Abstract
Many factors affect the integrity of messenger RNA from human autopsy tissues including postmortem interval (PMI) between death and tissue preservation and the pre-mortem agonal and disease states. In this communication, we describe RNA isolation and characterization of 389 samples from 18 different tissues from elderly donors who were participants in a rapid whole-body autopsy program located in Sun City, Arizona (www.brainandbodydonationprogram.org). Most tissues were collected within a PMI of 2–6 h (median 3.15 h; N = 455), but for this study, tissue from cases with longer PMIs (1.25–29.25 h) were included. RNA quality was assessed by RNA integrity number (RIN) and total yield (ng RNA/mg tissue). RIN correlated with PMI for heart (r = −0.531, p = 0.009) and liver (r = −558, p = 0.0017), while RNA yield correlated with PMI for colon (r = −485, p = 0.016) and skin (r = −0.460, p = 0.031). RNAs with the lowest integrity were from skin and cervix where 22.7 and 31.4 % of samples respectively failed to produce intact RNA; by contrast all samples from esophagus, lymph node, jejunum, lung, stomach, submandibular gland and kidney produced RNA with measurable RINs. Expression levels in heart RNA of 4 common housekeeping normalization genes showed significant correlations of Ct values with RIN, but only one gene, glyceraldehyde-3 phosphate dehydrogenase, showed a correlation of Ct with PMI. There were no correlations between RIN values obtained for liver, adrenal, cervix, esophagus and lymph node and those obtained from corresponding brain samples. We show that high quality RNA can be produced from most human autopsy tissues, though with significant differences between tissues and donors. The RNA stability and yield did not depend solely on PMI; other undetermined factors are involved, but these do not include the age of the donor.
Keywords: RNA integrity, Aging, Gene expression, Postmortem interval, Autopsy, Human, Tissue
Introduction
Since the decoding of the human genome and development of methods to survey transcripts of the whole genome, there has been an increasing need for high quality RNA from human tissue samples. Global gene expression profiling by microarrays or sequencing of RNA from normal and diseased human tissues has been a key approach for many years to identifying new drug targets (Tarvin and Sandusky 2014; Dawany et al. 2011; Kim and Webster 2009; Kampf et al. 2014). The amount of expression data accumulated during this period has been enormous, but a major problem has been reproducibility of results (Koppelkamm et al. 2011). Although inherent biological variability between human samples affects gene expression results (Opitz et al. 2010), the reliability of gene expression profiling for human studies has primarily been dependent on the quality of RNA and the availability of carefully banked human tissue samples (Brisco and Morley 2012; Fasold and Binder 2012; Opitz et al. 2010; Kiewe et al. 2009; Vermeulen et al. 2011). Difficulty in obtaining high quality human autopsy tissues has been one factor leading to the predominant use of animal models, but the latter have shortcomings in relation to identifying human disease mechanisms (Ruggeri et al. 2014; Beach 2013).
The integrity of human RNA is dependent on a number of factors, but how these interact and differences between different tissue type have not been well defined (Azevedo-Pouly et al. 2014). Assessment of the tissue storage temperature has produced conflicting results; recent studies have shown that storage at −80 °C is equivalent to or better than storage in liquid nitrogen (Andreasson et al. 2013; Auer et al. 2014). The Agilent Bioanalyzer RNA integrity number (RIN) has become the accepted standard for assessing the intactness of isolated RNA from all sources (Schroeder et al. 2006). RNA integrity appears to be dependent to some extent on the time after death that the tissue is collected and preserved, the postmortem interval (PMI), but the results obtained, mainly from brain samples, have been conflicting (Broniscer et al. 2010; Burke et al. 1991; Cummings et al. 2001; Ervin et al. 2007; Li et al. 2004; Preece and Cairns 2003; Tomita et al. 2004; Birdsill et al. 2011; Sun et al. 2016; Rudloff et al. 2010; Micke et al. 2006). In addition, the agonal state in the immediate premortem period is likely to be of at least equal importance as PMI, especially if there has been significant fever or hypoxia–ischemia events. Other factors include disease states, age and gender of the subject, type of tissue, storage conditions and length of time in storage (Koppelkamm et al. 2011; Kap et al. 2014). One study of forensic autopsies with PMI ranging from 4 to 94 h (mean 27.3 h) showed brain samples had lower mean integrity than cardiac muscle or skeletal muscle (Grabmuller et al. 2015). These authors showed mean RIN values for brain of 2.8, 3.8 for cardiac muscle, and 4.4 for skeletal muscle, which are considered indicative of poor quality and not suitable for research purposes. With these sample sets, an effect of PMI on RIN was only observed for cardiac muscle (Grabmuller et al. 2015). It was also recently shown that the isolation method used to extract RNA from frozen tissue had effects on integrity and yield. The widely-used Trizol (guanidine isothiocyanate/phenol/chloroform) method produced higher yields, but had poor integrity compared to column purification methods (Ahmed et al. 2015; Grabmuller et al. 2015), though this was not a uniform finding (Kap et al. 2014). A recent study examined RNA integrity in 37 different human tissues (mostly from tumors) that had been resected during surgery not at autopsy. In this series of 103 samples, they reported that 81.6 % had RIN values of 6.5 or greater, a parameter defined as acceptable for most gene expression studies (Kap et al. 2014).
In a previous study, we surveyed the features of RNA isolated from brain cerebellum samples collected by our rapid autopsy program. We showed a significant correlation between RIN and yield with PMI, and significant changes in gene expression with PMI (Birdsill et al. 2011). From this study, it was apparent that PMI alone is not the dominant factor as, even with tissue collected with very-short PMI, there were still large differences in RNA yield even when RIN values were high (Birdsill et al. 2011). The purpose of the present study was to extend our previous work to non-brain tissue samples taken over the last 7 years to determine the relative quality of these samples and their suitability for gene profiling studies. This study examined samples taken at autopsy from 18 different normal types of tissues. The integrity and yield varied significantly between tissues, and our results showed that the effect of PMI on RIN and RNA yield differs amongst tissue types. No correlations were observed between RIN values in certain tissues and RIN values from corresponding brain samples, which tended to have higher degrees of intactness. It can be concluded that other factors involved in RNA integrity in autopsied human tissue samples also need to be defined.
Materials and methods
Collection of human tissue
All tissue samples were from participants in the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND) and were autopsied by the Brain and Body Donation Program (BBDP) of the Banner Sun Health Research Institute, Sun City, Arizona. This longitudinal clinicopathological study has been running for 27 years firstly on the basis of brain-only donation, but since 2006 has accepted whole-body donation. The Program has had continuous Institutional Review Board approval. The goal of the Program since its inception has been to collect the highest quality tissue within as short a period of time as possible after death. The details of the Program and the demographics of the participants have been published (Beach et al. 2015, 2008). The median postmortem interval for the whole program is 3.0 h (N = 1654). For the purposes of this study, some tissues with longer PMI were deliberately included to provide a larger range of PMIs. Most participants had been studied clinically premortem, and were autopsied in a systematic manner. Tissue from selected organs were sampled and rapidly frozen on dry ice. All tissue samples were stored at −80 °C. For this study, weighed tissue samples (20–30 mg) were dissected from frozen tissue blocks and transferred to cooled microcentrifuge tubes. The samples did not thaw prior to extraction. The demographic details of all the samples analyzed are presented in Table 1. The majority of samples came from subjects that had shown no signs of defined neurodegenerative diseases. All tissues were considered to be histologically normal after examination by medically-certified pathologists. For comparative purposes with the results from the peripheral tissue samples, we have included data for brain RNA quality analyses. These samples were from a larger series of cerebellum tissue samples (n = 624).
Table 1.
