Abstract
Characterization of crystalline polymorphs and their quantitation has become an integral part of the pre-clinical drug development process. Raman spectroscopy is a powerful technique for the rapid identification of phases of pharmaceuticals. In the present work we demonstrate the use of low wavenumber Raman vibrational spectroscopy (including phonon measurement) for discrimination among polymorphs. A total of 10 polymorphic pharmaceuticals were employed to conduct a critical assessment. Raman scattering in the low frequency region (10–400 cm−1), which includes crystal lattice vibrations, has been analyzed and the results indicate lattice phonon Raman scattering can be used for rapid discrimination of polymorphic phases with additional discriminating power compared to conventional collection strategies. Moreover structural insight and conformational changes can be detected with this approach.
Keywords: Pharmaceuticals, Process analytical technology (PAT), Phonons, Vibrational spectroscopy
INTRODUCTION
Polymorphism, the ability of a molecule to exist in at least two distinct crystalline arrangements differing only in the assembly of molecules, carries many implications in pharmaceutical product development and intellectual property rights for industry.1,2 Different polymorphs display unique physiochemical properties, such as stability, solubility and bioavailability.3–5 Hence polymorph characterization from early stage drug discovery through drug product formulation is essential, and the importance of identifying polymorphs is recognized by several regulatory requirements.6 The appearance of undesired polymorphs during the manufacturing process can compromise performance and delay the drug manufacturing process. It is desirable to conduct polymorph screening during the different stages of drug product manufacturing, since process-induced transformation is possible from mechanical stress and environmental exposure, in addition to stochastic events that can influence the outcome of crystallization.7 Recently interest has surged in alternative technology which is fast, reliable, and suitable for pharmaceutical materials characterization, particularly as a process analytical technology (PAT) tool.8, 9 Polymorph characterization methods such as powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC), and solid state nuclear magnetic resonance (SS-NMR) spectroscopy are widely used, but these methods are relatively time consuming and not suitable for PAT application. More rapid spectroscopic techniques based on vibrational spectroscopy are therefore the methods of choice albeit with the potential limitation of less discriminating power.
Raman spectroscopy has increasingly gained in popularity as a vibrational spectroscopic technique for the identification of crystalline phases in the pharmaceutical industry due to its lack of a need for sample preparation and high sensitivity. The major advantages of the Raman scattering technique over other vibrational spectroscopy methods such as infrared (IR) or terahertz (THz) spectroscopy are efficiency and online monitoring capabilities in high throughput crystallization environments. Along with near-infrared (NIR) spectroscopy, Raman is already considered as PAT tool for at-line, in-line and on-line manufacturing processes.10 Here we demonstrate that the performance of Raman spectroscopy is remarkably improved when the low wavenumber region Raman active modes (10–400 cm−1), encompassing the lattice phonon region, are considered along with the conventional Raman spectroscopy region (200–4000 cm−1) and we propose low wavenumber Raman spectroscopy alone or in conjunction with conventional Raman as a PAT tool. Previous isolated reports11, 12 applying phonon mode Raman spectroscopy have been met with success, but we have now conducted a critical and comprehensive assessment of the utility of low wavenumber spectroscopy and found that this method can provide structural insight as well.
Low wavenumber, Raman active modes arise from the collective translation and deformation of the molecular skeleton inside the crystal lattice or deformation of the whole unit cell.13 Thus Raman bands observed in the 10–400 cm−1 region are expected to be more directly correlated with the crystal structure of the molecule because lattice phonons are involved.13–16 Hence, these low wavenumber vibrational modes might act as fingerprints for different phases of the same compound. Conventional Raman scattering (200–4000 cm−1) is dominated by characteristic intramolecular vibrational modes of the compounds that arise from functional groups present in the molecules. Since different polymorphs possess the same functional groups, standard Raman spectroscopy generally differentiates by the indirect method of a peak shift. In contrast, lattice phonon Raman scattering allows for the discrimination of polymorphs from direct vibrational measurements usually observed below 150 cm−1. Another major advantage of collecting low frequency Raman spectra is that it suffers from far less fluorescence interference because the stokes shift displaces emission outside of the spectral window.
