Abstract
Venetoclax (VEN, ABT-199/GDC-0199) is an orally bioavailable BH3-mimetic that specifically inhibits the anti-apoptotic B-cell lymphoma/leukemia 2 (BCL2) protein. Although BCL2 overexpression is not genetically driven in chronic lymphocytic leukemia (CLL), it is nearly universal and represents a highly important and prevalent mechanism of apoptosis evasion, making it an attractive therapeutic target. This review summarizes the role of BCL2 in CLL pathogenesis, the development path targeting its inhibition prior to VEN, and the preclinical and clinical data regarding the effectiveness and safety of VEN. We further strive to contextualize VEN in the current CLL treatment landscape and discuss potential mechanisms of resistance.
Keywords: ABT-199, B-cell chronic lymphocytic leukemia, venetoclax
Introduction
Chronic lymphocytic leukemia (CLL) is the most common type of leukemia in western countries, with an incidence of 4.1/100,000 inhabitants in the United States (US). Each year, about 15,000 patients are diagnosed with CLL, and about 4,500 die. The median age at diagnosis is 71, with nearly 70% of CLL patients over the age of 65, and 54% between the ages of 65–84 [NCI, 2016]. As incidence rates rise with age, the prevalence and mortality of CLL are likely to increase further in the coming decade, due to demographic changes in society, specifically the aging of the population [Hallek, 2015].
CLL is an extremely heterogeneous disease that can range from asymptomatic for decades to rapidly progressive. It is characterized by the clonal proliferation and accumulation of CD5/CD23-positive B-cells within the blood, bone marrow, lymph nodes and spleen. Leukemic transformation is likely initiated by specific genomic alterations that are most often clonal in CLL cells, such as 13q deletion, which results in the deletion of specific micro-RNA genes that increase the resistance of B-cells toward apoptosis [Lovat et al. 2015]. The progression of the disease is dependent, however, on an intricate relationship of anti-apoptotic and proliferative pathways. Cytogenetic aberrations (i.e. del(13q), del(17p), del(11)q, and trisomy 12) and recurrent somatic gene mutations (including NOTCH1, TP53, ATM, MYD88, SF3B1 and others) shed light on the pathogenesis of CLL, and have prognostic importance, that may change treatment selection. Specifically, del(17p) or TP53 mutation confers poor prognosis, with rapidly progressive disease, lower response rates to conventional chemoimmunotherapy and shorter progression-free and overall survival (OS) [Hallek, 2015].
The remarkable progress in the understanding of CLL biology has brought forth the development of molecularly-targeted drugs directed at specific cellular signaling pathways, thus transforming the therapeutic approach to CLL both in the relapsed and refractory (R/R) and first-line settings [Morabito et al. 2015]. Over the past decade, the combination of fludarabine, cyclophosphamide and rituximab (FCR) is considered the most effective first-line regimen in young physically-fit patients [Hallek et al. 2010], and may induce very long progression-free survival (PFS), especially in cases with mutated immunoglobulin heavy chain variable (IGHV) region genes [Fischer et al. 2016; Thompson et al. 2016]. The CLL10 trial demonstrates improved complete remission (CR) rate and minimal residual disease (MRD) negativity, and longer PFS in CLL patients randomized to FCR compared with bendamustine-rituximab (BR). There was no benefit, however, in subgroup analysis of older and less fit patients, making BR an adequate alternative in this group [Eichhorst et al. 2014]. Moreover, both regimens show inferior response and PFS rates in high risk CLL patients especially those with del(17p). Elderly unfit patients cannot tolerate FCR and have usually been treated with an alkylating agent, alone or combined with rituximab, but recent data from the CLL11 trial suggest a significant PFS increase with switching of the rituximab to obinutuzumab, a humanized type II anti-CD20 antibody, together with chlorambucil [Goede et al. 2014b]. Until recently, patients with relapsed disease have had a generally unfavorable outcome, with standard therapies achieving less disease control as compared with the treatment-naïve setting, and median PFS typically 15–18 months [Fischer et al. 2011]. Stem cell transplantation, still the only known curative therapy, produces durable remissions in about 50% of patients, but morbidity and mortality remain a problem in this generally older population.
The introduction of a number of novel agents, namely, the Bruton’s tyrosine kinase (BTK)-inhibitor, ibrutinib, and the phosphatidylinositol-3-kinase delta isoform (PI3Kδ)-inhibitor, idelalisib, has the potential to continue to improve therapy for R/R and high risk CLL patients [Woyach and Johnson, 2015]. Nonetheless, patients still progress after treatment with these novel agents or may not tolerate them. Thus, it is imperative to continue the search for other drugs for the treatment of CLL, and find out how to best implement them in the course of therapy. Since almost all CLL cells evade apoptosis by overexpression of B-cell lymphoma/leukemia 2 (BCL2) protein, inhibition of this pathway is an attractive therapeutic target. Venetoclax (VEN, ABT-199/GDC-0199), a small molecule that was engineered to specifically inhibit BCL2, is the subject of this review.
BCL2 over-expression in the pathogenesis of CLL
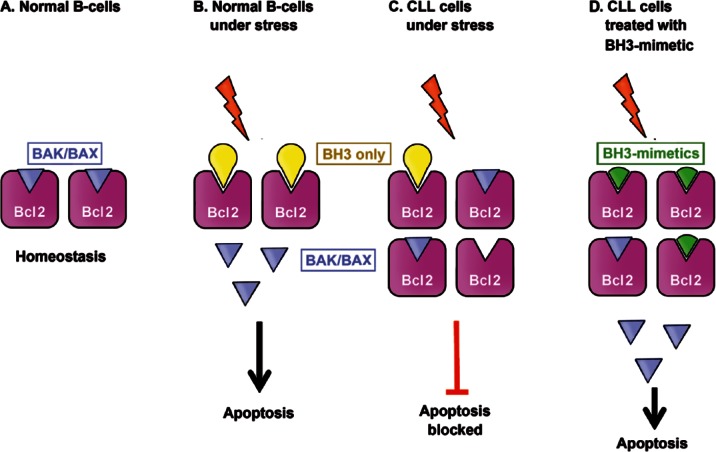
CLL cells are well known to be resistant to apoptosis, which is mainly mediated through overexpression of the proto-oncogene BCL2, although other mechanisms have been described [Huang et al. 2015]. The BCL2 family of proteins regulates the intrinsic or mitochondrial pathway of apoptosis, which is often perturbed in lymphoid malignancies. This family is considered to contain three subfamilies: prosurvival proteins (BCL2, BCL-XL, BCLw, MCL1, and BFL/A1) that share sequence motifs, termed BH domains; initiator BH3-only proteins (BIM, BAD, PUMA, NOXA); and cell death mediators (BAX and BAK). In healthy lymphoid tissues, under normal conditions, the prosurvival members (i.e. BCL2) constrain the essential cell death mediators, BAX and BAK. Under stress signals (including chemotherapy and irradiation), the BH3-only members (i.e. BIM) are activated, and thereby bind to and inactivate the prosurvival members. This allows the activation of BAX and BAK, which in turn, cause mitochondrial outer membrane permeabilization (MOMP), triggering the release of cytochrome c that activates the caspase cascade, and ending with apoptosis. Higher levels of BCL2 overcome the activation of the BH3-only proteins, and the release of cell death mediators is decreased, thus evading apoptosis [Anderson et al. 2014; Scarfò and Ghia, 2013] (Figure 1).
Figure 1.
B-cell lymphoma/leukemia 2 (BCL2) over-expression and its role in chronic lymphocytic leukemia (CLL). (a) In normal B-cells, the prosurvival members (i.e. BCL2) constrain the essential cell death mediators (BAX and BAK). (b) When exposed to stress signals, the BH3-only members (i.e. BIM) are activated, and thereby bind to and inactivate the prosurvival members. This allows the activation of BAX and BAK, which in turn, induces apoptosis. (c) In CLL cells, BCL2 over-expression prevents apoptosis when cell is stressed, by inhibiting cell death mediators. (d) BH3-mimetic agents bind and inhibit excess BCL2 thereby re-sensitizing cells to apoptotic stimuli.
As opposed to follicular lymphoma, CLL cells do not usually harbor the translocation t(14;18) in which the BCL2 gene at 18q21 is placed into juxtaposition with the immunoglobulin heavy-chain locus at 14q32. However, BCL2 expression is elevated in approximately 95% of patients with CLL and to a level similar to t(14;18)-bearing cells in 70%. Higher levels are seen in CLL cells derived from lymph nodes, compared with bone marrow or peripheral blood [Hanada et al. 1993; Papakonstantinou et al. 2001]. Over-expression in CLL appears to be explained by epigenetic mechanisms, including hypomethylation of the BCL2 gene; super-enhancer activity as demonstrated by H3K27 acetylation chromatin analysis [Ott et al. 2015]; or post-transcriptional regulation by the absence of microRNA miR-15a and miR-16-1, located on the long arm of chromosome 13, a region characteristically deleted in more than 50% of CLL patients [Hernández-Sánchez et al. 2016]. Elevated MCL1 protein expression may be another mechanism of apoptosis resistance, as it occurs in nearly half of CLL B-cells and was associated with fludarabine resistance [Pepper et al. 2008; Awan et al. 2009]. Other anti-apoptotic BCL2 family members also have a role in the pathogenesis of lymphoid malignancies, and the induction of both BCL-XL and A1 in CLL was associated with chemoresistance in preclinical models [Klein et al. 2000; Olsson et al. 2007].