Demographic details of cases used in study
| Tissue (n) | Sex (M/F) | Age (years) | Age range (years) (±SD) | PMI (h) | PMI range (h) (±SD) |
|---|---|---|---|---|---|
| Adrenal (24) | 15/9 | 87.4 ± 5.4 | 73–97 | 6.6 ± 6.3 | 2.4–29.2 |
| Cervix (16) | –/16 | 86.1 ± 8.5 | 67–103 | 4.5 ± 2.8 | 2.2–11.7 |
| Colon (24) | 13/11 | 88.5 ± 6.4 | 73–99 | 4.9 ± 5.8 | 1.2–29.2 |
| Esophagus (18) | 10/8 | 86.3 ± 5.3 | 73–93 | 6.0 ± 4.5 | 2.4 ± 18.0 |
| Heart (26) | 16/10 | 83.1 ± 10.4 | 49–95 | 4.8 ± 5.5 | 2.2–29.2 |
| Jejunum (21) | 12/9 | 86.7 ± 5.3 | 73–94 | 6.4 ± 6.1 | 2.4–29.2 |
| Kidney (26) | 15/11 | 88.4 ± 6.2 | 87–99 | 5.6 ± 6.3 | 1.2–29.2 |
| Liver (30) | 19/11 | 86.9 ± 6.9 | 73–99 | 4.9 ± 5.4 | 1.2–29.2 |
| Lymph node (21) | 12/9 | 86.7 ± 5.3 | 73–94 | 6.4 ± 6.1 | 2.4–29.2 |
| Lung (15) | 9/6 | 85.7 ± 11.9 | 49–97 | 3.8 ± 2.2 | 2.2–10.3 |
| Ovary | –/17 | 86.1 ± 8.3 | 67–103 | 5.9 ± 6.6 | 2.2–29.2 |
| Pancreas (26) | 16/10 | 88.3 ± 6.2 | 73–99 | 5.7 ± 6.2 | 1.2–29.2 |
| Prostate (19) | 19/– | 82.5 ± 11.5 | 49–94 | 4.1 ± 2.8 | 2.0–12.2 |
| Skin (22) | 13/9 | 86.9 ± 5.3 | 73–94 | 6.9 ± 6.5 | 2.4–29.2 |
| Stomach (20) | 12/8 | 86.7 ± 5.5 | 73–94 | 5.2 ± 3.3 | 2.4–12.2 |
| Submandibular (21) | 13/8 | 88.5 ± 6.6 | 75–99 | 5.2 ± 4.3 | 1.2–18.0 |
| Testis (18) | 18/– | 82.5 ± 12.1 | 49–96 | 4.6 ± 4.1 | 2.0–18.0 |
| Thyroid (25) | 14/11 | 88.7 ± 6.3 | 73–99 | 4.5 ± 3.8 | 1.2–18.0 |
| TOTAL (389) | 86.6 ± 6.3 | 5.4 ± 5.2 | (Median 3.25) | ||
| Brain (624) | 401/223 | 82.2 + 9.33 | 38–106 | 3.1 + 1.2 | 1.0–18.0 |
PMI post-mortem interval
RNA isolation
We employed the same method for RNA isolation for these different tissues as was used for our previously-published brain RNA isolation study, using the RNA EasyPlus Mini Kit (Qiagen, Valencia, CA) (Birdsill et al. 2011). Each tissue sample was dispersed in RNA-lysis buffer (containing 0.1 M β-mercaptoethanol) by mild sonication (10–15 s), and then processed according to the manufacturer’s instructions. In brief, extracted tissue is passed through a prefilter to remove DNA, adjusted with an equal volume of 70 % ethanol and applied to the RNA column. After washes with kit buffers, RNA is eluted from the column with 100 μl of water. A point to note was that for highest yield and integrity, sonication of tissue sample should be carried out for as brief a time as necessary to disrupt the tissue; excessive sonication significantly reduced the yield of RNA from tissues. Some tissues contained insoluble connective tissue or fat that did not dissolve in RNA lysis buffer, but were removed on the DNA prefilters prior to RNA isolation. This same method was used for all cerebellum brain samples.
RNA characterization
The concentration and purity of each RNA sample was assessed using a Nanodrop 1000 spectrometer (Nanodrop Inc—Thermo Fisher Scientific, Wilmington, DE). The RNA yield from each sample was calculated as ng/mg of tissue (wet weight). To assess RNA integrity, samples were measured using an Agilent 2100 Bioanalyzer with RNA 6000 Nano Kits (Agilent Inc, Wilmington, DE). This instrument produces an RNA integrity number (RIN) for each sample using a proprietary algorithm (Schroeder et al. 2006) with values ranging from 10 (RNA completely intact) to 1 (RNA extensively degraded). For a number of samples, no measurable RIN values could be obtained (RIN not available—N/A). Each sample producing a N/A assessment was analyzed 2 separate times to ensure this result was not due to technical issues.
Gene expression—reverse transcription and quantitative polymerase chain reaction
Samples derived from heart were analyzed for expression of a panel of housekeeping genes to determine how RIN and PMI affected the stability of gene expression levels. RNA from each sample (0.5 μg) was reverse transcribed using a Quantitect reverse transcription kit according to the manufacturer’s protocols (Qiagen). Prior to use in quantitative PCR (qPCR) analyses, each cDNA was diluted 1:1 with water. Appropriate numbers of no-reverse-transcriptase controls were prepared in parallel for each batch of samples. For qPCR, cDNA samples were amplified using Perfecta SYBR Green Fast Mix 2× reaction mixture (Quanta Biosciences, Gaithersburg, MD) and mRNA-specific primers for the following housekeeping genes: β-Actin: sense sequence: TCCT ATGTGGGCGACGAG; anti-sense: ATGGCTGGG GTGTTGAAG too produce fragment size 242 bp: Beta 2-macroglobulin; sense sequence GGGTTTCAT CCATCCGACA; and anti-sense sequence: ACACG GCAGGCATACTCATC (161 bp): GAPDH: sense sequence: CGGATTTGGTCGTATTGG; anti-sense sequence: GGAAGATGGTGATGGGATTT (206 bp): Transferrin receptor: sense sequence: ATGCTGCT TTCCCTTTCCTT: anti-sense sequence: CCCATTT CCTTTATGTCTGCTC (292 bp). All primer-pair sequences were verified for absence of hairpin formations or primer-dimers that interfere with amplification efficiency (NetPrimer, Premier Biosoft), and had qPCR amplification efficiencies in the range of 95–105 %. QPCR was carried out using a Stratagene Mx3000p machine. QPCR analyses followed most of the recommended criteria for minimal information for publication of quantitative real time PCR experiments (MIQE) (Bustin et al. 2009).
Statistical analysis
All statistical analyses were carried out using Graph-pad Prism v.6 (Graphpad Software, La Jolla, CA). Statistical significance between measures was accepted if p < 0.05.
Results
Study design and summary of results
RNA isolation and characterization was carried out for a total of 389 samples from 18 different tissues. The demographic details of the samples analyzed are shown in Table 1. The average age of the donors was 86.6 years with mean PMI of 5.3 h. The median PMI of tested samples was 3.25 h. The mean or median PMI of the group of samples was affected by the deliberate inclusion of samples with long PMI (up to 29.2 h) since one of the goals of this study was to examine in each tissue the effect of PMI on RNA integrity (RIN) and yield. The majority of the samples for each tissue were from subjects with no neurodegenerative diseases (NND) (Table 2). A summary of RIN and yields for each tissue is shown in Table 3. Some samples were extensively degraded and did not produce a measurable RIN value; these were excluded from mean RIN calculations but not from the mean yield calculations. The number of samples that produced RIN values compared to the total number analyzed is shown in the first column (n) (Table 3). The mean RIN values for each tissue are shown in the second column (RIN), but what is informative is the range of values (RIN range). Each tissue had samples with high RIN values as well as those that were extensively degraded even though most PMIs were relatively short. Considering RNA yield, there was also a high degree of variability for each tissue (Table 3—Yield Range). The tissues with the highest yields were liver, submandibular gland and pancreas. The tissues with large amounts of fibrous material or fat (skin, cervix and prostate) had lowest yields. It is possible that this material affected the efficiency of RNA extraction from these tissues, or, the lower yield may simply be due to lower overall cell density in samples with a large fraction of connective tissue. Using our methods, the tissue known to have the highest RNAse concentration—pancreas—did not produce RNA of inferior quality to any other tissue (Griffin et al. 2012). If the data are presented for each individual case used (Table 4), the wide range of RIN values, including samples where no RIN value (N/A) could be obtained, indicates that there must be tissue-specific factors, not just perimortem or postmortem donor conditions, which affect RNA integrity.