RESULTS AND DISCUSSION
In the present work we demonstrate the application of lattice phonon Raman scattering as a means to discriminate different pharmaceutical polymorphs and crystal phases. In our study we used a Near Excitation Tunable (NExT) filter accessory,17 which allows for high resolution spectral acquisitions in the 10–400 cm−1 region. A total of 10 polymorphic pharmaceutical compounds were chosen for the study. Polymorphs of the studied compounds were prepared either from the literature procedure or through polymer-induced heteronucleation (PIHn),18 as described in S1 of the Supporting Information, and the products were characterized by PXRD in order to confirm their identity. Low wavenumber Raman spectroscopy data for all the polymorphic samples were collected using 647 nm Kr+ laser excitation. The laser power varied from 5 mW to 30 mW at the sample. Laser exposure to the sample varied from 30 seconds to 90 seconds for each spectral collection, with the number of acquisitions ranging from 5–20 depending on the sample being collected. A quarter wave plate was used, sometimes in combination with a half wave plate, to scramble the incident and scattered light for the samples to minimize polarization effects from crystal orientation. Low wavenumber Raman scattering modes are reported in Table S1.
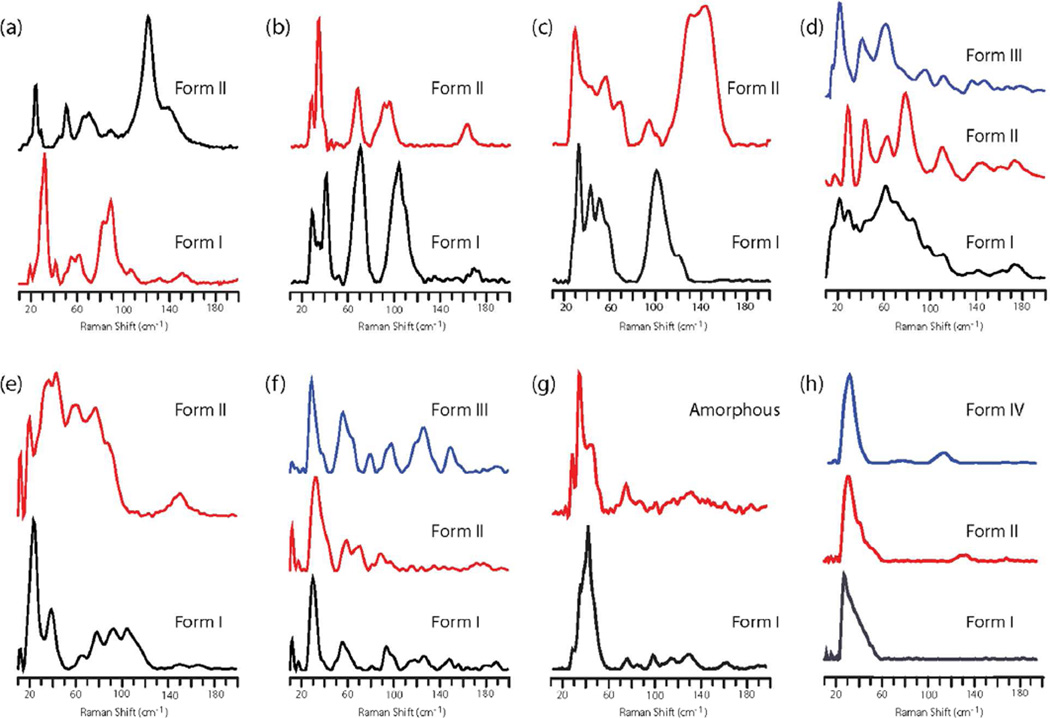
Acetaminophen
Acetaminophen mainly exists in two polymorphic forms, form I (monoclinic) and form II (orthorhombic).19 Raman and IR spectra are similar for both polymorphs due to their very similar molecular conformations. The polymorphic modifications can be easily differentiated from the low wavenumber Raman spectra as shown in Figure 2a. Form I has characteristic strong peaks at 32.7, 55.7, 63.0, 90.2 cm−1, whereas form II exhibits peaks at 25.4, 51.5, 67.2, 71.4, 122.6 cm−1.
Figure 2.
