-regulated kinase 1/2 involvement in the enhancement of contextual fear conditioning by nicotine. Behav Neurosci 121: 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. 2009. Nicotine withdrawal-induced deficits in trace fear conditioning in C57BL/6 mice—a role for high-affinity β2 subunit-containing nicotinic acetylcholine receptors. Eur J Neurosci 29: 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Room R, Graham K, Monteiro M, Gmel G, Sempos CT. 2003. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction 98: 1209–1228. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Gilstrap MG, Ramsey LA, See RE. 2014. Modafinil restores methamphetamine induced object-in-place memory deficits in rats independent of glutamate N-methyl-d-aspartate receptor expression. Drug Alcohol Depend 134: 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retailleau A, Dejean C, Fourneaux B, Leinekugel X, Boraud T. 2013. Why am I lost without dopamine? Effects of 6-OHDA lesion on the encoding of reward and decision process in CA3. Neurobiol Dis 59: 151–164. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Prasad A, MacDonald JF. 1992. Ethanol modulation of GABA receptor-activated Cl− currents in neurons of the chick, rat and mouse central nervous system. Eur J Pharmacol 224: 173–181. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. 1996. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Oakes RA. 1995. Nicotine-induced conditioned place preference and conditioned place aversion in mice. Pharmacol Biochem Behav 51: 457–461. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. 2008. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci 1141: 1–21. [DOI] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, Gruol DL. 2002. Long-term potentiation in the rat hippocampus is reversibly depressed by chronic intermittent ethanol exposure. J Neurophysiol 87: 2385–2397. [DOI] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, Brunelli M, Sanna PP, Gruol DL. 2003. The transient depression of hippocampal CA1 LTP induced by chronic intermittent ethanol exposure is associated with an inhibition of the MAP kinase pathway. Eur J Neurosci 17: 1646–1654. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. 1999. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse 33: 160–162. [DOI] [PubMed] [Google Scholar]
- Rogers JL, See RE. 2007. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem 87: 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, et al. 1999. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology 20: 322–339. [DOI] [PubMed] [Google Scholar]
- Rosenthal MZ, Kutlu MG. 2014. Translation of associative learning models into extinction reminders delivered via mobile phones during cue exposure interventions for substance use. Psychol Addict Behav 28: 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Patterson F, Lerman C. 2005. Increases in hyperactive–impulsive symptoms predict relapse among smokers in nicotine replacement therapy. J Subst Abuse Treat 28: 297–304. [DOI] [PubMed] [Google Scholar]
- Ryan C, Butters N. 1983. cognitive deficits in alcoholics. In The biology of alcoholism (pp. 485–538). Springer. [Google Scholar]
- Ryan C, Butters N, Montgomery K, Adinolfi A, Didario B. 1980. Memory deficits in chronic alcoholics: continuities between the “intact” alcoholic and the alcoholic Korsakoff patient. In Biological effects of alcohol (pp. 701–718). Springer. [DOI] [PubMed] [Google Scholar]
- Salmanzadeh F, Fathollahi Y, Semnanian S, Shafizadeh M. 2003. Dependence on morphine impairs the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Brain Res 965: 108–113. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, Flynn NM, Henik A, Pfefferbaum A, Sullivan EV. 2002. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res 111: 65–74. [DOI] [PubMed] [Google Scholar]
- Sandler D, Wilcox A, Everson R. 1985. Cumulative effects of lifetime passive smoking on cancer risk. Lancet 325: 312–315. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Simpson C, Lutjens R, Koob G. 2002. ERK regulation in chronic ethanol exposure and withdrawal. Brain Res 948: 186–191. [DOI] [PubMed] [Google Scholar]
- Santucci AC. 2008. Adolescent cocaine residually impairs working memory and enhances fear memory in rats. Exp Clin Psychopharmacol 16: 77. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Capodilupo S, Bernstein J, Gomez-Ramirez M, Milefsky R, Mitchell H. 2004. Cocaine in adolescent rats produces residual memory impairments that are reversible with time. Neurotoxicol Teratol 26: 651–661. [DOI] [PubMed] [Google Scholar]
