Abstract
Our 3-dimensional testis co-culture system (3D-TCS) represents a promising model of male reproductive toxicity which captures sensitive processes of male reproductive development and contains the main testes cell types (germ, Leydig and Sertoli cells). Macrophages are another cell type important for testicular function and help to modulate immuno-endocrine processes during testes development. Chemicals such as phthalate esters (PE’s) affect macrophage function and testosterone production in the testes in vivo. The aim of this study was to determine whether macrophages were present in the 3D-TCS and investigate responses in our model that may be related to immuno-endocrine functions. We observed consistent expression of the resident macrophage marker ED2 as well as increases in inflammatory cytokines produced by macrophages and testes cells (IL-6, TNF-α and KC/GRO) after exposure to toxic PE’s. Pathway analysis of gene expression changes after exposure to PE’s showed that IL-6 and TNF-α signaling pathways were enriched after treatment with reproductively toxic, but not non-reproductively toxic phthalates. These results indicate that macrophages and inflammatory processes are captured in the 3D-TCS and that these processes are impacted by exposure to reproductive toxicants. These processes represent a major mode of action for in vivo testis toxicity for a variety of compounds and our novel in vitro model is able to capture toxicant perturbation of immune function.
Keywords: reproductive toxicity, in vitro models, testis, macrophages, inflammation, cytokines, phthalate esters
Introduction
Environmental impacts on male reproductive endpoints are a significant public health concern and a majority of chemicals in commerce have not gone through adequate reproductive and developmental toxicity testing (Goldman and Koduru 2000, Judson et al. 2009, Neltner et al. 2013). Toxicity screening for reproductive and developmental endpoints is expensive, time-consuming and requires a large number of animals. Indeed, reproductive and developmental toxicity screening required by Europe’s REACH program was estimated to require 49 million animals and cost 6.9 billion or $9.3 billion (Scialli 2008, Rovida and Hartung 2009). Furthermore, the contribution of environmental chemicals to the multiple male reproductive pathologies that constitute the Testicular Dysgenesis Syndrome (TDS) first described by Skakkebaek, et al. is currently unclear (Skakkebaek, 2001). Due to these challenges and concerns, new in vitro models of testicular development are in demand. Such models could reduce the number of animals used for reproductive toxicity testing and provide critical insight into the molecular and cellular processes underpinning TDS. However, as with any new in vitro model of organ systems, these models must be evaluated for their ability to capture relevant in vivo biology.
Our lab has established a three dimensional testicular co-culture model (3D-TCS). The goal of this model is to provide an in vitro alternative to in vivo models of male reproductive toxicity by using a 3D (‘organotypic’) culture that resembles the testis in structure and function. This co-culture includes the major testes cell types, including germ, Sertoli and Leydig cells (Yu et al. 2005, Wegner et al. 2013). We have also demonstrated that the addition of a Matrigel™ overlay at an optimized concentration not only gave the testicular cells a three dimensional structure on which to grow, but also improved germ cell survival, enhanced cell survival pathways and reduced cell stress pathways (Yu et al. 2005). Taken together these data indicate that our co-culture system provides an in vivo-like niche for the testes cells. Treatment with a variety of toxicants has indicated that the 3D-TCS captures multiple modes of action for testicular toxicity. These include activation of cell death pathways (cadmium) as well as impacts on testosterone production and steroidogenic gene expression (phthalates) (Yu et al. 2008, 2009, Wegner et al. 2014, Harris et al. 2015).
Inflammatory pathways play a role in normal testicular development and represent an important mode of action for testes toxicity. Inflammatory cytokines are produced by multiple testis cell types including macrophages, Sertoli and Leydig cells. Cytokine signaling plays an important regulatory role in testes development and function (Lee et al. 1999, Richburg et al. 1999, Hedger and Meinhardt 2003). For example, pro-inflammatory cytokines like interleukin-1 and interleukin-6 are known to have direct effects on spermatogenic cell differentiation and testicular steroidogenesis (Boockfor et al. 1994, Hayes et al. 1996, Yan et al. 2000). Although the testes are considered to be “immune-privileged” due to their ability to tolerate autoantigens that are expressed by germ cells, inflammation can occur due to a number of factors like pathogenic infection or exposure to toxic compounds (Guazzone et al. 2009). There have been a number of recent reports demonstrating impacts on inflammatory responses in in vivo rat testes after exposure to various toxicants. Observed effects include increases in macrophages and macrophage produced cytokines after exposure to phthalate esters and benzo(a)pyrene, as well as effects on macrophage function after exposure to lead (Zheng et al. 2010, Shamim Ahmed S.K. Barbhuiya 2013, Murphy et al. 2014). Researchers have proposed that macrophages partially mediate the toxic action of phthalate esters in the testes in vivo (Murphy et al. 2014).