An intriguing interactive mechanism between BCL2 over-expression and activation of the nuclear factor-kB (NFkB) pathway was demonstrated in a transgenic mouse model of t(14;18) translocation or tumor necrosis factor (TNF)-receptor associated factor2 (TRAF2) over-expression, which mediates activation of NF-kB and c-Jun N-terminal kinase (JNK). Only mice with both t(14;18) and TRAF2 over-expression developed a CLL-like aggressive disease, whereas a single lesion was insufficient to initiate a disease process [Pekarsky et al. 2010]. This model likely mimics CLL, in which NF-kB is also constitutively activated [Doménech et al. 2012]. Hence, BCL2 over-expression may enhance other aberrant signaling pathways, thereby promoting survival and proliferation of CLL cells.
BCL2 over-expression prevents apoptosis despite endogenous and exogenous death stimuli. Paradoxically, these cells are ‘primed for death’, since it would hypothetically take a minor change in BCL2 activity for apoptosis to be induced. In a preliminary study using BH3-profiling, a functional assay which assesses the proximity of cells to the threshold of apoptosis, Davids and colleagues demonstrated that CLL cells from peripheral blood are highly primed. Although a small number of samples were studied, it was suggested that increased priming is associated with improved clinical response [Davids et al. 2012]. This has been realized by the unexpected tumor lysis syndrome (TLS) after ABT-199 VEN administration, as discussed below [Anderson et al. 2013].
BCL2 inhibition: preclinical data
Targeting the protein–protein interaction surface between BCL2 and BH3 mimetics is technically very complicated. Hence, the first compound focused on interfering with BCL2 gene expression using an antisense oligonucleotide that binds to BCL2 mRNA, oblimersen. However, this compound was never demonstrated to reach its target in vivo, and likely resulted in nonspecific cytokine release. Ultimately in a phase III trial comparing fludarabine/cyclophosphamide (FC) to FC plus oblimersen in relapsed refractory CLL, the evidence of clinical activity was insufficient to lead to US Food and Drug Administration (FDA) approval and clinical development was terminated [O’Brien et al. 2009].
Fabrication of BH3 mimetic agents that require targeting a protein–protein interaction surface was another approach. These agents antagonize the excess prosurvival BCL2, thus releasing the mediators of cell death (BAX and BAK) and enabling apoptosis. Since BAD is a BH3-only protein selective for BCL2/BCL-XL/BCLw, its stereotactic interaction with BCL2 was the focus of structure-based design of molecules that mimic its action. However, most designed compounds did not fully meet the major criteria for defining a bona fide BH3 mimetic (i.e. high-affinity binding to prosurvival BCL2 proteins and induction of BAX/BAK-dependent apoptosis) [Lessene et al. 2008]. Many of these compounds were cytotoxic in vitro, but likely, at least in part, through off-target effects.
Obatoclax (GX15-070) has been described as a pan-BCL2 family inhibitor, which binds to BCL2, BCL-XL, BCLw, and MCL1 with low micromolar affinity and reportedly kills cells via BAX and BAK. However, it seems rather to act through other mechanisms such as caspase-independent or autophagic cell death. The response rates as a single agent in clinical trials have been low, and dose escalation is limited by neurologic toxicity, hence its development has ceased [Billard, 2013]. The natural product gossypol and its synthetic derivative AT-101 bind BCL2, BCL-XL, and MCL1, yet the cell killing induced is not entirely mediated by BAX and BAK. As with obatoclax, these agents have demonstrated minimal single-agent activity in clinical trials, although some efficacy was observed in patients with follicular lymphoma when combined with rituximab [Anderson et al. 2014].
Of a few other compounds that progressed toward clinical trials, ABT-737 was the first BH3-mimetic with the required structure and that was validated to inhibit BCL2, BCL-XL and BCLw with high affinity (Ki < 1 nM) albeit with 500-fold weaker binding to MCL1 and A1. Its in vitro killing assay was entirely dependent on BAX/BAK and it remained active in cell lines with BCL2 over-expression [van Delft et al. 2006]. Since ABT-737 was not suitable for clinical development as an oral agent, its orally bioavailable relative, ABT-263 (navitoclax), was substituted for clinical trials. It is important to note; however, that ABT-737 was more potent than ABT-263 at inducing apoptosis in CLL cells, especially in whole blood, since ABT-263 was highly bound by albumin as compared with ABT-737 [Vogler et al. 2010].
Navitoclax induced a 35% overall response rate (ORR) in patients with R/R CLL in a phase I study (all partial responses), with a median PFS of 25 months. ORR and PFS were not affected by poor prognostic factors, including del(17p), bulky disease and fludarabine refractoriness [Roberts et al. 2012]. However, dose escalation in these trials was limited by acute, dose-dependent thrombocytopenia described below, and capping navitoclax dose due to this adverse event has perhaps mitigated its clinical effect.
Dose-dependent thrombocytopenia was demonstrated in several mammalian models for ABT-737, and in a canine model for navitoclax [Souers et al. 2013]. This is an on-target effect resulting from the increasing dependence of platelets on BCL-XL for prevention of apoptosis as they age. It can be observed within hours of drug administration, and is caused by platelet apoptosis and their rapid clearance from circulation by the reticuloendothelial system. Since older platelets are more susceptible, there is a selection for younger, larger platelets to persist in circulation. Clinically significant thrombocytopenia has been mitigated in clinical trials through institution of a lead-in lower dose of navitoclax and continuous dosing to stimulate platelet production, so when higher therapeutic doses are administered, the platelet nadir is not as low or prolonged [Roberts et al. 2012].
In a phase II study of previously untreated CLL patients who were randomized to either rituximab alone, rituximab with 12-week navitoclax or rituximab with navitoclax given until progression, a higher ORR was achieved in the long navitoclax arm (70% versus 55% in the short navitoclax arm versus 35% with rituximab alone). Although rates of thrombocytopenia of any grade were higher in the long navitoclax arm (33% versus 18% in the short navitoclax arm versus none with rituximab alone), there was only a single case where navitoclax was stopped due to thrombocytopenia. PFS was also longer with prolonged administration of navitoclax, but median PFS and remission duration data are lacking, since the study was terminated early, according to sponsor decision [Kipps et al. 2015]. The impact of thrombocytopenia, and the necessity to reduce the dosing of the drug, together with its pharmacokinetic profile influenced by its strong binding to albumin, all probably influenced the decision to switch the development to another BH3-mimetic with specific affinity to BCL2, ABT-199.
The generation of a BCL2-selective inhibitor is complicated by the degree of similarity within the BH3-binding domains of BCL2 and BCL-XL. To circumvent this challenge Souers and colleagues exploited a unique BCL2 small molecule co-crystal structure to guide the rational design of ABT-199 (GDC-0199/RG7601, VEN) [Souers et al. 2013] (Figure 2). VEN has a subnanomolar affinity for BCL2 (Ki < 0.01 nM) and bound over three orders of magnitude less avidly to BCL-XL and BCLw (Ki = 48 nM and 245 nM, respectively), and showed no measurable binding to MCL1. Selectivity has also been proven in a cellular context, using cell lines dependent on either BCL2 or BCL-XL. VEN was shown to selectively disrupt BCL2–BIM complexes and to induce caspase-dependent cell death. It was validated in various human non-Hodgkin’s lymphoma (NHL) cell lines, including those derived from diffuse large B-cell lymphoma (DLBCL), follicular lymphoma or mantle cell lymphoma (MCL), in addition to its activity against acute myelogenous leukemia and ALL lines. Furthermore, cell lines with a higher expression of BCL2 were more sensitive to its effect. Several groups have confirmed the ability of ABT-199 to induce BAX/BAK-mediated apoptosis that is triggered specifically by BIM [Vogler et al. 2009, 2013; Khaw et al. 2014]. Interestingly, Khaw and colleagues demonstrated that VEN has a similar effect on normal, untransformed mature B-cells, but largely spares B-cell precursors and cells of myeloid origin [Khaw et al. 2014]. Others have shown, however, that VEN has an effect on granulopoiesis, which explains both neutropenia, as its prevalent side effect, and its efficacy in acute myeloid leukemia [Leverson et al. 2015; Pan et al. 2014].
Figure 2.
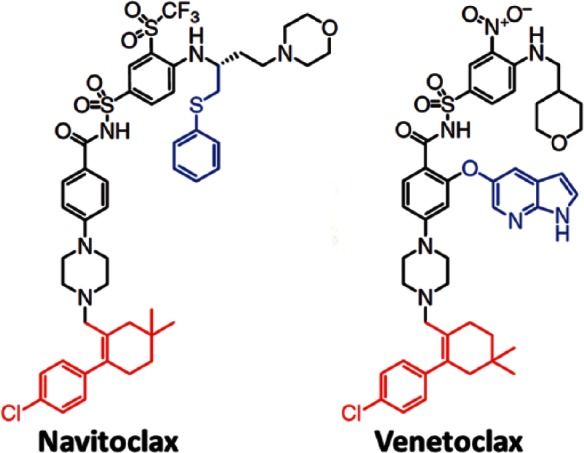
Molecular structure of navitoclax and venetoclax. Reprinted with permission from Macmillan Publishers Ltd: Nature Medicine [Souers et al. 2013].
The ability of VEN to suppress tumor growth in vivo was evaluated in a broad spectrum of hematological tumor xenografts established in immunocompromised mice. A single oral dose of VEN was able to inhibit and delay tumor growth, and more so when given continuously. Combination therapy with rituximab induced CR in all the mice tested, whereas neither compound alone was able to achieve that. The addition of one dose of bendamustine also greatly enhanced the VEN effect even in tumors which were only moderately sensitive to BCL2 inhibition. A different xenograft model employed intravenous injection of human lymphoid malignancy cell lines, rather than subcutaneous injection, in order to test drug efficacy on tumors arising in their ‘natural’ compartments. VEN was effective, in this model as well, at regressing tumors in distal sites, including the bone marrow, and combination therapy was effective at enhancing this response. Interestingly, systemic inoculation of a MCL cell line (Granta 519) induced disseminated disease to the central nervous system (CNS), with an enhanced response to the combination of BR with VEN (compared with BR alone), although neither single agent navitoclax or VEN had any effect on CNS disease [Ackler et al. 2015]. With regard to thrombocytopenia, VEN was compared with navitoclax in a canine model, and was shown to have a minimal effect on circulating platelets [Souers et al. 2013].