Low wavenumber Raman spectra of (a) acetaminophen (b) tolfenamic acid (c) nabumetone (d) furosemide (e) mefenamic acid (f) flurbiprofen (g) sulfamethazine and (h) sulindac.
Tolfenamic acid
Tolfenamic acid is pentamorphic.20 Among the five polymorphs, crystallization from solvent yields form I and form II.20 Both form I and form II are stable at room temperature and show several strong peaks below 200 cm−1 (Figure 2b). Form I has characteristics peaks at 28.9, 41.2, 70.8, 104.3 cm−1 and form II has peaks 28.9, 35.2, 68.7, 91.7, 95.9, 164.0 cm−1.
Nabumetone
Nabumetone exists in two polymorphic21, 22 modifications that can be easily discriminated using Raman scattering in the lattice phonon region. Form I has a characteristic strong broad peak at 100.9 cm−1 whereas form II shows a broad peak at 143.9 cm−1 with a shoulder at 131.3 cm−1 (Figure 2c).
Furosemide
Furosemide exists in three conformational polymorphs.23 It was observed that form I exhibits broad spectral features with poorly resolved peaks (Figure 2d). Raman scattering of form II and form III is shown to be more resolved than form I. Form II exhibits strong peaks at 18.2, 29.7, 44.4, 64.3, 80.0 cm−1, whereas form III shows peaks at 22.2, 42.1, 63.0 cm−1.
Mefenamic acid
Mefenamic acid exists in two polymorphs24 that can be distinguished from the lattice phonon region (Figure 2e). Form I has a strong peak at 33.3 cm−1 with a shoulder at 48.0 cm−1. Form II shows a broad scattering profile with peaks at 22.8, 30.1, 45.8, 52.1, 68.8, 84.5 cm−1.
Flurbiprofen
Three polymorphs of flurbiprofen25 were characterized with low wavenumber Raman spectroscopy. Form I, form II, and form III all exhibit distinct Raman scattering below 200 cm−1 (Figure 2f). Form I is characterized by strong peaks at 29.7, 56.9, 98.8 cm−1, while form II observes high intensity peaks at 13.0, 33.9, and 60.1 cm−1. The spectrum of form III exhibits a strong characteristic peak at 30.8 cm−1 along several other weaker peaks.
Sulfamethazine
Sulfamethazine exists in one stable crystalline form (form I) and an amorphous form. Both forms show strong Raman scattering below 60 cm−1. Form I exhibits a sharp peak at 43.3 cm−1, whereas the amorphous form has a peak 35.0 cm−1 with a shoulder at 28.7 cm−1 (Figure 2g).26
Sulindac
Three polymorphs of the anti-inflammatory drug sulindac25 were characterized with low wavenumber Raman spectroscopy. All the three polymorphs primarily show a single sharp Raman scattering peak in the low wavenumber region (Figure 2h). The peak for form I is at 28.7 cm−1, whereas for form II it is at 32.9 cm−1 and form IV at 33.7 cm−1. Apart from these sharp Raman signals, form II and form IV also have weak peaks at 132.3 cm−1 and 118.3 cm−1, respectively.
The polymorphic systems presented above demonstrate that facile discrimination among different phases through low wavenumber Raman scattering is achieved. Although the polymorphic samples discussed in the previous section can also be differentiated through PXRD and conventional Raman spectroscopy, low wave number Raman scattering presents a more direct approach to phase determination. Polymorphism in a compound can be distinguished by peak shifting of a few wavenumbers, broadening/sharpening of peaks, or from the change in shape of the peaks in the conventional Raman scattering range (200–4000 cm−1) whereas the peaks in the low wavenumber region are generally unique for the different polymorphs of the compound. For certain polymorphic compounds that have very similar conventional Raman spectra, low wavenumber Raman spectroscopy presents a more reliable method for polymorph discrimination. This is illustrated for two model polymorphic systems: carbamazepine and phenobarbital.
