Abstract
Purpose
To perform a two-cohort, phase 1 safety and immunogenicity study of IMA950 in addition to standard chemo-radiotherapy (CRT) and adjuvant temozolomide in patients with newly diagnosed glioblastoma (GBM). IMA950 is a novel GBM specific therapeutic vaccine containing 11 tumor-associated peptides (TUMAPs), identified on human leukocyte antigen (HLA) surface receptors in primary human GBM tissue.
Experimental Design
Patients were HLA-A*02 positive and had undergone tumor resection. Vaccination comprised 11 intradermal injections with IMA950 plus GM-CSF over a 24 week period, beginning 7-14 days prior to initiation of CRT (Cohort 1) or 7 days post CRT (Cohort 2). Safety was assessed according to NCI CTCAE Version 4.0 and TUMAP specific T-cell immune responses determined. Secondary observations included progression-free survival (PFS), pre-treatment regulatory T-cell (Treg) levels and the effect of steroids on T-cell responses.
Results
Forty five patients were recruited. Related adverse events included minor injection site reactions, rash, pruritus, fatigue, neutropenia and single cases of allergic reaction, anemia and anaphylaxis. Two patients experienced Grade 3 dose limiting toxicity of fatigue and anaphylaxis. Of 40 evaluable patients, 36 were TUMAP responders and 20 were multi-TUMAP responders, with no important differences between cohorts. No effect of pre-treatment Treg levels on IMA950 immunogenicity was observed and steroids did not affect TUMAP responses. PFS was 74% at 6 months and 31% at 9 months.
Conclusion
IMA950 plus GM-CSF was well tolerated with the primary immunogenicity endpoint of observing multi-TUMAP responses in at least 30% of patients exceeded. Further development of IMA950 is encouraged.
Keywords: IMA950, Phase I study, therapeutic cancer vaccine, glioblastoma, immunotherapy
Introduction
GBM, the most aggressive central nervous system tumor, develops from glial tissue of the brain and spinal cord (1). Newly diagnosed GBM is an orphan disease with 100% mortality and a median overall survival (OS) of only 14.6 months (2). Standard first-line therapy comprises maximal safe tumor resection, followed by concomitant chemo-radiotherapy (radiotherapy plus daily temozolomide; CRT) and six 28-day cycles of adjuvant temozolomide (TMZ) (2). Although the incidence is relatively low, around 3 to 4 cases per 100,000 population (3), GBM affects patients of all ages and there is a large unmet medical need for improved first-line therapy. Furthermore, there is evidence to suggest that the overall incidence of GBM is rising over time and will continue to increase in an ageing population (4, 5).
IMA950 is an immunotherapeutic multiple-peptide vaccine specifically developed to treat GBM (6). It contains 11 tumor-associated peptides (TUMAPs) that are presented by a majority of GBMs on human leukocyte antigen (HLA) surface receptors. IMA950 is designed to trigger the immune system by activation of TUMAP-specific cytotoxic T cells. Once activated, these cells are postulated to find and destroy malignant tumor cells presenting the cognate TUMAPs. By vaccinating with 11 TUMAPs simultaneously there is an increased probability that a multi-clonal, broad yet highly specific T-cell response can be mounted against tumor cells thus hindering potential tumor escape mechanisms.
The primary objectives of this first time in human study were to assess the safety, tolerability and immunogenicity of IMA950 plus GM-CSF given alongside standard therapy in newly diagnosed GBM patients.
Patients and Methods
Patients
Eligible patients had histologically or cytologically proven GBM, an operable tumor which had already been maximally resected, were at least 18 years of age, human leukocyte antigen (HLA) A*02 positive and hepatitis B core antigen seronegative; had a World Health Organization (WHO) performance status 0 or 1, a life expectancy of at least 30 weeks and were expected to complete standard CRT and six 28 day cycles of adjuvant TMZ. Key exclusion criteria included: receipt of any prior GBM treatment apart from surgery, vaccination within 2 weeks or having taken dexamethasone at a dose >4 mg/day within 7 days prior to the first IMA950 plus GM-CSF vaccination, a history of serious cardiac or autoimmune disease or any condition which might interfere with the patient’s ability to generate an immune response. This study was conducted in accordance with the principles of International Conference on Harmonisation (ICH) Good Clinical Practice (GCP), the requirements of the UK Clinical Trials regulations (SI 2004/1031 and SI 2006/1928), and the Declaration of Helsinki. The study protocol, patient information sheet and informed consent form were approved by the Sponsor’s Central Institutional Review Board, and the appropriate Research Ethics Committee prior to study conduct. After a full explanation of the study protocol, written informed consent was obtained from all patients before being enrolled.