Phthalate esters (PEs) are a class of industrial compounds that are used in various consumer products, including food packaging, make up and children’s toys (Wilkinson and Lamb 1999, Koo and Lee 2004, Lhuguenot 2009). The adverse effects of PEs on male reproductive development are well known and include endocrine disruption as well as inflammation (Granholm et al. 1992, Gray et al. 2000). The purpose of this study was to investigate the presence of immune cells in this co-culture as well as the capability of this testicular model to capture testes inflammatory responses. Using phthalates with differing levels of potency in regards to male reproductive toxicity, we assessed whether inflammatory markers in our model could be used to distinguish between potent testis toxicants from relatively non-toxic compounds within a similar chemical class.
Materials and Methods
Preparation of three dimensional testis co-culture model (3D-TCS) and chemical treatment
The protocol for the preparation of three dimensional testes co-cultures has been described in more detail previously (Wegner et al. 2013). Briefly, testes were dissected from 5-day-old male pups obtained from mated Sprague–Dawley rats (Charles River Laboratories, Wilmington, USA). Sequential enzymatic digestions were used to create a single cell suspension containing primarily Sertoli, germ and Leydig cells. Cells were suspended in a solution containing serum-free Eagle’s Minimal Essential Medium (Invitrogen, Carlsbad, CA) containing 0.1nM nonessential amino acids, 1mM sodium pyruvate, 3mM sodium lactate, 1% ITS culture supplement (BD Biosciences, Bedford, MA). Cells were then plated at a density of 1.6×106 cells/dish in 35mm plates, followed by the addition of extracellular matrix medium (Matrigel™) at a density of 200μg/mL. For chemical treatments, media was prepared at final concentration of 100μM of either dibutyl phthalate (DBP), diethylhexyl phthalate (DEHP) or diethyl phthalate (DEP) diluted in dimethyl sulfoxide (DMSO, 0.1% by volume). Vehicle control treatments contained DMSO only. Media was then added directly to cells 48 h after initial plating. Phthalate concentrations were selected based on those that caused minimal cytotoxicity in previous experiments. DBP (Sigma #D2270, 99% purity), DEHP (Sigma #4-8557, 99% purity), DEP (Sigma #524972, 99.5% purity) and DMSO (Sigma #D1435) were obtained from Sigma-Aldrich (MO, USA).
Western Blot for Presence of Macrophage Markers
Total protein was collected from testicular co-cultures at various timepoints from initial plating of cells to 7 days in culture using the following protocol. Cells were harvested in cell lysis buffer (Cell Signaling Technology #9803). Protein was isolated using three freeze-thaw cycles followed by centrifugation (16,300xG for 15 min). Protein concentration was measured using a Protein Assay kit (Bio-Rad Laboratories, #5000002). Protein samples were then diluted in Nupage LDS sample buffer (Novex ThermoFisher, NP0008) and reducing agent, then brought up to volume with cell lysis buffer, generating samples with equal protein concentrations. Samples were loaded in Nupage Novex 4–12% Bis-Tris Protein Gels (Invitrogen, NP0321BOX) and separated by running at 200V for 45 minutes in running buffer containing 500μL Nupage Antioxidant (Novex ThermoFisher, NP0005). Protein was then transferred to polyvinylidene difluoride nylon membranes (Bio-Rad Laboratories, #1704157) by running at 100V for 2 hours in transfer buffer at 4°C. Efficiency of transfer was confirmed using Coomassie SimplyBlue SafeStain (Novex ThermoFisher, LC6060) to stain gel after transfer was completed. Membranes were then rinsed in Tris-buffered saline (TBS) at pH 7.6 and blocked with 5% nonfat dry milk in TBS with 0.1% Tween 20 (TTBS) for one hour. Membranes were rinsed with TTBS and incubated overnight with primary antibodies for the macrophage markers ED1 (MCA341R, Bio-Rad) and ED2 (MCA324R, Bio-Rad) and for 2 hours with secondary antibody conjugated to horseradish peroxidase (554002, BD Pharmingen) (Dijkstra et al. 1985). Membranes were then washed 3 times for 5 minutes with TTBS followed by incubation with enhanced Amersham ECL Western Blotting Detection Reagent (RPN2106, GE Lifescience) and exposure to Blue Autorad film (F-9029, Genemate). Blots shown are representative of 3 biological replicates.
Cytokine assay
Proinflammatory Panel 1 (rat) kit was obtained from Meso Scale Discovery (Rockville, MD). Testis co-culture cells were incubated in 35mm tissue cultures dishes with one of three phthalates or vehicle control (DBP, DEHP or DEP) for 2, 8 or 24 hours. After incubation, cell media was collected and frozen at −80°C for future analysis. Cell media samples were thawed and 50μL of each sample was added to the Proinflammatory Panel 1 plate. This Meso Scale plate comes with wells pre-coated with a capture anti-body for 9 different cytokines/well (IFN-γ, IL-1β, IL-10, IL-13, IL-4, IL-5, IL-6, KC/GRO, TNF-α). Antibodies are conjugated with electrochemiluminescent labels. A voltage was then applied to the plate causing the captured analyte/antibody complexes to emit light at a 620nm. Luminescence was measured using an SECTOR Imager 2400 and analyzed with MSD Discovery Workbench software. Levels of cytokines in each sample were determined by comparing signals to known standards. Significant changes in cytokine levels by phthalate treatment or time were identified using two-way ANOVA with Bonferonni correction for multiple comparisons (p<0.05).