- Saucier D, Cain DP. 1995. Spatial learning without NMDA receptor-dependent long-term potentiation. Nature 378: 186–189. [DOI] [PubMed] [Google Scholar]
- Schummers J, Browning MD. 2001. Evidence for a role for GABA A and NMDA receptors in ethanol inhibition of long-term potentiation. Mol Brain Res 94: 9–14. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. 1957. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Richter-Levin G, Maggio N. 2010. Stress-induced dynamic routing of hippocampal connectivity: a hypothesis. Hippocampus 20: 1332–1338. [DOI] [PubMed] [Google Scholar]
- Sharifzadeh M, Tavasoli M, Naghdi N, Ghanbari A, Amini M, Roghani A. 2005. Post-training intrahippocampal infusion of nicotine prevents spatial memory retention deficits induced by the cyclo-oxygenase-2-specific inhibitor celecoxib in rats. J Neurochem 95: 1078–1090. [DOI] [PubMed] [Google Scholar]
- Sherman CB. 1992. The health consequences of cigarette smoking. Pulmonary diseases. Med Clin North Am 76: 355–375. [DOI] [PubMed] [Google Scholar]
- Sherwood N. 1993. Effects of nicotine on human psychomotor performance. Hum Psychopharmacol Clin Exp 8: 155–184. [Google Scholar]
- Shimizu K, Matsubara K, Uezono T, Kimura K, Shiono H. 1998. Reduced dorsal hippocampal glutamate release significantly correlates with the spatial memory deficits produced by benzodiazepines and ethanol. Neuroscience 83: 701–706. [DOI] [PubMed] [Google Scholar]
- Silber BY, Croft RJ, Papafotiou K, Stough C. 2006. The acute effects of d-amphetamine and methamphetamine on attention and psychomotor performance. Psychopharmacology 187: 154–169. [DOI] [PubMed] [Google Scholar]
- Simões PF, Silva AP, Pereira FC, Marques E, Grade S, Milhazes N, Borges F, Ribeiro CF, Macedo TR. 2007. Methamphetamine induces alterations on hippocampal NMDA and AMPA receptor subunit levels and impairs spatial working memory. Neuroscience 150: 433–441. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. 2000. Cognitive impairment in individuals currently using methamphetamine. Am J Addict 9: 222–231. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier CP, Sim T, Richardson K, Rawson RA, Ling W. 2001. Cognitive performance of current methamphetamine and cocaine abusers. J Addict Dis 21: 61–74. [DOI] [PubMed] [Google Scholar]
- Sinden JD, Le Magnen J. 1982. Parameters of low-dose ethanol intravenous self-administration in the rat. Pharmacol Biochem Behav 16: 181–183. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. 2006. Hippocampal place cells, context, and episodic memory. Hippocampus 16: 716–729. [DOI] [PubMed] [Google Scholar]
- Smith DA, Browning M, Dunwiddie TV. 1993. Cocaine inhibits hippocampal long-term potentiation. Brain Res 608: 259–265. [DOI] [PubMed] [Google Scholar]
- Snyder FR, Davis FC, Henningfield JE. 1989. The tobacco withdrawal syndrome: performance decrements assessed on a computerized test battery. Drug Alcohol Depend 23: 259–266. [DOI] [PubMed] [Google Scholar]
- Soetens E, D'Hooge R, Hueting JE. 1993. Amphetamine enhances human-memory consolidation. Neurosci Lett 161: 9–12. [DOI] [PubMed] [Google Scholar]
- Solowij N. 1995. Do cognitive impairments recover following cessation of cannabis use? Life Sci 56: 2119–2126. [DOI] [PubMed] [Google Scholar]
- Spain JW, Newsom GC. 1991. Chronic opioids impair acquisition of both radial maze and Y-maze choice escape. Psychopharmacology 105: 101–106. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. 1982. Cocaine-induced place preference conditioning: lack of effects of neuroleptics and 6-hydroxydopamine lesions. Brain Res 253: 195–203. [DOI] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. 2013. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol 18: 203–213. [DOI] [PubMed] [Google Scholar]
- Steinpreis RE, Rutell AL, Parrett FA. 1996. Methadone produces conditioned place preference in the rat. Pharmacol Biochem Behav 54: 339–341. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Babor TF, Kadden R, Miller M. 2002. The Marijuana Treatment Project: rationale, design and participant characteristics. Addiction 97Suppl 1: 109–124. [DOI] [PubMed] [Google Scholar]
- Stern CA, Gazarini L, Takahashi RN, Guimaraes FS, Bertoglio LJ. 2012. On disruption of fear memory by reconsolidation blockade: evidence from cannabidiol treatment. Neuropsychopharmacology 37: 2132–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinus L, Cador M, Zorrilla EP, Koob GF. 2005. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology 30: 90–98. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. 1995. The scientific case that nicotine is addictive. Psychopharmacology 117: 2–10. [DOI] [PubMed] [Google Scholar]