IMA950 Vaccine
IMA950 is a novel multi-peptide GBM specific vaccine comprising 11 HLA binding TUMAPs and one viral marker peptide, identified on HLA surface receptors in primary human GBM tissue, as described previously (6). Supplementary Table S1 gives an overview of the TUMAP source antigens and their respective expression levels found in primary GBM tumor samples. Selected TUMAPs are designed to activate TUMAP-specific CD8+ cytotoxic and CD4+ helper T lymphocytes, which then recognize cognate TUMAPs presented by GBM tumor cells and effect a targeted immune response. Nine of the 11 TUMAPs were selected on the basis of their functional relevance, association with the human leukocyte antigen HLA-A*02, over-expression in GBM and proven immunogenicity using in vitro T-cell assays. The other two TUMAPs contained in IMA950 are both HLA class II-binding peptides designated IMA-BIR-002 and IMA-MET-005. IMA-BIR-002 has the capacity to activate CD4+ helper T cells (7) and potentially cytotoxic T lymphocytes (CTLs). IMA-MET-005 contains a known HLA class I epitope, which was elongated based on the natural sequence of c-Met (known oncogene and potential marker of GBM stem cells (8), with the capacity to activate helper T cells (9) and, after processing, CTLs). An additional non-TUMAP (IMA-HBV-001) was included in IMA950 derived from Hepatitis B virus (HBV) core antigen, to act as a positive control from a “non-self” antigen in cases where no vaccine-induced T-cell responses to TUMAPs from “self” antigens are observed.
Study Design and Treatment
Vaccination comprised fixed doses of recombinant granulocyte macrophage-colony stimulating factor (GM-CSF; 75 μg), a commonly used immunomodulator (10), followed by IMA950 (4.96 mg, 413 μg each peptide) injected intradermally (i.d.) at 11 time points over a 24 week period. All patients received the same vaccination schedule comprising an “Induction Phase” (VIP) of six intensive vaccinations (V1-V6), followed by a "Maintenance Phase" (VMP) of five vaccinations (V7-V11) over a longer period. Forty five patients with newly diagnosed GBM were entered into one of two Cohorts that differed by virtue of the first vaccination given at different time points alongside standard therapy (rationale for recruiting at least 20 patients per cohort is given in Supplementary Table S2). In Cohort 1, the VIP started 7 to 14 days before the scheduled onset of CRT to ensure that at least the first three vaccinations (Days 1, 2, 3) were administered prior to the start of CRT. In Cohort 2, the VIP started a minimum of 7 days after the final dose of CRT and 28 days (+7 days) prior to the first scheduled dose of adjuvant TMZ. This ensured that all six vaccinations in the VIP were administered at least a week after the end of immunosuppressive CRT and completed a week prior to the start of adjuvant TMZ. Three safety observation periods of 21 days were included after 1, 3 and 6 patients had completed 21 days of treatment prior to opening to general recruitment. CRT comprised 54 to 60 Gray in 30 daily fractions over 6 weeks with concomitant daily TMZ, 75 mg/m2 throughout. Adjuvant TMZ, 150-200 mg/m2 for 5 days began 35 (+/-7 days) following the last fraction of radiotherapy, repeated every 28 days for a total of 6 cycles. See Supplementary Fig. S1 for a detailed overview of the treatment and assessment schedule.
Patient Monitoring and Assessment
The primary study endpoint of safety and tolerability was assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0. Disease was assessed using MacDonald criteria (11) with the secondary endpoint of progression free survival evaluated at 6 (PFS-6) and 9 months (PFS-9) from date of surgery. Any clinical complete (CR) or partial response (PR) to therapy was confirmed by an independent neuro-oncologist and radiologist. Although not an endpoint of the study, survival data was collected for two years after the final patient had received their first vaccination. The cut-off date for analysis was February 18, 2015.
