Abstract
Ichthyotoxic Karlodinium veneficum has become a persistent problem in the eutrophic Swan River Estuary (SRE) near Perth, Western Australia. Karlotoxin (KmTx) concentrations and K. veneficum were sampled from March to July 2005, spanning a bloom confirmed by microscopy and genetics (ITS sequence), and a fish kill coincident with end of the bloom. The objective of this study was to investigate K. veneficum cell and toxin dynamics, and water quality conditions, leading up to the bloom and fish kill in this estuarine system. Abundance of K. veneficum increased as diatom abundance decreased over a 3-month period (Jan-Mar) preceding the bloom. Low freshwater flow to the SRE characterized the bloom initiation period, while elevated seasonal flows altered water quality and preceded the end of the bloom and fish kill. The bloom of K. veneficum was localized over a bottom layer of hypoxic water in a stratified water column. Low nitrate levels, DIN:DIP (mol) near unity, and particulate C:N:P of K. veneficum-rich water samples were consistent with nitrogen limitation of phytoplankton. A KmTx 2 congener was present in the concentration range 0–1052 ng KmTx mL−1, levels that were sufficient to kill larval fish in the laboratory within 4 h. A KmTx cell quota of 2.8 pg KmTx cell−1 was estimated for the bloom, which is moderately high for the species. Gill histopathology of fish from this fish kill showed signs of damage similar to those caused by KmTx in the lab. Results from this study suggest that conditions in the SRE, including elevated K. veneficum abundance and KmTx cell quotas, as well as hypoxia in the upper SRE, likely contribute to seasonal fish kills observed in this system.
Keywords: Fish kill, Karlodinium veneficum, Karlotoxins, Swan River Estuary
1. Introduction
The Swan River Estuary (SRE) is a subtropical microtidal estuary with a Mediterranean climate and has been negatively impacted by anthropogenic nutrient loading (Thompson and Hosja, 1996) and hydrological alterations (Chan et al., 2002). The seasonal cycle of phytoplankton dynamics in the SRE is largely driven by nitrate supply and riverine runoff (Thompson, 1998; Chan and Hamilton, 2001; Horner-Rosser and Thompson, 2001). Dinoflagellate blooms (Gymnodinium spp., Prorocentrum) have been observed austral summer through autumn in stratified portions of the upper estuary where surface nitrate concentrations are low and bottom-water ammonium is high (Hamilton et al., 1999; Twomey and John, 2001), but no toxicity was reported associated with these blooms. Recently, the seasonal cycle of phytoplankton dynamics has been punctuated by harmful algal blooms (HAB). A major toxic bloom of Microcystis aeruginosa (cyanobacterium) in 2000 was associated with an anomalous, record-breaking summer rainfall event that freshened much of the SRE and dispersed the bloom population from the upper estuary into the lower basin (Hamilton, 2000; Robson and Hamilton, 2003). Ichthyotoxic blooms of the dinoflagellate, Karlodinium veneficum, had not been reported in the SRE before 2002, but have occurred periodically since that time.
The dinoflagellate Karlodinium veneficum (10–15 µm) is a cosmopolitan mixotrophic (Li et al., 1996; Adolf et al., 2006a; Calbet et al., 2011) protist responsible for numerous fish kills in temperate estuaries on the eastern seaboard of the United States (Deeds et al., 2002; Kempton et al., 2002; Fensin, 2004; Goshorn et al., 2004; Garces et al., 2006; Hall et al., 2008) as well as in other waters around the world (Bachvaroff et al., 2008; Place et al., 2012). Previously K. veneficum has been called Gyrodinium estuariale, Gymnodinium galatheanum, Gymnodinium veneficum, and K. micrum, all of which are now synonymous with K. veneficum (Bergholtz et al., 2005). Ichthyotoxic and cytotoxic bioactivity associated with K. veneficum was first reported by Abbot and Ballantine (1957), as Gymnodinium veneficum (Ballantine, 1956).
The linear polyketide toxins produced by Karlodinium veneficum (Van Wagoner et al., 2008, 2010; Peng et al., 2010), karlotoxins (KmTx), have hemolytic, cytotoxic and ichthyotoxic activities (Deeds et al., 2002; Kempton et al., 2002; Mooney et al., 2009). Strains of Karlodinium veneficum from geographically distinct regions produce different KmTx congeners that vary in chromatographic properties, UV absorption maxima (Deeds et al., 2004) and mass spectra (Deeds et al., 2004; Bachvaroff et al., 2008) although they are similar in bioactivity. The bioactivity of karlotoxin is membrane pore formation, dependent on the sterol composition of the target cell. The predominance of 4-α-methyl sterols (i.e. gymnodinosterol and brevesterol) in K. veneficum makes it immune to its own toxin, whereas organisms (i.e. fish, other protists) with a predominance of 4-des-methyl sterols, (i.e. cholesterol), are susceptible to KmTx bioactivity (Deeds and Place, 2006; Adolf et al., 2006b). Karlotoxins have anti-grazing/allelo-pathic activity (Adolf et al., 2007, 2008; Stoecker et al., 2008; Brownlee et al., 2008; Wagget et al., 2008; Vaque et al., 2006). Ichthyotoxicity of KmTx in situ appears to depend on cell lysis since intact, toxic K. veneficum has little effect on fish (Deeds et al., 2002; Mooney et al., 2010; Dorantes-Aranda et al., 2011). In fish exposed to free KmTx, lysis of gill epithelial cells leading to collapse of the gill lamellae is observed (Deeds et al., 2006), presumably impairing gas and ion exchange across the gills.
Records predating 2002 for the SRE indicate observations of Gyrodinium estuariale (April 1999 @ 32,926 cells per mL) and Gymnodinium galatheanum (March 2001 @ 6000 cells per mL) (Jeff Cosgrove, pers. comm.), which were likely Karlodinium veneficum (Bergholtz et al., 2005) but no ichthyotoxicity was reported. From April to July 2003, numerous fish kills occurred in both the Swan and Canning River estuaries located in Perth, Western Australia. These kills coincided with a persistent bloom of K. veneficum starting in the Swan River in early April and spreading to the Canning River in Mid-June. The bloom ended in early July following a large rainfall event. The bloom was preceded by unusually high rainfall in the area during early April followed by a prolonged period of calm, sunny weather. Karlodinium veneficum densities fluctuated during this period, often reaching 1.0 × 105 cells mL−1. Numerous fish species were affected, including black bream (Acanthopagrus butcheri), a popular local sport fish, Perth herring (Elops machnata), and Swan River gobies (Pseudogobius olorum). Most kills contained <1000 fish, but two kills were estimated to contain in excess of 100,000 fish each (P. Musk, pers. comm.). Observations of fish gill histopathology were consistent with effects observed during exposure of zebrafish (Danio rerio) to purified KmTx (Deeds et al., 2006).
Toxic blooms of Karlodinium veneficum were first observed in the SRE in 2002 but detailed measurements of karlotoxin and K. veneficum abundance in the SRE have not been published before. Cell concentrations of K. veneficum, karlotoxin levels and SRE water quality were sampled to better understand the role of K. veneficum in fish mortalities that were anticipated to occur between April and June (austral autumn) based on past years’ observations. It was hypothesized that increased karlotoxin concentrations, either through increased cell numbers or increases in cellular toxin cell quotas (pg toxin cell−1) will precede or accompany major fish kills. Karlotoxin (KmTx) concentration associated with the observed 2005 SRE fish kill was closely examined and compared values with levels of KmTx observed to kill fish in laboratory trials.
2. Materials and methods
2.1. Water sampling
Swan River Estuary (Fig. 1) water quality sampling and analysis was done weekly, in the morning, according to standard procedures of the Western Australia Dept. of Environment (WA DoE) and Swan River Trust (http://www.swanrivertrust.wa.gov.au/docs/monitoring-and-evaluation/water-quality-parameters.pdf), including all salinity, temperature, nutrients, and oxygen measurements (Hydrolab or YSI multiparameter sonde, 0.5 m depth intervals). Nutrient samples were forwarded to Queensland Health Scientific Services (http://www.health.qld.gov.au/qhcss/qhss/) for analysis on the day of collection, if not the following day with overnight storage a 4 °C. Phytoplankton cell count samples were collected from the same stations as nutrient samples and counts conducted weekly by WA DoE standard procedures on surface-collected water samples. River discharge data for the Avon (Station 616011, 116.068524205 E 31.750532184 S) and Bennet Brook (Station 616084, 115.959525641 E 31.877702539 S) were obtained from the State of Western Australia Department of Water.
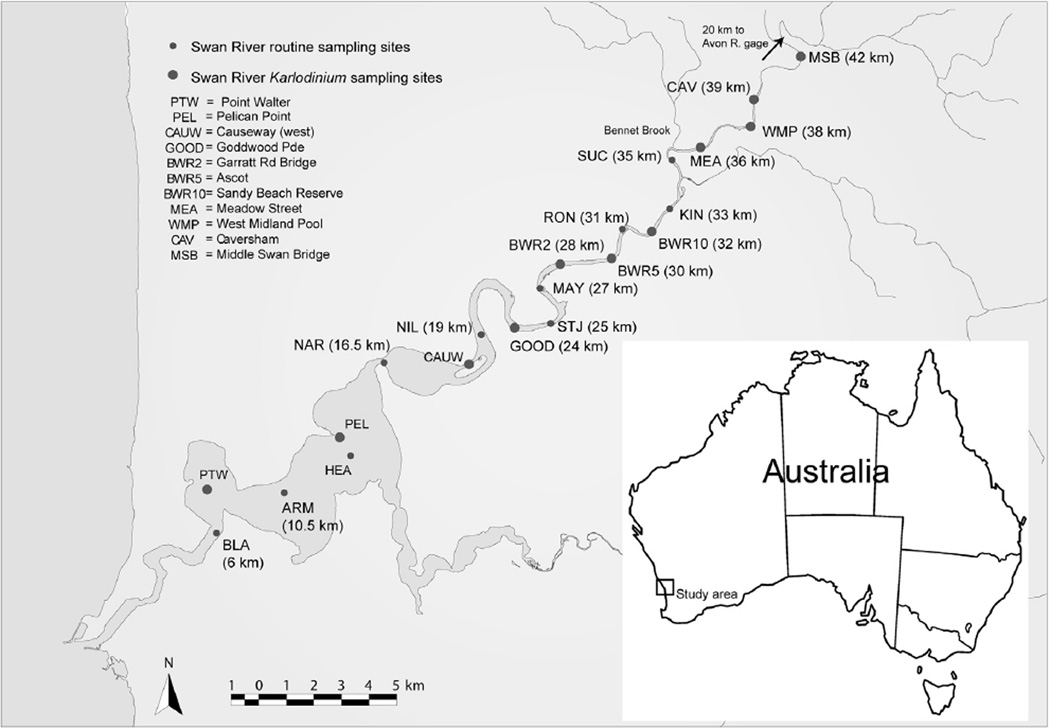
Fig. 1.
Sampling map of the Swan River Estuary (SRE). Sampling in the SRE was conducted March–July 2005. Individual site labels and distance from the ocean are indicated on the map, as well as the location of the Bennet Brook and Avon River gages.