Transcriptomic impacts from phthalate exposure
We analyzed a previously generated microarray dataset containing gene expression changes in the 3D-TCS after treatment with seven different phthalates. The seven treatments include the potent reproductive toxicants BBP (benzyl butyl phthalate), DBP (dibutyl phthalate), DEHP (bis[2-ethyl hexyl] phthalate) and DPP (dipentyl phthalate), the relatively weak or non-reproductive toxicants DEP (diethyl phthalate), DMP (dimethyl phthalate) and DOTP (dioctyl terephthalate) or vehicle control (DMSO). Methods for this experiment are reported in more detail by Yu, et al. (Yu et al. 2009). Microarray chips were obtained from Affymetrix (Rat 230 2.0 arrays). Cells in 3D-TCS were exposed to 100μM of each phthalate for 24 hours (n=3 for each treatment). Gene expression data were normalized using GeneChip RMA (GC-RMA) in ArrayAnalysis.org (Eijssen et al. 2013). Genes with significantly changed expression values were identified for each phthalate using t-test for phthalate treatment vs. vehicle control with false-discovery rate (FDR)<0.05. Expression data for significantly changed genes were analyzed using IPA (Ingenuity Pathway Analysis) software (Qiagen). Enriched canonical pathways (curated by IPA) for each phthalate treatment were identified by a Fisher’s exact test (p<0.05). For the purposes of this study, there was a focus on cytokine signaling, inflammation, macrophage function and related pathways. The map of the IL-6 signaling pathway available in IPA software was modified to visualize individual gene expression data across all seven phthalate treatments that were contained in the dataset.
Results
Presence of ED1 (macrophage marker) in the testicular co-culture
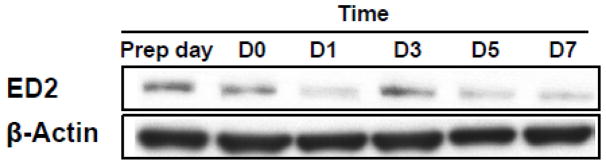
Using Western blots to probe for expression of the macrophage markers ED1 and ED2, we observed continuous expression of ED2 across from day 0 through day 7 in the 3D-TCS (Fig. 1). Presence of this marker indicates that resident macrophages are present in the testicular co-culture. Expression of ED1 was not detected, indicating the cells in the testicular co-culture were most likely the resident macrophage populations.
Fig. 1. Expression of macrophage marker ED2 in testicular co-culture (3D-TCS) over time.
Total protein was collected from testicular co-cultures at various timepoints from initial plating of cells (“Prep day”) to 7 days in culture. Using Western blots to probe for ED1 and ED2 expression, continuous expression of ED2 (but not ED1) was observed. Blot is representative of 3 independent experiments.
Effects of phthalate exposure on cytokine levels in cell media
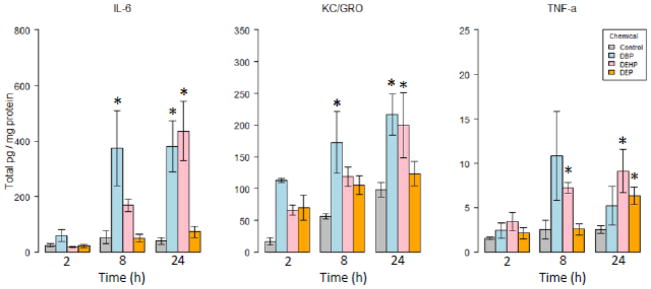
Three of the nine cytokines analyzed (IL-6, TNF-α and KC/GRO) were significantly increased compared to controls after exposure to reproductively toxic phthalate esters (DBP and DEHP). DEP, which is not known to be associated with male reproductive toxicity, generated a significant increase in TNF-α only at 24 hours post-treatment (Fig. 2). Two of the three responsive cytokines (IL-6, KC) were impacted by both of the reproductively toxic phthalates, while TNF-α levels were consistently increased after DEHP treatment only. Cytokine levels generally increased over 24 hours, with the exception of TNF-α in DBP treated cells, which showed a brief (non-significant) spike at 8 hours followed by a return to lower levels at 24 hours.
Fig. 2. Cytokines altered after exposure to phthalate esters.