Table 2.
Disease states of analyzed tissue samples
| Tissue (n) | Control | NDD |
|---|---|---|
| Adrenal (24) | 18 | 3 |
| Cervix (16) | 10 | 5 |
| Colon (24) | 18 | 2 |
| Esophagus (18) | 12 | 3 |
| Heart (26) | 17 | 1 |
| Jejunum (21) | 15 | 3 |
| Kidney (26) | 20 | 2 |
| Liver (30) | 21 | 3 |
| Lymph node (21) | 15 | 3 |
| Lung (15) | 11 | 0 |
| Ovary (17) | 11 | 4 |
| Pancreas (26) | 19 | 3 |
| Prostate (19) | 12 | 3 |
| Skin (22) | 16 | 3 |
| Stomach (20) | 15 | 2 |
| Submandibular (21) | 17 | 2 |
| Testis (18) | 12 | 1 |
| Thyroid (25) | 21 | 0 |
NDD neurodegenerative disease
Table 3.
RNA integrity and Yield Results for 18 human peripheral tissues compared to brain
| Tissue (n) | RIN (±SD) | RIN range (% <6.5) | Yield (ng/mg) | Yield range (ng/mg) |
|---|---|---|---|---|
| Adrenal (23/24) | 7.2 ± 1.8 | 3.0–9.0 (33) | 946.2 ± 668.2 | 50–2363 |
| Cervix (11/16) | 6.4 ± 1.8 | 2.4–8.3 (50) | 156.9 ± 144.0 | 0–578 |
| Esophagus (18/18) | 7.1 ± 2.3 | 2.4–8.5 (37.5) | 455.0 ± 399.4 | 23–767 |
| Heart (23/26) | 7.6 ± 2.0 | 2.6–9.0 (26.9) | 174.9 ± 136.2 | 1–488 |
| Jejunum (21/21) | 6.4 ± 1.5 | 2.5–8.8 (33) | 1010.9 ± 734.2 | 107–1935 |
| Kidney (26/26) | 7.0 ± 1.5 | 4.6–9.1 (30.8) | 868.8 ± 511.5 | 59–2914 |
| Liver (29/30) | 7.2 ± 2.2 | 1.1–9.4 (23.3) | 2288.3 ± 1204.5 | 17–4701 |
| Lymph node (21/21) | 7.6 ± 1.4 | 3.6–9.2 (14.3) | 1709.5 ± 773.0 | 684–2895 |
| Lung (15/15) | 6.5 ± 2.2 | 1.6–8.9 (33) | 410.3 ± 358.6 | 77–1159 |
| Ovary (15/17) | 5.9 ± 6.6 | 2.4–9.1 (52.9) | 546.4 ± 496.4 | 67–1795 |
| Pancreas (25/26) | 6.5 ± 1.6 | 3.9–9.1 (46.1) | 2225.9 ± 2152.2 | 14–6840 |
| Prostate (15/19) | 5.7 ± 1.9 | 2.2–8.3 (63.1) | 251.0 ± 617.3 | 12–2773 |
| Colon (23/24) | 6.7 ± 1.0 | 4.7–8.3 (37.5) | 737.2 ± 422.7 | 41–1370 |
| Skin (17/22) | 6.2 ± 1.8 | 2.4–8.2 (54.4) | 89.6 ± 75.4 | 0.1–347 |
| Stomach (20/20) | 6.0 ± 1.7 | 2.4–8.0 (45) | 1420.4 ± 938.9 | 9.7–3275 |
| Submandibular (20/21) | 7.8 ± 1.4 | 2.8–9.2 (9.5) | 2082.1 ± 1054.5 | 4 –3498 |
| Testis (16/18) | 7.5 ± 2.0 | 3.0–9.4 (33) | 342.2 ± 343.5 | 25–528 |
| Thyroid (23/25) | 7.0 ± 1.5 | 2.4–8.8 (28) | 400.2 ± 277.0 | 9–803 |
| Brain (401/223) | 8.6 + 1.2 | 2.4–10 (5.4) | 379.8 + 200 | 0–966.8 |
n number of samples with RIN/Total number of samples, RIN RNA integrity number
Table 4.
RNA integrity and yield results for individual cases
| Case # (n) | RIN range | Yield range (ng/mg) | Age/years | PMI/h |
|---|---|---|---|---|
| 1 (6) | 5.7–9.4 | 428–2434 | 88 | 1.25 |
| 2 (6) | 6.5–8.8 | 129–1812 | 97 | 1.87 |
| 3 (2) | 2.2–9.1 | 138–670 | 86 | 2 |
| 4 (6) | 5.9–9.1 | 31–1879 | 93 | 2 |
| 5 (3) | 6–9.2 | 142–664 | 88 | 2.2 |
| 6 (1) | 1.6 | 97 | 81 | 2.25 |
| 7 (7) | 7.5–8.3 (1 N/A) | 32–2496 | 81 | 2.25 |
| 8 (3) | 6.6–9.1 | 44–296 | 61 | 2.33 |
| 9 (3) | 2.9–88 | 14–350 | 94 | 2.33 |
| 10 (3) | 3–8.4 | 41–233 | 80 | 2.35 |
| 11 (2) | 3.3 (1 N/A) | 2–77 | 95 | 2.5 |
| 12 (8) | 4.1–8.5 (3 N/A) | 41–2235 | 90 | 2.45 |
| 13 (6) | 5.2–8.9 | 29–2863 | 97 | 2.5 |
| 14 (15) | 2.4–9.5 | 62–3310 | 87 | 2.5 |
| 15 (14) | 3.7–8.6 | 13–2929 | 73 | 2.5 |
| 16 (14) | 7–9.4 (1 N/A) | 347–5567 | 82 | 2.5 |
| 17 (6) | 7.1–9.1 | 553–2889 | 82 | 2.67 |
| 18 (6) | 4.4–8.2 | 63–1087 | 94 | 2.8 |
| 19 (9) | 7.1–9.1 | 159–2488 | 80 | 2.88 |
| 20 (7) | 1.1–9.0 | 17–4226 | 96 | 3 |
| 21 (4) | 8.9–9.0 (2 N/A) | 1–1154 | 93 | 3 |
| 22 (14) | 3.7–9.6 (1 N/A) | 19–3275 | 92 | 3 |
| 23 (3) | 6.6–9.0 | 65–3814 | 79 | 3 |
| 24 (2) | 7.9 (1 N/A) | 0–457 | 84 | 3 |
| 25 (9) | 7.7–9.8 | 0–5252 | 82 | 3 |
| 26 (14) | 2.4–9.5 | 58–4701 | 91 | 3.16 |
| 27 (6) | 7.4–9.4 | 548–3684 | 93 | 3.16 |