2.2. ITS sequence analysis
Karlodinium veneficum isolated from the SRE was grown in f/2 medium in a culture chamber at 100 µmol photons m−2 s−1,12:12 L:D, 20 °C, salinity 15. The unialgal culture (AUS#7) was isolated using single cell isolation by micropipette, from a dense sample collected at station SUC on March 29, 2005. Cell pellets from AUS #7 of 105–106 cells were obtained by centrifugation (IEC Centra CL2, Thermo Fisher Scientific, Waltham, MA, USA) at 3000 rpm (1600 g) and frozen at 20 °C prior to DNA extraction. DNA was isolated using a CTAB detergent method followed by chloroform extraction and precipitation of the aqueous phase with isopropanol (Doyle and Doyle, 1987). The DNA was diluted based on 230 nm absorbance, and 100 ng was used in a 20 µL PCR reaction in the following buffer: 3 mM MgCl, 500 mM Tris-HCl pH 8.3, 500 µg mL−1 BSA, 2 mM dNTPs, and 4 pmoles of primer (all concentrations final). The temperature was cycled from 15s denaturating at 94 °C to a 55 °C annealing for 15 s followed by 30 s of extension at 72 °C for 35 cycles. The primers used were based on primers suggested by Litaker et al. (2003): ITSFor CTGCGAAGC-TATCGCTATT and ITSRev TGAGGGAATCCTATTTAG designed to cross from ITS1 to the LSU rRNA including most of ITS1, 5.8S, and ITS2. PCR amplicons were purified with equal volumes of 20% w/v polyethylene glycol (mw 8000) containing 2.5 M NaCl, pelleted by centrifugation, and washed with 70% ethanol. Sequencing reactions were performed and run on an ABI 3130XL sequencer according to the manufacturer’s protocol. Sequences were edited using Sequencher 4.5 software (Genecodes Corp., Ann Arbor, MI, USA) and exported to MacClade 4.05 (Sinauer Associates Inc., Sunderland, MA, USA) for manual alignment using GenBank sequences AF352365–AF352367 for K. veneficum as a reference.
2.3. Large scale Karlotoxin isolation from a natural bloom
A total of 4.8 L of SRE water containing the bloom of Karlodinium veneficum, collected from sites SUC (March 31, 2005) and WMP, MEA, and SUC (April 4, 2005), were filtered through a GF/F (Whatman) filter, and the filtrate then passed through a 55 g tC18 cartridge (AnaLogix, Sorbtech Technologies) to collect dissolved organic material including toxin. The column was first activated by washing with 200 mL methanol followed by 200 mL HPLC-grade water. The column was stored at 4 °C and then transported in a cooler with an ice pack until further processing in Baltimore, MD, less than 2 weeks later. In Baltimore, the tC18 cartridge was washed successively with 200 mL volumes of dH2O, 20% MeOH, and 40% MeOH (all discarded after testing for hemolytic activity). The 60% MeOH and 80% MeOH eluates, anticipated to contain partially purified KmTx, were retained, dried under vacuum at 40 °C, and analyzed by LC–MS. The concentrated toxin from the pooled 60–80% tC18 eluent was then purified using reverse phase chromatography as on a semi-preparative scale C18 column (Phenomenex Hyperprep HS C18–80S, 250 × 10 mm, 5 µ ) subjected to a 4 mL min−1 binary methanol/water gradient from 10% to 95% methanol over 45 min using an Agilent 1100 Series LC/ MSD system, comprising of binary pump system, autosampler and diode array detector (DAD) with a micro high-pressure flow cell (6 mm path length, 1.7 µL volume), fraction collector and quadrupole mass spectrometer (G1956A SL) equipped with an electrospray ionization (ESI) interface. Toxin peaks were detected over the wavelength range 190–950 nm Based on the UV/vis spectra the absorption at 225 nm was used to detect KmTx 1 while absorption at 235 nm was used to detect KmTx 2. The entire UV/vis spectra were saved for each UV detectable peak. Fractions corresponding to KmTxPerth 2-1 and KmTxPerth 2–2 were collected using a fraction collector (61364A, AFC, Agilent) based on known retention times. Fractions were pooled as appropriate, dried under vacuum and resuspended in 80% methanol:20% water. From the total 4.8 L, 23 mg of KmTxPerth 2-1 and 1 mg of KmTxPerth 2-2 were obtained.
2.4. In situ Karlotoxin sampling and analysis
Routine sampling of KmTx from Swan River samples was done using a modification of the procedure described in Bachvaroff et al. (2008). 5 mL of SRE water collected for cell count sampling were pushed through 13 mm 0.2 µm pore size PTFE filters, labeled, wrapped in small manila envelopes and then stored at 4 °C, thus collecting both all toxin in the sample whether cell-associated or free. At the end of the sampling season, these syringe filters were shipped in a cooler with ice packs overnight from Perth to Baltimore. In Baltimore, the filters were eluted with 1 mL MeOH into 2 mL dH2O to yield a crude extract of KmTx in 33% MeOH. Quantification of KmTx used HPLC-MS methods detailed in Bachvaroff et al. (2008). Ions corresponding to KmTx 2 (1367.8 amu), and a 1365 amu ion that showed strong hemolytic activity, were summed to obtain KmTx abundance for this study. The 1365 ion was in lower abundance than 1367.8, averaging 24% (±20.7%) of total ion counts for KmTx.
2.5. Hemolytic assay and fish bioassay of KmTx
The HPLC fractions were collected for 20 s during the first 32 m of the chromatography gradient into 96 well plates. The hemolytic assay is described in detail elsewhere (Eschbach et al., 2001). Briefly, rainbow trout (Oncorhynchus mykis) erythrocytes were extracted from the caudal vein and the blood washed three times with three volumes of buffer mL−1 of packed erythrocytes using the incubation buffer described below minus CaCl2. Following the third wash the cells were resuspended in 3 mL of buffer mL−1 of packed cells. Resuspended cells were diluted 1:20 in buffer and 200 mL of diluted, washed erythrocytes were incubated in the presence of 10 mL of each HPLC fraction for 30 min in a buffer of 150 mM NaCl, 3.2 mM KCl, 1.25 mM MgSO4,12.2 mMTris Base and 3.75 mM CaCl2. Assays were run in 96 well, V-bottom, non-treated, polystyrene plates (Corning Inc.) sealed with Falcon 3073 pressure sensitive film (Becton Dickinson Labware). Assays were incubated on an orbital shaker (80–100 rpm)at 20 °C for 1 h. Plates were then centrifuged at 2500 g for 5 min and the supernatant (100 µL) was transferred to a flat bottom 96 well polystyrene plate, where the absorbance of released hemoglobin was read at 540 nm using a microtiter plate spectrophotometer (Model Spectramax 340, Molecular Devices). Saponin (10 µg) (from Quillaja bark; Sigma Chemical Co.) as used as a hemolytic positive control.
To examine icthyotoxicity of the isolated karlotoxins, sheeps-head minnows (Cyprinodon variegatus, purchased as larvae from Chesapeake Cultures, Inc., Hayes, Virginia, and raised at salinity 12 in-house following procedures described previously for zebrafish (Danio rerio)) were exposed to increasing concentrations of toxin. Sheepshead minnow (60–90 days old; 2 cm long) were exposed as described (Deeds et al., 2006) except that O2-saturated 12% artificial seawater (Instant Ocean Brand) was used as the exposure medium. Sheepshead minnows were exposed to control (0.4% methanol), 267, 668, 1338, 2675 KmTxPerth 2–1 and 0, 69.3, 173, 346, and 693 KmTxPerth 2-2 ng mL−1 (fish/beaker; n = 2). Fish were checked for mortality hourly, and any fish that did not die by 4 h were killed then, as described previously (Deeds et al., 2006). Whole gills were dissected, preserved and examined as described (Deeds et al., 2006).
3. Results
3.1. Molecular characterization of Karlodinium veneficum strain
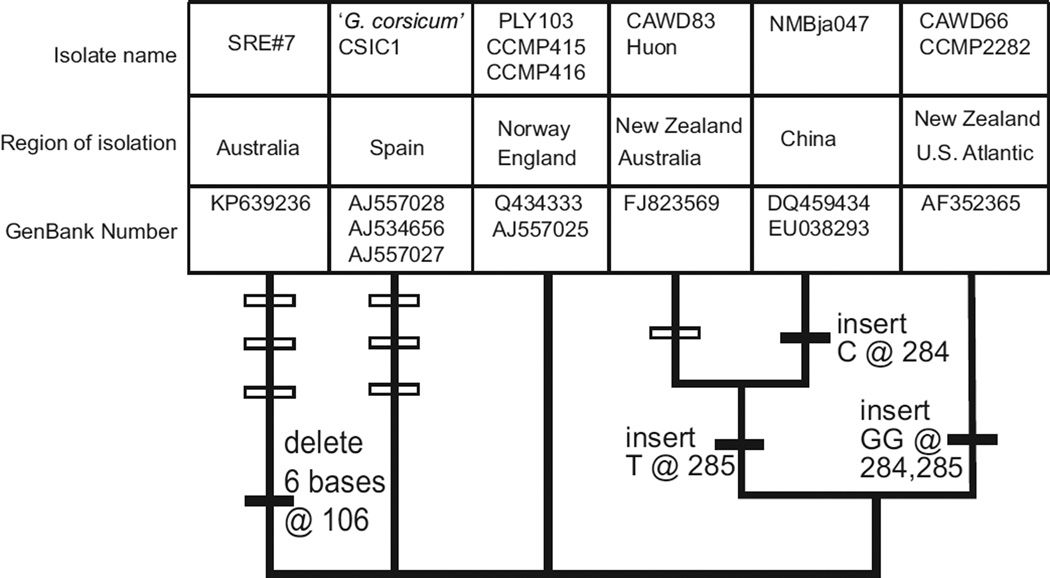
The 541 base ITS sequence from a cultured SRE Karlodinium veneficum (AUS #7, GENBANK# KP639236) had three single nucleotide differences and a six base deletion when compared to the ITS for CCMP 415, isolated from the North Sea (AJ557026) (Fig. 2). The ITS2 for K. veneficum was able to be folded into a typical secondary structure with four base paired ‘stems’ (http://its2.bioapps.biozentrum.uni-wuerzburg.de). The variant ITS2 sites could then be sorted into compensatory changes occurring in both bases of a base paired stem and non-compensatory changes found in single stranded loops (Gottschling and Plotner, 2004). Two single nucleotide differences occurred in the single stranded loop at the end of the third base paired stem in ITS2 and were thus not compensatory changes. The six base deletion and the third nucleotide substitution shortened the fourth ITS2 stem from 9 base pairs to five and were compensatory changes.
Fig. 2.
Comparisons of an approximately 500 base ITS region between different K. veneficum strains. The sequence spans part of ITS1, the 5.8S rRNA and ITS2. There were no differences in the 5.8 S region. The tree contains an unresolved polytomy at the base. Above each branch are (listed in order of the corresponding strains) strain or clone designations, geographic source, and GenBank accession numbers. Where there is a single accession number with more than one strain the sequences are identical. The isolate ‘Huon’ refers to strain HU01 from the Huon river (Mooney et al., 2009). Filled symbols on branches are insertions, open symbols on branches are mutations.
