Abstract
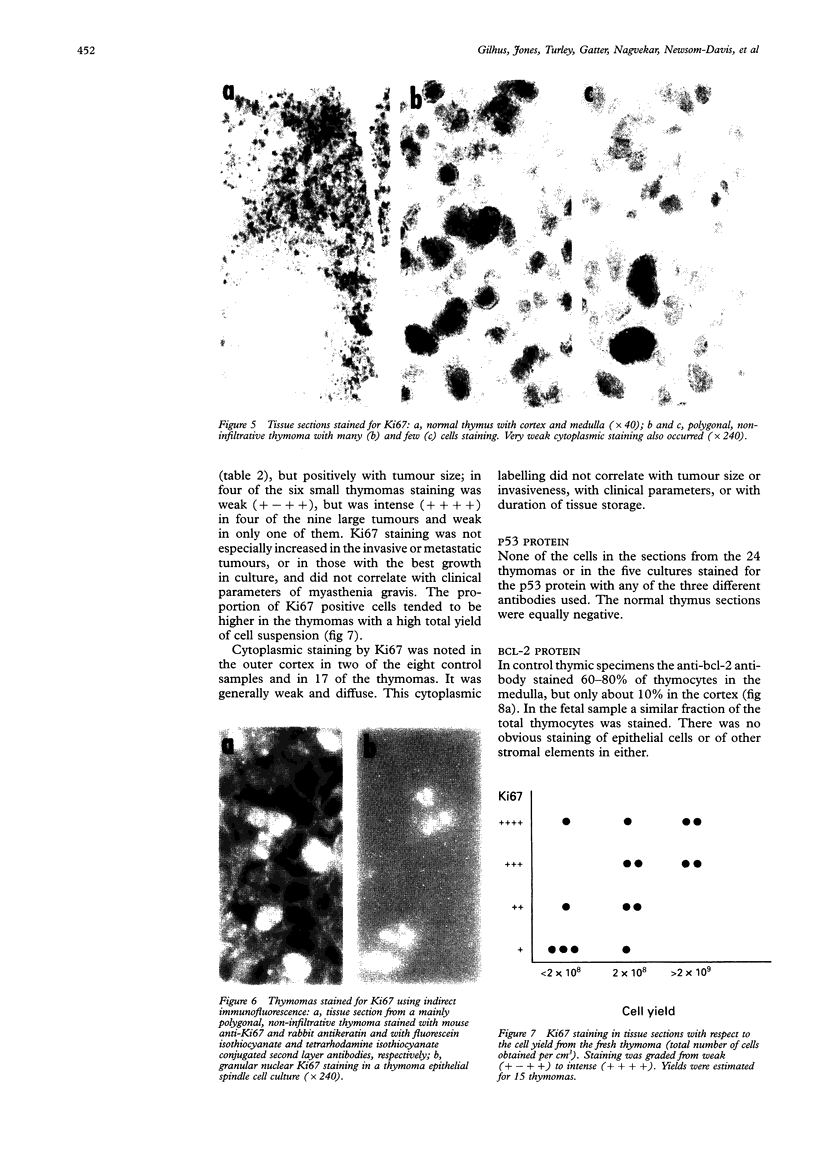
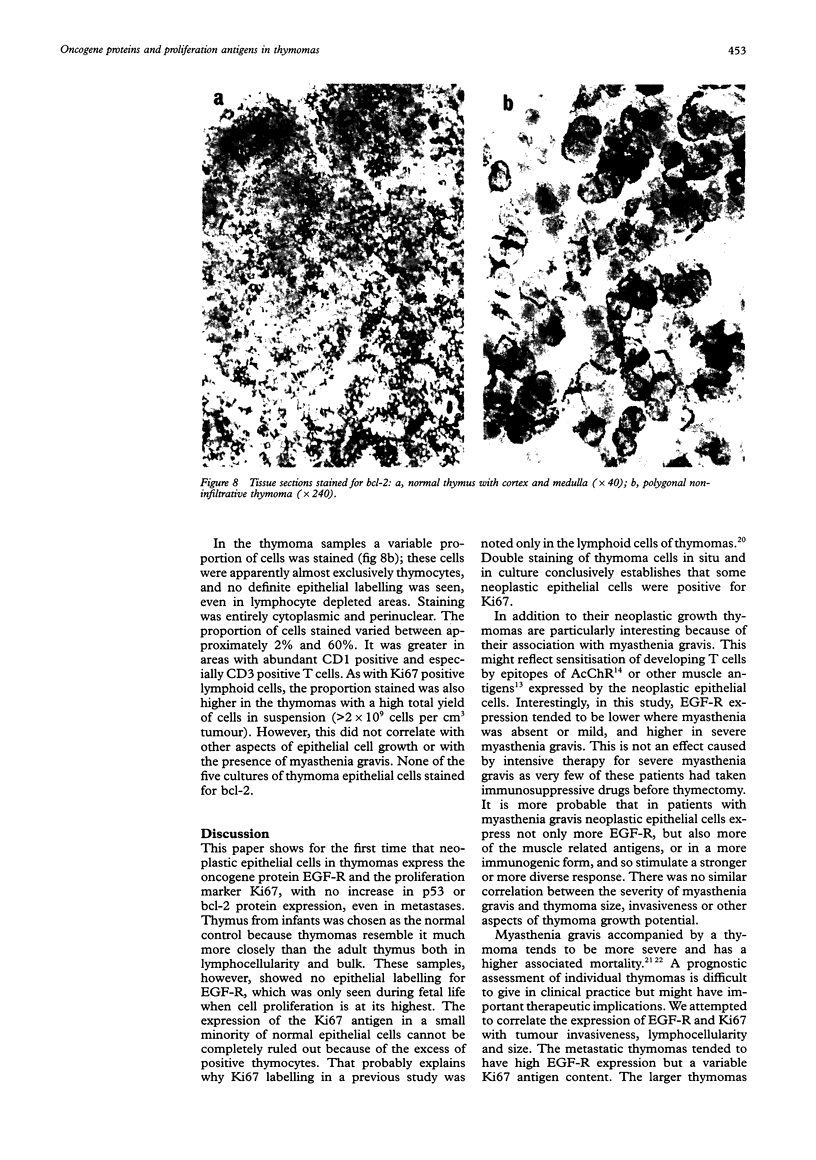
AIMS--To examine thymomas for proteins encoded by oncogenes and to determine whether their presence correlates with tumour growth and associated myasthenia gravis. METHODS--Sections of 24 thymomas were incubated with anti-EGF receptor (EGF-R), anti-Ki67 antigen, anti-p53, and anti-bcl-2 antibodies, and then stained using the alkaline phosphatase/anti-alkaline phosphatase (APAAP) technique. Cell suspensions and epithelial cell cultures from some of the tumours were also studied. RESULTS--Whereas EGF-R expression was not detected in any of the controls (but only in a 20 week old fetus), it was detected in neoplastic epithelial cells of all thymomas, and was most strongly expressed in metastases and in samples from donors with severe myasthenia gravis. Ki67 labelling was also increased, especially in the larger thymomas. Epithelial expression of both of these markers was confirmed in fresh cell suspensions and monolayer cultures from the five available cases. In contrast, p53 and bcl-2 were not detected in the neoplastic cells, but bcl-2 was present in the intermingling thymocytes. CONCLUSIONS--Neoplastic thymoma cells express EGF-R and Ki67, but there is no concomitant increase in the expression of p53 and bcl-2 proteins. Increased EGF-R expression may result in increased proliferation of neoplastic cells and also in myasthenia gravis. Measurement of EGF-R concentrations may be of prognostic value. The bcl-2 staining pattern in T lymphocytes illustrates the broad spectrum of maturational stages in thymoma lymphocytes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Salmon M., Savill J., Janossy G. A possible role for bcl-2 in regulating T-cell memory--a 'balancing act' between cell death and survival. Immunol Today. 1993 Nov;14(11):526–532. doi: 10.1016/0167-5699(93)90181-J. [DOI] [PubMed] [Google Scholar]
- Apte S. S. Ki-67 monoclonal antibody (MAb) reacts with a proliferation associated nuclear antigen in the rabbit Oryctolagus cuniculus. Histochemistry. 1990;94(2):201–204. doi: 10.1007/BF02440188. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Brada M. BCL2 gene: current relevance to clinical oncology. Eur J Cancer. 1992;28(1):270–272. doi: 10.1016/0959-8049(92)90431-z. [DOI] [PubMed] [Google Scholar]
- Brown D. C., Gatter K. C. Monoclonal antibody Ki-67: its use in histopathology. Histopathology. 1990 Dec;17(6):489–503. doi: 10.1111/j.1365-2559.1990.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Charpin C., Devictor B., Bonnier P., Andrac L., Lavaut M. N., Allasia C., Piana L. Epidermal growth factor receptor in breast cancer: correlation of quantitative immunocytochemical assays to prognostic factors. Breast Cancer Res Treat. 1993;25(3):203–210. doi: 10.1007/BF00689834. [DOI] [PubMed] [Google Scholar]
