Abstract
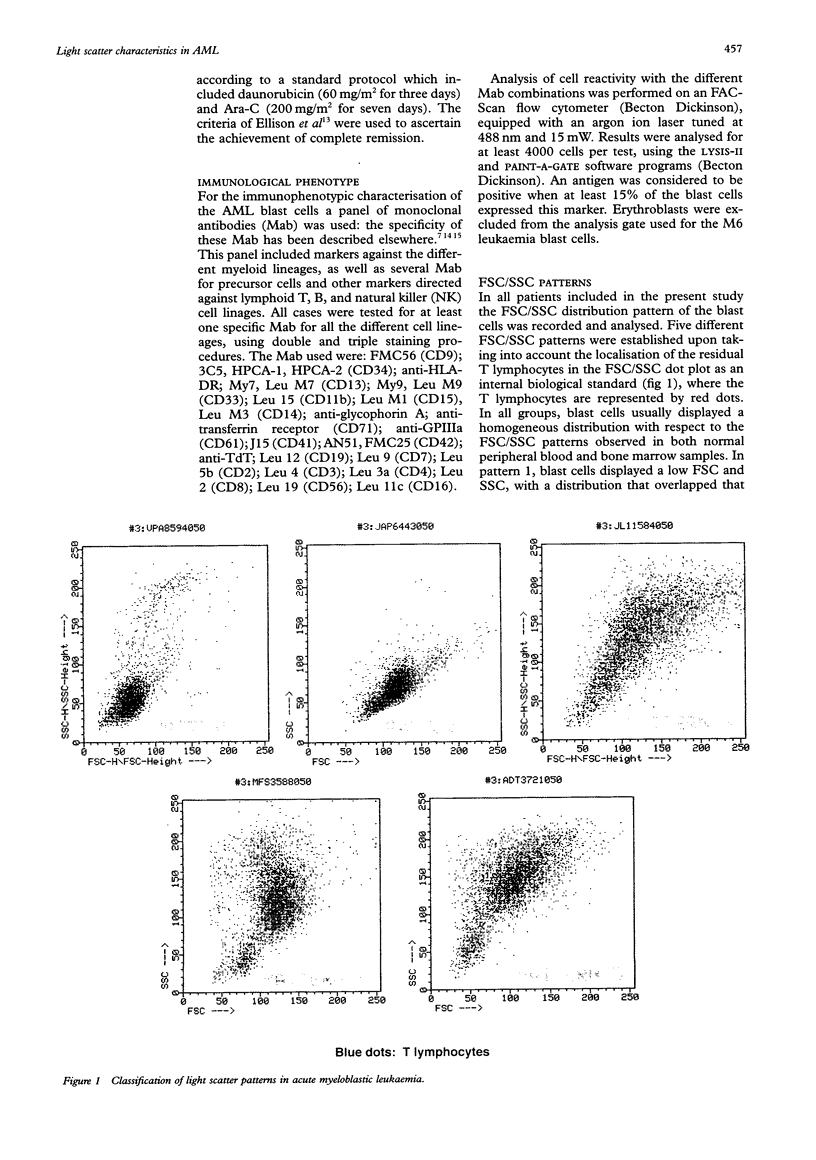
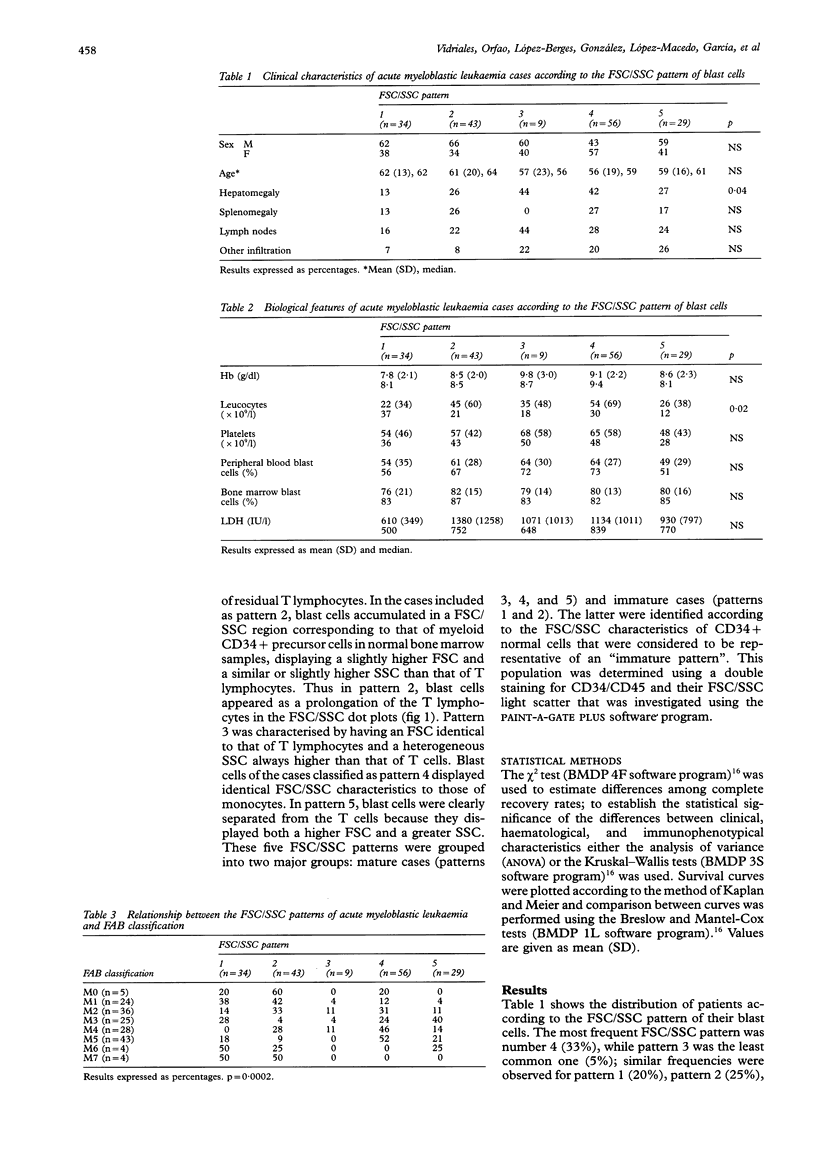
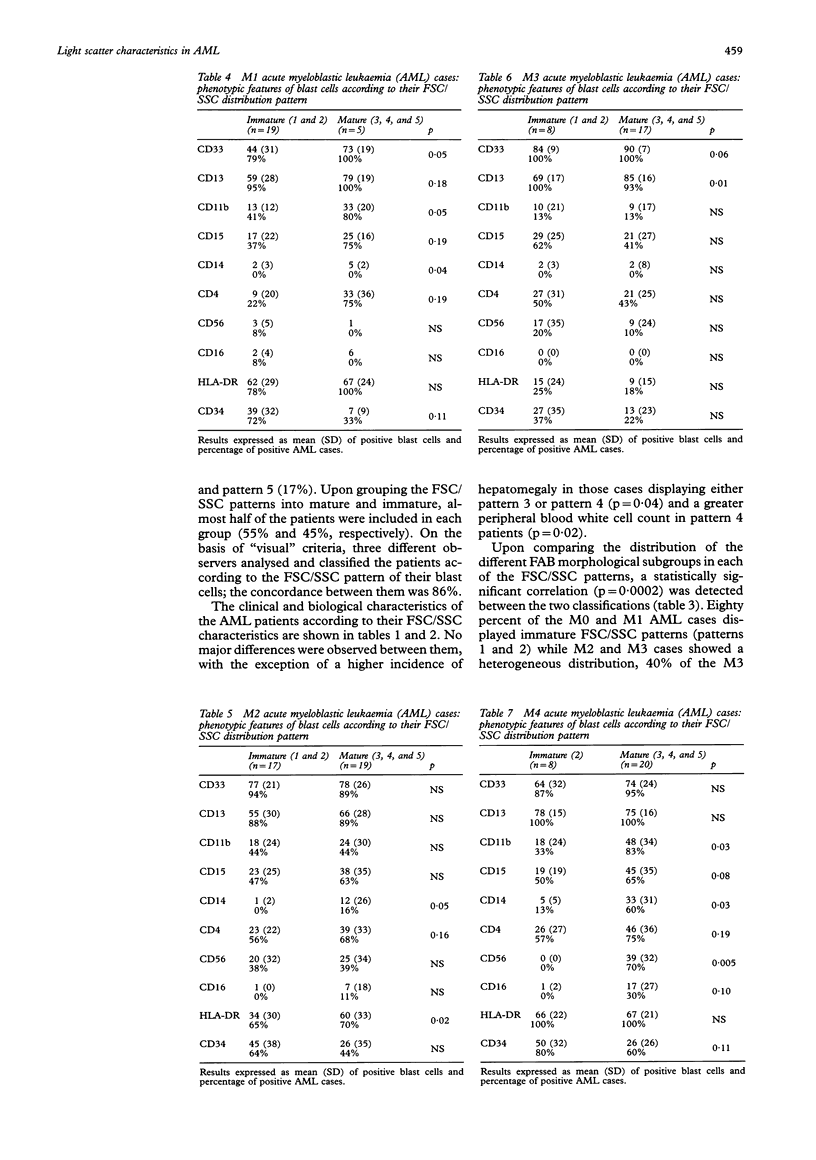
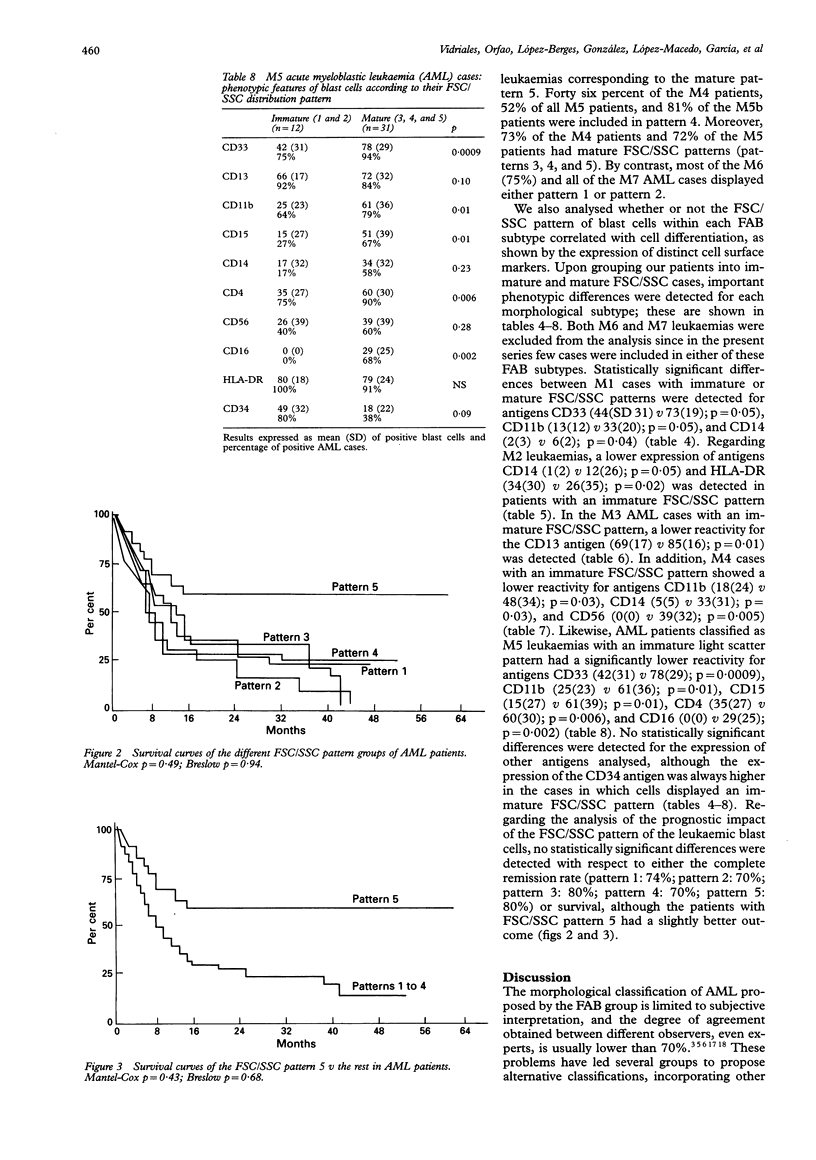
AIMS--To analyse the forward scatter/side scatter (FSC/SSC) distribution of acute myeloblastic leukaemia (AML) blast cells in order to assess whether it correlates with their morphology, immunophenotype, and clinical and biological disease characteristics. METHODS--FSC/SSC patterns were established upon taking into account the localisation of the residual T lymphocytes in the FSC/SSC dot plot as an internal biological standard. One hundred and seventy one newly diagnosed AML patients were analysed and five different FSC/SSC patterns were established. These five patterns could be grouped into two major categories taking into account the FSC/SSC distribution of normal cells in a bone marrow aspirate: immature patterns (1 and 2) and mature patterns (3, 4, and 5). These FSC/SSC patterns were correlated with different clinical and biological characteristics of AML patients. RESULTS--No significant associations were detected in relation to the clinical and haematological disease characteristics and the prognosis of these patients. By contrast there was a significant correlation between the FSC/SSC pattern of the AML blast cells and the FAB