Pharmacodynamic Analysis
A co-primary endpoint was determining the number of patients showing patient individual T-cell responses directed against TUMAPs contained in IMA950 at one or more post-vaccination time points, as determined by HLA multimer analysis (12, 13). Individual patient peripheral blood mononuclear cell (PBMC) samples were pooled in order to ensure sufficient viable PBMCs for multimer analysis as follows: “Pre-vaccination” (PBMC samples 1 and 2), “post-vaccination 1” (PBMC samples 3 and 4), “post-vaccination 2” (PBMC samples 5 and 6) and “post-vaccination 3” (samples 7 and 8). See Supplementary Fig. S1 for further details. Tetramer staining for each TUMAP and control antigens were performed after an in vitro sensitization as described previously (13). Exemplary gating is shown in Supplementary Fig. S2. A positive vaccine-induced multimer CD8 T-cell response for any specific post-vaccination time point of a given antigen and patient was assigned if the following criteria were met: an above threshold immune response (assessed by five independent, trained and blinded jurors and according to Association for Cancer Immunotherapy recommendations (14)) and an at least four-fold higher frequency of multimer positive CD8 T cells (normalized to total CD8 T cells) compared to the respective pre-vaccination time point. Based on prior clinical experience, at the time of study inception, with similar multi-peptide vaccines (13), study success criteria were defined as either ≥ 30% multi-TUMAP response or > 60% single TUMAP response in the study population. Further development would be recommended if either criterion was met. Secondary outcome measures included Treg levels (defined as CD3+CD4+CD8-CD25highCD127lowFoxp3+ lymphocytes (15)) pre- and post-vaccination, and correlation of steroid dose with observed T-cell responses. Research analysis examined the kinetics of TUMAP immunogenicity, effect of O6-Methylguanine DNA methyltransferase (MGMT) promoter methylation status on PFS and exploring the possible effects of vaccination on observed disease pseudo-progression and pseudo-regression measured using a standardized diffusion-weighted (DWI) and perfusion-weighted (PWI) magnetic resonance imaging (MRI) protocol. Pseudo-progression was defined as an apparent increase in the enhancing tumor (>25%) on an early reference scan followed by a reduction in subsequent scans (assessed at Week 25 onwards), with no associated clinical deterioration. Pseudo-regression was assessed using an inverse definition. It was recommended that patients continue on therapy until the true clinical diagnosis was clarified. Although this design pre-dated that of recently published guidance, suggesting that patients continue the immunotherapy regimen for 3 months prior to PD confirmation (16), it is generally in line with these recommendations.
Statistical Analysis
For the pharmacodynamic analysis, several different methods were used to calculate statistical significance depending on the type of data being examined. All statistical analysis was performed using Prism version 6.02 software (GraphPad Software Inc., La Jolla, California, USA). Two-tailed non-parametric Mann-Whitney test was used to determine differences between independent groups under examination. This included, for example, the number of vaccine induced TUMAP responses per patient between Cohorts and frequency of Treg as a percentage of total lymphocytes for a given patient compared between Cohorts. Fisher’s exact test was used to analyze contingency tables. This included a comparison of the proportion of patients showing a TUMAP response between Cohorts. Non-parametric Spearman’s rank correlation test was used to analyze dependence between two variables such as immune responses and regulatory T cell levels.
The Kaplan-Meier method was used to generate survival curves and estimate OS rates. Log rank test was used to compare the survival distributions between groups of patients that included censored data.
Statistical analysis of imaging parameters was performed using a one-way ANOVA analysis with post hoc intergroup analysis using Tukey’s test, due to a significant number of datasets being unavailable for analysis.
Results
Patient Demographics
Table 1 provides an overview of patient demographics. Of 138 patients screened, 53% were HLA-A*02 positive, which is in the expected range for a United Kingdom population (17). Reasons for non-entry of 26 HLA-A*02 patients is given in Supplementary Table S3. Forty five patients were recruited into the study; 22 in Cohort 1 and 23 in Cohort 2. Forty patients were immune evaluable, with 39 evaluable for clinical activity assessment. This discrepancy is a result of two patients being lost for follow up between blood sample 6 and week 25 scan (see Supplementary Fig. S1), including one patient that was immune evaluable. The overall median age was 53 years (range 20-75 years) with no meaningful difference between cohorts. All patients had WHO performance status (PS) 0 or 1 at recruitment. A larger proportion of patients in Cohort 2 (65%) had a PS of 1 compared to Cohort 1 (27%), most likely due to Cohort 2 patients having undergone treatment with CRT. As expected, the lymphocyte count on patient entry was lower in Cohort 2 (0.80x109/L) compared to Cohort 1 (1.49x109/L) reflecting the effect of concomitant TMZ in the former. Of the 38 patients evaluable for MGMT promoter methylation testing, 11 (29%) were positive for methylated promoter, 8 of 19 (42%) in Cohort 1 compared to 3 of 19 (16%) in Cohort 2.