| 28 (6) | 6.1–9.2 | 559–3498 | 93 | 3.16 |
| 29 (4) | 5.3–8.6 (1 N/A) | 25–2410 | 74 | 3.25 |
| 30 (2) | 6.7 (1 N/A) | 64–386 | 80 | 3.25 |
| 31 (4) | 5.2–7.4 (1 N/A) | 28–3771 | 75 | 3.3 |
| 32 (2) | 7.4 (1 N/A) | 0–67 | 79 | 3.3 |
| 33 (15) | 5.9–8.9 (2 N/A) | 112–2773 | 89 | 3.3 |
| 34 (4) | 4.2–8.5 (1 N/A) | 7.3–1069 | 97 | 3.5 |
| 35 (6) | 2.5–7.7 (1 N/A) | 12–2157 | 86 | 3.5 |
| 36 (6) | 3.2–7.6 | 37–3020 | 89 | 3.5 |
| 37 (8) | 7.1–9.2 (2 N/A) | 30–2450 | 82 | 3.66 |
| 38 (6) | 4.9–8.5 | 415–3965 | 99 | 3.75 |
| 39 (2) | 6.8–7.7 | 88–115 | 91 | 4 |
| 40 (7) | 6.5–9.0 (1 N/A) | 63–2288 | 91 | 4.25 |
| 41 (14) | 2.4–8.5 | 23–4122 | 84 | 4.25 |
| 42 (13) | 2.8–8.6 | 4–2663 | 90 | 4.25 |
| 43 (4) | 4.5–9.1 | 252–1216 | 49 | 4.5 |
| 44 (2) | 2.4–6.7 | 255–889 | 67 | 5 |
| 45 (13) | 4.4–9.3 | 66–2423 | 91 | 7.25 |
| 46 (13) | 3–9.2 (1 N/A) | 14–1813 | 93 | 7.33 |
| 47 (10) | 2.4–8.5 | 31–2335 | 84 | 10 |
| 48 (14) | 2.2–7.9 (1 N/A) | 17–1958 | 87 | 10.3 |
| 49 (15) | 2.4–8.1 | 9–2285 | 88 | 11.66 |
| 50 (12) | 3.2–8.2 | 38–4378 | 84 | 12.25 |
| 51 (8) | 5.1–8.5 (1 N/A) | 1–3844 | 90 | 18 |
| 52 (10) | 2.6–8.4 (2 N/A) | 51–2655 | 87 | 29.25 |
n number of different tissues from each case, N/A no RIN value available
RNA “fit for purpose”
There is limited consensus on what are the acceptable criteria for RNA quality for downstream applications. The concept of “fit for purpose” came from a similar study as this one where a large number of different tissues were surveyed for RNA integrity. The major difference between the studies was the analysis of postmortem tissue in this study compared to surgical samples in the cited study (Kap et al. 2014). The cutoff criteria of RIN values of less than 6.5 as being unacceptable was established by these authors (Kap et al. 2014). In their tissue bank, 18.4 % of 103 surgical samples (mostly tumors) or surgical biopsy samples had RIN less than 6.5 (including samples where RIN could not be determined (ND). Overall, we had 34.4 % of samples with RIN values of less than 6.5, while 41.6 % of samples had RIN values of greater than 7.5. The percentage of samples with RIN values less than 6.5 for each tissue is shown in Table 3, varying from 14.3 % for lymph node to 63.1 % for prostate. By comparison, in a series of 624 brain samples, only 5.4 % had RIN values less than 6.5 and 15.5 % had RIN values less than 7.5 (Table 3).
Does PMI affect RIN and yield?
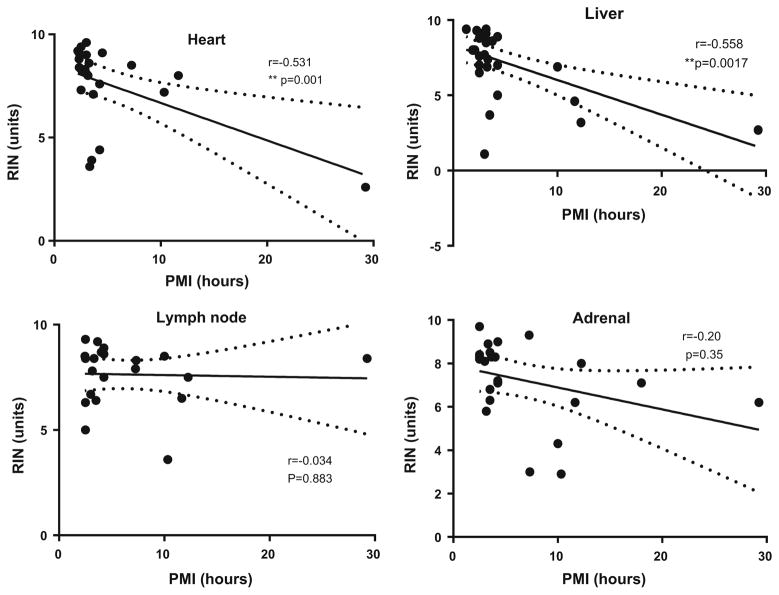
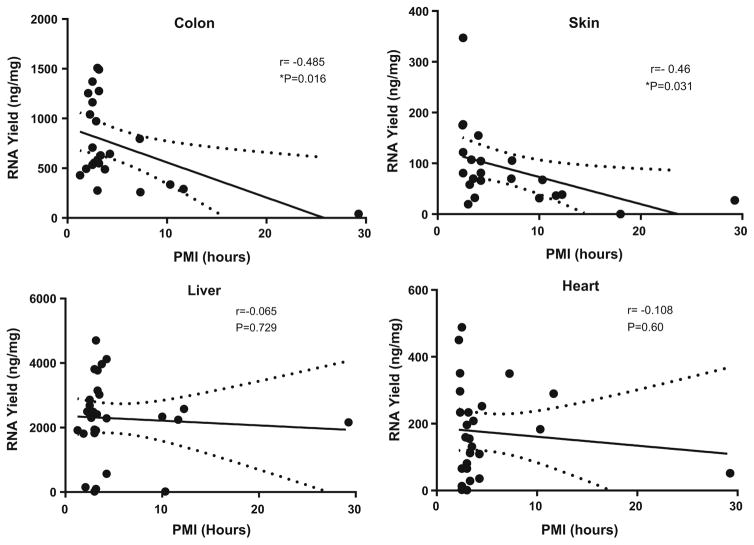
Pearson correlation analyses were carried out for all tissues, comparing PMI and RIN; PMI and Yield; RIN and Yield; Age and RIN; and Age and Yield (Table 5). For the great majority of tissue types, both RIN and RNA yield decreased with increasing PMI (decreasing RIN for 15/17 tissue types and decreasing RNA yield for 13/17 tissue types; p = 0.002 and 0.03, respectively. (One Sample Goodness of Fit Test). Correlation analyses of all tissue RIN values with PMI showed significance (r = −0.2251, p <0.0001). However, due to high variability in RIN, for individual tissue types, the correlation of PMI with RIN reached significance level only for heart (r = −0.531; p = 0.0092) and liver (r = −0.559; p = 0.0016)(Fig. 1), while the correlation of PMI with RNA yield was significant only for colon and skin (respectively, r = −0.485, p = 0.016; r = −0.460, p = 0.031) (Fig. 2). Correlation analysis of RIN and yield for all tissue samples from individual cases (Table 4) did not produce statistical significance (data not shown).
Table 5.