Interestingly, Sylvan and colleagues investigated the killing capacity of 31 different small therapeutic molecules and 29 conventional chemotherapeutic agents on fresh CLL cells from 42 patients with indolent or progressive CLL, in a fluorescence survival assay. Among small molecules tested, ABT-737 and VEN had one of the highest direct killing capacities, with mean killing efficacy of about 70%, which was significantly higher than that of ibrutinib and idelalisib (<12%), as well as that of conventional chemotherapy. Moreover, BCL2 inhibitors were even more effective in cells with TP53 disruption. The advantage of these agents was demonstrated also in CLL cells aspirated from involved bone marrow (9 patients) and lymph-nodes (3 patients), and did not differ between samples from patients with indolent or progressive CLL. Although the clinical significance of this observation is unknown, it is somewhat unique, and suggestive of the potential for very potent clinical activity [Sylvan et al. 2015].
Venetoclax (ABT-199): clinical data in chronic lymphocytic leukemia patients
The first in-human trial started with three patients with refractory CLL. Within 24 hours of a single dose (100 mg or 200 mg) they had rapid reduction in palpable lymphadenopathy and over 95% reduction in peripheral blood lymphocytosis, with evident laboratory TLS. Thrombocytopenia was minimal. Thus, ABT-199 induced a much greater immediate antileukemic effect than that previously observed with single dose navitoclax [Souers et al. 2013].
After two patients had died due to TLS in two subsequent trials, a dose step-up modification with weekly dose increases from 20, 50, 100, 200 mg to the final recommended dose of 400 mg daily was implemented. Moreover, a new TLS assessment tool was designed based on the observed data and validated initially on a small cohort; this tool is now used in all VEN trials and in the US label, without further evidence of clinical TLS. Patients with any mass ⩾ 10 cm or a mass ⩾ 5 cm and absolute lymphocyte count (ALC) ⩾ 25,000 cells/mcl are considered at high risk. Patients with lymphadenopathy between 5–10 cm or ALC ⩾ 25,000 cells/mcl are at moderate risk, and the others are at low risk. All risk group patients start at a dose of 20 mg/day for a week, although high risk patients are admitted to the hospital. All patients receive at least oral or possibly intravenous hydration, and have laboratory values checked at baseline prior to dosing, at 8 and 24 hours post-dosing, before administration of a second dose, and at each dose escalation. Uric acid reducing agents should be given 72 hours prior to therapy and rasburicase is mandatory in all high risk patients. With these modifications there were no cases of clinical TLS, and many fewer instances of laboratory TLS [Seymour et al. 2014]. All trial results are summarized in Table 1.
Table 1.
Venetoclax in chronic lymphocytic leukemia: clinical trial data summary.
| Study | Treatment arms | Design | Patient characteristics | Response rates | Survival measures | Main AEs (>10%) |
|---|---|---|---|---|---|---|
| [Roberts et al. 2016] | Monotherapy phase I/II phase I (n = 56). After protocol change, trial continued as phase II. Total n = 116 |
Phase I. Single dose of VEN on week 1, day –3 or –7 followed by continuous once-daily dosing from week 1, day1, until disease progression or unacceptable toxicity | R/R Del(17p) – 31/102 (30%) F-refractory - 70 (60%) |
ORR 79% CR/CRi 20% (expansion cohort data have not matured) |
Med f/u 14.7 months Med PFS 25 months (56 pts in dose-escalating cohort) |
G3/4 AEs: neutropenia (41%), anemia (12%), thrombocytopenia (12%). TLS: 10 pts (18%), clinical in 3, including 1 death |
| Trial continued as phase II, with weekly dose increases from 20, 50, 100, 200 mg to the final recommended dose of 400 mg daily | ||||||
| [Ma et al.
2015] |
VEN-R phase Ib n = 49 |
VEN: ramp-up dose schedule, to a final dose of 200–600 mg/day R: every 4 weeks for a total of 6 doses; 400 mg selected to move forward |
Age 68y (50–88) 2 (1–5) prior Tx R-ref 29% F-ref 18% Del(17p) 20% Unm. IGHV 70% |
ORR 86% CR/CRi 41% BM MRD neg 53% (75% in pts with CR) |
Med f/u 17.5 months PFS 87% at 12 months PFS 84% at 24 months OS 94% at 12 months 5/6 pts with PD had RT |
1 fatal TLS prior to protocol modification G3/4 AEs: neutropenia (53%), thrombocytopenia (16%), anemia (14%), febrile neutropenia (12%) |
| [Salles et al. 2015] | VEN-BR phase I |
After dose-finding phase, daily VEN dose at 400 mg Schedule A: VEN→BR Schedule B: BR→VEN Benda 70 mg/m2 in R/R; 90 mg/m2 in 1L R 375 mg/m2 C1; 500 mg/m2 C2–6. Finally, schedule B selected. |
R/R (n = 20) 1L (n = 10) Age 63y (22–77) |
No TLS events (lab and clinical) Schedule A is as safe as B in R/R pts. G3/4 AEs: neutropenia (57%), thrombocytopenia (17%) |
||
| [Flinn et al. 2015] | VEN-G phase I |
3 + 3 design, VEN ramp-up dose schedule and TLS prophylaxis, to a final dose of 100–400 mg/d (600 mg was finally not explored). Schedule A: VEN→G Schedule B: G→VEN 6 cycles R/R– VEN till PD 1L VEN for another 6 months |
Age 62.5y (45–80), R/R (n = 26), 1L (n = 6) |
Only in R/R pts (n = 17), ORR 100% CR/CRi 23% |
G3/4 AEs: Lab TLS (12.5%), neutropenia (47%), infections (19%). 1 death (acute respiratory failure) |
|
| [Fischer et al. 2015] | VEN-G versus chl-G CLL14 trial phase III safety run-in period |
6 cycles of VEN-G + 6 additional cycles of VEN G administered according to CLL11. A ramp-up schedule of VEN up to 400 mg/d starting d22 of cycle 1 |
1L unfit pts (n = 13) CIRS > 6 or CrCl <70 ml/min Age 75y (59–88) |
G3/4 AEs: neutropenia (23%), all other: 15% (2 pts): infusion-related reaction, lab TLS, thrombocytopenia, infections |
||
| [Jones et al. 2015a] | VEN monotherapy after ibrutinib or idelalisib failure | Ramp-up schedule to 400 mg/d | R/R after: ibrutinib (n = 41), median time 19w (0.5–39) or idelalisib (n = 13), median time 10w (0.5–29) |
Of evaluable pts: Ibrutinib, ORR 61% (3 CRs); idelalisib, ORR 50% (all PR) |
10 pts discontinued VEN, 5 d/t PD | |
| [Stilgenbaueret al. 2015] | VEN monotherapy in del(17p) CLL pts n = 107 |
Dose ramp-up escalation and TLS prophylaxis. VEN at 400 mg/d |
R/R Age 67y (37–85); 34/78 F-refractory; 38/54 B-refractory |
Med f/u 12.1 months ORR 79% CR/CRi 7.5% 18/45 (17%) MRD-neg. |
PFS at 12 months 72% OS at 12 months 87% 22 pts progressed, 9 with RT |
G3/a AEs: neutropenia 40%, anemia 18%, thrombocytopenia 15%, infections 20% |
1L, first line; AEs, adverse events; BM, bone marrow; BR, bendamustine-rituximab; C, cycle; CIRS, Cumulative Illness Rating Scale; CLL, chronic lymphocytic leukemia; CR, complete remission; CRi, CR with incomplete bone marrow recovery; f/u, follow up; IGHV, immunoglobulin heavy chain variable; Med, median; MRD, minimal residual disease; neg., negative; ORR, overall response rate; OS, overall survival; PD, progressive disease; pts, patients; PFS, progression-free survival; PR, partial response; R, rituximab; R/R, relapsed and refractory; RT, Richter’s transformation; TLS, tumor lysis syndrome; Tx, treatment; Unm.; unmutated; VEN, venetoclax.
Phase I/II trials
The first report was of a phase I/II trial that examined the safety and efficacy of VEN in patients with high risk, R/R CLL or small lymphocytic lymphoma (SLL). Of 56 patients treated at the phase I dose-escalating stage of the trial, 10 (18%) had TLS. Overall, three of them had clinical TLS, including one patient with acute renal failure requiring dialysis, and another patient with sudden death on the second day after stepping up to 1200 mg per day. After resolution of the TLS, 9 of 10 patients resumed taking VEN. Of these patients, 8 had no recurrence of the syndrome at subsequent doses.
After temporary suspension, the trial continued to its expansion phase with weekly dose increases as described above and a maximum dose of 400 mg daily, in order to avoid TLS. At the latest report, 116 patients had been enrolled with a median time on study of 17 months. A total of 30% of patients had del(17p) and 60% were fludarabine-refractory. Most common adverse events (AEs) of any grade were diarrhea (52%), upper respiratory tract infection (48%), nausea (47%), neutropenia (45%), and fatigue (40%). Grade 3/4 AEs were neutropenia (41%), anemia (12%) and thrombocytopenia (12%). Severe adverse events (SAEs) included TLS (3% including 1 death), febrile neutropenia (6%), and pneumonia (4%). Overall, eight patients discontinued therapy due to AEs. The ORR was 79%, and was comparable among high risk groups. The CR rate was 20%, although data from the expansion cohort have not yet matured, and CRs tend to develop over time, after 6 months of therapy. In the dose-escalating cohort, the median PFS was 25 months. The median PFS for patients with del(17p) across all doses was 16 months, whereas 71% of patients without del(17p) were progression-free at 15 months. Disease progression occurred in 41 patients (35%), including Richter’s transformation (RT) in 18 (16%), which was more common among patients with del(17p) [Roberts et al. 2016].