3.2. Physical and chemical conditions in the Swan River Estuary
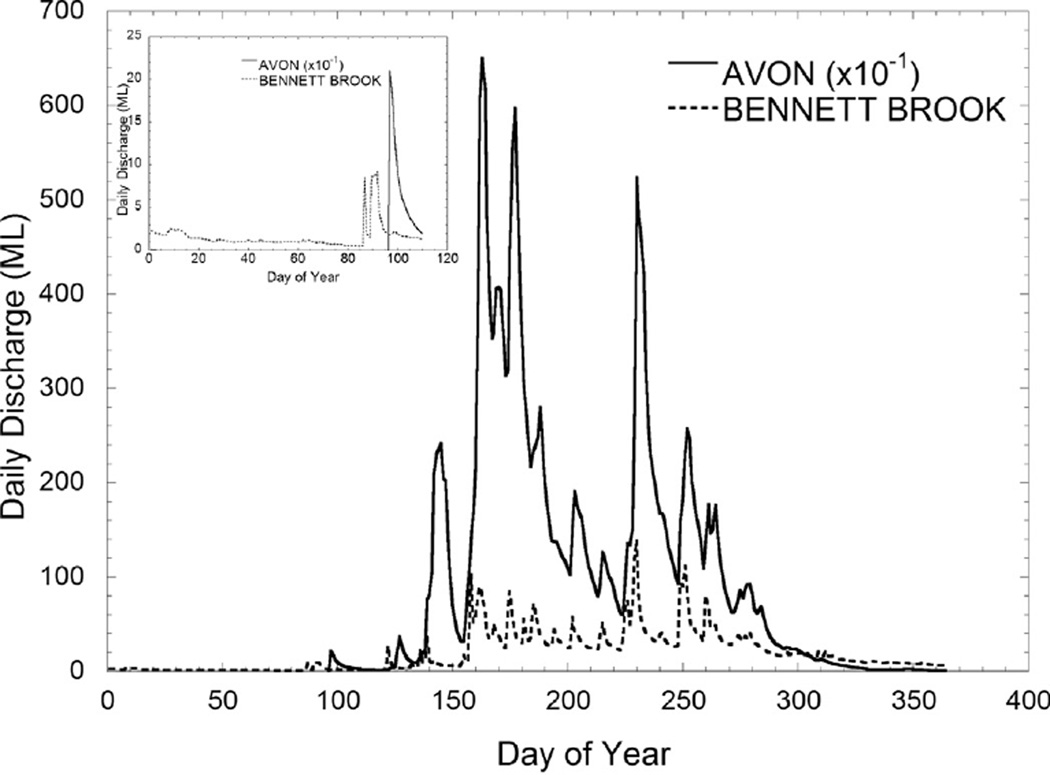
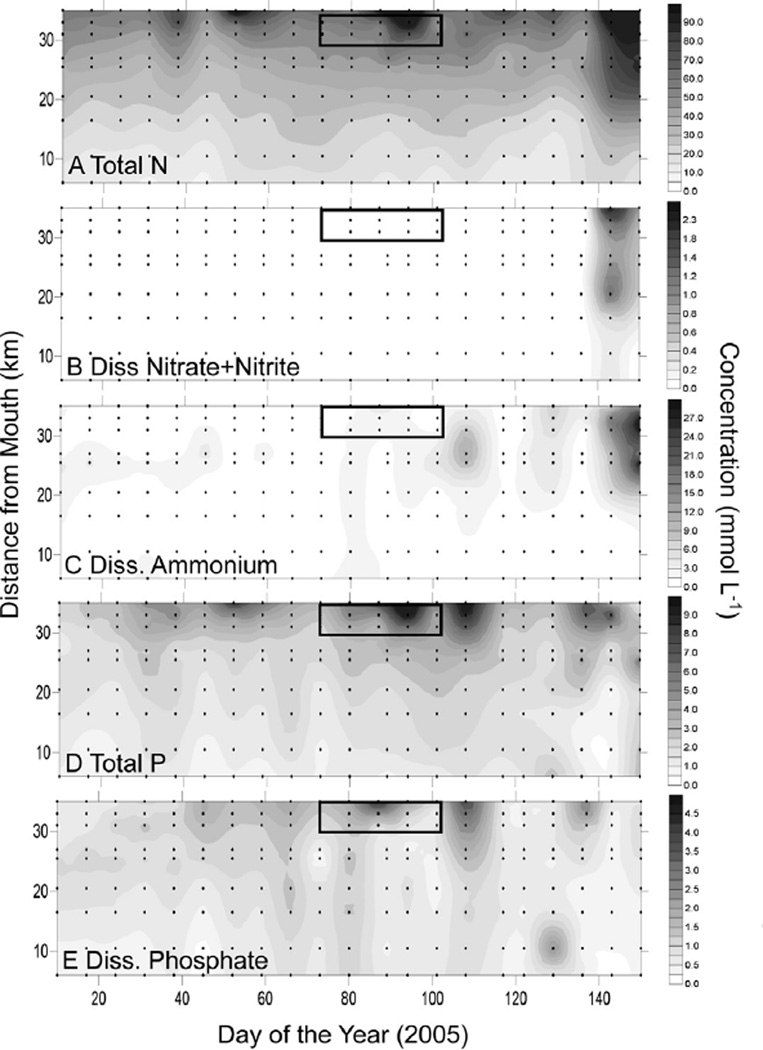
Discharge from the Avon River and Bennet Brook (confluence with the SRE just above SUC, Fig. 1) was negligible through DOY 84, followed by elevated flow in Bennet Brook (DOY 86) and the Avon River (DOY 96) (Fig. 3). Total N in the surface SRE declined from 60 µmol L−1 or greater at the 35 km station (SUC), to 10–20 µmol L−1 by the 6 km station (BLA) (Fig. 4A). Nitrate was low (<0.2 µmol L−1) throughout the surface SRE except DOY >120 (May) when values increase throughout most of the estuary (Fig. 4B). Ammonium averaged 0.83 µmol L−1 throughout the surface SRE between DOY 1 and 90 (Jan-March), then showed elevated values associated with the bloom (average 2 µmol L−1) and thereafter (Fig. 4C). Total P showed a pattern similar to that of total N, with values of 8–10 µmol L−1 in the surface waters of the upper SRE declining to <1 µmol L−1 downstream, and some of the highest values of the sampling period associated with the bloom (Fig. 4D). Dissolved phosphate was generally 1–2 µmol L−1 in the surface waters of the upper SRE and declined downstream, with elevated values of 2–4 µmol L−1 observed at the bloom (Fig. 4E). Particulate elemental analyses of high Karlodinium veneficum density water samples yielded average C:N (mol) of 8.0, and C:P (mol) of 97.3 (Table 1).
Fig. 3.
Hydrographs (2005) for the Avon River and Bennet Brook, the latter of which has a confluence with the Swan just upstream from station SUC (please see Fig. 1).
Fig. 4.
Surface N and P nutrient conditions in the upper SRE. (A) Total N, (B) dissolved nitrate and nitrite, (C) dissolved ammonium, (D) total phosphorus (E) dissolved phosphate. The box represents the time/place of the bloom of K. veneficum.
Table 1.
Particulate C, N, and P composition of samples taken in high-density K. veneficum waters.
| Date (2005) |
DOY | Site | km | C:N (mol) |
C:P (mol) |
K. veneficum (cells mL−1) |
|---|---|---|---|---|---|---|
| 3/31 | 90 | MSB | 42 | 7.5 | 78.6 | 12,044 |
| 3/31 | 90 | WMP | 38 | 7.4 | 121.6 | 42,420 |
| 3/31 | 90 | MEA | 36 | 8.2 | 191.6 | 154,429 |
| 3/31 | 90 | SUC | 35 | 8.8 | 85.2 | 116,847 |
| 3/31 | 90 | KIN | 33 | 8.0 | 140.6 | 51,712 |
| 4/4 | 94 | MSB | 42 | 7.7 | 89.2 | 4621 |
| 4/4 | 94 | CAV | 39 | 8.2 | 46.1 | 29,189 |
| 4/4 | 94 | WMP | 38 | 7.8 | 65.9 | 46,864 |
| 4/4 | 94 | SUC | 35 | 8.7 | 66.2 | 104,737 |
| 4/4 | 94 | KIN | 33 | 8.7 | 95.0 | 79,586 |
| 4/4 | 94 | BWR 10 | 32 | 7.7 | 97.5 | 21,513 |
| 4/4 | 94 | RON | 31 | 7.5 | 89.9 | 34,239 |
| Avg. (±sd) | 8.0 (±0.51) | 97.3 (±38.80) |
DOY, day of year; km, kilometers from mouth of estuary. Site abbreviations are given in Fig. 1.
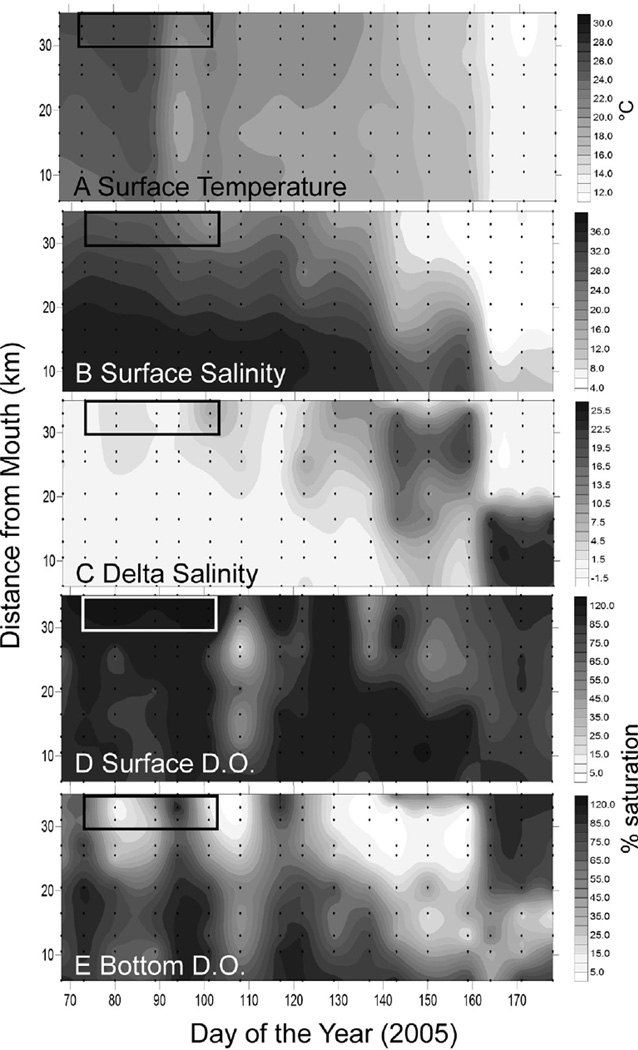
At the beginning of the bloom (DOY 76–104 and 30–42 km upstream), surface temperature was 25–26 °C, but dropped to 20– 21 °C between DOY 89 and 94 (March 30-April 4) (Fig. 5A). A drop in salinity from ~27 to ~21 between DOY 90 and 101 (March 31-April 11) was observed (Fig. 5B), associated with a brief stratification of the water column observed most strongly (delta salinity 6–9) on DOY 101 (April 11) (Fig. 5C). A pronounced drop in salinity occurred toward the end of the study period (after DOY 140, Fig. 5B), associated with elevated flow in the Avon River (Fig. 3). Surface D.O. (Fig. 5D) was supersaturated (9–11 mgL−1) initially, while bottom water D.O. (Fig. 5E) was low (<1–6 mg L−1) at the site of the bloom as well as at stations downstream (to 25 km upstream) of the main Karlodinium veneficum biomass accumulation. Low values of surface D.O. first appeared between DOY 101–108 (April 11 and April 18), centered at the 27 km station (MAY) but including most of the SRE from 35 km to the mouth (Fig. 5D).
Fig. 5.
Salinity, temperature and dissolved oxygen conditions in the upper SRE. The box represents the time/place of the bloom of K. veneficum. Sampling stations are represented by the points on the graphs.