Correlation analyses of RNA integrity, yield and demographic
| Tissue | PMI and RIN
|
PMI and yield
|
RIN and yield
|
Age and RIN
|
Age and yield
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| Adrenal | −0.200 | 0.3486 | −0.392 | 0.058 | 0.565 | 0.004* | −0.165 | 0.4403 | −0.3801 | 0.067 |
| Cervix | −0.297 | 0.276 | −0.075 | 0.783 | 0.048 | 0.903 | 0.578 | 0.049* | −0.176 | 0.514 |
| Colon | −0.314 | 0.145 | −0.485 | 0.016* | −0.105 | 0.634 | −0.025 | 0.634 | −0.105 | 0.906 |
| Esophagus | −0.090 | 0.721 | −0.199 | 0.427 | 0.503 | 0.033* | 0.023 | 0.929 | 0.004 | 0.988 |
| Heart | −0.531 | 0.009** | −0.108 | 0.599 | 0.537 | 0.008** | −0.296 | 0.171 | 0.077 | 0.708 |
| Jejunum | −0.168 | 0.467 | −0.325 | 0.151 | −0.308 | 0.174 | 0.016 | 0.960 | −0.392 | 0.078 |
| Kidney | 0.273 | 0.177 | −0.341 | 0.086 | 0.133 | 0.516 | 0.071 | 0.730 | 0.217 | 0.516 |
| Liver | −0.558 | 0.0017** | −0.065 | 0.729 | 0.232 | 0.226 | 0.000 | 0.991 | −0.274 | 0.143 |
| Lymph node | −0.034 | 0.883 | 0.059 | 0.797 | 0.642 | 0.0017** | −0.105 | 0.651 | −0.059 | 0.798 |
| Lung | 0.399 | 0.215 | 0.302 | 0.915 | 0.320 | 0.245 | 0.196 | 0.484 | −0.168 | 0.549 |
| Ovary | −0.013 | 0.964 | 0.192 | 0.461 | 0.039 | 0.891 | −0.156 | 0.581 | −0.043 | 0.868 |
| Pancreas | −0.328 | 0.110 | −0.219 | 0.281 | −0.033 | 0.874 | −0.134 | 0.522 | −0.319 | 0.112 |
| Prostate | 0.198 | 0.480 | 0.093 | 0.970 | 0.213 | 0.452 | −0.068 | 0.811 | 0.074 | 0.765 |
| Skin | −0.412 | 0.098 | −0.460 | 0.031* | −0.025 | 0.925 | 0.209 | 0.421 | −0.206 | 0.357 |
| Stomach | −0.429 | 0.059 | −0.433 | 0.057 | 0.331 | 0.154 | 0.108 | 0.652 | −0.106 | 0.655 |
| SMG | −0.224 | 0.328 | 0.309 | 0.172 | 0.232 | 0.027* | 0.099 | 0.668 | 0.099 | 0.670 |
| Testis | −0.055 | 0.839 | −0.184 | 0.464 | 0.576 | 0.019* | 0.019 | 0.943 | −0.213 | 0.397 |
| Thyroid | −0.08 | 0.715 | −0.311 | 0.130 | −0.042 | 0.850 | 0.048 | 0.835 | −0.215 | 0.301 |
Numbers in bold indicate values reached statistical significance
Asterisk on figures indicate statistical significance
Fig. 1.
Linear regression analysis for RNA integrity compared to postmortem interval for heart, liver, lymph node and adrenal. Results show linear regression lines for each tissue for RIN compared to PMI with 95 % confidence limits. Results show Pearson r values with p values indicating degree of significance. The distribution for samples with significant correlations (heart and liver) compared to those with no correlation (lymph node and adrenal) can be compared
Fig. 2.
Linear regression analysis for RNA Yield compared to postmortem interval for colon, skin, liver and heart. Results show linear regression lines for each tissue for RNA yield compared to PMI with 95 % confidence limits. Results show Pearson r values with p values indicating degree of significance. The distribution for samples with significant correlations (colon and skin) compared to those with no correlation (liver and heart) can be compared
Comparison of RNA quality from peripheral tissues with matching brain samples
As part of our tissue banking procedures, we also have collected RNA quality data on 624 brain samples (cerebellum) as a continuation of a previously published study (Birdsill et al. 2011). The demographics of these brain samples are included in Table 1, and RNA quality data in Table 3. It can be seen that RNA quality was higher in brain samples than other peripheral tissue samples in terms of mean RIN values and percentage of samples with RIN values less than 6.5. Analyses carried out showed no correlations of RIN values between brain, and liver (p = 0.401, Pearson r = 0.217), adrenal (p = 0.777, r = 0.091), cervix (p = 0.584, r = 0.197), esophagus (p = 0.776, r = 0.103), kidney (p = 0.219, r = 0.325) or lymph node (p = 305, r = 0.3385). For the other tissues, there were insufficient numbers of corresponding brain values to make comparisons.
Assessment of gene expression of housekeeping genes
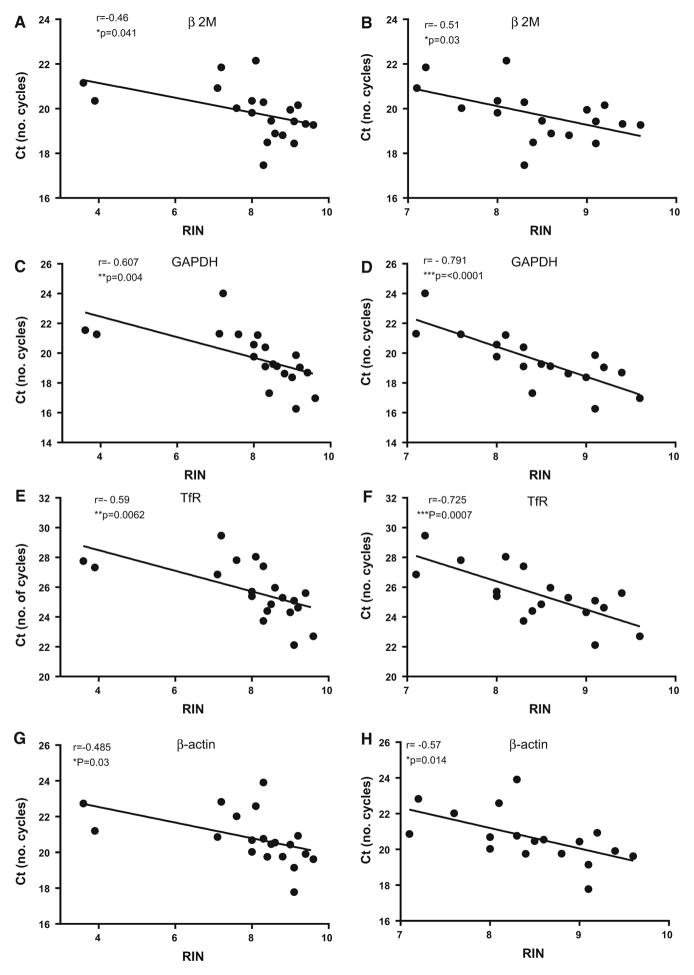
A central question for gene expression studies with RNA produced from human tissue samples is performance in quantitative assays. We selected RNA samples (n = 23) derived from heart, as RNA from this tissue had shown significant correlation of RIN with PMI, to examine how changes in these factors affected levels of gene expression. We analyzed expression of 4 “housekeeping” genes—beta-2-microglobulin (β2 M), glyceraldehyde-3 phosphate dehydrogenase (GAPDH), transferrin receptor (TfR) and β-actin—that are widely used in assays for normalizing purposes (Barber et al. 2005; Huggett et al. 2005; Dheda et al. 2004; Hellemans and Vandesompele 2014; Vandesompele et al. 2002). As such, the expression of these genes should not differ between samples from the same tissue. In Fig. 3, it can be seen that there was significant correlation between RIN and Ct values for all of the genes. In the left hand column, all 23 of the samples were included in the analyses, while in the right hand column, only samples with RIN>6.5 were analyzed. In both cases, there are significant correlations. This indicated that integrity does affect levels of gene expression in this widely-used assay. In these samples, there was marginally significant correlation between Ct values and PMI only for GAPDH gene expression (r = 0.446, p = 0.049).
Fig. 3.
Effect of RIN on expression values of housekeeping normalization genes in RNA samples derived from heart. The results show linear correlation analyses for Ct values compared to RIN values for the genes β2 microglobulin (β2 M) (a and b), glyceraldehyde-3 phosphate dehydrogenase (GAPDH) (c and d), transferrin receptor (e and f), and β-actin (g and h). Results on left show analyses for these genes using the samples with complete range of RIN values (a, c, e, g), while the panels on right side show matching analyses for samples with RIN values meeting the criteria of>6.5 (b, d, f, h). In both cases, significant correlation of Ct values with RIN was shown
Discussion
Human tissue banking has become an important component of the process of discovering new causes and treatments of diseases. The collection and storage of donated human autopsy tissue is complicated due to the unique medical condition of each human donor along with a myriad of ethical and legal requirements (Mora et al. 2014; Ravid and Ferrer 2012; Beach et al. 2015). The applications for human tissue principally involve analysis of alterations of protein expression and post-translational modifications (proteomics); small molecules (metabolomics), lipids (lipidomics), DNA (genomics and epigenetics) and changes in expression of mRNA or other RNA species, including small miRNA. Over the last decade, gene expression profiling studies were the most common disease screening applications of tissue, first with gene profiling microarrays and more recently with RNA sequencing procedures, and these methods identified a critical need for high quality RNA from well-characterized human tissue (Buesa et al. 2004).