The second study is a phase Ib trial that examined the safety and efficacy of VEN plus rituximab in 49 patients with R/R CLL (1 with SLL). It used the ramp-up dose schedule with the final dose set at 400 mg/day in the safety expansion phase, a dose that was well tolerated in the combination. Rituximab was given every 4 weeks for a total of 6 doses, after the VEN dose escalation. Patients had a median age of 68 years (range 50–88), with a median of 2 (range 1–5) prior therapies, and most had received prior rituximab (90%), 14 (29%) were rituximab-refractory and 9 (18%) were fludarabine-refractory. Overall, 33% had del(17p) or TP53 mutation and 70% expressed unmutated IGHV. The ORR was 86%, with 47% CR/CRi. A total of nine patients with partial response (PR) or stable disease (SD) at the 7-month assessment achieved CR after an additional median of 6 months. Bone marrow MRD-negativity was achieved in 17/23 evaluable patients who achieved a CR or CR with incomplete bone marrow recovery (CRi) and 27/49 (55%) overall, which is rather remarkable compared with other agents in the relapsed setting. Actuarial PFS was 83% at 24 months (median follow up of 17.5 months). A total of 94% were alive at 12 months. Response and survival measures were not significantly impacted by high-risk subgroups. A total of 11 patients stopped VEN after achieving CR/CRi or MRD negativity (9 patients), and remained off VEN for a median of 16 (2–29) months. Overall, two MRD-positive CR/CRi patients had asymptomatic relapse after 14 and 24 months off therapy. In the last update, 12 patients discontinued the study, 6 due to progressive disease (PD), of whom 5 had Richter’s transformation, and 3 due to AEs. One treatment-emergent TLS led to death, prior to the protocol modification to enhance monitoring, perform risk stratification and slow dose escalation. Grade 3/4 AEs were neutropenia (53%), thrombocytopenia (16%), anemia (14%) and febrile neutropenia (12%) [Ma et al. 2015].
In work conducted in parallel with the phase I clinical trials of VEN monotherapy, or in combination with rituximab in R/R CLL, most patients had evidence of TP53 abnormality. In vitro study of these cells showed high sensitivity to ABT-199, similar to cells without TP53 abnormality [Anderson et al. 2013]. A phase II study of VEN monotherapy in 107 patients with del(17p) R/R CLL was recently presented, and 60/83 (72%) also had mutated TP53. A ramp-up dose schedule up to 400 mg daily and TLS prophylaxis were used. The median patient age was 67 (range 37–85), with a median number of 2 (1–10) prior regimens, 34/78 (44%) fludarabine-refractory and 38/54 (70%) bendamustine-refractory. With a median follow up of 12.1 months (range 0.03–21.5), ORR was 79% with CR/CRi of 7.5%. In 45 patients with MRD assessment, 18 (17%) were MRD negative in the peripheral blood, and 6/10 in the bone marrow as well. Actuarial 12-months PFS and OS were 72% and 86.7%. Grade 3–4 AEs were mainly manageable hematological AEs (neutropenia in 40%) and infections. Overall, 37 patients discontinued VEN; 22 due to PD, of whom 11 had Richter’s transformation; and 9 due to AEs. A total of 18 patients died, 14 due to disease progression and 4 due to AEs [Stilgenbauer et al. 2015].
Combination of VEN with BR is also feasible both in R/R patients (20 patients) and as a first-line treatment (10 patients), as reported by Salles and colleagues [Salles et al. 2015]. A dose finding schedule for VEN recommended the 400 mg daily dose. Although no safety differences were observed when starting with either VEN or BR, the latter was chosen for the safety expansion cohort due to its potential for tumor debulking and reducing the risk of tumor lysis. Main toxicities were hematological with grade 3/4 neutropenia in 57% and thrombocytopenia in 17%. No TLS events were observed [Salles et al. 2015]. A phase III open-label randomized trial of VEN-R versus VEN-BR in R/R CLL patients is currently recruiting and will serve as the confirmatory registration for the US FDA approval.
It seems that response rates with BCL2 inhibitors are more robust in CLL compared with other R/R NHL patients. The phase I VEN trial also includes an arm for patients with NHL. Preliminary data from this heterogeneous group of patients indicate an ORR of 53%, with CR of 8%. An early signal for efficacy has been suggested for MCL (8 patients) and lymphoplasmacytic lymphoma (3 patients), with ORR of 82% and 100%, respectively. Results of other subtypes of NHL have been less impressive, with ORR of 27% and 38% in the follicular lymphoma (11 patients) and DLBCL (8 patients) subgroups, respectively [Seymour et al. 2013]. Results with navitoclax were even less remarkable in NHL [Roberts et al. 2015; Wilson et al. 2010].
Comparison with other targeted agents in chronic lymphocytic leukemia
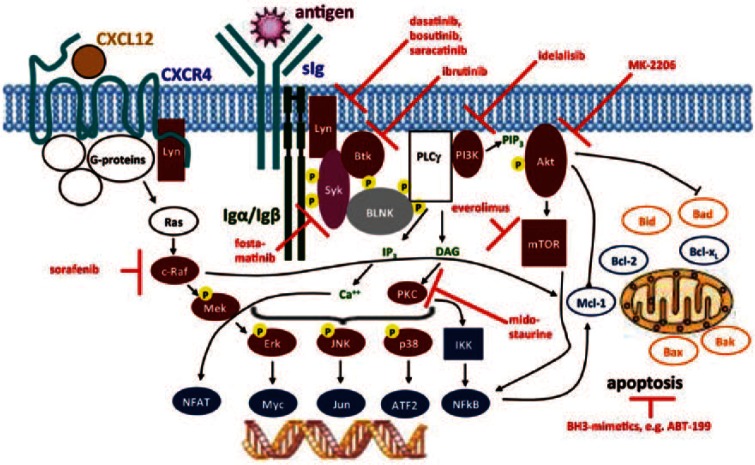
As mentioned earlier, during the last few years, there has been a proliferation of highly effective therapies in the therapeutic landscape of CLL. Although great care must be taken when comparing trial results from individual studies, it seems that VEN has an advantage over several novel agents with regard to complete remission rates and achievement of MRD negativity. We will briefly review three innovative drugs in CLL that are relevant to our discussion: the humanized anti-CD20, obinutuzumab and the kinase inhibitors, ibrutinib and idelalisib. Other approved agents in the treatment of CLL, like the fully humanized type I anti-CD20 antibody, ofatumumab, and the anti-CD52 antibody, alemtuzumab are beyond the scope of this review. Figure 3 illustrates B-cell receptor (BCR) signaling pathways and new therapeutic agents, some of which will be discussed here.
Figure 3.
Targeted therapies in chronic lymphocytic leukemia. Figure created by Dr Gunter Krause, Cologne; used with permission of the author.
Obinutuzumab (GA101), a humanized glycoengineered monoclonal antibody that showed impressive results in vitro with higher rates of direct cell killing of B-cells in comparison with rituximab [Patz et al. 2011], with increased antibody-dependent cell-mediated cytotoxicity (ADCC), low complement-mediated cytotoxicity (CDC) and increased direct cell death induction [Mössner et al. 2010]. The GAUGUIN trial evaluated the safety and efficacy of obinutuzumab monotherapy in patients with R/R CLL [Cartron et al. 2014]. ORR was only 30% in the phase II trial involving 20 patients, with median PFS of 10.7 months, although strict computed tomography (CT)-based criteria were used in this study, which has not been the case historically in studies of single-agent antibodies in CLL. Based on these results, combination therapy with obinutuzumab was tested in the CLL11 trial for previously untreated elderly or unfit patients, in which patients were randomized to either chlorambucil (Chl) alone or in combination with rituximab (Chl-R) or obinutuzumab (Chl-G). The latter combination achieved an ORR of 77% (CR 22%), with higher blood and bone marrow MRD (37.7 and 19.5, respectively) compared with Chl-R. There was also a statistically significant PFS benefit of Chl-G versus Chl-R (29.2 versus 15.4 months) and a trend towards improved OS (hazard ratio [HR] 0.7, p = 0.0632) [Goede et al. 2014b]. Obinutuzumab was associated with grade 3/4 infusion-related reactions during the first infusion, and more grade 3/4 neutropenia was reported with Chl-G compared with Chl-R, yet the infectious complication rate was similar. A subset analysis of the CLL11 trial showed that Chl-G combination was also active in patients with CLL refractory to previous single-agent chlorambucil treatment [Goede et al. 2014a, 2015].