Table 1.
Patients’ Baseline Characteristics
| Characteristic | Cohort 1 | Cohort 2 | Overall |
|---|---|---|---|
| Age, years | |||
| Median | 54 | 49 | 53 |
| Range | 21 – 75 | 20 – 68 | 20 – 75 |
| Sex, No. (%) | |||
| Male | 15 (68%) | 15 (65%) | 30 (67%) |
| Female | 7 (32%) | 8 (35%) | 15 (33%) |
| Total | 22 | 23 | 45 |
| WHO performance status, No. (%) | |||
| 0 | 16 (73%) | 8 (35%) | 24 (53%) |
| 1 | 6 (27%) | 15 (65%) | 21 (47%) |
| MGMT methylation status, No. (%†) | |||
| Methylated | 8 (42%) | 3 (16%) | 11 (29%) |
| Unmethylated | 11 (58%) | 16 (84%) | 27 (71%) |
| Unavailable | 3 | 4 | 7 |
| Lymphocyte count, x109/L | |||
| Median | 1.49* | 0.80* | 1.12 |
| Range | 0.88 – 2.50 | 0.35 – 1.91 | 0.35 – 2.50 |
| Concomitant steroid use, No. (%) | |||
| Yes | 16 (73%) | 17 (74%) | 43 (73%) |
| Entry concomitant steroid dose, mg | |||
| Median | 2.0 | 1.5 | 2.0 |
| Range | 0 – 4.0 | 0 – 4.0 | 0 – 4.0 |
Abbreviations: WHO, World Health Organization; MGMT, O6-Methylguanine DNA methyltransferase.
Percentages calculated excluding those patients whose MGMT methylation status was unavailable.
Significantly different lymphocyte counts between the two cohorts; p < 0.0001, two-tailed Man-Whitney test.
Safety
All patients received at least one vaccination and were evaluated for safety (see Table 2 for the most commonly reported adverse events (AEs), regardless of causality). Injection site reaction (ISR) was the most frequent AE, and most common study drug related AE with 81 instances reported in 26 patients (12/22 patients in Cohort 1 and 14/23 patients in Cohort 2). The majority of ISRs were grade 1 (24 out of 26 patients) with only two instances of grade 2 events. Thirty one patients experienced at least one serious adverse event (SAE), one of which was a death unrelated to the study drug. The most frequently reported SAEs were seizure in 8 patients followed by thromboembolic events in 6 patients, none was study drug related. Investigators considered 4 SAEs to be related to the study drug including two cases of grade 4 neutrophil count decrease and one case each of grade 3 fatigue and anaphylaxis. The related SAEs of anaphylaxis and fatigue were both considered dose limiting toxicities. There were no unexpected differences in the safety profiles observed in the two cohorts.
Table 2.
Most Common Adverse Events Occurring in >20% Patients (regardless of causality)
| Grade, No. |
|||||
|---|---|---|---|---|---|
| Symptom* | 1 | 2 | 3 | 4 | Total No. (% pts) |
| Nausea | 21 | 6 | 0 | 0 | 27 (60%) |
| Injection Site Reaction | 24 | 2 | 0 | 0 | 26 (58%) |
| Fatigue | 16 | 5 | 4 | 0 | 25 (56%) |
| Headache | 20 | 2 | 0 | 0 | 22 (49%) |
| Vomiting | 16 | 4 | 1 | 0 | 21 (47%) |
| Alopecia | 8 | 8 | 0 | 0 | 16 (36%) |
| Dizziness | 11 | 3 | 0 | 0 | 14 (31%) |
| Seizure | 4 | 4 | 3 | 2 | 13 (29%) |
| Cough | 9 | 2 | 0 | 0 | 11 (24%) |
Abbreviations: pts, patients.
Patients may have experienced multiple AEs of the same type.