- Stramiello M, Wagner JJ. 2010. Cocaine enhancement of long-term potentiation in the CA1 region of rat hippocampus: lamina-specific mechanisms of action. Synapse 64: 644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strupp BJ, Bunsey M, Levitsky D, Kesler M. 1991. Time-dependent effects of post-trial amphetamine treatment in rats: evidence for enhanced storage of representational memory. Behav Neural Biol 56: 62–76. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies (SAMHSA). 2005. The NSDUH report: methamphetamine use, abuse, and dependence: 2002, 2003, and 2004. SAMHSA, Rockville, MD. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies (SAMHSA). 2006a. The DASIS report: trends in methamphetamine/amphetamine admissions to treatment, 1993–2003. SAMHSA, Rockville, MD. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). 2006b. Results from the 2005 National Survey on Drug Use and Health: National Findings. (Office of Applied Studies, NSDUH Series H-30, DHHS Publication No. SMA 06-4194). SAMHSA, Rockville, MD. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). 2011. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. SAMHSA, Rockville, MD. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). 2014. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. (HHS Publication No. (SMA) 14-4887. NSDUH Series H-49). Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Sullivan JM. 2000. Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learn Mem 7: 132–139. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L. 2003. Hippocampal volume deficits in alcoholic Korsakoff's syndrome. Neurology 61: 1716–1719. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. 2009. Neuroimaging of the Wernicke–Korsakoff syndrome. Alcohol Alcohol 44: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, Rudy JW. 1989. Configural association theory: the role of the hippocampal formation in learning, memory, and amnesia. Psychobiology 17: 129–144. [Google Scholar]
- Swant J, Chirwa S, Stanwood G, Khoshbouei H. 2010. Methamphetamine reduces LTP and increases baseline synaptic transmission in the CA1 region of mouse hippocampus. PLoS One 5: e11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi KA, Castillo PE. 2006. The CB1 cannabinoid receptor mediates glutamatergic synaptic suppression in the hippocampus. Neuroscience 139: 795–802. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Singer G. 1979. Self-administration of Δ 9-tetrahydrocannabinol by rats. Pharmacol Biochem Behav 11: 737–740. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Michaud JC, Le Fur G, Soubrié P. 1995. Inhibition of long-term potentiation in rat hippocampal slices by anandamide and WIN55212-2: reversal by SR141716 A, a selective antagonist of CB1 cannabinoid receptors. Naunyn Schmiedebergs Arch Pharmacol 352: 576–579. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, DiScenna P. 1987. Long-term potentiation. Annu Rev Neurosci 10: 131–161. [DOI] [PubMed] [Google Scholar]
- Thewissen R, Van Den Hout M, Havermans RC, Jansen A. 2005. Context-dependency of cue-elicited urge to smoke. Addiction 100: 387–396. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Everitt BJ. 2001. Limbic-cortical-ventral striatal activation during retrieval of a discrete cocaine-associated stimulus: a cellular imaging study with γ protein kinase C expression. J Neurosci 21: 2526–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KL, Arroyo M, Everitt BJ. 2003. Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. Eur J Neurosci 17: 1964–1972. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. 2008. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol 154: 327–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AM, Gosnell BA, Wagner JJ. 2002. Enhancement of long-term potentiation in the rat hippocampus following cocaine exposure. Neuropharmacology 42: 1039–1042. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Swant J, Gosnell BA, Wagner JJ. 2004. Modulation of long-term potentiation in the rat hippocampus following cocaine self-administration. Neuroscience 127: 177–185. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Swant J, Wagner JJ. 2005. Cocaine-induced modulation of long-term potentiation in the CA1 region of rat hippocampus. Neuropharmacology 49: 185–194. [DOI] [PubMed] [Google Scholar]
- Thorn DA, Winter JC, Li JX. 2012. Agmatine attenuates methamphetamine-induced conditioned place preference in rats. Eur J Pharmacol 680: 69–72. [DOI] [PubMed] [Google Scholar]