Ibrutinib is an orally-active BTK-inhibitor that blocks downstream activation from the BCR through NF-kB and mitogen-activated protein (MAP)-kinases, thereby inducing apoptosis of B-cell lymphomas and CLL. In the phase I trial in R/R CLL and lymphoma patients, the ORR with ibrutinib was 60%, including 16% CRs and median PFS of 13.6 months. Results varied by histology, and of 16 CLL/SLL patients, 11 responded with 2 achieving CR [Advani et al. 2013]. A unique pattern of response frequently encountered with BCR-targeting small molecules, including ibrutinib, is transient and reversible lymphocytosis, often associated with rapid reduction in lymphadenopathy. This is thought to reflect a redistribution of malignant lymphocytes between compartments, probably because of a disruption of BCR-mediated stromal interactions [Kim and Dhillon, 2015]. This was demonstrated in a pivotal phase II trial of ibrutinib in R/R CLL patients. On the first publication, ORR was 71% and another 15–20% of patients were reported as PR with lymphocytosis [Byrd et al. 2013]. At three-year follow up, ORR was 90% with 7% CR and only 3% PR with lymphocytosis. At 30 months, PFS was 69% and OS 79%. High risk patients, specifically those with del(17p), had a shorter PFS and OS at 30 months of 48% and 65%, respectively [Byrd et al. 2013, 2015]. Ibrutinib was approved based on these phase II data in the US in R/R CLL. The phase III RESONATE trial was the confirmatory registration trial and compared the fully humanized anti-CD20 antibody, ofatumumab, with ibrutinib in the R/R setting. Ibrutinib resulted in a superior ORR of 63% (no CRs) and improved PFS and OS (HR of 0.22 and 0.43, respectively) compared with ofatumumab, which held true in subgroup analysis of high-risk CLL patients and was more significant at a later update [Byrd et al. 2014; Brown et al. 2014b]. Ibrutinib has also demonstrated a survival benefit compared with chlorambucil in previously-untreated CLL patients in the RESONATE-2 trial (OS at 2 years of 98% versus 85%, respectively, HR 0.16, p = 0.001), which led to its FDA approval for frontline therapy of CLL [Burger et al. 2015]. Another phase II study designed specifically for patients with TP53 aberration, reported 97% ORR in 35 previously-untreated CLL patients and 80% in 16 R/R patients, but there were no CRs. Other than grade 3/4 hematological AEs in <20% of patients, notable AEs were pyrexia, atrial fibrillation (7%, all grades) and a bleeding tendency with ibrutinib [Farooqui et al. 2015]. An acquired unique resistance mechanism to ibrutinib was found using whole-exome sequencing of either a mutation in the BTK-binding site or gain-of-function mutations in PLCγ2, leading to autonomous BCR activity [Woyach et al. 2014]. After discontinuation of ibrutinib, many patients suffer from a rapid flare-up of their disease, leading to caution about stopping ibrutinib even in patients who are progressing on it. Although patients who stop ibrutinib due to toxicity may benefit from other kinase inhibitors [Mato et al. 2015], for those patients who do progress (about 40% of patients who discontinue ibrutinib), survival has been reported to be very short, and especially for those relapsing with Richter’s transformation [Jain et al. 2015; Maddocks et al. 2015]. Thus, at present, in relapsed refractory patients given a single agent, it seems that ibrutinib should be administered continuously until progression [Hallek, 2015].
Idelalisib is an oral PI3Kδ-inhibitor. In CLL, the PI3K pathway is constitutively activated and dependent on the δ isoform which is specific for B-cells. Idelalisib promotes apoptosis in primary CLL cells in a time- and dose-dependent manner employing several mechanisms, including chemotaxis inhibition, reduction of survival signals from BCR, inhibition of AKT/MAPK activation and interference with integrin-mediated adhesion to the microenvironment. Idelalisib treatment resulted in ORR of 72% of R/R CLL patients, with a median PFS of 15.8 months [Brown et al. 2014a]. In a phase III registration trial of the idelalisib-rituximab combination versus rituximab-placebo, the combination resulted in 81% ORR and superior PFS and OS at 12 months compared with rituximab alone [Furman et al. 2014]. In this trial, patients with del(17p) or TP53 mutation responded at a similar rate, and at least in the phase III registration trial with rituximab, had similar PFS to patients without del(17p) [Furman et al. 2014]. Notable side effects with idelalisib were rash, elevated transaminases, pneumonitis, and late-onset colitis. Importantly, although grade ⩾3 diarrhea/colitis was reported in only 14% of R/R CLL patients, in the first-line setting it occurred in 42% of patients at a median of 9.5 months. Other AEs such as grade 3 or above rash and pneumonia were also more frequent [O’Brien et al. 2014]. This was corroborated in a small phase II single-arm study of first-line idelalisib and ofatumumab in treatment-naïve patients, and was associated with a larger decrease in regulatory T-cells (Tregs) in patients with grade 3 or above toxicity [Lampson et al. 2016].
It is interesting to know whether VEN could salvage CLL patients relapsing or progressing after ibrutinib or idelalisib. Preliminary results from a phase II trial evaluating VEN efficacy in 41 CLL patients relapsing after ibrutinib and 13 after idelalisib were recently reported [Jones et al. 2015a]. With indeed, a short follow up with a median of 19 (range 0.5–39) weeks for prior ibrutinib and 10 (range 0.5–29) weeks for prior idelalisib, 10 patients had discontinued VEN, half due to disease progression. Of evaluable patients, ORR was 61% in patients previously treated with ibrutinib (including 3 CRs), and 50% with prior idelalisib treatment. No new safety signals for VEN were observed.
Resistance to BCL2 inhibitors
Patients do progress under treatment with BCL2-inhibitors. Resistance mechanisms have not been elucidated in a clinical setting, although several options may come into play. The specificity of VEN may very well be its Achilles heel, as upregulation and over-expression of other anti-apoptotic BCL2 family members may salvage these cells from apoptosis. Correspondingly, cell viability assays with NHL cell lines have shown that VEN has limited efficacy in BCL-XL and MCL-1-dependent hematopoietic malignancies [Woyach and Johnson, 2015; Phillips et al. 2015; Khaw et al. 2014]. A reliance on MCL-1 has also been demonstrated in a minority of cell samples from CLL patients that may confer a resistance advantage to VEN [Davids et al. 2012]. Other putative mechanisms are constitutive intracellular signaling (i.e. downstream of BCR) and stroma-mediated treatment resistance due to prosurvival signals from the microenvironment [Woyach and Johnson, 2015].
Clinically, retrospective data from phase I/II VEN studies in 70 R/R CLL patients, reported outcomes in 28 (40%) patients who ceased VEN for reasons other than voluntary drug hold after achieving CR. These patients had received a median 7.5 (range, 1–38) months of VEN. Overall, 7 (25%) had CLL progression, 16 (57%) developed RT and 5 (18%) ceased for other reasons. They had high-risk CLL with TP53 aberration in 70% and un-mutated IGHV in 88% and a median of 4 (range, 1–12) prior lines of therapy. The post-progression survival for patients with progressive CLL was 69% at 1 year, and about 50% for those with RT. Post-progression survival did not statistically differ by response to VEN, progression on VEN before or after 12 months, cytogenetic risk, or the number of prior therapies. Patients with progressive CLL were able to be salvaged with BTK inhibitors. Thus, failure on VEN does not necessarily portend a poor prognosis, and survival after VEN failure may be superior to that reported for failure on BTK-inhibitors [Tam et al. 2015].
Data regarding BCL2-inhibitor resistance come from in vitro/ex vivo studies. ABT-737 was shown to induce resistance in a lymph node model by upregulation of BCL-XL and BFL2/A1 [Vogler et al. 2009]. Using another lymph node model, CLL cells which upregulated BCL-XL after stimulation with CD40 and IL4 were resistant to high doses of VEN [Pascutti et al. 2013]. In follicular lymphoma cell lines, and primary cells with induced resistance to VEN, increased levels of MCL-1 as well as increased autophagy were observed [Bodo et al. 2014]. In DLBCL cell lines with low MCL1 and BCL-XL expression that were initially sensitive to VEN, resistant subclones emerged after chronic exposure to VEN. These subclones were not found prior to VEN treatment. They demonstrated a higher expression of MCL-1 and BCL-XL, caused by elevated mRNA levels and enhanced MCL1 protein stability that was regulated through the AKT pathway. These proteins were shown to sequester BIM that was displaced from BCL2. Moreover, VEN displaced BIM from BCL2 only in parental, but not in VEN-resistant cells [Choudhary et al. 2015a]. Interestingly, in mouse and lymphoma cells continuously exposed to VEN, resistance was found to evolve as a consequence of new mutations in the BCL2 BH3 domain impeding VEN binding to BCL2 (mouse), and a mutation in BAX abrogating its attachment to mitochondria, thus blocking VEN-induced apoptosis (human) [Fresquet et al. 2014].
The post-translational status of BCL2-family members, and specifically their phosphorylation status, has been associated with resistance to various BH3-mimetics. The ERK pathway is activated in CLL cells through a B-cell activating factor of the TNF family (BAFF) or a proliferation-inducing ligand (APRIL) receptor or BCR stimulation, which could generate pBCL2 in CLL patients. Song et al. demonstrated that response to various BCL2 inhibitors is predicted by the (MCL1 + pBCL2)/BCL2 ratio in CLL cells from primary CLL patients. Mechanistically, pBCL2 interfered with BCL2-inhibitor displacement of BAX and BIM from BCL2, thereby suppressing mitochondrial apoptosis [Song et al. 2016]. The post-translational phosphorylation of MCL1, which is cyclin E/CDK2-dependent, is also important as it enhances its stability and reduces cellular sensitivity to BH3-mimetics, including VEN [Choudhary et al. 2015b].
These studies may be useful in analyzing resistance mechanisms of patients who relapse after VEN and guide physicians in selection of another therapeutic line, whether a single agent or combination therapy. In vitro data suggest that distinct mechanisms of resistance to VEN may be successfully targeted by dasatinib, ibrutinib, idelalisib or inhibition of CDK9 [Choudhary et al. 2015a, 2015b; Pascutti et al. 2013; Chen et al. 2014]. An example of CDK9 inhibition is the use of dinaciclib, a CDK-inhibitor, to overcome resistance by MCL1 up-regulation in DLBCL cells treated with VEN [Li et al. 2015]. Importantly, flavopiridol, another CDK-inhibitor, showed interest in phase II trials in R/R CLL patients, with significant TLS as an AE [Lin et al. 2009; Lanasa et al. 2015]. Inhibition of other targets may be used to salvage patients who progress under these novel agents or enhance efficacy or prevent resistance in patients treated with BH3-mimetics. Examples of such agents, for which there is preclinical data, are HSP90-inhibitors, the XPO/CRM1-inhibitor (selinexor), SYK-inhibitors [Blunt et al. 2015; Bojarczuk et al. 2015] and the anti-BAFF antibody (belimumab) [Schmidt et al. 2015]. Of course, other treatment modalities can also be employed, especially immunotherapy with chimeric antigen receptor (CAR) T-cell therapy or allogeneic stem cell transplantation.