Pharmacodynamics
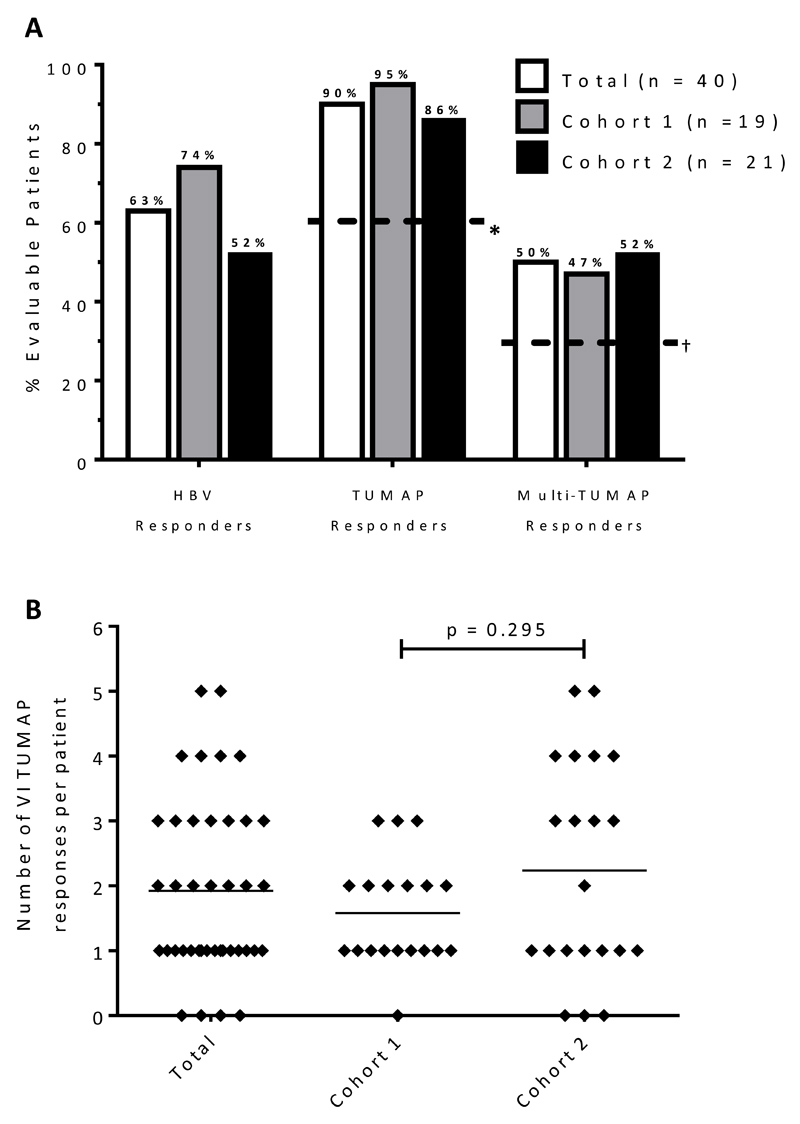
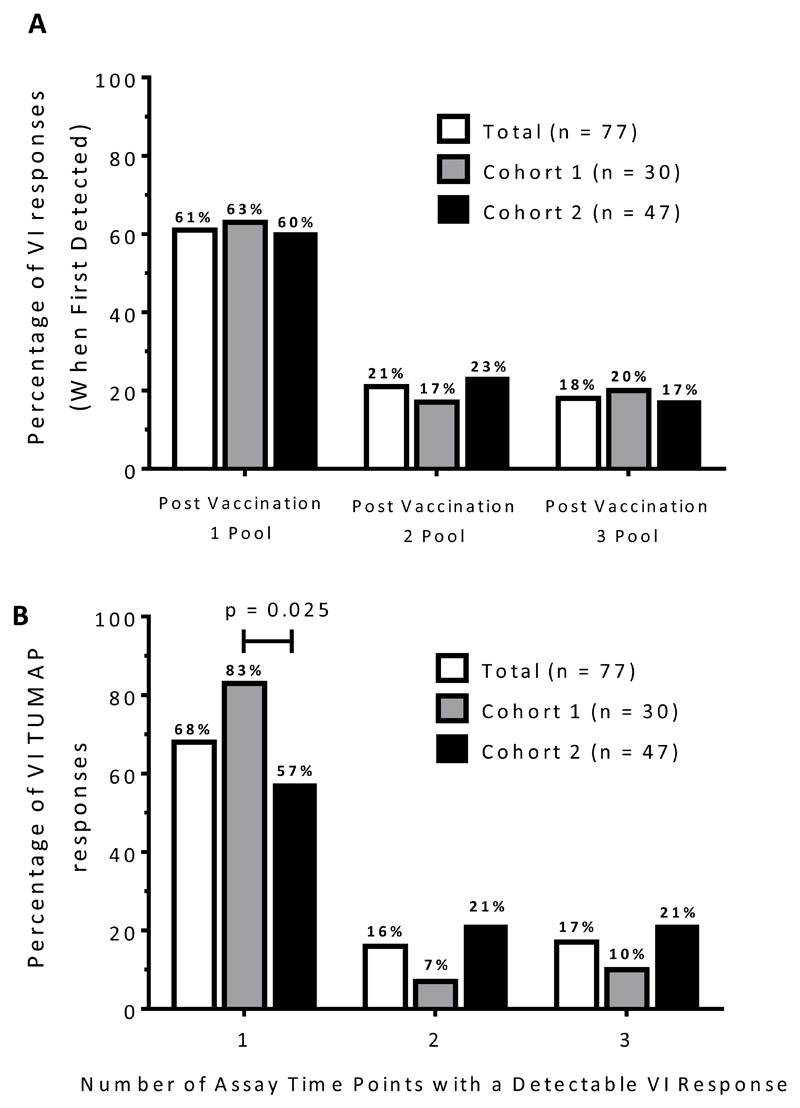
Thirty six of 40 immune evaluable patients (90%) were TUMAP responders, with 20 (50%) responding to more than one TUMAP (Fig. 1A). The pre-defined primary immunologic endpoint for recommending further development (≥60% single or ≥30% multi TUMAP responders) was therefore reached for the total immune evaluable study population and each of the two individual study cohorts. In Cohort 1, 9/19 (47%) evaluable patients responded to multiple TUMAPs, with a further 9 (47%) responding to a single TUMAP. Similarly, in Cohort 2, 11/21 (52%) evaluable patients had multiple TUMAP responses and a further 7 (33%) had a single response. Although the number of vaccine induced responses per patient in Cohort 2 appeared to be greater than in Cohort 1 (an arithmetic mean of 2.2 in Cohort 2 versus 1.6 in Cohort 1), this was not statistically significant (p=0.3; Mann Whitney test; Fig. 1B). Immune response kinetics showed a predominant onset of vaccine-induced TUMAP responses in the post-vaccination 1 sample PBMC pool, with 47 (61%) being detected at this time point (Fig. 2A). This was also true for each cohort. In addition, 24 out of 77 (31%) of vaccine-induced TUMAP responses were already detectable pre-vaccination and were boosted at least four-fold by administration of IMA950 plus GM-CSF (data not shown). The majority of vaccine-induced TUMAP responses were detectable at one post-vaccination assay time point only (61%, 52/77 immune responses; Fig. 2A) and