Levels of cytokines were analyzed in testes co-culture media via ELISA after exposure to several phthalate esters. Several cytokines produced by both macrophages and testes cells were altered after exposure to toxic (DBP, DEHP), but not non-toxic (DEP) phthalates. Graphs show mean +/− standard error for 3 replicate experiments *:p<0.05 vs. control at corresponding timepoint
Pathway based analysis of gene changes induced by DBP and DEHP treatments
For the potent male reproductive toxicants BBP, DBP, DEHP and DPP there were 1256, 2212, 2240 and 2380 genes changed, respectively. For the relatively weak reproductive toxicants, there were 0, 0 and 13 significant genes identified at FDR<0.05 for DEP, DMP and DOTP, respectively. As shown in Table 1, pathway analysis revealed that pathways related to cytokine function (e.g. IL-6, IL-8 and TNF-α) and/or Vegf signaling (a process partially mediated by IL-6, see discussion) were enriched among genes significantly changed by each of the four reproductively toxic phthalates. Consistent with results observed in the cytokine analysis, the canonical IL-6 signaling pathway was found to be enriched for both DBP and DEHP. When responses in the IPA curated IL-6 signaling pathway were analyzed across the seven phthalate treatments, we observed increases in IL-6, Cyp19a1, Mdr1, A2m, Vegf and Nf-IL6 expression for potent reproductive toxicant (BBP, DBP, DEHP and DPP) but not the weak reproductive toxicant (DEP, DMP and DOTP) phthalate treatments.
Table 1.
Selected pathways enriched in testes co-culture after exposure to multiple reproductively toxic phthalates
| Pathway | Number of genes changed (pathway enrichment p-value) | |||
|---|---|---|---|---|
| BBP | DBP | DEHP | DPP | |
| CD27 Signaling in Lymphocytes | - | - | 12 (0.003) | - |
| CXCR4 Signaling | - | 24 (0.014) | - | |
| Role of Macrophages, Fibroblasts and Endothelial Cells in Rheumatoid Arthritis | 31 (0.05) | 46 (0.003) | 41 (0.022) | - |
| IGF-1 Signaling | - | - | 16 (0.031) | - |
| IL-1 Signaling | - | - | 15 (0.036) | - |
| IL-6 Signaling | - | 18 (0.035) | 19 (0.021) | - |
| IL-8 Signaling | - | 29 (0.008) | - | - |
| IL-9 Signaling | - | - | 8 (0.017) | - |
| IL-15 production | 6 (0.008) | - | - | - |
| Leukocyte extravasation signaling | 25 (0.01) | - | - | - |
| LPS/IL-1 Mediated Inhibition of RXR Function | - | 39 (0.0004) | - | - |
| NF-kB Activation by Viruses | - | 15 (0.005) | - | - |
| TNFR1 Signaling | - | - | 12 (0.003) | - |
| TGF-β Signaling | - | - | 15 (0.025) | 21 (0.02) |
| PI3K Signaling in B Lymphocytes | - | - | 21 (0.016) | - |
| VEGF Signaling | - | 21 (0.0002) | - | 23 (0.02) |
Note: Testicular co-cultures were treated with 100μM of seven phthalate esters. Genes with significantly changed expression (FDR<0.05) were analyzed using IPA pathway analysis software. Significantly enriched pathways (p<0.05) were identified, with a focus on pathways related to cytokine signaling, inflammation, macrophage function or other immune processes. For three of the phthalates evaluated, pathway data were not included, either because no significantly altered genes were identified at FDR<0.05 (DEP, DMP) or no pathways related to cytokine signaling or related functions were enriched (DOTP). (- indicates pathway not enriched)
Discussion
Processes mediated by immune capable cells and inflammatory cytokines play an important role in normal testicular development in vivo as well as in response to toxicants. In the current study, we confirmed the presence of immune cells in our testicular co-culture system and demonstrated a quantifiable immune response following phthalate exposure. The presence of macrophages in our testicular co-culture indicates the presence of an important cell type that may aid in maintaining cell signaling processes that are important components of in vivo testes physiology. Furthermore, the presence of macrophages highlights the potential to investigate toxicity responses that are mediated by this inflammatory cell type in our model. The ability of our in vitro model to capture such important in vivo testicular toxicity pathways makes it a promising model for high-content screening of testicular toxicants.