3.3. Phytoplankton community structure leading up to the bloom of K. veneficum
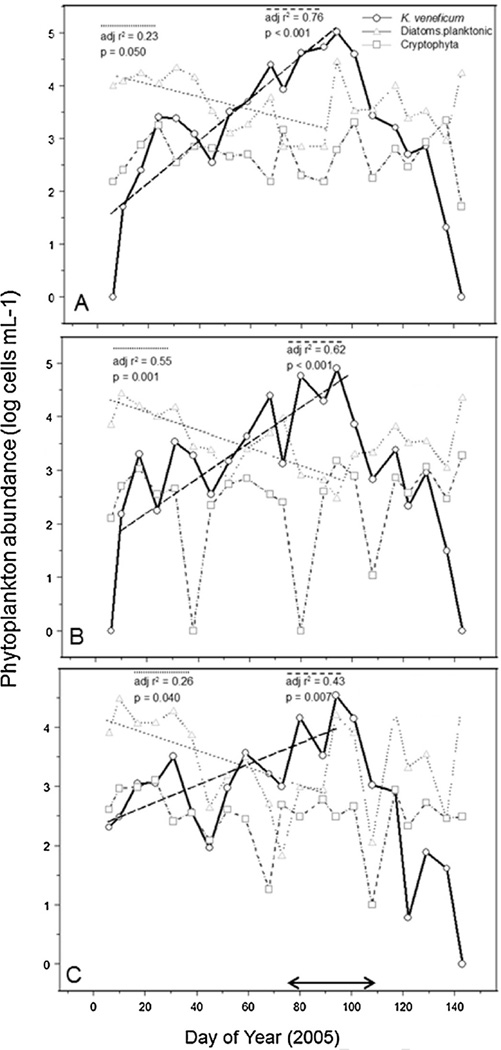
Shifts in phytoplankton community composition over DOY 1– 94 (Jan–March) were observed at three stations (SUC, KIN, and RON), showing a decline in planktonic diatoms coincident with increasing Karlodinium veneficum cell abundance (Fig. 6, DOY 1–94). The decline in diatoms involved a shift in dominant forms within that group, from chain-forming Thalassiosira/Skeletonema to small cf. Cyclotella by early March (WA DOE, data not shown). Cryptophytes, a potential food source of K. veneficum, were present but showed no trend with time or correlations with K. veneficum abundance (Fig. 6). No significant trends were observed for other phytoplankton groups (chloro-phytes, prasinophytes, euglenophytes, chrysophytes) over the time period leading up to the bloom of K. veneficum.
Fig. 6.
Time series plots of K. veneficum, planktonic diatoms, and cryptophytes for the period spanning the 2005 K. veneficum bloom at three SRE sites, (A) SUC, (B) KIN, (C) RON. The double arrow along the X-axis of (C) indicates the time period of the K. veneficum bloom and fish kill. Regression lines and statistics are shown for K. veneficum and planktonic diatoms, whereas trends for cryptophytes were not significant.
3.4. LC-MS characterization of KmTx from the Swan River Estuary
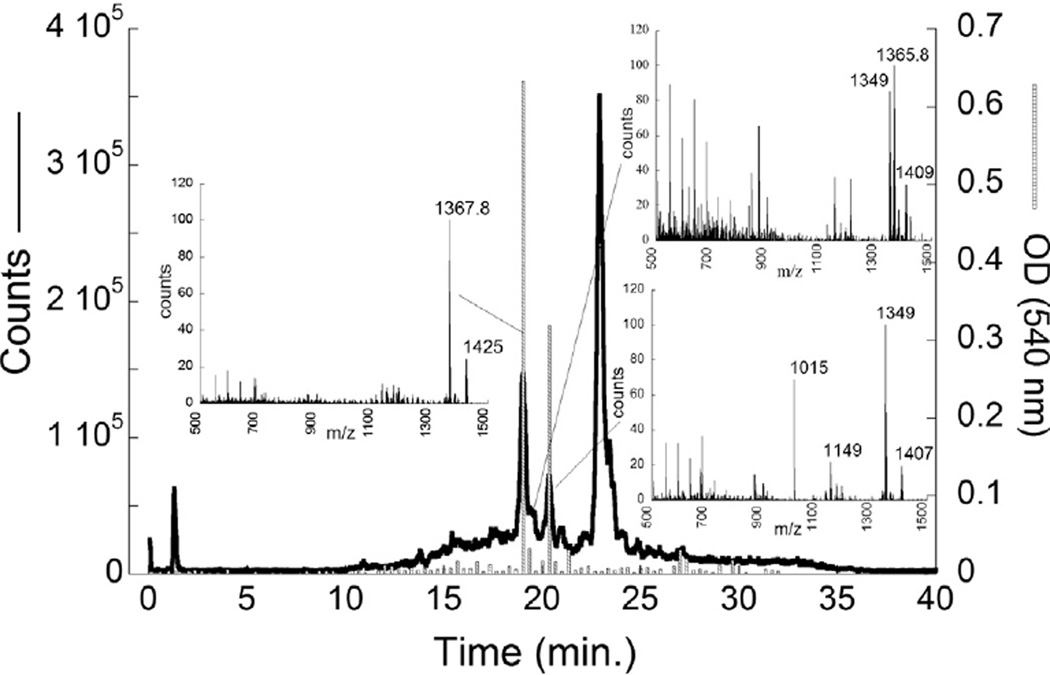
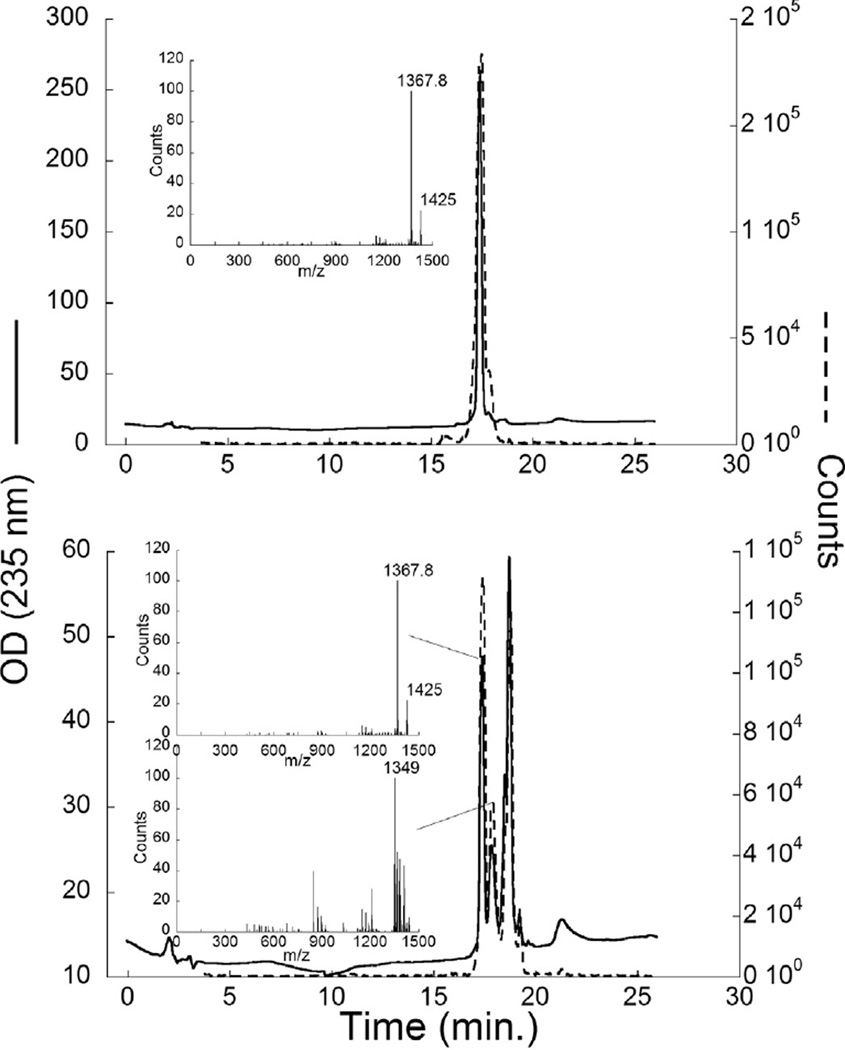
The total ion chromatograph (TIC) for partially purified material showed three major peaks, two associated with activity in the hemolytic assay (Fig. 7). The major ions associated with these peaks were in order of elution: 1367.8,1365.8 and 1349 amu(Fig. 7 insets). Further purification of these peaks resulted in two fractions, ‘Perth 1’ or KmTxPerth2-1 (Fig. 8A) dominated by the 1367.8 amu ion, and ‘Perth 2’ or KmTxPerth2-2 containing a mixture of the 1367.8, 1349 and 1365.8 ions (Fig. 8B). Both toxin peaks were classified as KmTx2 types due to higher absorbance at 235 nm on UV absorption spectra (data not shown).
Fig. 7.
Partially purified KmTx from the SRE, characterized by LC-MS. The thick line is the total ion chromatogram (left axis). The histograms are from the red blood cell hemolysis assay for each 20 s fraction of the LC run. Insets 1,2, and 3 show the major ions in each of the peaks with hemolytic activity. Inset 1 corresponds to the toxin KmTxPerth2-1, and inset 3 to the toxin KmTxPerth 2-2.
Fig. 8.
Further purified KmTx (A. ‘Perth 1′; B. ‘Perth 2′) characterization by LC–MS. Solid line is the OD at 235 nm, dashed line is the total ion chromatogram, insets show the major ions associated with peaks.