- Tian S, Huang F, Li P, Li Z, Zhou S, Deng H, Yang Y. 2011. Nicotine enhances contextual fear memory reconsolidation in rats. Neurosci Lett 487: 368–371. [DOI] [PubMed] [Google Scholar]
- Tidy HL. 1938. Discussion on benzedrine: uses and abuses. Proc R Soc Med 32: 385–398. [Google Scholar]
- Tinklenberg JR, Melges FT, Hollister LE, Gillespie HK. 1970. Marijuana and immediate memory. Nature 226: 1171–1172. [DOI] [PubMed] [Google Scholar]
- Tramullas M, Martínez-Cué C, Hurlé MA. 2008. Chronic administration of heroin to mice produces up-regulation of brain apoptosis-related proteins and impairs spatial learning and memory. Neuropharmacology 54: 640–652. [DOI] [PubMed] [Google Scholar]
- Tremwel MF, Hunter BE. 1994. Effects of chronic ethanol ingestion on long-term potentiation remain even after a prolonged recovery from ethanol exposure. Synapse 17: 141–148. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, Mons N, Micheau J. 2006. Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn Mem 13: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea TF, Kosofsky BE, Rajadhyaksha AM. 2008. Enhanced CREB and DARPP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. J Neurochem 106: 1780–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ. 1997. Episodic and declarative memory: role of the hippocampus. Hippocampus 8: 198–204. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. 1995. N-methyl-D-aspartic acid-receptor antagonists block morphine-induced conditioned place preference in rats. Neurosci Lett 193: 37–40. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. 2011. Results from the 2010 National Survey on Drug Use and Health: Summary of national findings. Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- US Department of Health and Human Services. 2014. The health consequences of smoking—50 years of progress: a report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health 17, Atlanta, GA. [Google Scholar]
- Valjent E, Maldonado R. 2000. A behavioural model to reveal place preference to Δ9-tetrahydrocannabinol in mice. Psychopharmacology 147: 436–438. [DOI] [PubMed] [Google Scholar]
- Valzachi MC, Teodorov E, Marcourakis T, Bailey A, Camarini R. 2013. Enhancement of behavioral sensitization, anxiety-like behavior, and hippocampal and frontal cortical CREB levels following cocaine abstinence in mice exposed to cocaine during adolescence. PLoS One 8: e78317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandergriff JL, Matthews DB, Best PJ, Simson PE. 1995. Effects of ethanol and diazepam on spatial and non-spatial tasks in rats on an 8-arm maze. Alcohol Clin Exp Res 19: 64. [Google Scholar]
- Vandrey RG, Budney AJ, Moore BA, Vandrey RG, Budney AJ, Moore BA, Hughes JR. 2005. A cross-study comparison of cannabis and tobacco withdrawal. Am J Addict 14: 54–63. [DOI] [PubMed] [Google Scholar]
- Vandrey RG, Budney AJ, Hughes JR, Liguori A. 2008. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depen 92(1): 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. 2002. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav 77: 107–114. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Hall JM, Savage LM. 2011. Alcohol-related amnesia and dementia: animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiol Learn Mem 96: 596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna MR, Alonso M, Viola H, Quevedo J, de Paris F, Furman M, de Stein ML, Medina JH, Izquierdo I. 2000. Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat. Learn Mem 7: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Infer A, Arenas MC, Daza-Losada M, Aguilar MA, Miñarro J, Rodríguez-Arias M. 2012. High novelty-seeking predicts greater sensitivity to the conditioned rewarding effects of cocaine. Pharmacol Biochem Behav 102: 124–132. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. 1999. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol 13: 337–345. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SR. 2014. Adverse health effects of marijuana use. N Engl J Med 370: 2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. 2001. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science 292: 1175–1178. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cui H, Wang W, Zhao B, Lai J. 2012. The region-specific activation of Ca2+/calmodulin dependent protein kinase II and extracellular signal-regulated kinases in hippocampus following chronic alcohol exposure. Brain Res Bull 89: 191–196. [DOI] [PubMed] [Google Scholar]
- Warburton DM, Wesnes K, Shergold K, James M. 1986. Facilitation of learning and state dependency with nicotine. Psychopharmacology 89: 55–59. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. 2000. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res 2: 19–37. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. 2004. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience 129: 11–24. [DOI] [PubMed] [Google Scholar]
- Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, Fuchs RA. 2011. Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem 18: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsby P, Rowan M, Anwyl R. 2006. Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. Eur J Neurosci 24: 3109–3118. [DOI] [PubMed] [Google Scholar]
- Welsby PJ, Rowan MJ, Anwyl R. 2007. Beta-amyloid blocks high frequency stimulation induced LTP but not nicotine enhanced LTP. Neuropharmacology 53: 188–195. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. 1983. Effects of smoking on rapid information processing performance. Neuropsychobiology 9: 223–229. [DOI] [PubMed] [Google Scholar]
- West R, Gossop M. 1994. Overview: a comparison of withdrawal symptoms from different drug classes. Addiction 89: 1483–1489. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. 2001. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry 158: 86–95. [DOI] [PubMed] [Google Scholar]
- White NM. 1996. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction 91: 921–950. [PubMed] [Google Scholar]
- White AM, Simson PE, Best PJ. 1997. Comparison between the effects of ethanol and diazepam on spatial working memory in the rat. Psychopharmacology 133: 256–261. [DOI] [PubMed] [Google Scholar]
- White AM, Elek TM, Beltz TL, Best PJ. 1998. Spatial performance is more sensitive to ethanol than nonspatial performance regardless of cue proximity. Alcohol Clin Exp Res 22: 2102–2107. [PubMed] [Google Scholar]
- Wikler A. 1948. Opiate addiction. Am J Psychiatry 105: 74–75. [DOI] [PubMed] [Google Scholar]
- Wikler A. 1973. Dynamics of drug dependence: implications of a conditioning theory for research and treatment. Arch Gen Psychiatry 28: 611–616. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S. 2008. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry 47: 21–31. [DOI] [PubMed] [Google Scholar]
- Wilkinson DS, Gould TJ. 2013. Withdrawal from chronic nicotine and subsequent sensitivity to nicotine challenge on contextual learning. Behav Brain Res 250: 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DS, Turner JR, Blendy JA, Gould TJ. 2013. Genetic background influences the effects of withdrawal from chronic nicotine on learning and high-affinity nicotinic acetylcholine receptor binding in the dorsal and ventral hippocampus. Psychopharmacology 225: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder DG, Martin KC, Muzzio IA, Rohrer D, Chruscinski A, Kobilka B, Kandel ER. 1999. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by β-adrenergic receptors. Neuron 24: 715–726. [DOI] [PubMed] [Google Scholar]
- Wing VC, Shoaib M. 2008. Contextual stimuli modulate extinction and reinstatement in rodents self-administering intravenous nicotine. Psychopharmacology 200: 357–365. [DOI] [PubMed] [Google Scholar]
- Wise LE, Varvel SA, Selley DE, Wiebelhaus JM, Long KA, Middleton LS, Sim-Selley LJ, Lichtman AH. 2011. Δ 9-Tetrahydrocannabinol-dependent mice undergoing withdrawal display impaired spatial memory. Psychopharmacology 217: 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SC, Fay J, Sage JR, Anagnostaras SG. 2007. Cocaine and Pavlovian fear conditioning: dose–effect analysis. Behav Brain Res 176: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2014. Global status report on alcohol and health. [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. 2010. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology 208: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, Hori T, Asada T. 2004. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann N Y Acad Sci 1025: 351–362. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Liu J, Arai H, Hori T, Shiga T. 2005. Decreased cell proliferation in the dentate gyrus of rats after repeated administration of cocaine. Synapse 58: 63–71. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Fujii S, Jia Y, Sumikawa K. 2006. Nicotine withdrawal suppresses nicotinic modulation of long-term potentiation induction in the hippocampal CA1 region. Eur J Neurosci 24: 2903–2916. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. 2013. The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behav Brain Res 254: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeuws I, Soetens E. 2007. Verbal memory performance improved via an acute administration of D-amphetamine. Hum Psychopharmacol 22: 279–287. [DOI] [PubMed] [Google Scholar]