Quality control of tissue samples has focused in many studies on the quality of extracted tissue RNA. Many of these investigations have studied the isolation and stability of RNA from human brains; there have been relatively few studies on other tissues. The factors that could affect the quality of tissue and tissue-derived products include disease conditions of the subject, agonal state of the subject immediately premortem, postmortem interval, pH of the tissue, tissue handling and preservation techniques, storage conditions and length of time in storage. Although the majority of studies of brain RNA have concluded that PMI does not have an overall significant effect on RNA stability over the time periods under consideration, the methods used to assess this have varied considerably (Auer et al. 2014; Burke et al. 1991; Condelli et al. 2014; Fajardy et al. 2009; Gonzalez-Herrera et al. 2013; Hatzis et al. 2011; Birdsill et al. 2011; Burke et al. 1991; Cummings et al. 2001; Heinrich et al. 2007; Koppelkamm et al. 2011; Stan et al. 2006). It is apparent that some mRNAs, for example cyclooxygenase-2 mRNA in brain (Lukiw and Bazan 1997), are lost quickly while others are more resistant (Nagy et al. 2015; Birdsill et al. 2011). The central issue with respect to studies of gene expression in human disease is the need to compare expression level of particular genes in a large number of different human samples with the goal of associating changes in expression with disease processes. If the changes in expression are significantly affected by tissue RNA quality, the variance could be too large to identify disease association. For this reason, it is important to understand possible sources of variability in the postmortem preservation of RNA. The published data have not been consistent, but overall, there are convincing studies showing that for reliable gene expression data from microarray or qPCR, RNA quality affects the validity of findings (Abasolo et al. 2011; Popova et al. 2008; Atz et al. 2007). Although recent advances in expression profiling using RNA sequencing methods, particular using degraded RNA extracted from formalin fixed-paraffin embedded tissues, have suggested reliable expression profiling results can be obtained, it is noticeable that the manufacturers of RNA sequencing platforms still recommend the use of RNA with high degree of intactness as starting material (Hedegaard et al. 2014).
In our study of brain samples collected in this rapid autopsy program, we found a significant correlation between RIN and PMI (r = 0.34, p = 0.002) (Birdsill et al. 2011). In a similar study, 58 % of all samples of RNA derived from normal human brain tissue from 33 autopsied subjects with a median PMI of 7.7 h had a cutoff RIN value above 7.5—defined as “minimally-degraded” RNA (Broniscer et al. 2010). Seventy percent of samples from the short-PMI autopsies had a RIN greater than or equal to 7.5 compared to only 21 % of the samples from the longer-PMI autopsies (p < 0.01). In our series of brain samples, 94.6 % of samples had RIN values greater than 6.5 and 84.7 % had RIN values of greater than 7.5 %. All of these findings are in contrast to other studies suggesting no PMI effect on brain mRNA integrity (Cummings et al. 2001; Ervin et al. 2007; Preece and Cairns 2003). It appears likely from the preponderance of inverse correlations presented here and in our prior publication (Birdsill et al. 2011) that there is a progressive loss of RNA integrity and yield with increasing PMI, but most of the variability in these RNA measures is due to other, as-yet uncertain factors. For many individual comparisons, a statistically significant result between RIN and PMI was not obtained. Overall, our results indicate that PMI, RIN and RNA yield are not interrelated in a straightforward fashion and are affected by other variables not accounted for here.
When procuring and handling human bio-specimens, one has to consider what the standards for acceptable quality of samples are; what factors are affecting this and how to improve on the quality of processed tissues if possible? RNA integrity represents a useful marker for assessing tissue quality in large series of samples compared to more intensive methods such as histology or protein modifications (Shabihkhani et al. 2014). In addition, there is still a demand for human tissue for preparation of RNA for gene expression profiling studies. These studies have produced valuable data for most diseases. Overall, tissues analyzed in the present study, chosen to represent a wide range of PMI, 65.6 % had RIN >6.5 while 41.6 % had RIN>7.5.
From this study, we have shown that relatively high quality RNA can be isolated from all human postmortem tissues but with significant variability in results from case to case and between tissue types. The standard parameters of RNA quality, RIN and yield, illustrate major sample-to-sample differences for reasons that are not immediately apparent. In our autopsy program, the collection, handling, storage and processing of tissue is handled in a highly reproducible manner according to defined protocols. Although, for the great majority of tissue types, RIN and RNA yield both declined with increasing PMI, there was so much variability that this correlation reached significance level for only two individual tissue types. In a series of forensic cardiac samples, it was shown that, as in our study, heart RNA integrity was affected by PMI; this study used materials with PMI up to 50 h (Koppelkamm et al. 2011). This study concluded, however, that heart RNA was significantly more stable than brain RNA. One other study concluded that heart RNA quality was not related to PMI (Gonzalez-Herrera et al. 2013).
It is not clear from these results what additional factors are responsible for variability in RNA quality and yield. One critical factor, not measured here, is the ambient temperature during cadaver storage prior to autopsy (Ferrer et al. 2007). This is a crucial question to address as it will aid in sample selection prior to their use in gene expression profiling studies. It is expensive and labor-intensive to screen a large number of samples to find many to be unacceptable for downstream processing. We also showed for heart that the raw gene expression values (Ct) for a range of housekeeping normalization genes could be affected by RIN. These genes are widely used to normalize for expression of genes of interest with the assumption that they remain relatively constant (Barber et al. 2005; Dheda et al. 2004; Gonzalez-Herrera et al. 2013; Vandesompele et al. 2002). A recent study reported that expression of common housekeeping genes is actually more stable with increased PMI than many other transcripts (Nagy et al. 2015).
In summary, RNA prepared from a range of human autopsy tissue had large variability in intactness, but the majority of samples (65.6 %) produced RNA with acceptable integrity. We have shown from these analyses that there are tissues that have a high success of producing acceptable RNA—submandibular gland (90.5 %)—and tissues with a low success—prostate (36.9 %). Our results show considerable variability between donors and between tissues from the same donor. Production of high quality RNA from brains from our donors was achieved in a more consistent manner (Birdsill et al. 2011) (this study). Further analyses will demonstrate whether there are correlations between RNA integrity for different tissues from an individual donor or not, but larger numbers of samples need to be analyzed for such a study. Our preliminary estimation showed no correlation between brain RNA RIN values and those from 6 other tissues. It remains an accepted practice and goal to prepare RNA of high integrity for downstream studies, and having as short a PMI as possible is still advisable for this purpose (Abasolo et al. 2011; Popova et al. 2008; Atz et al. 2007).
Acknowledgments
The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
Contributor Information
Douglas G. Walker, Email: douglas.g.walker@asu.edu, Banner Sun Health Research Institute, Sun City, AZ, USA. Present Address: Laboratory of Neuroinflammation, Biodesign Neurodegenerative Disease Research Center, Arizona State University, Room 538, School of Life Sciences – E Wing, 427 E. Tyler Mall, Tempe, AZ 85287, USA
Alexis M. Whetzel, Banner Sun Health Research Institute, Sun City, AZ, USA
Geidy Serrano, Banner Sun Health Research Institute, Sun City, AZ, USA.
Lucia I. Sue, Banner Sun Health Research Institute, Sun City, AZ, USA
Lih-Fen Lue, Present Address: Laboratory of Neuroinflammation, Biodesign Neurodegenerative Disease Research Center, Arizona State University, Room 538, School of Life Sciences – E Wing, 427 E. Tyler Mall, Tempe, AZ 85287, USA.