3.5. Biomass of Karlodinium veneficum and KmTx concentrations in the Swan River Estuary
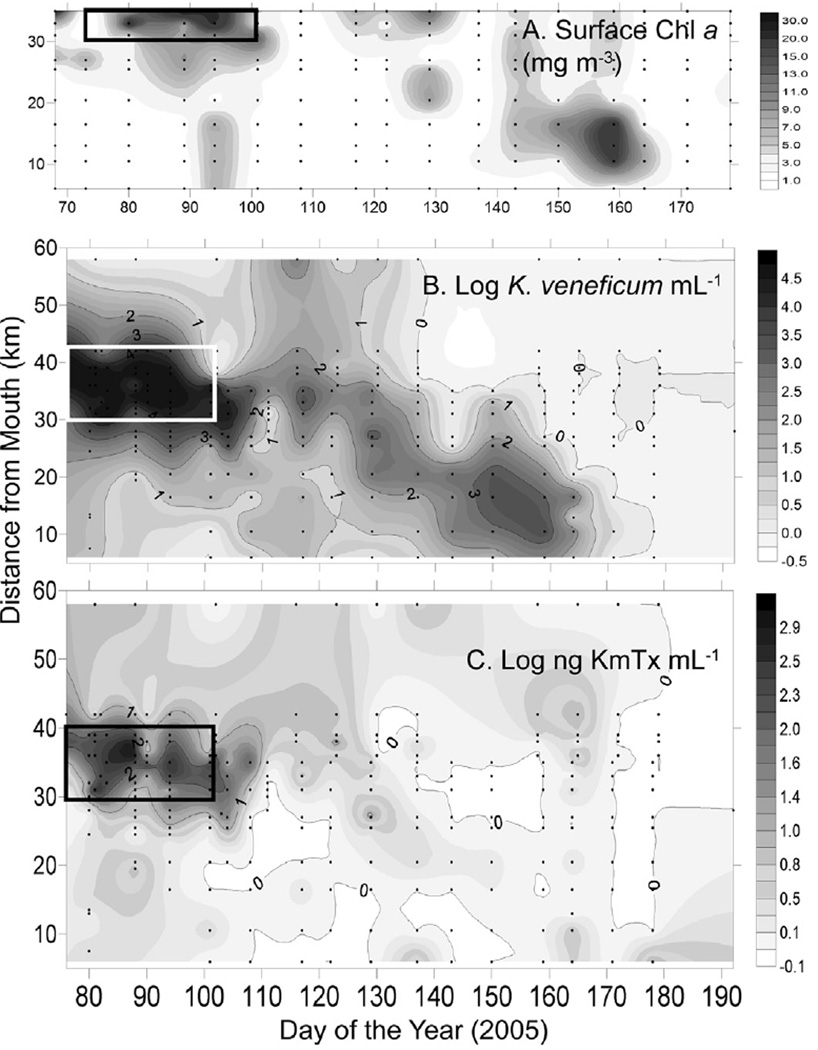
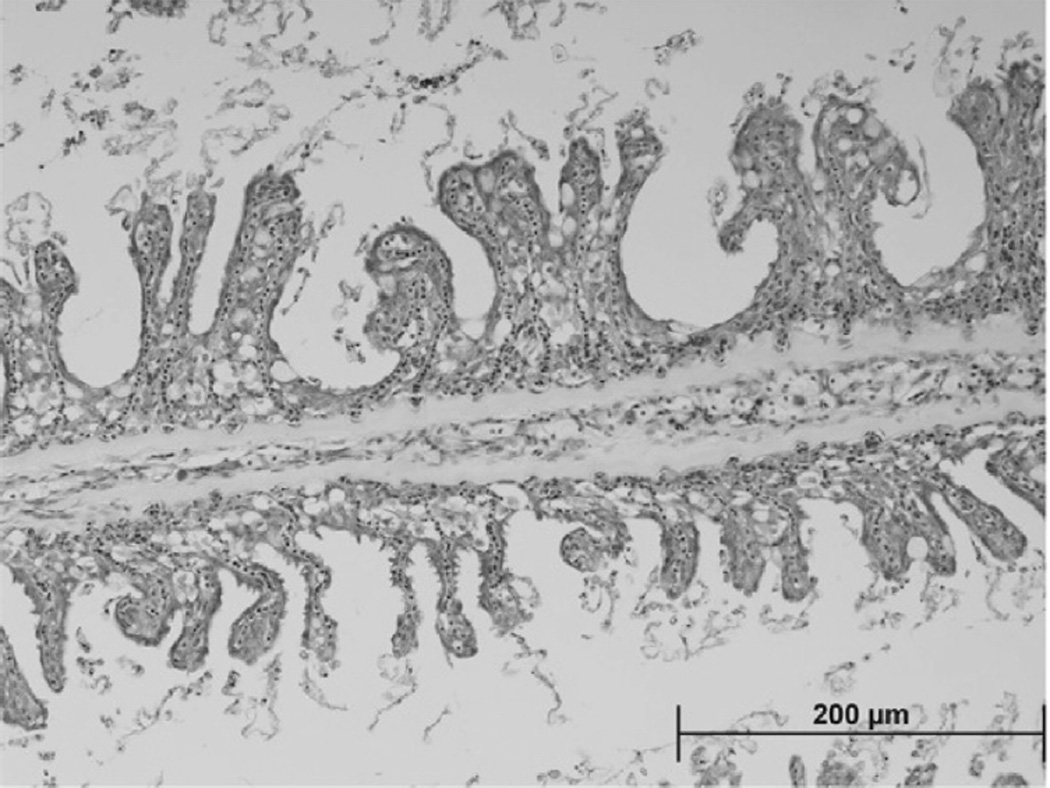
Elevated phytoplankton biomass as Chl a (Fig. 9A) and Karlodinium veneficum cell concentrations (Fig. 9B; ≥103 mL−1) were observed DOY 76–104 (March 17–April 14,2005) and between 30 and 42 km from the mouth of the SRE (Fig. 9B). Karlotoxin levels up to 1052 ng mL−1 were observed during this time period in the same region (Fig. 9C). Following the fish kill, low levels of K. veneficum (102–101 cells mL−1) and KmTx were detectable in the lower SRE with the center of the population moving approximately 20 km downstream as the time series progressed (Fig. 9B). Swan River Trust (SRT) crews observed a fish kill on the morning of April 13, 2005 (DOY 103) after reports of sluggish and dying fish from residents (SRT Media Statement, April 13, 2005). Examination of hematoxylin and eosin stained gill arches from a moribund black seabream (Acanthopagrus butcheri) collected at the Swan River fish kill on April 10, 2005 showed loss of intra-lamellar spacing and secondary lamellar fusion with extensive hyperplasia of gill epithelial tissue, similar to damage caused exposure to pure KmTx2 in laboratory studies (Deeds et al., 2006) (Fig. 10).
Fig. 9.
Surface (A) Chl a, (B) K veneficum cell numbers, and (C) KmTx concentrations in the SRE. The box represents the time/place of the K veneficum bloom. Sampling stations are represented by points.
Fig. 10.
H&E (hematoxylin and eosin) stained gill arches from a moribund black seabream (Acanthopagrus butcheri) collected at the Swan River fishkill on April 10, 2005. Note loss of intra-lamellar spacing and secondary lamellar fusion with extensive hyperplasia of gill epithelial tissue. This pathology is identical to that observed when pure KmTx 2 is exposed to fish the laboratory (Deeds et al., 2006).
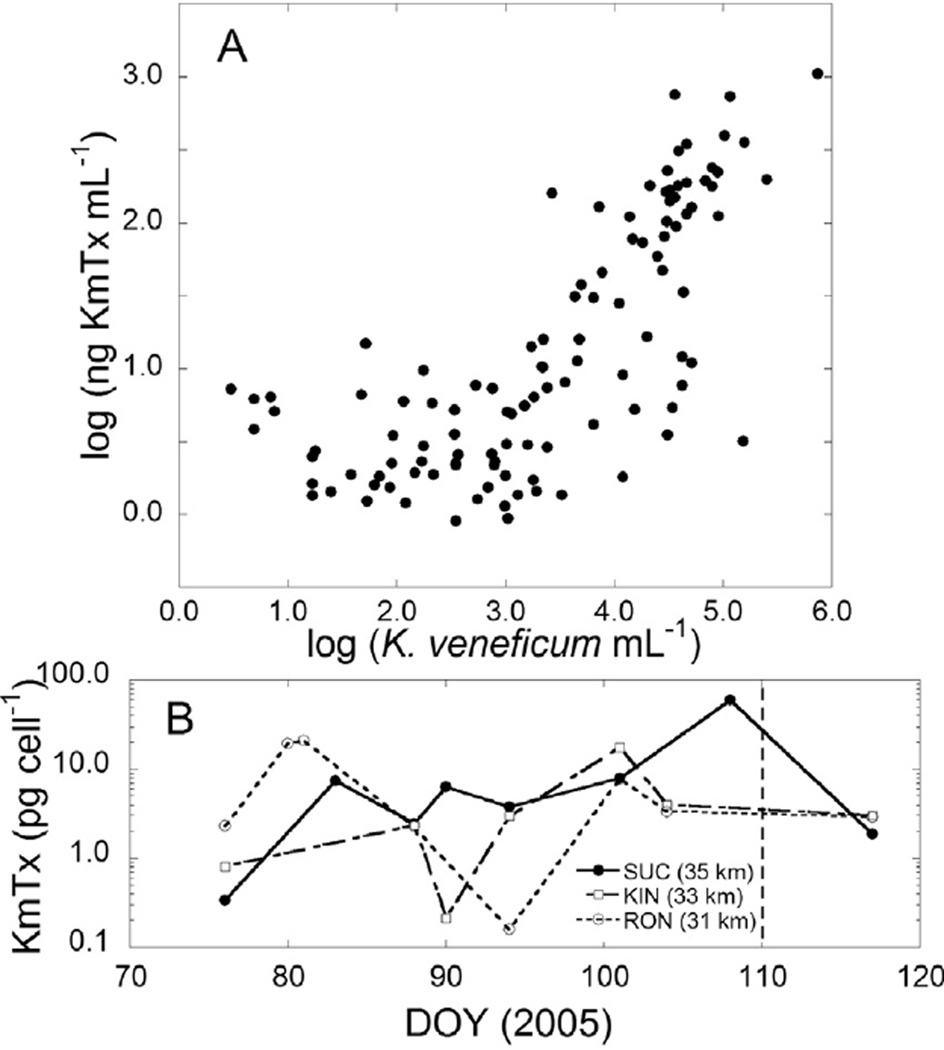
Log toxin concentration (ng KmTx 2 m−1) correlated with log Karlodinium veneficum concentration (cells mL−1) (Fig. 11A, Pearson r = 0.73, p < 0.0001). Considering only samples where log K. veneficum mL−1 was ≥103, cellular toxin quota (pg KmTx cell−1) averaged 4.5 ±7.50 (median 2.8) pg KmTx cel−1. Cellular KmTx (pg cel−1) showed a weak positive but significant regression with total KmTx (ng mL−1) (r2 = 0.14, p = 0.004). Time series plots of KmTx cel−1 (Fig. 11B) for stations with cell density >100 mL−1 show a high degree of variability, with a spike in cellular toxicity at SUC on DAY 108, when K. veneficum abundance was 2702 cells mL−1.
Fig. 11.
(A) Scatter plot of log KmTx mL−1 versus log K. veneficum mL −1. (B) Time series plot of KmTx (pg cell−1) for stations SUC, KIN and MEA.
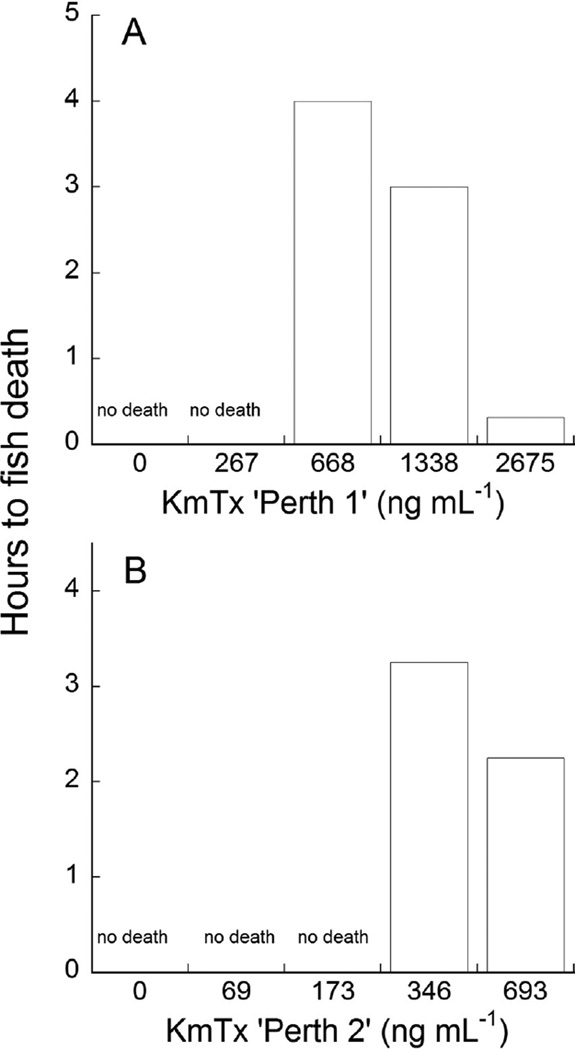
Both purified karlotoxins exhibited dose-dependent ichthyotoxicity against juvenile sheepshead minnows (Cyprinodon variegatus) (Fig. 12A, B). For KmTx ‘Perth 1’, the lethal dose was between 267 and 668 ng mL−1, while for KmTx ‘Perth 2’ the lethal dose was between 173 and 346 ng mL−1.