Phthalate exposure increased expression of several pro-inflammatory cytokines. For example, treatment with DBP or DEHP resulted in upregulation of IL-6. IL-6 is produced by Sertoli cells, Leydig cells and macrophages. However, it has been reported that in an experimental model of orchitis, only the ED1 macrophages produced IL-6 (Guazzone et al. 2009). Since ED2 (and not ED1) was detected in the 3D-TCS, this suggests that either the IL-6 in the 3D-TCS was produced by one of the primary testicular cells (Sertoli or Leydig cells) or that the inflammatory response induced by phthalates is somehow distinct from that of the orchitis model. IL-6 production is stimulated in the testes in response to a number of endogenous signals including testosterone and FSH as well as under conditions of inflammation (Hedger and Meinhardt 2003). Exposure to phthalate metabolites of DBP (MnBP and MBzP) has been shown to induce the release of IL-6 from cultured human epithelial cells (Jepsen et al. 2004). Cells in the testes co-culture responded in a similar manner with levels of IL-6 increasing over time after exposure to DBP and DEHP. Interestingly, Jepsen et al. found that in cultured epithelial cells, phthalates with fewer carbon atoms in their side chains tended to be less potent in regards to IL-6 stimulation when compared to those with more carbon atoms (Jepsen et al. 2004). A similar trend was observed with IL-6 responses in the current study. Consistent with observed cytokine levels in the 3D-TCS, pathway analysis of gene expression data showed enrichment of IL-6 signaling after 24 hours of exposure to toxic phthalate esters. Activation of the IL-6 signaling pathway is usually indicative of a pro-inflammatory response with the potential source of the cytokine coming from the testicular somatic cells, interstitial macrophages and ED1+ monocytes (Guazzone et al. 2009). However, non-inflammatory IL-6 expression and signaling can occur within the testes, as IL-6 has been shown to be an integral part of the regulation of Sertoli cell steroidogenesis, germ cell differentiation and spermatogonial proliferation (Hedger and Meinhardt 2003). Taken together, these responses provide important mechanistic information regarding male reproductive toxicity signals in our model.
We also observed a significant elevation of TNF-α at the transcript level and increased cytokine concentrations in the media of treated cultures. TNF-α is a cytotoxic cell death mediator as well as an inhibitor of testosterone secretion by Leydig cells. TNF-α has been shown to be expressed by testicular macrophages as well as spermatocytes and spermatids (Hedger and Meinhardt 2003, Theas et al. 2008). It is thought that TNF-α may play a role in the regulation of the normal apoptotic processes that occur during normal spermatogenic development. In the current study, it was not possible to deduce whether the observed increase in TNF-α was from the resident macrophages in the culture, the germ cells, or both. TNF-α levels have important relevance for detecting mechanisms of male reproductive injury in the 3D-TCS, as TNF-α levels in the reproductive tract have been reported to be associated with lower levels of sperm production and TNF-α has been shown to decrease sperm motility in vitro (Eisermann et al. 1989, Azenabor et al. 2015).
KC/GRO (otherwise known as CXCL1) is a chemokine that is also expressed by multiple cell types in the testes, including macrophages, Sertoli and Leydig cells (Guazzone et al. 2009). KC plays a role in the attraction of neutrophils and its expression is stimulated by TNF-α. In addition, KC/GRO has been hypothesized to be involved in germ cell proliferation, due to its role as a growth stimulator in certain cells such as human umbilical vein endothelial cells and melanocytes (Aubry et al. 2000). Consistent with the current study, short term DEHP exposure (3 hours) has been shown to upregulate gene expression of KC in human macrophage-like THP-1 cells in vitro (Nishioka et al. 2012). Similarly, 3 hours after DBP exposure (500mg/kg), Johnson, et al. observed increased KC gene expression in rat testis in vivo (Johnson et al. 2007). In the 3D-TCS, increased levels of KC protein were observed at early timepoints (significant impacts detected at 8 hours) suggesting that KC mediates early responses to phthalate exposure in the 3D-TCS in manner similar to what is observed in vivo. It is important to note that phthalate effects across all three cytokines were observed at early time points and at a concentration (100μM) that has been shown to be non-cytotoxic in the 3D-TCS, suggesting that increases in cytokine levels are a more sensitive endpoint than cytotoxicity in the 3D-TCS (Yu et al. 2009, Wegner et al. 2014).