were of relatively low magnitude (see Supplementary Fig. S3 for exemplary data). The proportion of vaccine induced TUMAP responses detected at only one post-vaccination assay time point was significantly higher (p=0.025; Fisher’s exact test) in Cohort 1 (25/30 immune responses; 83%) than in Cohort 2 (27/47 immune responses; 57%) (Fig. 2B). No apparent differences in TUMAP responses were noted between patients who were and were not receiving concomitant steroid treatment (data not shown).
Figure 1. Primary Immune Response Summary.
(A) Further development is based on: * >60% of patients being single or f †30% of patients being multi-TUMAP responders. (B) The number of vaccine-induced TUMAP responses is shown for the overall immune evaluable patient population (n=40) as well as for study cohorts. Black lines indicate mean values. For statistical analysis the Mann-Whitney test was used.
Abbreviations: HBV, hepatitis B virus-derived vaccinated marker peptide; TUMAP, tumor associated peptide; VI, vaccine induced.
Figure 2. Onset and sustainability of vaccine induced immune responses.
(A) Onset (first appearance) of vaccine-induced immune responses to IMA950 TUMAPs (n=77 total detected vaccine-induced responses in n=40 immune evaluable patients). (B) The percentage of vaccine-induced responses to IMA950 TUMAPs with detection at one, two or three post-vaccination assay time points. p-values were calculated using the Fisher’s exact test (only significant results are shown).
Abbreviations: TUMAP, tumor associated peptide; VI, vaccine induced.