Carbamazepine
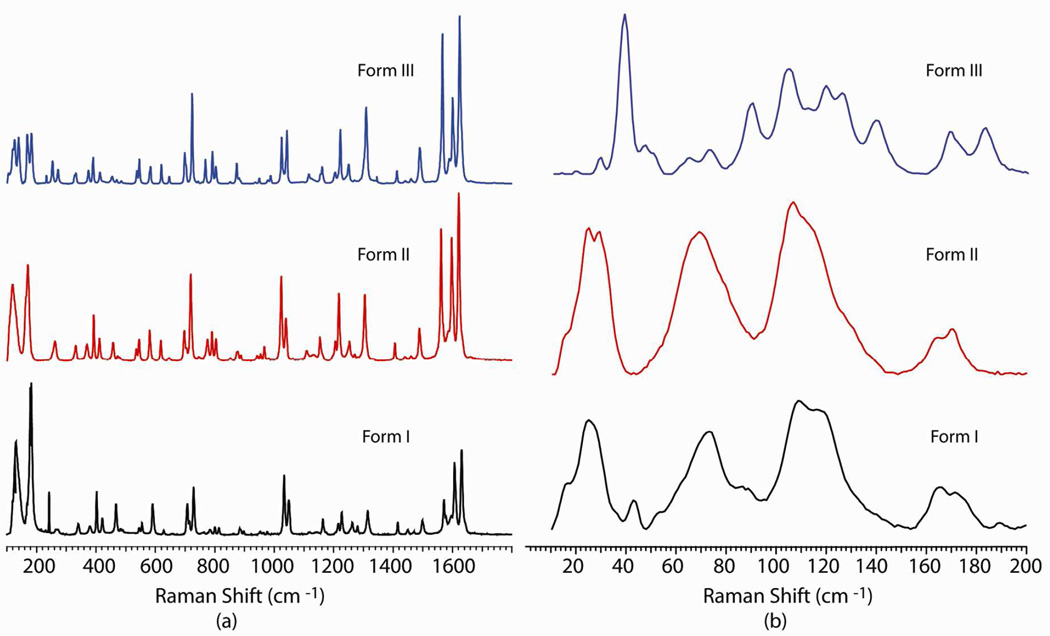
The anticonvulsant drug carbamazepine (5H-dibenz[b,f]azepine-5-carboxamide, CBZ) has been shown to be highly polymorphic.27–29 Among the five polymorphs of CBZ, three polymorphic modifications (forms I, II, and III) are most studied. Structural analysis shows that though form I and form II crystallize in different crystal systems (form I triclinic P-1, form II hexagonal R-3) they have similar packing arrangements.28 Both form I and form II have a similar dimer hydrogen bonding motif with π–π interactions among the benzene rings of different molecules (Figure S1). Form III has a completely different structure and is the most stable at room temperature.
The low wavenumber Raman spectra of form I and form II of CBZ are very similar: four broad peaks appear below 200 cm−1 (Figure 3b). Form III has a completely distinct Raman spectrum with well resolved Raman scattering peaks in the phonon region and several strong peaks. Although forms I and II are distinguishable by Raman spectroscopy, the similarity of the low wavenumber spectra reflects the similarity in lattice structure. This raises the possibility that comparison of Raman spectra in this region for new forms might be an approach to ascertain the degree of structural similarity in the absence of crystallographic information. If general, this could prove valuable in cases where phase changes destroy single crystallinity making structural elucidation more challenging.
Figure 3.
Raman spectra of carbamazepine polymorphs form I, form II and form III in the (a) conventional and (b) low wavenumber region.