- Chilosi M., Iannucci A., Menestrina F., Lestani M., Scarpa A., Bonetti F., Fiore-Donati L., DiPasquale B., Pizzolo G., Palestro G. Immunohistochemical evidence of active thymocyte proliferation in thymoma. Its possible role in the pathogenesis of autoimmune diseases. Am J Pathol. 1987 Sep;128(3):464–470. [PMC free article] [PubMed] [Google Scholar]
- Collins V. P., James C. D. Gene and chromosomal alterations associated with the development of human gliomas. FASEB J. 1993 Jul;7(10):926–930. doi: 10.1096/fasebj.7.10.8344489. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Fukai I., Masaoka A., Hashimoto T., Yamakawa Y., Mizuno T., Tanamura O. Cytokeratins in normal thymus and thymic epithelial tumors. Cancer. 1993 Jan 1;71(1):99–105. doi: 10.1002/1097-0142(19930101)71:1<99::aid-cncr2820710116>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Gaidano G., Ballerini P., Gong J. Z., Inghirami G., Neri A., Newcomb E. W., Magrath I. T., Knowles D. M., Dalla-Favera R. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5413–5417. doi: 10.1073/pnas.88.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de Palazzo I. E., Adams G. P., Sundareshan P., Wong A. J., Testa J. R., Bigner D. D., Weiner L. M. Expression of mutated epidermal growth factor receptor by non-small cell lung carcinomas. Cancer Res. 1993 Jul 15;53(14):3217–3220. [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Gilhus N. E., Aarli J. A., Christensson B., Matre R. Rabbit antiserum to a citric acid extract of human skeletal muscle staining thymomas from myasthenia gravis patients. J Neuroimmunol. 1984 Nov;7(1):55–64. doi: 10.1016/s0165-5728(84)80006-5. [DOI] [PubMed] [Google Scholar]
- Gilhus N. E., Willcox N., Harcourt G., Nagvekar N., Beeson D., Vincent A., Newsom-Davis J. Antigen presentation by thymoma epithelial cells from myasthenia gravis patients to potentially pathogenic T cells. J Neuroimmunol. 1995 Jan;56(1):65–76. doi: 10.1016/0165-5728(94)00134-a. [DOI] [PubMed] [Google Scholar]
- Gill G. N. Regulation of EGF receptor expression and function. Mol Reprod Dev. 1990 Sep;27(1):46–53. doi: 10.1002/mrd.1080270110. [DOI] [PubMed] [Google Scholar]
- Gillitzer R., Pilarski L. M. In situ localization of CD45 isoforms in the human thymus indicates a medullary location for the thymic generative lineage. J Immunol. 1990 Jan 1;144(1):66–74. [PubMed] [Google Scholar]
- Hirokawa K., Utsuyama M., Moriizumi E., Hashimoto T., Masaoka A., Goldstein A. L. Immunohistochemical studies in human thymomas. Localization of thymosin and various cell marker. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;55(6):371–380. doi: 10.1007/BF02896596. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld R. Myasthenia gravis and thymoma: paraneoplastic failure of neuromuscular transmission. Lab Invest. 1990 Mar;62(3):241–243. [PubMed] [Google Scholar]