Combination therapy of venetoclax with novel agents: clinical and preclinical data
Combination therapy may yield higher and more durable responses, and prevent the occurrence of resistance. Combination of VEN with rituximab and BR was discussed above, and phase III trials are ongoing. Importantly, available data imply that VEN in combination with BR may be stopped after achieving CR or at 18 months in treatment-naïve patients [Seymour et al. 2014; Salles et al. 2015].
The combination of VEN and obinutuzumab (VEN-G) was tested in a phase I trial in first line and R/R CLL patients (6 and 26 patients, respectively). Grade 3/4 AEs consisted of TLS in 4 (12.5%), neutropenia in 15 (47%), febrile neutropenia in 2 (6%) and infectious complications in 6 (19%). Overall, one patient died with acute respiratory failure. A total of 17 patients with R/R disease were evaluable for efficacy. ORR was 100% with 23% CR/CRi, and 76% PRs, of whom 3 patients have improved to CR after completing six cycles. MRD data are not yet available [Flinn et al. 2015]. Based on this trial, a phase III randomized trial was initiated to evaluate the efficacy and safety of the combination of obinutuzumab/VEN compared with obinutuzumab/chlorambucil in previously untreated unfit patients (CLL 14 trial). Preliminary data from the safety run-in phase were presented at the American Society of Hematology (ASH) meeting in 2015, including 13 patients, 12 of whom had completed the VEN ramp-up period. Overall, one patient developed a grade 4 infusion reaction related to obinutuzumab and was withdrawn. All 12 patients had rapid reduction in lymphocytosis. No clinical, and two cases of laboratory TLS occurred. One grade 4 elevation in transaminases was related to obinutuzumab. The trial is currently recruiting [Fischer et al. 2015].
Treatment of CLL patients with VEN and a kinase inhibitor together is new terrain, but substantial laboratory work supports its use. As mentioned earlier, there is an intricate interaction between BCL2 over-expression and signaling pathways downstream of the BCR, suggesting a synergistic effect to inhibition of both pathways. In the DLBCL VEN-resistant cell lines mentioned above, the addition of a dual PI3K/mTOR-inhibitor (NVP-BEZ235) to VEN was able to overcome VEN resistance. Similarly, a combination of VEN with idelalisib was able to sensitize VEN-resistant cell lines to VEN, although idelalisib alone had only a moderate effect in these cells. A less robust effect was gained when combining VEN with the mTOR inhibitor, RAD001. Others have shown that there is indeed an in vitro synergy between VEN and idelalisib on peripheral blood CLL cells [Jones et al. 2015b]. In addition to the intracellular benefit of these combinations, PI3K blockade mobilizes CLL cells into the blood, where, without constitutive survival signals from the microenvironment, they would be more ‘primed’ to die [Choudhary et al. 2015a].
Recent studies have demonstrated promising preclinical efficacy for the combination of ibrutinib with VEN in CLL and MCL patients. Gene expression profiling in MCL cell lines exposed to either or both agents, suggests that the combination induces unique transcriptional changes and potentiates existing ones. Emergent genes revealed activation of apoptosis via p53 and BIM as mechanisms of synergy [Portell et al. 2014]. Ex vivo incubation of cells from CLL patients on ibrutinib with several agents (including idelalisib, bendamustine, ABT-737 and VEN), have demonstrated high cell death rates with BCL2-inhibitors, and especially with VEN. This was corroborated in a corresponding in vitro study [Cervantes-Gomez et al. 2015]. Recent data, using a modified functional assay of ‘mitochondrial priming’ (the proximity of a cell to the threshold of apoptosis), suggest that while VEN increases the overall level of mitochondrial priming of CLL cells, ibrutinib selectively increases the dependence of CLL cells on BCL2. This was confirmed ex vivo in primary CLL cells from three patients treated with ibrutinib. These data suggest that ibrutinib makes mitochondria more BCL2-dependent, and lays biologic grounds for clinical combination trials [Deng et al. 2015]. Another concern is the importance of microenvironment-related resistance. Idelalisib pretreatment in vivo abrogated stromal resistance and improved the killing ability of navitoclax in cells that were resistant to either drug alone [Davids et al. 2012]. Others have shown that CD40-induced resistance to VEN in CLL cell lines, may be counteracted by addition of anti-CD20 antibodies (rituximab and obinutuzumab) or kinase inhibitors (dasatinib and imatinib) [Thijssen et al. 2015]. However, the work of Jayappa and colleagues emphasizes the importance of microenvironment-related resistance, as they demonstrated that even the toxicity of VEN/ibrutinib on CLL cells in an in vitro model, could be abrogated by signaling from CD40, IL10R and TLRs through NF-kB, JAK/STAT or PI3K signaling [Jayappa et al. 2015]. To overcome stroma-related resistance, a novel therapy would need to bypass dysfunctional upstream cell signaling such as absent or mutant TP53. It would also need to be tolerated at a dose high enough to overcome prosurvival signals in sanctuary sites like the bone marrow [Davids et al. 2013].
Conclusion and future directions
VEN is a novel, orally-bioavailable and potent agent in the treatment of CLL that addresses one of the key mechanisms in the pathogenesis of the disease. VEN achieves remissions in the high majority of patients, including high risk groups, and results in a substantial rate of CRs and MRD negativity, a significant feature among novel agents. Results with VEN seem superior in CLL than in some other lymphoid malignancies, although evidence in MCL and lymphoplasmacytic lymphoma is encouraging. Although continuous VEN administration yielded longer PFS in a clinical trial and is the accepted convention today, stopping VEN after achieving CR/CRi and MRD negativity, or after a fixed duration of therapy, has been shown preliminarily to be feasible for further study. This finding is in contrast to current data on single agent ibrutinib, where there is still concern regarding a flare-up reaction when stopping. Many small clinical trials currently underway will help us understand the best way of using VEN in various settings, before large phase III trials can provide the appropriate evidence for its benefit and address concerns regarding TLS, neutropenia and a suggestion of a higher incidence of Richter’s transformation that arose in the heavily pretreated population of patients in phase I/II clinical trials.
Ultimately, combinations of novel biologically synergistic nonchemotherapeutic agents, such as VEN and BCR-signaling inhibitors, will be pushed to first line. If they prove effective in achieving complete remissions and MRD negativity, it may allow for a defined duration of treatment followed by a long PFS, while at progression, patients who have been off drug for some time may not be resistant, and may therefore even be treated with the same combination. If this possibility holds true in large clinical trials, it will also be of immense importance with regard to the cost-effectiveness of CLL treatment in a growing world of molecularly-targeted costly therapeutic agents which are currently being studied as continuous therapies. The potential for deep remissions with VEN combinations, and the opportunity for defined duration therapy that this raises make VEN one of the most exciting agents in the treatment of CLL today. Optimizing the use of VEN and BCR inhibitors is a great challenge that nonetheless provides incredible hope for CLL patients and the physicians who treat them.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Gilad Itchaki, Beilinson Hospital, Rabin Medical Center, Petah Tikva, Israel.
Jennifer R. Brown, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215, USA.