Thomas G. Beach, Banner Sun Health Research Institute, Sun City, AZ, USA
References
- Abasolo N, Torrell H, Roig B, Moyano S, Vilella E, Martorell L. RT-qPCR study on post-mortem brain samples from patients with major psychiatric disorders: reference genes and specimen characteristics. J Psychiatr Res. 2011;45:1411–1418. doi: 10.1016/j.jpsychires.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Shaffer A, Geddes T, Studzinski D, Mitton K, Pruetz B, Long G, Shanley C. Evaluation of optimal RNA extraction method from human carotid atherosclerotic plaque. Cardiovasc Pathol. 2015;24:187–190. doi: 10.1016/j.carpath.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Andreasson A, Kiss NB, Juhlin CC, Hoog A. Long-term storage of endocrine tissues at −80 °C does not adversely affect RNA quality or overall histomorphology. Biopreserv Biobank. 2013;11:366–370. doi: 10.1089/bio.2013.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atz M, Walsh D, Cartagena P, Li J, Evans S, Choudary P, Overman K, Stein R, Tomita H, Potkin S, Myers R, Watson SJ, Jones EG, Akil H, Bunney WE, Jr, Vawter MP. Methodological considerations for gene expression profiling of human brain. J Neurosci Methods. 2007;163:295–309. doi: 10.1016/j.jneumeth.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer H, Mobley JA, Ayers LW, Bowen J, Chuaqui RF, Johnson LA, Livolsi VA, Lubensky IA, McGarvey D, Monovich LC, Moskaluk CA, Rumpel CA, Sexton KC, Washington MK, Wiles KR, Grizzle WE, Ramirez NC. The effects of frozen tissue storage conditions on the integrity of RNA and protein. Biotech Histochem. 2014;89:518–528. doi: 10.3109/10520295.2014.904927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo-Pouly AC, Elgamal OA, Schmittgen TD. RNA isolation from mouse pancreas: a ribonuclease-rich tissue. J Vis Exp. 2014;90:e51779. doi: 10.3791/51779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics. 2005;21:389–395. doi: 10.1152/physiolgenomics.00025.2005. [DOI] [PubMed] [Google Scholar]
- Beach TG. Alzheimer’s disease and the “Valley Of Death”: not enough guidance from human brain tissue? J Alzheimers Dis. 2013;33(Suppl 1):S219–S233. doi: 10.3233/JAD-2012-129020.:S219-S233. [DOI] [PubMed] [Google Scholar]
- Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The sun health research institute brain donation program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, Lue L, Roher AE, Dugger BN, Maarouf C, Birdsill AC, Intorcia A, Saxon-Labelle M, Pullen J, Scroggins A, Filon J, Scott S, Hoffman B, Garcia A, Caviness JN, Hentz JG, Driver-Dunckley E, Jacobson SA, Davis KJ, Belden CM, Long KE, Malek-Ahmadi M, Powell JJ, Gale LD, Nicholson LR, Caselli RJ, Woodruff BK, Rapscak SZ, Ahern GL, Shi J, Burke AD, Reiman EM, Sabbagh MN. Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology. 2015;35(4):354–389. doi: 10.1111/neup.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsill AC, Walker DG, Lue L, Sue LI, Beach TG. Postmortem interval effect on RNA and gene expression in human brain tissue. Cell Tissue Bank. 2011;12:311–318. doi: 10.1007/s10561-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisco MJ, Morley AA. Quantification of RNA integrity and its use for measurement of transcript number. Nucleic Acids Res. 2012;40:e144. doi: 10.1093/nar/gks588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broniscer A, Baker JN, Baker SJ, Chi SN, Geyer JR, Morris EB, Gajjar A. Prospective collection of tissue samples at autopsy in children with diffuse intrinsic pontine glioma. Cancer. 2010;116:4632–4637. doi: 10.1002/cncr.25405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buesa C, Maes T, Subirada F, Barrachina M, Ferrer I. DNA chip technology in brain banks: confronting a degrading world. J Neuropathol Exp Neurol. 2004;63:1003–1014. doi: 10.1093/jnen/63.10.1003. [DOI] [PubMed] [Google Scholar]
- Burke WJ, O’Malley KL, Chung HD, Harmon SK, Miller JP, Berg L. Effect of pre- and postmortem variables on specific mRNA levels in human brain. Brain Res Mol Brain Res. 1991;11:37–41. doi: 10.1016/0169-328x(91)90018-s. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Condelli V, Lettini G, Patitucci G, D’Auria F, D’Amico M, Vita G, Musto P, Cuomo C, Landriscina M. Validation of vacuum-based refrigerated system for biobanking tissue preservation: analysis of cellular morphology, protein stability, and RNA quality. Biopreserv Biobank. 2014;12:35–45. doi: 10.1089/bio.2013.0065. [DOI] [PubMed] [Google Scholar]
- Cummings TJ, Strum JC, Yoon LW, Szymanski MH, Hulette CM. Recovery and expression of messenger RNA from postmortem human brain tissue. Mod Pathol. 2001;14:1157–1161. doi: 10.1038/modpathol.3880451. [DOI] [PubMed] [Google Scholar]
- Dawany NB, Dampier WN, Tozeren A. Large-scale integration of microarray data reveals genes and pathways common to multiple cancer types. Int J Cancer. 2011;128:2881–2891. doi: 10.1002/ijc.25854. [DOI] [PubMed] [Google Scholar]
- Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, Zumla A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 2004;37:112–119. doi: 10.2144/04371RR03. [DOI] [PubMed] [Google Scholar]
- Ervin JF, Heinzen EL, Cronin KD, Goldstein D, Szymanski MH, Burke JR, Welsh-Bohmer KA, Hulette CM. Postmortem delay has minimal effect on brain RNA integrity. J Neuropathol Exp Neurol. 2007;66:1093–1099. doi: 10.1097/nen.0b013e31815c196a. [DOI] [PubMed] [Google Scholar]
- Fajardy I, Moitrot E, Vambergue A, Vandersippe-Millot M, Deruelle P, Rousseaux J. Time course analysis of RNA stability in human placenta. BMC Mol Biol. 2009;10:21. doi: 10.1186/1471-2199-10-21.:21-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasold M, Binder H. Estimating RNA-quality using GeneChip microarrays. BMC Genom. 2012;13:186. doi: 10.1186/1471-2164-13-186.:186-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Santpere G, Arzberger T, Bell J, Blanco R, Boluda S, Budka H, Carmona M, Giaccone G, Krebs B, Limido L, Parchi P, Puig B, Strammiello R, Ströbel T, Kretzschmar H. Brain protein preservation largely depends on the postmortem storage temperature: implications for study of proteins in human neurologic diseases and management of brain banks: a BrainNet Europe Study. J Neuropathol Exp Neurol. 2007;66:35–46. doi: 10.1097/nen.0b013e31802c3e7d. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Herrera L, Valenzuela A, Marchal JA, Lorente JA, Villanueva E. Studies on RNA integrity and gene expression in human myocardial tissue, pericardial fluid and blood, and its postmortem stability. Forensic Sci Int. 2013;232:218–228. doi: 10.1016/j.forsciint.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Grabmuller M, Madea B, Courts C. Comparative evaluation of different extraction and quantification methods for forensic RNA analysis. Forensic Sci Int Genet. 2015;16C:195–202. doi: 10.1016/j.fsigen.2015.01.006.:195-202. [DOI] [PubMed] [Google Scholar]