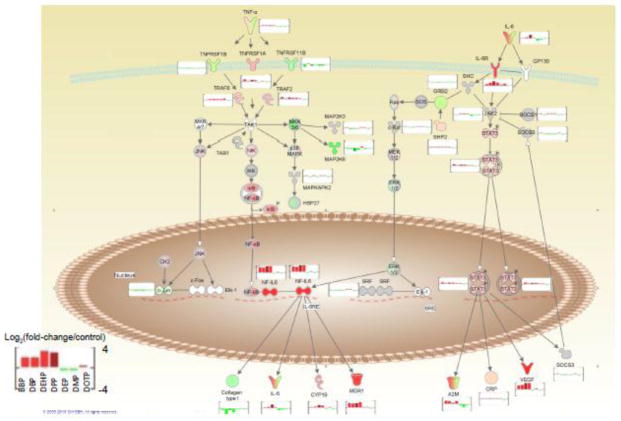
Pathway analysis of gene expression data showed that pathways associated with cytokine signaling, inflammation or related processes were affected by phthalate exposure across all four of the potent male reproductive toxicants at the same concentration (100μM). Furthermore, this analysis confirmed phthalate induced increases in IL-6 in the 3D-TCS are part of signaling processes associated with testicular tumor formation and testicular endocrine disruption in vivo. As shown in Fig. 3, IL-6 and TNF-α both are both part of the IL-6 signaling pathway which leads to the upregulation of Vegf and Cyp19a1. Vegf is a growth factor that serves multiple functions including vasculogenesis, angiogenesis and inhibition of apoptosis. Vegf is known to be involved with cancer progression and has been shown to be induced by xenoestrogens in breast cancer cells (Buteau-Lozano et al. 2008). Upregulation of Vegf has important implications for testes development, as it is highly expressed in germ cell tumors and angiogenesis is an important aspect of normal testes development (Samson et al. 2004). Cyp19a1 (otherwise known as aromatase) also serves important functions in the testes, notably the conversion of testosterone to estrogen. The upregulation of this enzyme after exposure to phthalates has direct functional relevance to the physiological role of the testes in male reproductive development, as increased levels of Cyp19a1 could lead to a decrease in testis testosterone levels. Altered aromatase levels and activity have been shown to be associated with multiple male reproductive effects in vivo and is a well-known target of endocrine disruptors (Li et al. 2001, Sanderson 2006). These results give us key insight into the mechanism behind the toxicity signals demonstrated in our testes co-culture model.
Fig. 3. Phthalate ester effects on the IL-6/TNF-α signaling pathway.
Microarray gene expression in testes co-culture was evaluated after exposure to 7 different phthalates and Ingenuity Pathway Analysis software were used to identify canonical pathways enriched among differentially expressed genes. The enriched pathway “IL-6 signaling” is shown with associated up or downregulated genes (up- or downregulation represented by bar graphs next to each gene).
Inflammation is emerging as an important mode of action for phthalate induced testis injury in vivo. As we have shown, we can use gene expression changes in inflammatory pathways and in cytokine protein expression to distinguish relative potencies of reproductively toxic phthalate esters in the 3D-TCS. Taken together the presence of macrophage markers, the induction of pro-inflammatory cytokines and the enrichment of inflammatory pathways by toxic phthalates in the 3D-TCS demonstrate that this in vitro testicular co-culture captures early responses in cytokines that are potentially mediated by macrophages and that correlate with testes injury or disease states in vivo. These results suggest a promising role for the 3D-TCS in providing high content data on chemical injury in the testes which will aid in the elucidation of the cellular and molecular responses underlying the Testicular Dysgenesis Syndrome.
Highlights.
Presence of macrophages and inflammatory cytokines were evaluated in an in vitro model of testis development
The marker protein ED2 was detected in culture, indicating presence of resident macrophages
Levels of inflammatory cytokines were increased after exposure to phthalate esters in culture
Analysis of microarray data indicated that inflammatory pathways were activated by phthalate exposure
Results indicate that this testis model captures responses in inflammatory pathways similar to in vivo testis
Acknowledgments
This work was supported by in part by the UW NIEHS Center for Ecogenetics and Environmental Health (5 P30 ES007033), NIEHS Training Grant (T32ES007032), UW EPA Center for Predictive Toxicology (RD-83573801), US-FDA (FDA: 1U01FD004242), and the NIH Center on Human Development and Disability (1 U54 HD083091-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works cited
- Aubry F, Habasque C, Satie AP, Jegou B, Samson M. Expression and regulation of the CXC-chemokines, GRO/KC and IP-10/mob-1 in rat seminiferous tubules. Eur Cytokine Netw. 2000;11(4):690–698. [PubMed] [Google Scholar]
- Azenabor A, Ekun AO, Akinloye O. Impact of Inflammation on Male Reproductive Tract. J Reprod Infertil. 2015;16(3):123–129. [PMC free article] [PubMed] [Google Scholar]
- Boockfor FR, Wang D, Lin T, Nagpal ML, Spangelo BL. Interleukin-6 secretion from rat Leydig cells in culture. Endocrinology. 1994;134(5):2150–2155. doi: 10.1210/endo.134.5.8156916. [DOI] [PubMed] [Google Scholar]