References
- Ackler S., Oleksijew A., Chen J., Chyla B., Clarin J., Foster K., et al. (2015) Clearance of systemic hematologic tumors by venetoclax (ABT-199) and navitoclax. Pharmacol Res Perspect 3: e00178 DOI: 10.1002/prp2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani R., Buggy J., Sharman J., Smith S., Boyd T., Grant B., et al. (2013) Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 31: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M., Huang D., Roberts A. (2014) Targeting BCL2 for the treatment of lymphoid malignancies. Semin Hematol 51: 219–227. [DOI] [PubMed] [Google Scholar]
- Anderson M., Tam C., Seymour J., Bell A., Westerman D., Juneja S., et al. (2013) Selective BCL-2 inhibition with ABT-199 is highly active against chronic lymphocytic leukemia (CLL) irrespective of TP53 mutation or dysfunction. Blood (ASH Annual Meeting Abstracts): abstract 1304. [Google Scholar]
- Awan F., Kay N., Davis M., Wu W., Geyer S., Leung N., et al. (2009) MCL-1 expression predicts progression-free survival in chronic lymphocytic leukemia patients treated with pentostatin, cyclophosphamide, and rituximab. Blood 113: 535–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard C. (2013) BH3 mimetics: status of the field and new developments. Mol Cancer Ther 12: 1691–1700. [DOI] [PubMed] [Google Scholar]
- Blunt M., Parnell J., Larrayoz M., Smith L., Dobson R., Strefford J., et al. (2015) The SYK\JAK inhibitor cerdulatinib (PRT062070) shows promising preclinical activity in chronic lymphocytic leukemia by antagonising B-cell receptor and microenvironmental signaling. Blood 126: 1716. [Google Scholar]
- Bodo J., Zhao X., Smith M., Hsi E. (2014) Activity of ABT-199 and acquired resistance in follicular lymphoma cells. Blood 124: 3635. [Google Scholar]
- Bojarczuk K., Sasi B., Gobessi S., Innocenti I., Laurenti L., Efremov D. (2015) Inhibition of SYK more effectively overcomes MCL-1 mediated ABT-199 resistance of BCR-stimulated CLL cells than inhibition of BTK or PI3Kdelta. Blood 126: 489. [Google Scholar]
- Brown J., Byrd J., Coutre S., Benson D., Flinn I., Wagner-Johnston N., et al. (2014a) Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood 123: 3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J., Hillmen P., O’Brien S., Barrientos J., Reddy N., Coutre S., et al. (2014b) Updated efficacy including genetic and clinical subgroup analysis and overall safety in the phase III RESONATETM trial of ibrutinib versus ofatumumab in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood 124: 3331. [Google Scholar]
- Burger J., Tedeschi A., Barr P., Robak T., Owen C., Ghia P., et al. (2015) Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 373: 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J., Brown J., O’Brien S., Barrientos J., Kay N., Reddy N., et al. (2014) Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 371: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J., Furman R., Coutre S., Burger J., Blum K., Coleman M., et al. (2015) Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 125: 2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J., Furman R., Coutre S., Flinn I., Burger J., Blum K., et al. (2013) Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 369: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron G., de Guibert S., Dilhuydy M., Morschhauser F., Leblond V., Dupuis J., et al. (2014) Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood 124: 2196–2202. [DOI] [PubMed] [Google Scholar]
- Cervantes-Gomez F., Lamothe B., Woyach J., Wierda W., Keating M., Balakrishnan K., et al. (2015) Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res 21: 3705–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Jin S., Tapang P., Tahir S., Smith M., Xue J., et al. (2014) CDK9 inhibition reverses resistance to ABT-199 (GDC-0199) by down-regulating MCL-1. Blood 124: 2161.25278562 [Google Scholar]
- Choudhary G., Al-Harbi S., Mazumder S., Hill B., Smith M., Bodo J., et al. (2015a) MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis 6: e1593 DOI: 10.1038/cddis.2014.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary G., Tat T., Misra S., Hill B., Smith M., Almasan A., et al. (2015b) Cyclin E/Cdk2-dependent phosphorylation of Mcl-1 determines its stability and cellular sensitivity to BH3 mimetics. Oncotarget 6: 16912–16925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids M., Deng J., Wiestner A., Lannutti B., Wang L., Wu C., et al. (2012) Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood 120: 3501–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids M., Letai A., Brown J. (2013) Overcoming stroma-mediated treatment resistance in chronic lymphocytic leukemia through BCL-2 inhibition. Leuk Lymphoma 54: 1823–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Isik E., Fernandes S., Brown J., Letai A., Davids M. (2015) Ibrutinib therapy increases BCL-2 dependence and enhances sensitivity to venetoclax in CLL. Blood 126: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doménech E., Gómez-López G., Gzlez-Peña D., López M., Herreros B., Menezes J., et al. (2012) New mutations in chronic lymphocytic leukemia identified by target enrichment and deep sequencing. PLoS One 7: e38158 DOI: 10.1371/journal.pone.0038158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorst B., Fink A., Busch R., Kovacs G., Maurer C., Lange E., et al. (2014) Frontline chemoimmunotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) shows superior efficacy in comparison to bendamustine (B) and rituximab (BR) in previously untreated and physically fit patients (pts) with advanced chronic lymphocytic leukemia (CLL): final analysis of an international, randomized study of the German CLL Study Group (GCLLSG) (CLL10 Study). Blood 19. [Google Scholar]
- Farooqui M., Valdez J., Martyr S., Aue G., Saba N., Niemann C., et al. (2015) Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol 16: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K., Bahlo J., Fink A., Goede V., Herling C., Cramer P., et al. (2016) Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood 127: 208–215. [DOI] [PubMed] [Google Scholar]
- Fischer K., Cramer P., Busch R., Stilgenbauer S., Bahlo J., Schweighofer C., et al. (2011) Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 29: 3559–3566. [DOI] [PubMed] [Google Scholar]
- Fischer K., Fink A., Bishop H., Dixon M., Bahlo J., Choi M., et al. (2015) Results of the safety run-in phase of CLL14 (BO25323): a prospective, open-label, multicenter randomized phase III trial to compare the efficacy and safety of obinutuzumab and venetoclax (GDC-0199/ABT-199) with obinutuzumab and chlorambucil in patients with previously untreated CLL and coexisting medical conditions. Blood 126: 496. [Google Scholar]
- Flinn I., Brunvand M., Choi M., Dyer M., Gribben J., Hillmen P., et al. (2015) Safety and efficacy of a combination of venetoclax (GDC-0199/ABT-199) and obinutuzumab in patients with relapsed/refractory or previously untreated chronic lymphocytic leukemia - results from a phase 1b study (GP28331). Blood 126: 494.25987658 [Google Scholar]
- Fresquet V., Rieger M., Carolis C., García-Barchino M., Martinez-Climent J. (2014) Acquired mutations in BCL2 family proteins conferring resistance to the BH3 mimetic ABT-199 in lymphoma. Blood 123: 4111–4119. [DOI] [PubMed] [Google Scholar]
- Furman R., Sharman J., Coutre S., Cheson B., Pagel J., Hillmen P., et al. (2014) Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 370: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goede V., Engelke A., Fischer K., Lopez Jimenez J., Kuzmin A., Dyer M., et al. (2014a) Salvage therapy with obinutuzumab (GA101) plus chlorambucil (Clb) after treatment failure of Clb alone in patients with chronic lymphocytic leukemia (CLL) and comorbidities: results of the CLL11 study. Blood 124: 3327. [Google Scholar]
- Goede V., Fischer K., Busch R., Engelke A., Eichhorst B., Wendtner C., et al. (2014b) Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 370: 1101–1110. [DOI] [PubMed] [Google Scholar]
- Goede V., Fischer K., Engelke A., Schlag R., Lepretre S., Montero L., et al. (2015) Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study. Leukemia 29: 1602–1604. [DOI] [PubMed] [Google Scholar]
- Hallek M. (2015) Chronic lymphocytic leukemia: 2015 update on diagnosis, risk stratification, and treatment. Am J Hematol 90: 446–460. [DOI] [PubMed] [Google Scholar]
- Hallek M., Fischer K., Fingerle-Rowson G., Fink A., Busch R., Mayer J., et al. (2010) Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 376: 1164–1174. [DOI] [PubMed] [Google Scholar]
- Hanada M., Delia D., Aiello A., Stadtmauer E., Reed J. (1993) Bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood 82: 1820–1828. [PubMed] [Google Scholar]
- Hernández-Sánchez M., Rodríguez-Vicente A., Hernández J., Lumbreras E., Sarasquete M., Martín A., et al. (2016) MiRNA expression profile of chronic lymphocytic leukemia patients with 13q deletion. Leuk Res 46: 30–36. [DOI] [PubMed] [Google Scholar]
- Huang Y., Wu J., Li J., Xu W. (2015) Know the enemy as well as the weapons in hand: the aberrant death pathways and therapeutic agents in chronic lymphocytic leukemia. Am J Cancer Res 5: 2361–2375. [PMC free article] [PubMed] [Google Scholar]
- Jain P., Keating M., Wierda W., Estrov Z., Ferrajoli A., Jain N., et al. (2015) Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood 125: 2062–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayappa K., Portell C., Gordon V., Williams M., Petricoin E., Bender T., et al. (2015) Ligands that mimic the tissue microenvironment of replicating chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL) protect ex vivo patient cell samples from the cytotoxicity of combined treatment with ibrutinib and venetoclax (ABT-199). Blood 126: 448. [Google Scholar]
- Jones J., Mato A., Coutre S., Wierda W., Choi M., Davids M., et al. (2015a) Preliminary results of a phase II, open-label study of venetoclax (ABT-199/GDC-0199) monotherapy in patients with chronic lymphocytic leukemia relapsed after or refractory to ibrutinib or idelalisib therapy. Blood 126: 715. [Google Scholar]
- Jones R., Axelrod M., Tumas D., Quéva C., Di Paolo J. (2015b) Combination effects of B cell receptor pathway inhibitors (entospletinib, ONO/GS-4059, and idelalisib) and a BCL-2 inhibitor in primary CLL cells. Blood 126: 1749. [Google Scholar]
- Khaw S., Merino D., Anderson M., Glaser S., Bouillet P., Roberts A., et al. (2014) Both leukaemic and normal peripheral B lymphoid cells are highly sensitive to the selective pharmacological inhibition of prosurvival Bcl-2 with ABT-199. Leukemia 28: 1207–1215. [DOI] [PubMed] [Google Scholar]
- Kim E., Dhillon S. (2015) Ibrutinib: a review of its use in patients with mantle cell lymphoma or chronic lymphocytic leukaemia. Drugs 75: 769–776. [DOI] [PubMed] [Google Scholar]
- Kipps T., Eradat H., Grosicki S., Catalano J., Cosolo W., Dyagil I., et al. (2015) A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk Lymphoma 56: 2826–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A., Miera O., Bauer O., Golfier S., Schriever F. (2000) Chemosensitivity of B cell chronic lymphocytic leukemia and correlated expression of proteins regulating apoptosis, cell cycle and DNA repair. Leukemia 14: 40–46. [DOI] [PubMed] [Google Scholar]
- Lampson B., Kasar S., Matos T., Morgan E., Rassenti L., Davids M., et al. (2016) Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. DOI: 10.1182/blood-2016-03-707133 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanasa M., Andritsos L., Brown J., Gabrilove J., Caligaris-Cappio F., Ghia P., et al. (2015) Final results of EFC6663: a multicenter, international, phase 2 study of alvocidib for patients with fludarabine-refractory chronic lymphocytic leukemia. Leuk Res 39: 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessene G., Czabotar P., Colman P. (2008) BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov 7: 989–1000. [DOI] [PubMed] [Google Scholar]
- Leverson J., Phillips D., Mitten M., Boghaert E., Diaz D., Tahir S., et al. (2015) Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med 7: 279ra40 DOI: 10.1126/scitranslmed.aaa4642. [DOI] [PubMed] [Google Scholar]
- Li L., Pongtornpipat P., Tiutan T., Kendrick S., Park S., Persky D., et al. (2015) Synergistic induction of apoptosis in high-risk DLBCL by BCL2 inhibition with ABT-199 combined with pharmacologic loss of MCL1. Leukemia 29: 1702–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T., Ruppert A., Johnson A., Fischer B., Heerema N., Andritsos L., et al. (2009) Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol 27: 6012–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovat F., Fassan M., Gasparini P., Rizzotto L., Cascione L., Pizzi M., et al. (2015) miR-15b/16–2 deletion promotes B-cell malignancies. Proc Natl Acad Sci USA 112: 11636–11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Brander D., Seymour J., Kipps T., Barrientos J., Davids M., et al. (2015) Deep and durable responses following venetoclax (ABT-199 / GDC-0199) combined with rituximab in patients with relapsed/refractory chronic lymphocytic leukemia: results from a phase 1b study. Blood 126: 830. [Google Scholar]
- Maddocks K., Ruppert A., Lozanski G., Heerema N., Zhao W., Abruzzo L., et al. (2015) Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol 1: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato A., Nabhan C., Barr P., Ujjani C., Hill B., Lamanna N., et al. (2015) Favorable outcomes in CLL Pts with alternate kinase inhibitors following ibrutinib or idelalisib discontinuation: results from a large multi-center study. Blood 126: 719. [Google Scholar]
- Morabito F., Gentile M., Seymour J., Polliack A. (2015) Ibrutinib, idelalisib and obinutuzumab for the treatment of patients with chronic lymphocytic leukemia: three new arrows aiming at the target. Leuk Lymphoma 56: 3250–3256. [DOI] [PubMed] [Google Scholar]
- Mössner E., Brünker P., Moser S., Püntener U., Schmidt C., Herter S., et al. (2010) Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 115: 4393–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute (NCI) (2016) Surveillance Epidemiology and End Results Cancer Statistics Review. Available at: http://seer.cancer.gov/statfacts/html/clyl.html (accessed 27 February 2016).