- Iggo R., Gatter K., Bartek J., Lane D., Harris A. L. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990 Mar 24;335(8691):675–679. doi: 10.1016/0140-6736(90)90801-b. [DOI] [PubMed] [Google Scholar]
- Kirchner T., Schalke B., Buchwald J., Ritter M., Marx A., Müller-Hermelink H. K. Well-differentiated thymic carcinoma. An organotypical low-grade carcinoma with relationship to cortical thymoma. Am J Surg Pathol. 1992 Dec;16(12):1153–1169. [PubMed] [Google Scholar]
- Kirchner T., Schalke B., Marx A., Müller-Hermelink H. K. Evaluation of prognostic features in thymic epithelial tumors. Thymus. 1989;14(1-3):195–203. [PubMed] [Google Scholar]
- Kitadai Y., Yasui W., Yokozaki H., Kuniyasu H., Haruma K., Kajiyama G., Tahara E. The level of a transcription factor Sp1 is correlated with the expression of EGF receptor in human gastric carcinomas. Biochem Biophys Res Commun. 1992 Dec 30;189(3):1342–1348. doi: 10.1016/0006-291x(92)90221-6. [DOI] [PubMed] [Google Scholar]
- Koenders P. G., Beex L. V., Kienhuis C. B., Kloppenborg P. W., Benraad T. J. Epidermal growth factor receptor and prognosis in human breast cancer: a prospective study. Breast Cancer Res Treat. 1993;25(1):21–27. doi: 10.1007/BF00662397. [DOI] [PubMed] [Google Scholar]
- Lloyd S. N., Brown I. L., Leake R. E. Ki-67 antibody immunostaining in benign and malignant human prostatic disease. Int J Biol Markers. 1992 Oct-Dec;7(4):256–259. doi: 10.1177/172460089200700411. [DOI] [PubMed] [Google Scholar]
- Magno W. B., Hirschfield L., Bhuiya T., Harrison G., Mir R. Correlation of proliferative index (PCNA reactivity and Ki-67 reactivity) in primary breast carcinoma with hormone status, lymph node status, and disease-free survival. Conn Med. 1992 Dec;56(12):667–669. [PubMed] [Google Scholar]
- Marshall C. J. Tumor suppressor genes. Cell. 1991 Jan 25;64(2):313–326. doi: 10.1016/0092-8674(91)90641-b. [DOI] [PubMed] [Google Scholar]
- Maruo T., Yamasaki M., Ladines-Llave C. A., Mochizuki M. Immunohistochemical demonstration of elevated expression of epidermal growth factor receptor in the neoplastic changes of cervical squamous epithelium. Cancer. 1992 Mar 1;69(5):1182–1187. doi: 10.1002/cncr.2820690519. [DOI] [PubMed] [Google Scholar]
- McCormick F. Signal transduction. How receptors turn Ras on. Nature. 1993 May 6;363(6424):15–16. doi: 10.1038/363015a0. [DOI] [PubMed] [Google Scholar]
- Modjtahedi H., Eccles S., Box G., Styles J., Dean C. Immunotherapy of human tumour xenografts overexpressing the EGF receptor with rat antibodies that block growth factor-receptor interaction. Br J Cancer. 1993 Feb;67(2):254–261. doi: 10.1038/bjc.1993.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monden Y., Tanioka T., Maeda M., Masaoka A., Nakahara K., Kawashima Y., Kitamura H. Malignancy and differentiation of neoplastic epithelial cells of thymoma. J Surg Oncol. 1986 Feb;31(2):130–138. doi: 10.1002/jso.2930310212. [DOI] [PubMed] [Google Scholar]