- Buteau-Lozano H, Velasco G, Cristofari M, Balaguer P, Perrot-Applanat M. Xenoestrogens modulate vascular endothelial growth factor secretion in breast cancer cells through an estrogen receptor-dependent mechanism. J Endocrinol. 2008;196(2):399–412. doi: 10.1677/JOE-07-0198. [DOI] [PubMed] [Google Scholar]
- Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Eijssen LM, Jaillard M, Adriaens ME, Gaj S, de Groot PJ, Muller M, Evelo CT. User-friendly solutions for microarray quality control and pre-processing on ArrayAnalysis.org. Nucleic Acids Res. 2013;41(Web Server issue):W71–76. doi: 10.1093/nar/gkt293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisermann J, Register KB, Strickler RC, Collins JL. The effect of tumor necrosis factor on human sperm motility in vitro. J Androl. 1989;10(4):270–274. doi: 10.1002/j.1939-4640.1989.tb00100.x. [DOI] [PubMed] [Google Scholar]
- Goldman LR, Koduru S. Chemicals in the environment and developmental toxicity to children: a public health and policy perspective. Environ Health Perspect. 2000;108(Suppl 3):443–448. doi: 10.1289/ehp.00108s3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm T, Creasy DM, Pollanen P, Soder O. Di-n-pentyl phthalate-induced inflammatory changes in the rat testis are accompanied by local production of a novel lymphocyte activating factor. J Reprod Immune. 1992;21(1):1–14. doi: 10.1016/0165-0378(92)90036-4. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Sty J, Fur J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58(2):350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Guazzone VA, Jacobo P, Theas MS, Lustig L. Cytokines and chemokines in testicular inflammation: A brief review. Microsc Res Tech. 2009;72(8):620–628. doi: 10.1002/jemt.20704. [DOI] [PubMed] [Google Scholar]
- Harris S, Hermsen SA, Yu X, Hong SW, Faustman EM. Comparison of toxicogenomic responses to phthalate ester exposure in an organotypic testis co-culture model and responses observed in vivo. Reprod Toxicol. 2015;58:149–159. doi: 10.1016/j.reprotox.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes R, Chalmers SA, Nikolic-Paterson DJ, Atkins RC, Hedger MP. Secretion of bioactive interleukin 1 by rat testicular macrophages in vitro. J Androl. 1996;17(1):41–49. [PubMed] [Google Scholar]
- Hedger MP, Meinhardt A. Cytokines and the immune-testicular axis. J Reprod Immune. 2003;58(1):1–26. doi: 10.1016/s0165-0378(02)00060-8. [DOI] [PubMed] [Google Scholar]
- Jepsen KF, Abildtrup A, Larsen ST. Monophthalates promote IL-6 and IL-8 production in the human epithelial cell line A549. Toxicol In Vitro. 2004;18(3):265–269. doi: 10.1016/j.tiv.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Johnson KJ, Hensley JB, Kelso MD, Wallace DG, Gaido KW. Mapping gene expression changes in the fetal rat testis following acute dibutyl phthalate exposure defines a complex temporal cascade of responding cell types. Biol Reprod. 2007;77(6):978–989. doi: 10.1095/biolreprod.107.062950. [DOI] [PubMed] [Google Scholar]
- Judson R, Richard A, Dix DJ, Houck K, Martin M, Kavlock R, Dellarco V, Henry T, Holderman T, Sayre P, Tan S, Carpenter T, Smith E. The toxicity data landscape for environmental chemicals. Environ Health Perspect. 2009;117(5):685–695. doi: 10.1289/ehp.0800168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo HJ, Lee BM. Estimated exposure to phthalates in cosmetics and risk assessment. J Toxicol Environ Health A. 2004;67(23–24):1901–1914. doi: 10.1080/15287390490513300. [DOI] [PubMed] [Google Scholar]
- Lee J, Richburg JH, Shipp EB, Meistrich ML, Boekelheide K. The Fas system, a regulator of testicular germ cell apoptosis, is differentially up-regulated in Sertoli cell versus germ cell injury of the testis. Endocrinology. 1999;140(2):852–858. doi: 10.1210/endo.140.2.6479. [DOI] [PubMed] [Google Scholar]
- Lhuguenot JC. Recent European Food Safety Authority toxicological evaluations of major phthalates used in food contact materials. Mol Nutr Food Res. 2009;53(8):1063–1070. doi: 10.1002/mnfr.200800076. [DOI] [PubMed] [Google Scholar]
- Li X, Nokkala E, Yan W, Streng T, Saarinen N, Warri A, Huhtaniemi I, Santti R, Makela S, Poutanen M. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology. 2001;142(6):2435–2442. doi: 10.1210/endo.142.6.8211. [DOI] [PubMed] [Google Scholar]
- Murphy CJ, Stermer AR, Richburg JH. Age- and species-dependent infiltration of macrophages into the testis of rats and mice exposed to mono-(2-Ethylhexyl) phthalate (MEHP) Biol Reprod. 2014;91(1):18. doi: 10.1095/biolreprod.113.115527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neltner TG, Alger HM, Leonard JE, Maffini MV. Data gaps in toxicity testing of chemicals allowed in food in the United States. Reprod Toxicol. 2013;42:85–94. doi: 10.1016/j.reprotox.2013.07.023. [DOI] [PubMed] [Google Scholar]