Phenobarbital
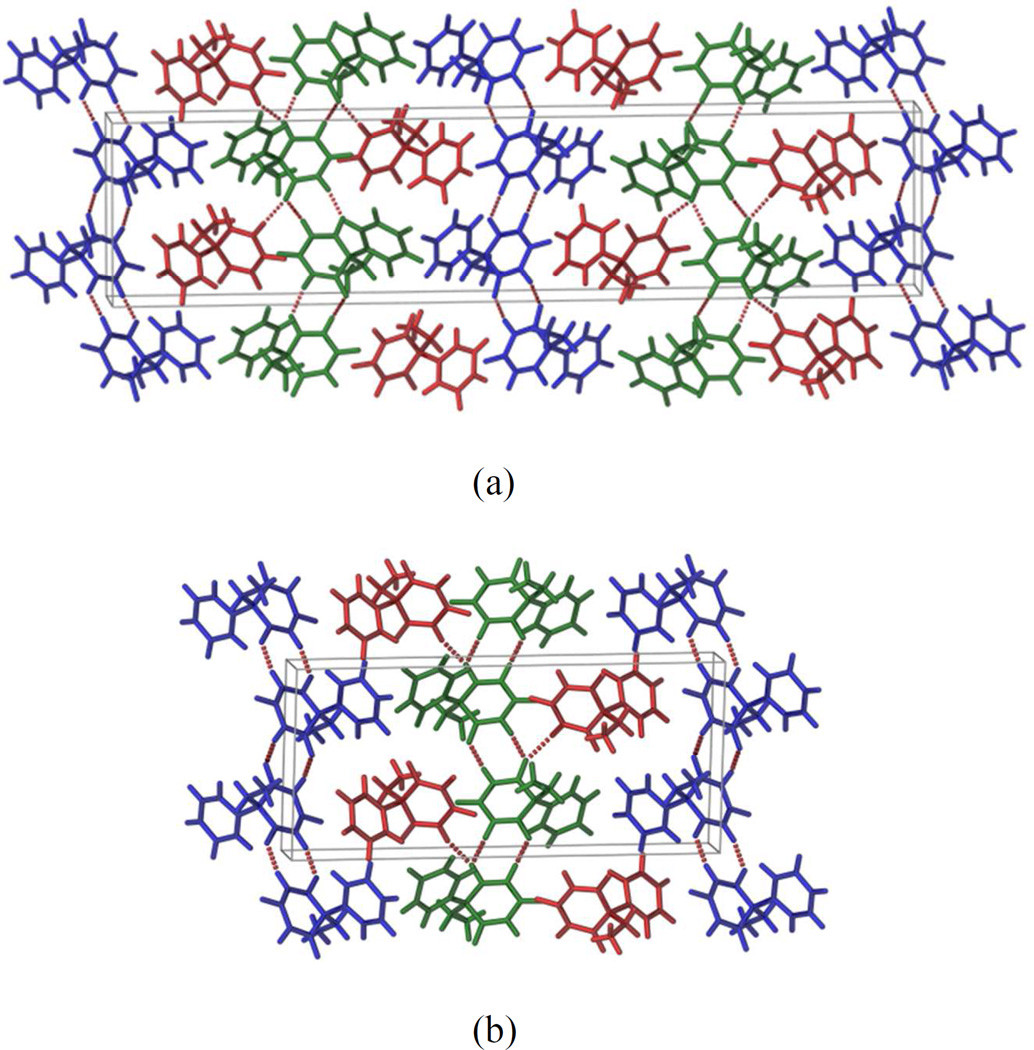
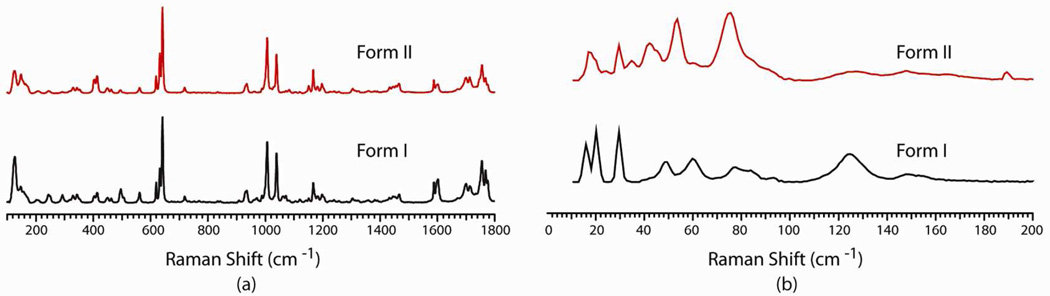
Phenobarbital (5-ethyl-5-phenylpyrimidine-2,4,6(1H,3H,5H)-trione, PB) is an anticonvulsant drug used for the treatment of epilepsy. Several polymorphs of PB are reported in the literature, among them form I and form II have been widely studied.30, 31 Both form I (Z′=3) and form II (Z′=3) exhibit N–H⋯O hydrogen bonded dimers with adjacent molecules. These dimers propagate through symmetry (inversion, glide or screw axis) to form a 1D ribbon and two independent chains form through two symmetry independent molecules (blue and green molecule in Figure 4a and 4b) and a third molecule (red) acts as a bridge between these chains.30, 31 Overall the packing of form I and form II are almost the same except for one crystallographic axis (axis b) is doubled in form I.30, 31 Due to similarity in the crystal structures, it is very difficult to differentiate between the two forms by PXRD or conventional Raman spectroscopy (Figure 5a). These two forms only can be differentiated by single crystal X-ray diffraction and DSC measurements (Figure S3). As shown in Figure 5b, this subtle difference in crystal packing can be clearly identified from the low wavenumber region of the Raman spectrum.