classification. An increased reactivity for the antigens associated with myeloid differentiation such as CD13, CD33, CD11b, CD15, CD14, CD4, CD56, and/or CD16 was detected among cases showing a mature FSC/SSC pattern (3, 4, and 5), both in the whole series and even within each of the FAB AML subtypes. By contrast, the reactivity for the CD34 precursor cell associated antigen was higher among those cases displaying an immature FSC/SSC pattern, this being observed even within each FAB subgroup. CONCLUSIONS--The FSC/SSC pattern distribution of AML blast cells not only provides an additional objective and reproductible system for the classification of these leukaemias but it may also represent a connection between the FAB morphological groups and the immunophenotypic classification of AML patients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argyle J. C., Benjamin D. R., Lampkin B., Hammond D. Acute nonlymphocytic leukemias of childhood. Inter-observer variability and problems in the use of the FAB classification. Cancer. 1989 Jan 15;63(2):295–301. doi: 10.1002/1097-0142(19890115)63:2<295::aid-cncr2820630215>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985 Oct;103(4):620–625. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- Dick F. R., Armitage J. O., Burns C. P. Diagnostic concurrence in the subclassification of adult acute leukemia using French-American-British criteria. Cancer. 1982 Mar 1;49(5):916–920. doi: 10.1002/1097-0142(19820301)49:5<916::aid-cncr2820490515>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Ellison R. R., Holland J. F., Weil M., Jacquillat C., Boiron M., Bernard J., Sawitsky A., Rosner F., Gussoff B., Silver R. T. Arabinosyl cytosine: a useful agent in the treatment of acute leukemia in adults. Blood. 1968 Oct;32(4):507–523. [PubMed] [Google Scholar]
- Head D. R., Cerezo L., Savage R. A., Craven C. M., Bickers J. N., Hartsock R., Hosty T. A., Saiki J. H., Wilson H. E., Morrison F. S. Institutional performance in application of the FAB classification of acute leukemia. The Southwest Oncology Group experience. Cancer. 1985 May 1;55(9):1979–1986. doi: 10.1002/1097-0142(19850501)55:9<1979::aid-cncr2820550925>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- San Miguel J. F., Gonzalez M., Cañizo M. C., Anta J. P., Zola H., Lopez Borrasca A. Surface marker analysis in acute myeloid leukaemia and correlation with FAB classification. Br J Haematol. 1986 Nov;64(3):547–560. doi: 10.1111/j.1365-2141.1986.tb02211.x. [DOI] [PubMed] [Google Scholar]