- O’Brien S., Lamanna N., Kipps T., Flinn I., Zelenetz A., Burger J., et al. (2014) Update on a phase II study of idelalisib in combination with rituximab in treatment-naïve patients ⩾65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). ASH: Blood 436. [Google Scholar]
- O’Brien S., Moore J., Boyd T., Larratt L., Skotnicki A., Koziner B., et al. (2009) 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. J Clin Oncol 27: 5208–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A., Norberg M., Okvist A., Derkow K., Choudhury A., Tobin G., et al. (2007) Upregulation of bfl-1 is a potential mechanism of chemoresistance in B-cell chronic lymphocytic leukaemia. Br J Cancer 97: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott C., Federation A., Kasar S., Klitgaard J., Fernandes S., Brown J., et al. (2015) Enhancer landscapes reveal transcription factor network dependencies in chronic lymphocytic leukemia. ASH: Blood 436. [Google Scholar]
- Pan R., Hogdal L., Benito J., Bucci D., Han L., Borthakur G., et al. (2014) Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov 4: 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakonstantinou G., Verbeke C., Hastka J., Bohrer M., Hehlmann R. (2001) Bcl-2 expression in non-Hodgkin’s lymphomas is not associated with bcl-2 gene rearrangements. Br J Haematol 113: 383–390. [DOI] [PubMed] [Google Scholar]
- Pascutti M., Jak M., Tromp J., Derks I., Remmerswaal E., Thijssen R., et al. (2013) IL-21 and CD40L signals from autologous T cells can induce antigen-independent proliferation of CLL cells. Blood 122: 3010–3019. [DOI] [PubMed] [Google Scholar]
- Patz M., Isaeva P., Forcob N., Müller B., Frenzel L., Wendtner C., et al. (2011) Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br J Haematol 152: 295–306. [DOI] [PubMed] [Google Scholar]
- Pekarsky Y., Zanesi N., Croce C. (2010) Molecular basis of CLL. Semin Cancer Biol 20: 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper C., Lin T., Pratt G., Hewamana S., Brennan P., Hiller L., et al. (2008) MCL-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood 112: 3807–3817. [DOI] [PubMed] [Google Scholar]
- Phillips D., Xiao Y., Lam L., Litvinovich E., Roberts-Rapp L., Souers A., et al. (2015) Loss in MCL-1 function sensitizes non-Hodgkin’s lymphoma cell lines to the BCL-2-selective inhibitor venetoclax (ABT-199). Blood Cancer J 5: e368 DOI: 10.1038/bcj.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portell C., Axelrod M., Brett L., Gordon V., Capaldo B., Xing J., et al. (2014) Synergistic cytotoxicity of ibrutinib and the BCL2 antagonist, ABT-199(GDC-0199) in mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL): molecular analysis reveals mechanisms of target interactions, Blood 124: 509. [Google Scholar]
- Roberts A., Advani R., Kahl B., Persky D., Sweetenham J., Carney D., et al. (2015) Phase I study of the safety, pharmacokinetics, and antitumour activity of the BCL2 inhibitor navitoclax in combination with rituximab in patients with relapsed or refractory CD20+ lymphoid malignancies. Br J Haematol 170: 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Davids M., Pagel J., Kahl B., Puvvada S., Gerecitano J., et al. (2016) Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 374: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Seymour J., Brown J., Wierda W., Kipps T., Khaw S., et al. (2012) Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol 30: 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles G., Boyd T., Morschhauser F., Wendtner C., Hallek M., Eichhorst B., et al. (2015) Updated safety and preliminary efficacy data from a phase Ib study combining venetoclax (GDC-0199, ABT-199) with bendamustine/rituximab in patients with relapsed/refractory or previously untreated chronic lymphocytic leukemia. Blood 126: 829.26272046 [Google Scholar]
- Scarfò L., Ghia P. (2013) Reprogramming cell death: BCL2 family inhibition in hematological malignancies. Immunol Lett 155: 36–39. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Wild J., Strunz B., Kanz L., Salih H. (2015) Belimumab, a neutralizing BAFF (B-cell activating factor) antibody, enhances the susceptibility of chronic lymphoid leukemia (CLL) cells to ABT-199 treatment. Blood 126: 2947. [Google Scholar]
- Seymour J., Davids M., Pagel J., Kahl B., Wierda W., Puvvada S., et al. (2014) ABT-199 (GDC-0199) in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL): High complete-response rate and durable disease control. Journal of Clinical Oncology 32: abstract 7015. [Google Scholar]
- Seymour J., Gerecitano J., Kahl B., Pagel J., Wierda W., Anderson M., et al. (2013) The single-agent Bcl-2 inhibitor ABT-199 (GDC-0199) in patients with relapsed/refractory (R/R) non-Hodgkin lymphoma (NHL): responses observed in all mantle cell lymphoma (MCL) patients. Blood 122: 1789.23869085 [Google Scholar]
- Song T., Chai G., Liu Y., Yu X., Wang Z., Zhang Z. (2016) Bcl-2 phosphorylation confers resistance of chronic lymphocytic leukemia cells to the BH3 mimetic ABT-737, ABT-263 and ABT-199 by impeding direct binding. Br J Pharmacol 173: 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souers A., Leverson J., Boghaert E., Ackler S., Catron N., Chen J., et al. (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19: 202–208. [DOI] [PubMed] [Google Scholar]
- Stilgenbauer S., Eichhorst B., Schetelig J., Coutre S., Seymour J., Munir T., et al. (2015) Venetoclax (ABT-199/GDC-0199) pumonotherapy induces deep remissions, including complete remission and undetectable MRD, in ultra-high risk relapsed/refractory chronic lymphocytic leukemia with 17p deletion: results of the pivotal international phase II study. Blood 126: LBA-6. [Google Scholar]
- Sylvan S., Skribek H., Norin S., Muhari O., Österborg A., Szekely L. (2015) Sensitivity of chronic lymphocytic leukemia cells to small targeted therapeutic molecules: an in vitro comparative study. Exp Hematol 44: 38–49, e1. DOI: 10.1016/j.exphem.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Tam C., Anderson M., Ritchie D., Januszewicz E., Carney D., Roberts A., et al. (2015) Favorable patient survival after failure of venetoclax (ABT-199/ GDC-0199) therapy for relapsed or refractory chronic lymphocytic leukemia (CLL). Blood 126: 2939. [Google Scholar]
- Thijssen R., Slinger E., Weller K., Geest C., Beaumont T., van Oers M., et al. (2015) Resistance to ABT-199 induced by microenvironmental signals in chronic lymphocytic leukemia can be counteracted by CD20 antibodies or kinase inhibitors. Haematologica 100: e302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P., Tam C., O’Brien S., Wierda W., Stingo F., Plunkett W., et al. (2016) Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood 127: 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft M., Wei A., Mason K., Vandenberg C., Chen L., Czabotar P., et al. (2006) The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via BAK/BAX if MCL-1 is neutralized. Cancer Cell 10: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler M., Butterworth M., Majid A., Walewska R., Sun X., Dyer M., et al. (2009) Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood 113: 4403–4413. [DOI] [PubMed] [Google Scholar]
- Vogler M., Dinsdale D., Dyer M., Cohen G. (2013) ABT-199 selectively inhibits BCL2 but not BCL2L1 and efficiently induces apoptosis of chronic lymphocytic leukaemic cells but not platelets. Br J Haematol 163: 139–142. [DOI] [PubMed] [Google Scholar]
- Vogler M., Furdas S., Jung M., Kuwana T., Dyer M., Cohen G. (2010) Diminished sensitivity of chronic lymphocytic leukemia cells to ABT-737 and ABT-263 due to albumin binding in blood. Clin Cancer Res 16: 4217–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W., O’Connor O., Czuczman M., LaCasce A., Gerecitano J., Leonard J., et al. (2010) Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol 11: 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyach J., Furman R., Liu T., Ozer H., Zapatka M., Ruppert A., et al. (2014) Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med 370: 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyach J., Johnson A. (2015) Targeted therapies in CLL: mechanisms of resistance and strategies for management. Blood 126: 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]