- Mygland A., Aarli J. A., Matre R., Gilhus N. E. Ryanodine receptor antibodies related to severity of thymoma associated myasthenia gravis. J Neurol Neurosurg Psychiatry. 1994 Jul;57(7):843–846. doi: 10.1136/jnnp.57.7.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomori H., Horinouchi H., Kaseda S., Ishihara T., Torikata C. Evaluation of the malignant grade of thymoma by morphometric analysis. Cancer. 1988 Mar 1;61(5):982–988. doi: 10.1002/1097-0142(19880301)61:5<982::aid-cncr2820610521>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Oosterhuis H. J. The natural course of myasthenia gravis: a long term follow up study. J Neurol Neurosurg Psychiatry. 1989 Oct;52(10):1121–1127. doi: 10.1136/jnnp.52.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H. F. Tumours of the thymus and their nomenclature. Virchows Arch A Pathol Anat Histopathol. 1991;419(4):257–260. doi: 10.1007/BF01606515. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Tse A. G., Cordell J. L., Pulford K. A., Gatter K. C., Mason D. Y. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990 Aug;137(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Pezzella F., Turley H., Kuzu I., Tungekar M. F., Dunnill M. S., Pierce C. B., Harris A., Gatter K. C., Mason D. Y. bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med. 1993 Sep 2;329(10):690–694. doi: 10.1056/NEJM199309023291003. [DOI] [PubMed] [Google Scholar]
- Rao J. Y., Hemstreet G. P., 3rd, Hurst R. E., Bonner R. B., Jones P. L., Min K. W., Fradet Y. Alterations in phenotypic biochemical markers in bladder epithelium during tumorigenesis. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8287–8291. doi: 10.1073/pnas.90.17.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateyama H., Mizuno T., Tada T., Eimoto T., Hashimoto T., Masaoka A. Thymic epithelial tumours: evaluation of malignant grade by quantification of proliferating cell nuclear antigen and nucleolar organizer regions. Virchows Arch A Pathol Anat Histopathol. 1993;422(4):265–269. doi: 10.1007/BF01608334. [DOI] [PubMed] [Google Scholar]
- Willcox N. Myasthenia gravis. Curr Opin Immunol. 1993 Dec;5(6):910–917. doi: 10.1016/0952-7915(93)90105-2. [DOI] [PubMed] [Google Scholar]
- Willcox N., Schluep M., Ritter M. A., Schuurman H. J., Newsom-Davis J., Christensson B. Myasthenic and nonmyasthenic thymoma. An expansion of a minor cortical epithelial cell subset? Am J Pathol. 1987 Jun;127(3):447–460. [PMC free article] [PubMed] [Google Scholar]
- Yu C. C., Woods A. L., Levison D. A. The assessment of cellular proliferation by immunohistochemistry: a review of currently available methods and their applications. Histochem J. 1992 Mar;24(3):121–131. doi: 10.1007/BF01047461. [DOI] [PubMed] [Google Scholar]