- San Miguel J. F., Hernández J. M., González-Sarmiento R., González M., Sánchez I., Orfao A., Cañizo M. C., López Borrasca A. Acute leukemia after a primary myelodysplastic syndrome: immunophenotypic, genotypic, and clinical characteristics. Blood. 1991 Aug 1;78(3):768–774. [PubMed] [Google Scholar]
- San Miguel J. F., Ojeda E., Gonzalez M., Orfao A., Cañizo M. C., Sanchez J., Lopez-Borrasca A. Prognostic value of immunological markers in acute myeloblastic leukemia. Leukemia. 1989 Feb;3(2):108–111. [PubMed] [Google Scholar]
- Terstappen L. W., Könemann S., Safford M., Loken M. R., Zurlutter K., Büchner T., Hiddemann W., Wörmann B. Flow cytometric characterization of acute myeloid leukemia. Part 1. Significance of light scattering properties. Leukemia. 1991 Apr;5(4):315–321. [PubMed] [Google Scholar]
- Terstappen L. W., Safford M., Unterhalt M., Könemann S., Zurlutter K., Piechotka K., Drescher M., Aul C., Büchner T., Hiddemann W. Flow cytometric characterization of acute myeloid leukemia: IV. Comparison to the differentiation pathway of normal hematopoietic progenitor cells. Leukemia. 1992 Oct;6(10):993–1000. [PubMed] [Google Scholar]
- Vidriales M. B., Orfao A., González M., Hernández J. M., López-Berges M. C., García M. A., Cañizo M. C., Caballero M. D., Macedo A., Landolfi C. Expression of NK and lymphoid-associated antigens in blast cells of acute myeloblastic leukemia. Leukemia. 1993 Dec;7(12):2026–2029. [PubMed] [Google Scholar]