Figure 4.
Crystal packing of phenobarbital (a) form I and (b) form II.
Figure 5.
Raman spectra of phenobarbital polymorphs form I and form II in the (a) conventional and (b) low wavenumber region.
Both form I and form II of PB display characteristic low wavenumber spectra (Table S1). Form I has three strong peaks below 40 cm−1 (16.0, 20.1, 29.6 cm−1), form II exhibits two strong peaks at 17.0 and 29.6 cm−1. Form I and form II show a characteristic broad strong peak at 124.7 and 75.5 cm−1 respectively (Figure 5b). Though the low wavenumber Raman spectra for the isostructural form I and form II of PB have some similarity, they can be easily differentiated from their peak intensity and shape. Overall, unlike conventional Raman spectra, form I and form II have distinct differences in the low wavenumber region.
To understand the lattice vibrations of CBZ and PB polymorphs, a comparative conformational analysis was conducted. The CBZ molecule is rigid and it is found that deviations of torsion angles (Table S2, Figure S5) among the different symmetry independent molecules are within a range of 2°. The CBZ molecule stays rigid during the lattice vibrations and π–π interactions among benzene rings remain almost constant for both form I and form II. However the PB molecule is flexible and although form I and form II are isostructural they have subtle difference in conformations (Table S2, Figure S5). Our hypothesis is that due to conformational flexibility and difference in conformations, PB form I and form II have distinguishable lattice vibrations.
CONCLUSION
The Raman spectra of 10 polymorphic compounds were recorded over a broad range of frequencies including the region below 150 cm−1. In some cases reliable differentiation was only possible by considering the low energy modes and these findings support the idea that a relatively narrow spectral region can greatly bolster differentiating power. Moreover, the similarity in the phonon modes among different polymorphs was correlated with structural similarity suggesting that Raman spectroscopy can be used both in the traditional mode of looking at changes in specific intermolecular interactions and conformation (high wavenumber) as well as in assessing gross packing similarities (low wavenumber). This finding could be especially useful in supporting PAT where the observation of solid form transformations during the manufacturing process is desired. Due to recent advances in filter technology and optics, it is now possible to incorporate ultra-narrow holographic filters into existing Raman systems, allowing for Raman data collection to reach as low as ~10 cm−1 to the laser line.14 Based on the results present here, incorporating such changes into Raman systems for PAT would provide considerable advantages in terms of additional discriminating power.