Fig. 12.
Ichthyotoxicity assay of purified KmTx from the Swan River Estuary, Perth, Australia against Sheepshead minnow (Cyprinodon variegatus). (A) KmTx ‘Perth 1′ toxin; (B) KmTx ‘Perth 2’ toxin.
4. Discussion
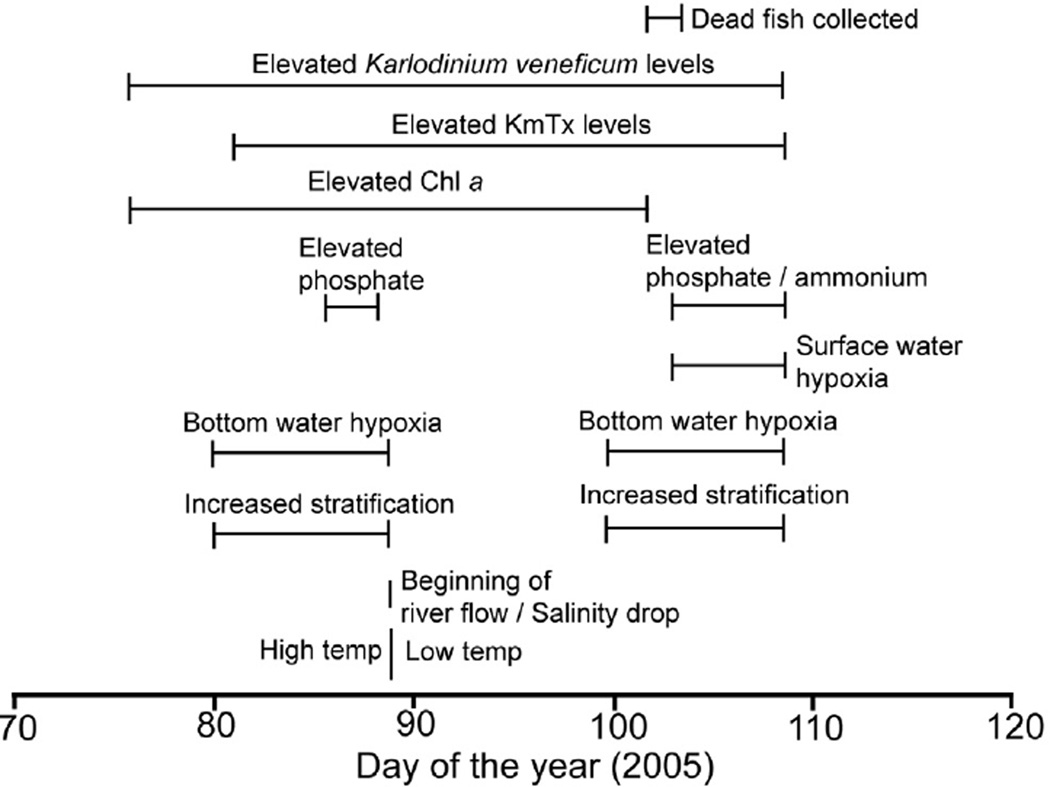
The occurrence of toxic algal blooms is a relatively recent phenomenon in the SRE (Thompson, 1998; Chan and Hamilton, 2001; Horner-Rosser and Thompson, 2001). The bloom described here is one of a series of recurrent ichthyotoxic bloom of Karlodinium veneficum observed in the SRE 2002–2006, 2010, and 2012. Gill histopathology (Fig. 10) observed on fish collected from the fish kill described here was consistent with damage from KmTx as described by Deeds et al. (2006). Water quality properties measured in association with this bloom, including bottom layer hypoxia, suggest nutrient over-enrichment, which is considered a key factor driving the proliferation of HAB in new systems (Glibert et al., 2005; Heisler et al., 2008). The results of this study allow us to better understand the ecological context of ichthyotoxic blooms of K. veneficum, including co-occurrence of karlotoxin-containing cells and hypoxia (Fig. 13), both of which likely contributed to the observed fish kill.
Fig. 13.
Composite time-line of events associated with the K. veneficum bloom and subsequent fish kill.
4.1. The role of freshwater inputs in blooms of K. veneficum in the SRE
Results of this study are consistent with observations that blooms of Karlodinium veneficum develop under low flow conditions in the upper SRE, and that elevated flow, typically beginning in May/June, dissipates upper SRE blooms of K. veneficum. Abloom of K. veneficum documented in the Neuse River Estuary (Hall et al., 2008) similarly occurred under low flow, high temperature/salinity conditions. Linkages between river flow and phytoplankton succession have been made previously in the SRE, including Twomey and John (2001) who showed that rainfall, river flow and the positioning of the salt wedge in the estuary dictates the typical seasonal succession of phytoplankton in the SRE. Toxic blooms Microcyctis were linked to anomalously high summer river flows (Robson and Hamilton, 2003). The displacement of the K. veneficum population down estuary 10–20 km (Fig. 9B), to the mouth of the Canning estuary, is another potentially important role of river flow in this system that deserves further attention. Whether or not K. veneficum is transported back up estuary in the lower layer upon relaxation of seasonal flows (i.e. Tyler and Seliger, 1978) should be investigated. Ecosystem managers can use rainfall and river flow data to help forecast bloom location along the estuary and imminent fish kills during years when a dense bloom of K. veneficum in present in the upper SRE. Further, understanding the linkages between ichthyotoxic blooms and river flow in the SRE will aid understanding of how changing patterns of rainfall predicted for Western Australia (Hughes, 2011, CSIRO State of the Climate Reports 2010, 2012 (http://www.csiro.au/resources/State-of-the-Climate)) might impact the timing and/or magnitude of blooms of K. veneficum in this system.
4.2. Phytoplankton community composition shift leading to the bloom of K. veneficum
Results of this study and continuing observations of annual blooms of Karlodinium veneficum (2010, 2012) suggest that while conditions in the SRE in late summer–autumn have historically been favorable for dinoflagellates, recently toxic K. veneficum has become a more prominent member of the dinoflagellate assemblage in the SRE. The bloom of K. veneficum and fish kill observed in March-April was preceded by a 3-month period over which planktonic diatoms were displaced by K. veneficum (Fig. 6) in the upper SRE. Physical/chemical conditions likely disfavored large diatoms over this time period, as freshwater (and thereby nitrate) inputs were low (Fig. 3), and the inorganic nutrient pools in surface waters suggested N-limitation (Thompson and Hosja, 1996; Twomey and John, 2001). The observed shift from chain-forming to small solitary diatoms (Cyclotella) over this time period points to low nutrients/turbulence in the surface layer. A similar shift over the period January-March, from diatoms dominated by S. costatum to dinoflagellates, was also reported by Thompson (1998) and Hamilton et al. (1999), but the dinoflagellates in these reports were numerically dominated by Scrippsiella sp. Or Gymnodinium and Prorocentrum with no mention of K. veneficum or ichthyotoxicity. Further research is required to determine what contributes to the differential success of K. veneficum over other flagellates during late summer-autumn in the upper SRE, including investigation of the anti-grazing/allelopathic activity of karlotoxins (Adolf et al., 2007, 2008; Stoecker et al., 2008; Brownlee et al., 2008; Wagget et al., 2008; Vaque et al., 2006) or mixotrophic nutrition (Li et al., 1996). Although mixotrophic grazing by K. veneficum was not quantified in this study, feeding by K. veneficum upon addition of cultured cryptophytes to SRE water collected at the bloom was observed (JEA, Pers. Obs.), indicating at least potential mixotrophic growth of SRE K. veneficum populations. Work by Griffin et al. (2001) suggests that anti-grazing properties of KmTx would be advantageous as dinoflagellate blooms in the upper SRE are strongly top-down regulated, and escape from grazing pressure is increasingly recognized as a strategy permitting some species to form blooms (Iriogen et al., 2005; Mitra and Flynn, 2006; Sunda et al., 2006; Smayda, 2008).
4.3. Physical and chemical conditions associated with the SRE bloom
By the time the bloom of Karlodinium veneficum was fully developed in the upper SRE, elevated Chl a and K. veneficum cell counts (DOY 76–90) occurred in warm surface waters that were weakly salinity-stratified (Fig. 5). Surface nutrient concentrations were low preceding and during the bloom, with molar DIN:DIP close to 1, typical for the SRE (Thompson and Hosja, 1996). The bloom was initially located over a patch of hypoxic bottom water that stretched from above the 35 km station to the 25 km station (Fig. 5E), environmental conditions that are not uncommon in this portion of the SRE (Stephens and Imberger, 1996; Thompson, 1998; Hamilton et al., 2001) associated with strong salinity stratification (Kurup and Hamilton, 2002) and elevated inorganic nutrient availability (Hamilton et al., 1999). Although not specifically addressed in this study, the ability of K. veneficum to undergo vertical migrations (sensu Hall and Paerl, 2011) may give this species an advantage over other forms in the stratified upper SRE environment, although this would not explain the differential success of K. veneficum over other flagellates capable of vertical migrations.
Lysis of Karlodinium veneficum cells is an important step leading to toxin release and ichthyotoxicity (Deeds et al., 2002; Mooney et al., 2010; Dorantes-Aranda et al., 2011), and the beginning of seasonal rainfall that occurred during the bloom period may have played an important role in cell disruption leading to the fish kill investigated here. After DOY 84, flow in the Avon River and Bennet Brook increased with an associated drop in surface salinity (5–10) and water temperatures of 4 °C, which could have contributed to osmotic lysis of the cells. Between DOY 90 and 100, bottom water oxygen levels increased compared to the prior period, but subsequently returned to hypoxic levels after DOY 101 until after DOY 108 (Fig. 5E). Low surface oxygen levels coincided with relaxation of salinity stratification and the crash of the bloom of K. veneficum on DOY 107 (Fig. 5E). Thus, in the narrow and shallow upper SRE, fish would have been exposed to both water column hypoxia and free KmTx simultaneously at the time of the observed fish kill event. While it is speculated here that changes in salinity and temperature led to K. veneficum disruption, positive identification of the factors leading to K. veneficum disruption in situ is an important area for future research to better understand the role of K. veneficum in fish kills.
4.4. Cellular toxicity of K. veneficum during the bloom period
The KmTx congener isolated from large-volume SRE samples (Fig. 8) was identical to KmTXPerth 2-1 described from a clonal culture of Karlodinium veneficum isolated from the SRE in 2005 (strain KVSR01) with major ions at 1367 and 1425 amu (Mooney et al., 2009). While the Australian SRE K. veneficum strains were considered conspecific with other isolates based on LSU sequences (Mooney 2009), the putative presence of compensatory changes in ITS2 structure that reported here (Fig. 2) and cellular toxicity (~0.1 pg KmTx cell−1) (J.E. Adolf, pers. obs.) of the SRE isolate (AUS #7) relative to CCMP 415 suggests cryptic species may be present (Gottschling and Plotner, 2004), although further investigation is needed. A number of Karlodinium species have recently been described from Australia (de Salas et al., 2005,2008), but hemolytic karlotoxins were only present in K. veneficum (Mooney et al., 2009). However, immobilization and ingestion of copepods by K. armiger suggests uncharacterized toxins may be present in other species of the Karlodinium genus (Berge et al., 2012).