- Griffin M, Abu-El-Haija M, Abu-El-Haija M, Rokhlina T, Uc A. Simplified and versatile method for isolation of high-quality RNA from pancreas. Biotechniques. 2012;52:332–334. doi: 10.2144/0000113862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzis C, Sun H, Yao H, Hubbard RE, Meric-Bernstam F, Babiera GV, Wu Y, Pusztai L, Symmans WF. Effects of tissue handling on RNA integrity and microarray measurements from resected breast cancers. J Natl Cancer Inst. 2011;103:1871–1883. doi: 10.1093/jnci/djr438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard J, Thorsen K, Lund MK, Hein AM, Hamilton-Dutoit SJ, Vang S, Nordentoft I, Birkenkamp-Demtroder K, Kruhoffer M, Hager H, Knudsen B, Andersen CL, Sorensen KD, Pedersen JS, Orntoft TF, Dyrskjot L. Next-generation sequencing of RNA and DNA isolated from paired fresh-frozen and formalin-fixed paraffin-embedded samples of human cancer and normal tissue. PLoS One. 2014;9:e98187. doi: 10.1371/journal.pone.0098187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich M, Matt K, Lutz-Bonengel S, Schmidt U. Successful RNA extraction from various human postmortem tissues. Int J Legal Med. 2007;121:136–142. doi: 10.1007/s00414-006-0131-9. [DOI] [PubMed] [Google Scholar]
- Hellemans J, Vandesompele J. Selection of reliable reference genes for RT-qPCR analysis. Methods Mol Biol. 2014;1160:19–26. doi: 10.1007/978-1-4939-0733-5_3.:19-26. [DOI] [PubMed] [Google Scholar]
- Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- Kampf C, Mardinoglu A, Fagerberg L, Hallstrom BM, Edlund K, Lundberg E, Ponten F, Nielsen J, Uhlen M. The human liver-specific proteome defined by transcriptomics and antibody-based profiling. FASEB J. 2014;28:2901–2914. doi: 10.1096/fj.14-250555. [DOI] [PubMed] [Google Scholar]
- Kap M, Oomen M, Arshad S, de JB, Riegman P. Fit for purpose frozen tissue collections by RNA integrity number-based quality control assurance at the Erasmus MC tissue bank. Biopreserv Biobank. 2014;12:81–90. doi: 10.1089/bio.2013.0051. [DOI] [PubMed] [Google Scholar]
- Kiewe P, Gueller S, Komor M, Stroux A, Thiel E, Hofmann WK. Prediction of qualitative outcome of oligonucleotide microarray hybridization by measurement of RNA integrity using the 2100 Bioanalyzer capillary electrophoresis system. Ann Hematol. 2009;88:1177–1183. doi: 10.1007/s00277-009-0751-5. [DOI] [PubMed] [Google Scholar]
- Kim S, Webster MJ. Postmortem brain tissue for drug discovery in psychiatric research. Schizophr Bull. 2009;35:1031–1033. doi: 10.1093/schbul/sbp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelkamm A, Vennemann B, Lutz-Bonengel S, Fracasso T, Vennemann M. RNA integrity in post-mortem samples: influencing parameters and implications on RT-qPCR assays. Int J Legal Med. 2011;125:573–580. doi: 10.1007/s00414-011-0578-1. [DOI] [PubMed] [Google Scholar]
- Li JZ, Vawter MP, Walsh DM, Tomita H, Evans SJ, Choudary PV, Lopez JF, Avelar A, Shokoohi V, Chung T, Mesarwi O, Jones EG, Watson SJ, Akil H, Bunney WE, Jr, Myers RM. Systematic changes in gene expression in postmortem human brains associated with tissue pH and terminal medical conditions. Hum Mol Genet. 2004;13:609–616. doi: 10.1093/hmg/ddh065. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Bazan NG. Cyclooxygenase 2 RNA message abundance, stability, and hypervariability in sporadic Alzheimer neocortex. J Neurosci Res. 1997;50:937–945. doi: 10.1002/(SICI)1097-4547(19971215)50:6<937::AID-JNR4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Micke P, Ohshima M, Tahmasebpoor S, Ren ZP, Ostman A, Ponten F, Botling J. Biobanking of fresh frozen tissue: RNA is stable in nonfixed surgical specimens. Lab Invest. 2006;86:202–211. doi: 10.1038/labinvest.3700372. [DOI] [PubMed] [Google Scholar]
- Mora M, Angelini C, Bignami F, Bodin AM, Crimi M, Di Donato JH, Felice A, Jaeger C, Karcagi V, LeCam Y, Lynn S, Meznaric M, Moggio M, Monaco L, Politano L, de la Paz MP, Saker S, Schneiderat P, Ensini M, Garavaglia B, Gurwitz D, Johnson D, Muntoni F, Puymirat J, Reza M, Voit T, Baldo C, Bricarelli FD, Goldwurm S, Merla G, Pegoraro E, Renieri A, Zatloukal K, Filocamo M, Lochmuller H. The EuroBioBank Network: 10 years of hands-on experience of collaborative, transnational biobanking for rare diseases. Eur J Hum Genet. 2014;23:1116–1123. doi: 10.1038/ejhg.2014.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy C, Maheu M, Lopez JP, Vaillancourt K, Cruceanu C, Gross JA, Arnovitz M, Mechawar N, Turecki G. Effects of postmortem interval on biomolecule integrity in the brain. J Neuropathol Exp Neurol. 2015;74:459–469. doi: 10.1097/NEN.0000000000000190. [DOI] [PubMed] [Google Scholar]
- Opitz L, Salinas-Riester G, Grade M, Jung K, Jo P, Emons G, Ghadimi BM, Beissbarth T, Gaedcke J. Impact of RNA degradation on gene expression profiling. BMC Med Genomics. 2010;3:36. doi: 10.1186/1755-8794-3-36.:36-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova T, Mennerich D, Weith A, Quast K. Effect of RNA quality on transcript intensity levels in microarray analysis of human post-mortem brain tissues. BMC Genom. 2008;9:91. doi: 10.1186/1471-2164-9-91.:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preece P, Cairns NJ. Quantifying mRNA in postmortem human brain: influence of gender, age at death, postmortem interval, brain pH, agonal state and inter-lobe mRNA variance. Brain Res Mol Brain Res. 2003;118:60–71. doi: 10.1016/s0169-328x(03)00337-1. [DOI] [PubMed] [Google Scholar]
- Ravid R, Ferrer I. Brain banks as key part of biochemical and molecular studies on cerebral cortex involvement in Parkinson’s disease. FEBS J. 2012;279:1167–1176. doi: 10.1111/j.1742-4658.2012.08518.x. [DOI] [PubMed] [Google Scholar]
- Rudloff U, Bhanot U, Gerald W, Klimstra DS, Jarnagin WR, Brennan MF, Allen PJ. Biobanking of human pancreas cancer tissue: impact of ex vivo procurement times on RNA quality. Ann Surg Oncol. 2010;17:2229–2236. doi: 10.1245/s10434-010-0959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri BA, Camp F, Miknyoczki S. Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem Pharmacol. 2014;87:150–161. doi: 10.1016/j.bcp.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabihkhani M, Lucey GM, Wei B, Mareninov S, Lou JJ, Vinters HV, Singer EJ, Cloughesy TF, Yong WH. The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings. Clin Biochem. 2014;47:258–266. doi: 10.1016/j.clinbiochem.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, Tamminga CA. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Sun R, Hao M, Wang Y, Zhang X, Liu Y, Cong X. Effect of duration of ex vivo ischemia time and storage period on RNA quality in biobanked human renal cell carcinoma tissue. Ann Surg Oncol. 2016;23:297–304. doi: 10.1245/s10434-014-4327-9. [DOI] [PubMed] [Google Scholar]
- Tarvin KA, Sandusky GE. Using molecular profiled human tissue to accelerate drug discovery. Expert Opin Drug Discov. 2014;9:1383–1387. doi: 10.1517/17460441.2014.959926. [DOI] [PubMed] [Google Scholar]
- Tomita H, Vawter MP, Walsh DM, Evans SJ, Choudary PV, Li J, Overman KM, Atz ME, Myers RM, Jones EG, Watson SJ, Akil H, Bunney WE., Jr Effect of agonal and postmortem factors on gene expression profile: quality control in microarray analyses of postmortem human brain. Biol Psychiatry. 2004;55:346–352. doi: 10.1016/j.biopsych.2003.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De PK, Pattyn F, Poppe B, Van RN, De PA, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen J, De PK, Lefever S, Nuytens J, De VF, Derveaux S, Hellemans J, Speleman F, Vandesompele J. Measurable impact of RNA quality on gene expression results from quantitative PCR. Nucleic Acids Res. 2011;39:e63. doi: 10.1093/nar/gkr065. [DOI] [PMC free article] [PubMed] [Google Scholar]