Supplementary Material
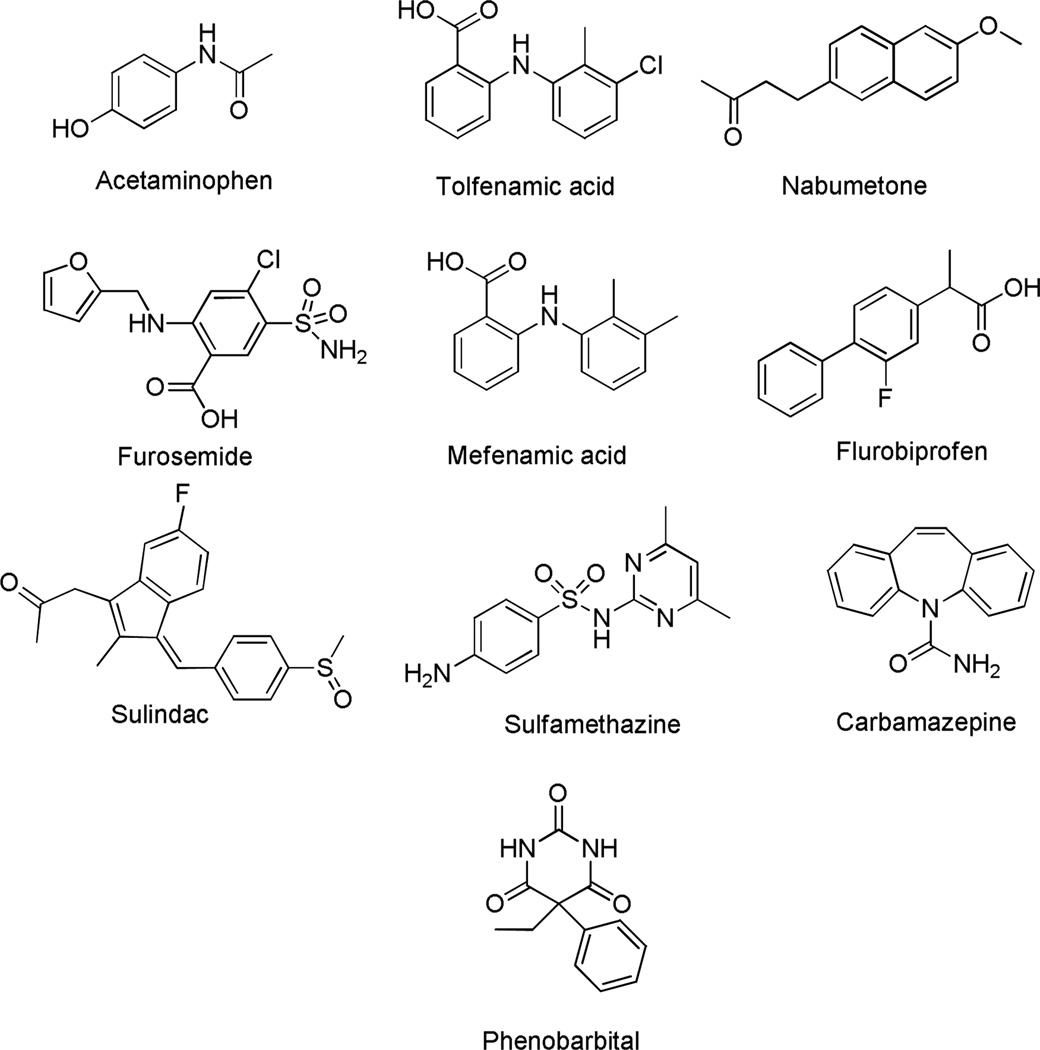
Figure 1.
Chemical structures of the polymorphic compounds studied.
Acknowledgments
Funding Sources
This work was supported by the National Institute of Health Grant Number GM072737.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Sample preparations, tabular data of Raman spectra, packing diagram of CBZ, PXRD of PB polymorphs, and conformational analysis of CBZ and PB polymorphs are available in Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Hilfiker R, Blatter F, Raumer MV. Polymorphism in the Pharmaceutical Industry. Weinheim: Wiley-VCH, Weinheim; 2006. pp. 1–19. [Google Scholar]
- 2.Byrn SR, Pfeiffer RR, Stowell JG. Solid-State Chemistry of Drugs. West Lafayette, IN: SSCI; 1999. [Google Scholar]
- 3.Aguiar AJ, Krc J, Kinkel AW, Samyn JC. J. Pharm. Sci. 1967;56(7):847–853. doi: 10.1002/jps.2600560712. [DOI] [PubMed] [Google Scholar]
- 4.Amidon GL, Lennernas H, Shah VP, Crison JR. Pharm. Res. 1995;12(3):413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 5.Singhal D, Curatolo W. Adv. Drug Deliver Rev. 2004;56(3):335–347. doi: 10.1016/j.addr.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Byrn S, Pfeiffer R, Ganey M, Hoiberg C, Poochikian G. Pharm. Res. 1995;12(7):945–954. doi: 10.1023/a:1016241927429. [DOI] [PubMed] [Google Scholar]
- 7.Wardrop J, Law D, Qiu Y, Engh K, Faitsch L, Ling C. J. Pharm. Sci. 2006;95(11):2380–2392. doi: 10.1002/jps.20679. [DOI] [PubMed] [Google Scholar]
- 8.Scott B, Wilock A. J. Pharm. Sci. Technol. 2006;60(1):17–53. [PubMed] [Google Scholar]
- 9.Watts C. PAT - A framework for Innovative Pharmaceutical Development Manufacturing and Quality Assurance, FDA/RPSGB Guidance Workshop; 2004. [Google Scholar]
- 10.Webster S, Baldwin KJ. Pharm. Tech. Europe. 2005;17(8):30–43. [Google Scholar]
- 11.Al-Dulaimi S, Aina A, Burley J. Cryst Eng Comm. 2010;12(4):1038–1040. [Google Scholar]
- 12.Ayala AP. Vib. Spectrosc. 2007;45(2):112–116. [Google Scholar]
- 13.Brillante A, Bilotti I, Della Valle RG, Venuti E, Girlando A. Cryst Eng Comm. 2008;10(8):937–946. [Google Scholar]