The level of cellular toxicity calculated for Karlodinium veneficum in this bloom (mean 4.4, median 2.8 pg KmTx cell−1) is within the previously reported range of laboratory cultures (Deeds et al., 2004; Adolf et al., 2009; Bachvaroff et al., 2009) including an SRE strain of K. veneficum (KmTXSwan 2, Mooney et al., 2009). Cellular toxin in the SRE was similar to values reported for 2005–2007 blooms observed in the Inner Harbor of Baltimore, MD (Adolf et al., 2008). Cellular toxicity of K. veneficum varies among strains grown under nutrient-replete conditions (Bachvaroff et al., 2009), and also as a result of inorganic N or P limitation (Adolf et al., 2009, Mooney et al., 2010). The particulate C, N, and P measurements measured for high density K. veneficum water samples from the SRE {C:N (8.0) and C:P (97.3)} suggest N-limitation when compared to nutrient replete averages from six cultured K. veneficum strains, where C:N averaged 4.8 and C:P averaged 136 (Adolf etal., 2009). Li et al. (2002) reported molar C:N values of 6.4 and C:P values of 164.4 for single strain (CCMP 1974) grown under 27 variable N and P conditions. The DIN:DIP values near unity surrounding the bloom also suggest N-limitation. Thus, N-limitation in surface waters at the time of the bloom likely increased the cellular toxicity of K. veneficum 2- to 5-fold (Adolf et al., 2009) compared to non-nutrient limited values, contributing to the total concentrations of KmTx observed during this event.
Some of the observations of elevated cellular toxicity levels in this study might have resulted from low Karlodinium veneficum cell abundance and a loss of precision in cell counts at low cell abundance. The in situ toxin concentrations leveled off below 103 cells mL−1, leading to cellular toxicity estimates that increased over two orders of magnitude at cell abundance below 103 cells mL−1. This most likely represents loss of precision in the ability to accurately quantify low cell abundance relative to the high precision with which KmTx can be measured by LC-MS (~1.2 ng KmTx mL−1; Bachvaroff et al., 2008). Alternatively, although KmTx is labile and not likely to last more than one day after cell senescence (Deeds et al., 2002), some of the anomalously high values for cellular toxicity may represent senescent regions of a bloom where high free toxin concentrations remained but cell abundance was diminished. For instance, the spike in cellular toxicity observed on DOY 108 at SUC (Fig. 11B) coincided with relatively low cell counts (2702 cell mL−1).
4.5. Ichthyotoxicity associated with the 2005 SRE bloom of K. veneficum: KmTx and hypoxia
Determination of acute ichthyotoxicity levels of between 173 and 346 ng mL−1 for purified KmTx ‘Perth2-1’, and between 267 and 668 ng mL−1 for purified KmTx ‘Perth2-2’ (Fig. 12) is in agreement with the conservative environmental threshold for ichthyotoxicity of 500 ng mL−1 suggested by Deeds et al. (2006). KmTx levels observed in the SRE (where >1000 Karlodinium veneficum mL−1 were present) were low comparably, with a median KmTx concentration of 33.4 ng m−1 (Table 2) and average cell densities of 42,000 cells mL−1, and thus were unlikely to be acutely ichthyotoxic on their own. However, Deeds et al. (2006) observed sub-lethal gill damage in the laboratory at 50 ng ml−1 KmTx2 (Deeds et al., 2006), and that examination of fish recovered from this SRE fish kill determined gill damage consistent with KmTx (Fig. 10), which may have exacerbated effects of hypoxia on fish.
Table 2.
Cell-specific and water toxin statistics for records with cell abundance >103 cells mL−1 and detectable toxin levels.
| n | Mean | s.d. | Min. | Q1 | Median | Q3 | Max. | |
|---|---|---|---|---|---|---|---|---|
| pg KmTx cell −1 | 71 | 4.4 | 7.48 | 0.02 | 1 | 2.8 | 4.8 | 59.1 |
| ng KmTx mL−1 | 71 | 113.6 | 185.4 | 0.9 | 5.1 | 33.4 | 162.6 | 1052.5 |
n, sample size; s.d., standard deviation; Min., minimum; Q1, first quartile; Q3, third quartile; Max., maximum.
Results from the SRE show a sequence of events involving two known ichthyotoxic factors co-occurring in a eutrophic estuary-high levels of KmTx (Deeds et al., 2002, 2006) and water column hypoxia (Diaz and Rosenberg, 2008). Hypoxia ranks with HAB as a global symptom of ecosystem decline (Cloern, 2001; Kemp et al., 2005; Diaz and Rosenberg, 2008) and can co-occur spatially and temporally with HAB, including Karlodinium veneficum (Hall et al., 2008). Hypoxic or anoxic conditions are common in the upper SRE (Stephens and Imberger, 1996; Hamilton et al., 2001), associated with water column stratification (Kurup and Hamilton, 2002). Further, night time oxygen levels were likely lower than the daytime values that were measured, potentially increasing their role in this fish kill. Karlotoxin affects fish by damaging cells along the exposed region of the gills (Deeds et al., 2006), resulting in death by suffocation in acute cases, but impaired oxygen uptake in sub-acute exposures that can potentially exacerbate sensitivity of fishes to hypoxic conditions. Thus, observations of toxin levels and DO in the SRE suggest several important lines of research including an examination of the effects of hypoxia on K. veneficum cell lysis (necessary for ichthyotoxicity, Deeds et al., 2002), the dose dependency of KmTx on fish exposed to hypoxia (sensu Deeds et al., 2006), and the effects of hypoxia on the lability of free KmTx that has been released from cells.
Acknowledgements
The authors thank all the staff and crew of the WA DOE and SRT, particularly Ashrafi Begum, Wasele (Vas) Hosja, Tarren Reitsema, Petra Ringeltaube, Malcolm Robb, and Laura Cunningham for speedy delivery of phytoplankton count data. We thank Nancy Feissner who worked at University of Maryland COMB as a summer technician and processed the >250 toxin samples received from Australia in that time period. Parts of this work have been supported by NOAA ECOHAB, CDC, and a NOAA CSCOR travel award. This is contribution #xxx from the University of Maryland Center for Environmental Sciences and contribution # xxx from the ECOHAB program.[SS]
Footnotes
Uncited references
Contributor Information
Jason E. Adolf, Email: jadolf@hawaii.edu.
Tsvetan R. Bachvaroff, Email: bachvarofft@gmail.com.
Jonathan R. Deeds, Email: jonathan.deeds@fda.hhs.gov.
Allen R Place, Email: place@umces.edu.
References
- Abbot BC, Ballantine D. The toxin from Gymnodinium veneficum Ballantine. J. Mar. Biol. Assoc. U.K. 1957;36:169–189. [Google Scholar]
- Adolf JE, Stoecker DK, Harding LW., Jr The balance of autotrophy and heterotrophy during mixotrophic growth of Karlodinium micrum. J. Plank. Res. 2006a;28:737–751. [Google Scholar]
- Adolf JE, Bachvaroff TR, Krupatkina DN, Nonogaki H, Brown PJP, Lewitus AJ, Harvey HR, Place AR. Species specificity and potential roles of Karlodinium micrum toxin. Afr. J. Mar. Sci. Harmful Algae. 2006b;28:415–421. [Google Scholar]
- Adolf JE, Bachvaroff TR, Krupatkina DN, Place AR. Karlotoxin mediates grazing by Oxyrrhis marina on strains of Karlodinium veneficum . Harmful Algae. 2007;6:400–412. [Google Scholar]
- Adolf JE, Bachvaroff TR, Place AR. Can cryptophyte abundance trigger toxic Karlodinium veneficum blooms in eutrophic estuaries? Harmful Algae. 2008;8:119–128. [Google Scholar]
- Adolf JE, Bachvaroff TR, Place AR. Environmental modulation of Karlotoxin levels in strains of the cosmopolitan dinoflagellate, Karlodinium veneficum (Dinophyceae) J. Phycol. 2009;45:176–192. doi: 10.1111/j.1529-8817.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- Bachvaroff TR, Adolf JE, Place AR. Strain variation in Karlodinium veneficum (Dinophyceae): toxin profiles, pigments, and growth characteristics. J. Phycol. 2009;45:137–153. doi: 10.1111/j.1529-8817.2008.00629.x. [DOI] [PubMed] [Google Scholar]
- Bachvaroff TR, Adolf JE, Squier A, Harvey HR, Place AR. Characterization and quantification of karlotoxins by liquid chromatography-mass spectrometry. Harmful Algae. 2008;7:473–484. [Google Scholar]
- Ballantine D. Two new marine species of Gymnodinium isolated from the Plymouth area. J. Mar. Biol. Assoc. U.K. 1956;35:473–484. [Google Scholar]
- Berge T, Poulsen LK, Moldrup M, Daugberg N, Hansen PJ. Marine microalgae attack and feed on metazoans. ISME J. 2012;2012:1–11. doi: 10.1038/ismej.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergholtz T, Daugbjerg N, Moestrup Ø, Fernandez-Tejedor M. On the identity of Karlodinium veneficum and description of Karlodinium armiger Sp. Nov. (Dinophyceae), based on light and electron microscopy, nuclear-encoded LSU rDNA, and pigment composition. J. Phycol. 2005;42:170–193. [Google Scholar]
- Brownlee EF, Sellner SG, Sellner KG, Nonogaki H, Adolf JE, Bachvaroff TR, Place AR. Responses of Crassostrea virginica (Gmelin) and C. ariakensis (Fujita) to bloom-forming phytoplankton including ichthyotoxic Karlodinium veneficum (Ballantine) J. Shellfish Res. 2008;27:581–591. [Google Scholar]
- Calbet A, Bertos M, Fuentes-Grunewald C, Alacid E, Figueroa R, Renom B, Garcia E. Intraspecific variability in Karlodinium veneficum: growth rates, mixotrophy and lipid composition. Harmful Algae. 2011;10:654–667. [Google Scholar]
- Chan TU, Hamilton DP. Effect of freshwater flow on the succession and biomass of phytoplankton in a seasonal estuary. Mar. Freshwater Res. 2001;52:869–884. [Google Scholar]
- Chan TU, Hamilton DP, Robson BJ, Hodges BR, Dallimore C. Impacts of hydrological changes on phytoplankton succession in the Swan River, Western Australia. Estuaries. 2002;25:1406–1415. [Google Scholar]
- Cloern JE. Our evolving conceptual model of the cultural eutrophication problem. Mar. Ecol. Progr. Ser. 2001;210:223–253. [Google Scholar]
- Deeds JR, Terlizzi DE, Adolf JE, Stoecker DK, PLace AR. Toxic activity from cultures of Karlodinium micrum (=Gyrodinium galatheanum) (Dinophyceae) - a dinoflagellate associated with fish mortalities in an estuarine aquaculture facility. Harmful Algae. 2002;1:169–189. [Google Scholar]
- Deeds JR, Kibler SR, Tester PA, Place AR. Geographic strain variation in toxin production in Karlodinium micrum (Dinophyceae) from Southeastern United States. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Harmful Algae 2002 Proceedings. St. Petersberg, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO. 2004. pp. 145–147. [Google Scholar]
- Deeds JR, Reimschussel R, Place AR. Histopathological effects in fish exposed to the toxins from Karlodinium micrum (Dinophyceae) J. Aquat. Animal Health. 2006;18:136–148. [Google Scholar]