- Nishioka J, Iwahara C, Kawasaki M, Yoshizaki F, Nakayama H, Takamori K, Ogawa H, Iwabuchi K. Di-(2-ethylhexyl) phthalate induces production of inflammatory molecules in human macrophages. Inflamm Res. 2012;61(1):69–78. doi: 10.1007/s00011-011-0390-x. [DOI] [PubMed] [Google Scholar]
- Richburg JH, Nanez A, Gao H. Participation of the Fas-signaling system in the initiation of germ cell apoptosis in young rat testes after exposure to mono-(2-ethylhexyl) phthalate. Toxicol Appl Pharmacol. 1999;160(3):271–278. doi: 10.1006/taap.1999.8786. [DOI] [PubMed] [Google Scholar]
- Rovida C, Hartung T. Re-evaluation of animal numbers and costs for in vivo tests to accomplish REACH legislation requirements for chemicals - a report by the transatlantic think tank for toxicology (t(4)) ALTEX. 2009;26(3):187–208. [PubMed] [Google Scholar]
- Samson M, Peale FV, Jr, Frantz G, Rioux-Leclercq N, Rajpert-De Meyts E, Ferrara N. Human endocrine gland-derived vascular endothelial growth factor: expression early in development and in Leydig cell tumors suggests roles in normal and pathological testis angiogenesis. J Clin Endocrinol Metab. 2004;89(8):4078–4088. doi: 10.1210/jc.2003-032024. [DOI] [PubMed] [Google Scholar]
- Sanderson JT. The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol Sci. 2006;94(1):3–21. doi: 10.1093/toxsci/kfl051. [DOI] [PubMed] [Google Scholar]
- Scialli AR. The challenge of reproductive and developmental toxicology under REACH. Regul Toxicol Pharmacol. 2008;51(2):244–250. doi: 10.1016/j.yrtph.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Barbhuiya Shamim Ahmed SK, SC, Sengupta Mahuya. Studies of lead toxicity on inflammatory damage and innate immune functions in testicular macrophages of male Swiss albino mice. Modern Research in Inflammation. 2013;2(4):75–81. [Google Scholar]
- Skakkebaek N, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16(5):972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Theas MS, Rival C, Jarazo-Dietrich S, Jacobo P, Guazzone VA, Lustig L. Tumour necrosis factor-alpha released by testicular macrophages induces apoptosis of germ cells in autoimmune orchitis. Hum Reprod. 2008;23(8):1865–1872. doi: 10.1093/humrep/den240. [DOI] [PubMed] [Google Scholar]
- Wegner S, Hong S, Yu X, Faustman EM. Preparation of rodent testis co-cultures. Curr Protoc Toxicol. 2013;Chapter 16(Unit 16):10. doi: 10.1002/0471140856.tx1610s55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner S, YX, Kim HY, Harris S, Griffith WC, Hong S, Faustman EM. Effect of dipentyl phthalate in 3-dimensional in vitro testis co-culture is attenuated by cyclooxygenase-2 inhibition. Journal of Toxicology and Environmental Health Sciences. 2014;6(8):161–169. [Google Scholar]
- Wilkinson CF, Lamb JCt. The potential health effects of phthalate esters in children’s toys: a review and risk assessment. Regul Toxicol Pharmacol. 1999;30(2 Pt 1):140–155. doi: 10.1006/rtph.1999.1338. [DOI] [PubMed] [Google Scholar]
- Yan W, Suominen J, Toppari J. Stem cell factor protects germ cells from apoptosis in vitro. J Cell Sci. 2000;113( Pt 1):161–168. doi: 10.1242/jcs.113.1.161. [DOI] [PubMed] [Google Scholar]
- Yu X, Hong S, Faustman EM. Cadmium-induced activation of stress signaling pathways, disruption of ubiquitin-dependent protein degradation and apoptosis in primary rat Sertoli cell-gonocyte cocultures. Toxicol Sci. 2008;104(2):385–396. doi: 10.1093/toxsci/kfn087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Hong S, Moreira EG, Faustman EM. Improving in vitro Sertoli cell/gonocyte co-culture model for assessing male reproductive toxicity: Lessons learned from comparisons of cytotoxicity versus genomic responses to phthalates. Toxicol Appl Pharmacol. 2009;239(3):325–336. doi: 10.1016/j.taap.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Sidhu JS, Hong S, Faustman EM. Essential role of extracellular matrix (ECM) overlay in establishing the functional integrity of primary neonatal rat Sertoli cell/gonocyte co-cultures: an improved in vitro model for assessment of male reproductive toxicity. Toxicol Sci. 2005;84(2):378–393. doi: 10.1093/toxsci/kfi085. [DOI] [PubMed] [Google Scholar]
- Zheng SJ, Tian HJ, Cao J, Gao YQ. Exposure to di(n-butyl)phthalate and benzo(a)pyrene alters IL-1beta secretion and subset expression of testicular macrophages, resulting in decreased testosterone production in rats. Toxicol Appl Pharmacol. 2010;248(1):28–37. doi: 10.1016/j.taap.2010.07.008. [DOI] [PubMed] [Google Scholar]