- 14.Lebedkin S, Blum C, Stürzl N, Hennrich F, Kappes MM. Rev. Sci. Instrum. 2011;82(1):013705-1–013705-6. doi: 10.1063/1.3520137. [DOI] [PubMed] [Google Scholar]
- 15.Carteret C, Dandeu A, Moussaoui S, Muhr H, Humbert B, Plasari E. Cryst. Growth Des. 2009;9(2):807–812. [Google Scholar]
- 16.Surovtsev NV, Malinovsky VK, Boldyreva EV. J. Chem. Phys. 2011;134(4):045102-1–045102-5. doi: 10.1063/1.3524342. [DOI] [PubMed] [Google Scholar]
- 17.Renishaw plc, Polymorph Discrimination Using Low Wavenumber Direct Lattice Information. Molecular Spectroscopy: The Application Notebook. 2007:24–26. [Google Scholar]
- 18.Price CP, Grzesiak AL, Matzger AJ. J. Am. Chem. Soc. 2005;127(15):5512–5517. doi: 10.1021/ja042561m. [DOI] [PubMed] [Google Scholar]
- 19.Lang M, Grzesiak AL, Matzger AJ. J. Am. Chem. Soc. 2002;124(50):14834–14835. doi: 10.1021/ja0286526. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Mejias V, Kampf JW, Matzger AJ. J. Am..Chem..Soc. 2009;131:4554–4555. doi: 10.1021/ja806289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price CP, Grzesiak AL, Lang M, Matzger AJ. Cryst. Growth Des. 2002;2(6):501–503. [Google Scholar]
- 22.Chyall LJ, Tower JM, Coates DA, Houston TL, Childs SL. Cryst. Growth Des. 2002;2(6):505–510. [Google Scholar]
- 23.Matsuda Y, Tatsumi E. Int. J. Pharm. 1990;60(1):11–26. [Google Scholar]
- 24.Gilpin RK, Zhou W. J. Pharm. Biomed. Anal. 2005;37(3):509–515. doi: 10.1016/j.jpba.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Grzesiak AL, Matzger AJ. J. Pharm. Sci. 2007;96(11):2978–2986. doi: 10.1002/jps.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang SS, Guillory JK. J. Pharm. Sci. 1972;61(1):26–40. doi: 10.1002/jps.2600610104. [DOI] [PubMed] [Google Scholar]
- 27.Arlin JB, Price LS, Price SL, Florence AJ. Chem. Commun. 2011;47(25):7074–7076. doi: 10.1039/c1cc11634g. [DOI] [PubMed] [Google Scholar]
- 28.Grzesiak AL, Lang M, Kim K, Matzger AJ. J. Pharm. Sci. 2003;92(11):2260–2271. doi: 10.1002/jps.10455. [DOI] [PubMed] [Google Scholar]
- 29.Lang M, Kampf JW, Matzger AJ. J. Pharm. Sci. 2002;91(4):1186–1190. doi: 10.1002/jps.10093. [DOI] [PubMed] [Google Scholar]
- 30.Zencirci N, Gelbrich T, Apperley DC, Harris RK, Kahlenberg V, Griesser UJ. Cryst. Growth Des. 2010;10(1):302–313. [Google Scholar]
- 31.Platteau C, Lefebvre J, Hemon S, Baehtz C, Danede F, Prevost D. ActaCrystallogr., Sect. B: Struct. Sci. 2005;B61(1):80–88. doi: 10.1107/S0108768104031143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.