- Deeds JR, Place AR. Sterol specific membrane interactions with the toxins from Karlodinium micrum (Dinophyceae) - a strategy for self protection? Afr. J. Mar. Sci. Harmful Algae 2004. 2006;28:421–427. [Google Scholar]
- Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321:926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- Dorantes-Aranda JJ, Waite TD, Godrant A, Rose AL, Tovar CD, Woods G, Hallegraeff GM. Novel application of a fish gill cell line assay to assess ichthyotoxicity of harmful marine microalgae. Harmful Algae. 2011;10:366–373. [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987;19:11–15. [Google Scholar]
- Eschbach E, Scharsack J, John U, Medlin L. Improved erythrocyte lysis assay in microtitre plates for sensitive detection and efficient measurement of haemolytic compounds from ichthyotoxic algae. J. Appl. Toxicol. 2001;21:513–539. doi: 10.1002/jat.797. [DOI] [PubMed] [Google Scholar]
- Fensin EE. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Occurrence and ecology of the dinoflagellate Karlodinium micrum in estuaries of North Carolina, USA; Harmful Algae 2002 Proceedings. St. Petersberg, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO; 2004. pp. 62–64. [Google Scholar]
- Garces E, Fernandez M, Penna A, Van Lenning K, Gutierrez A, Camp J, Zapata M. Characterization of NW Mediterranean Karlodinium spp. (Dinophyceae) strains using morphological, molecular, chemical, and physiological methodologies. J. Phycol. 2006;42:1096–1112. [Google Scholar]
- Glibert PM, Seitzinger S, Heil CA, Burkholder JM, Parrow MW, Codispoti LA, Kelly V. The role of eutrophication in the global proliferation of harmful algal blooms. Oceanography. 2005;18:198–209. [Google Scholar]
- Goshorn D, Deeds J, Tango P, Poukish C, Place AR, McGinty M, Butler W, Luckett C, Magnien R. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Occurrence of Karlodinium micrum and its association with fish kills in Maryland estuaries; Harmful Algae 2002 Proceedings. St. Peters-berg, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO; 2004. pp. 361–363. [Google Scholar]
- Gottschling M, Plotner J. Secondary structure models of the nuclear internal transcribed spacer regions and 5.8S rRNA in Calciodinelloideae (Peridiniaceae) and other dinoflagellates. Nucleic Acids Res. 2004;32:307–315. doi: 10.1093/nar/gkh168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin SL, Herzfeld M, Hamilton DP. Modeling the impact of zooplankton grazing on phytoplankton biomass during a dinoflagellate bloom in the Swan River Estuary, Western Australia. Ecol. Eng. 2001;16:373–394. [Google Scholar]
- Hall NS, Litaker RW, Fensin E, Adolf JE, Bowers HA, Place AR, Paerl HW. Environmental factors contributing to the development and demise of a toxic dinoflagellate (Karlodinium veneficum) bloom in a shallow, eutrophic, lagoonal estuary. Estuaries Coasts. 2008;31:402–418. [Google Scholar]
- Hall NS, Paerl HW. Vertical migration patterns of phytoflagellates in relation to light and nutrient availability in a shallow microtidal estuary. Mar. Ecol. Progr. Ser. 2011;425:1–9. [Google Scholar]
- Hamilton D. Record summer rainfall induced the first recorded major cyanobacterial bloom in the Swan River. J. Environ. Eng. Soc. Inst. Eng. 2000;1:25. [Google Scholar]
- Hamilton DP, Thompson PA, Kurup R. Dynamics of dinoflagellate blooms in the Swan River Estuary. Proceedings of the Vth International Wetlands. 1999 [Google Scholar]
- Hamilton DP, Chan T, Robb MS, Pattiaratchi CB, Herzfeld M. The hydrology of the upper Swan River Estuary with focus on an artificial destra-tification trial. Hydrol. Process. 2001;15:2465–2480. [Google Scholar]
- Heisler J, Glibert PM, Burkholder JM, Anderson DM, Cochlan W, Dennison WC, Dortch Q, Gobler CJ, Heil CA, Humphries E, Lewitus A, Magnien R, Marshall HG, Sellner K, Stockwell DA, Stoecker DK, Suddleson M. Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae. 2008;8:3–13. doi: 10.1016/j.hal.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner-Rosser SMJ, Thompson PA. Phytoplankton of the Swan-Canning Estuary: a comparison of nitrogen uptake by different bloom assemblages. Hydrol. Process. 2001;15:2579–2594. [Google Scholar]
- Hughes L. Climate change and Australia: key vulnerable regions. Reg. Environ. Change. 2011;11(Suppl 1):S189–S195. [Google Scholar]
- Iriogen X, Flynn KJ, Harris RP. Phytoplankton blooms: a ‘loophole’ in microzooplankton grazing impact? J. Plank. Res. 2005;27:313–321. [Google Scholar]
- Kemp WM, Boynton WR, Adolf JE, Boesch DF, Boicourt WC, Brush G, Cornwell JC, Fisher TR, Glibert PM, Hagy JD, Harding LW, Houde ED, Kimmel DG, Miller WD, Newell RIE, Roman MR, Smith EM, Stevenson JC. Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Mar. Ecol. Progr. Ser. 2005;303:1–29. [Google Scholar]
- Kempton JW, Lewitus AJ, Deeds JR, Law JM, Place AR. Toxicity of Karlodinium micrum (Dinophyceae) associated with a fish kill in a South Carolina brackish retention pond. Harmful Algae. 2002;1:233–241. [Google Scholar]
- Kurup RG, Hamilton DP. Flushing of dense, hypoxic water from a cavity of the Swan River Estuary, Western Australia. Estuaries. 2002;25:908–915. [Google Scholar]
- Li A, Stoecker DK, Coats DW, Adam EJ. Ingestion of fluorescently labelled and phycoerythrin-containing prey by mixotrophic dinoflagellates. Aquat. Microb. Ecol. 1996;10:139–147. [Google Scholar]
- Li A, Stoecker DK, Coats WD. Use of the ‘food vacuole content’ method to estimate grazing by the mixotrophic dinoflagellate Gyrodinium galtheanum on cryptophytes. J. Plank. Res. 2002;23:303–318. [Google Scholar]
- Litaker RW, Vandersea MW, Kibler SR, Reece KS, Stokes NA, Steidinger KA, Millie DF, Bendis BJ, Pigg RJ, Tester PA. Identification of Pfiesteria piscicida (Dinophyceae) and Pfiesteria-like organsims using internal transcribed spacer-specific PCR assays. J. Phycol. 2003;39:754–761. [Google Scholar]
- Mitra A, Flynn KJ. Promotion of harmful algal blooms by zooplankton predator activity. Biol. Lett. 2006;2:194–197. doi: 10.1098/rsbl.2006.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney BD, de Salas M, Hallegraeff GM, Place AR. Survey of karlotoxin production in 15 species of gymnodinoid dinfoflagellates (Kareniaceae, Dinophyta) J. Phycol. 2009;45:164–175. doi: 10.1111/j.1529-8817.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- Mooney BD, Hallegraeff GM, Place AR. Ichthyotoxicity of four species of gymnodinoid dinoflagellates (Kareniaceae, Dinophyta) and purified karlotoxins to larval sheepshead minnow. Harmful Algae. 2010;9:557–562. [Google Scholar]
- Peng J, Place AR, Yoshida W, Anklin C, Hamann MT. Structure and absolute configuration of Karlotoxin, an ichthyotoxin with global distribution from the marine dinoflagellate Karlodinium veneficum . J. Am. Chem. Soc. 2010;132:3277–3279. doi: 10.1021/ja9091853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place AR, Bowers HA, Bachvaroff TR, Adolf JE, Deeds JE, Sheng J. Karlodinium veneficum - the little dinoflagellate with a big bite. Harmful Algae. 2012;14:179–195. [Google Scholar]
- Robson BJ, Hamilton DP. Summer flow event induces a cyanobacterial bloom in a seasonal Western Australian estuary. Mar. Freshwater Res. 2003;54:139–151. [Google Scholar]
- de Salas MF, Bolch CJS, Hallegraeff GM. Karlodinium australe sp. nov. (Gymnodiniales, Dinophyceae), a new potentially ichthyotoxic unarmoured dinoflagellate from lagoonal habitats of south-eastern Australia. Phycologia. 2005;44:640–650. [Google Scholar]
- de Salas MF, Laza-Martinez A, Hallegraeff GM. Novel unarmored dinoflagellates from the toxigenic family Kareniaceae (Gymnodiniales): five new species of Karlodinium and one new Takayama from the Australian sector of the Southern Ocean. J. Phycol. 2008;44:241–257. doi: 10.1111/j.1529-8817.2007.00458.x. [DOI] [PubMed] [Google Scholar]
- Sheng J, Malkiel E, Katz J, Adolf JE, Place AR. A dinoflagellate exploits toxins to immobilize prey prior to ingestion. Proc. Natl. Acad. Sci. U.S. A. 2010;107:2082–2087. doi: 10.1073/pnas.0912254107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smayda TJ. Complexity in the eutrophication-harmful algal bloom relationship, with comment on the importance of grazing. Harmful Algae. 2008;8:140–151. [Google Scholar]
- Stephens R, Imberger J. Dynamics of the Swan River Estuary: the seasonal variability. Mar. Freshwater Res. 1996;47:517–529. [Google Scholar]
- Stoecker DK, Adolf JE, Place AR, Glibert PM, Merrit DW. Effects of the dinoflagellates Karlodinium veneficum and Prorocentrum minimum on early life history stages of the eastern oyster (Crassostrea virginica) Mar. Biol. 2008;154:81–91. [Google Scholar]
- Sunda WG, Graneli E, Gobler CJ. Positive feedback and the development and persistence of ecosystem disruptive algal blooms. J. Phycol. 2006;42:963–974. [Google Scholar]
- Thompson PA, Hosja W. Nutrient limitation of phytoplankton in the upper Swan River Estuary, Western Australia. Mar. Freshwater Res. 1996;47:659–667. [Google Scholar]
- Thompson PA. Spatial and temporal patterns of factors influencing phytoplankton in a salt wedge estuary, the Swan River, Western Australia. Estuaries. 1998;21:801–817. [Google Scholar]
- Twomey L, John J. Effects of rainfall and salt-wedge movement on phytoplankton succession in the Swan-Canning Estuary, Western Australia. Hydrol. Process. 2001;15:2655–2669. [Google Scholar]
- Tyler MA, Seliger HH. Annual subsurface transport of a red tide dinoflagellate to its bloom area: water circulation patterns and organism distributions in Chesapeake Bay. Limnol. Oceanogr. 1978;23:227–246. [Google Scholar]
- Van Wagoner RM, Deeds JR, Satake M, Ribeiro AA, Place AR, Wright JLC. Isolation and characterization of karlotoxin 1, a new amphipathic toxin from Karlodinium veneficum . Tetrahedron Lett. 2008;49:6457–6461. doi: 10.1016/j.tetlet.2008.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wagoner RM, Deeds JR, Tatters AO, Place AR, Tomas CR, Wright JLC. Structure and relative potency of several karlotoxins from Karlodinium veneficum . J. Nat. Prod. 2010;73:1360–1365. doi: 10.1021/np100158r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagget RJ, Tester PA, Place AR. Anti-grazing properties of the toxic dinoflagellate Karlodinium veneficum during predator-prey interactions with the copepod Acartia tonsa. Mar. Ecol. Progr. Ser. 2008;366:31–42. [Google Scholar]
- Vaque D, Felipe J, Sala MM, Calbet A, Estrada M, Alcaraz M. Effects of the toxic dinoflagelate Karlodinium sp. (cultured at different N/P) on micro and mesozooplankton. Sci. Mar. 2006;70:59–65. [Google Scholar]