Twenty five immune evaluable patients (63%) responded to the “non-self” viral antigen, IMA-HBV-001 (13) and was by trend, associated with the number of vaccine-induced TUMAP responses (p=0.117 by Wilcoxon test; data not shown). There was also a trend for the proportion of IMA-HBV-001 responders to be enriched within the multi-TUMAP responder fraction of patients (p=0.191 by Fisher’s exact test; data not shown).
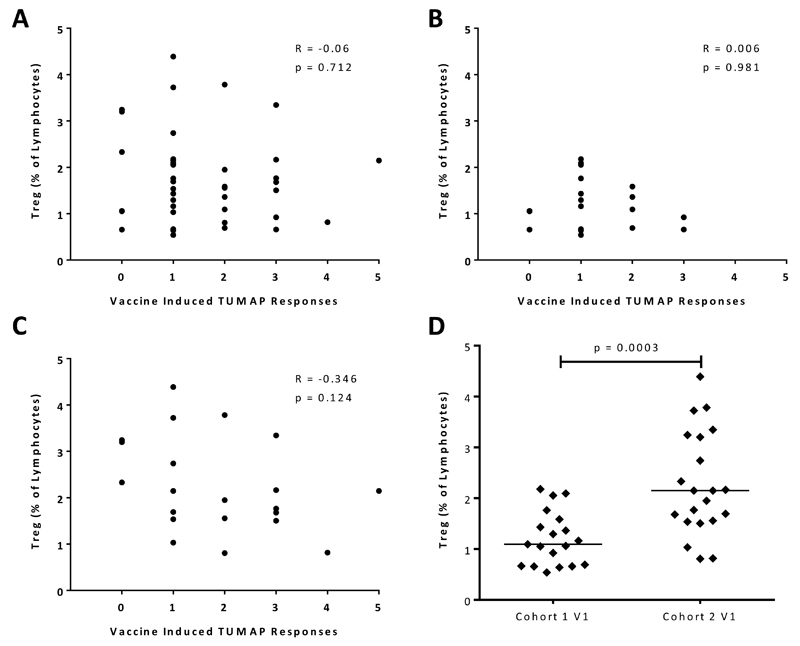
There was no correlation between pre-treatment Treg levels and number of vaccine-induced TUMAP responses overall (Fig. 3A) or within either cohort of patients (Fig 3B and C). A comparative analysis of study cohorts revealed that pre-treatment Treg levels normalized to lymphocytes were significantly increased (p=0.0003 by Wilcoxon test) in Cohort 2 compared to Cohort 1 (Fig. 3D).
Figure 3. Correlation of pre-treatment levels of regulatory T cells with vaccine-induced immune responses to IMA950 TUMAPs.
Treg (CD4+/CD25hi/CD127lo/FoxP3+) levels, normalized to lymphocytes, at V1 were analyzed in correlation with vaccine-induced CD8 T-cell responses to IMA950 TUMAPs in (A) all immune evaluable patients with n=40, (B) study Cohort 1 with n=19 and (C) study Cohort 2 with n=21. Correlation coefficients and p-values, calculated using Spearman’s correlation, are indicated on each graph. (D) Cohort comparison of pre-treatment Treg levels on the first vaccination day for immune evaluable patients. For statistical analysis the Mann-Whitney test was used.
Abbreviations: Treg, regulatory T cells; TUMAP, tumor associated peptide; V1, vaccination 1.
In order to explore possible effects of vaccination on observed pseudo-progression and pseudo-regression of disease, DWI and PWI was performed alongside standard gadolinium MRI scans. Cohort 1 patients showed increases in apparent diffusional coefficient (p<0.05), following CRT (see Supplementary Fig. S4). Over the same period, PWI parameters showed a trend (albeit not statistically significance) towards increased T1 values, contrast transfer coefficient (Ktrans) and total enhancing volume (ve) with an associated decrease in plasma volume (vp) between scans 1 and 2 (data not shown).
Clinical Activity
Twenty nine of 39 evaluable patients were progression free at 6 months (PFS-6 of 74.4%) with 12 continuing to be progression free at 9 months (PFS-9 of 30.8%). Stable disease (SD) was confirmed for 11 evaluable patients (28.2%) at Week 40. One patient with residual disease at baseline had a partial response (PR) at Week 40, with tumor size decreasing from 357 mm2 at baseline to 25 mm2 at week 17, being maintained until they went off study. Four patients with SD and the patient with PR at Week 40 had MGMT promoter methylation (5/11 patients with a methylated MGMT promoter; 45.5%). Five other patients with SD at Week 40 had unmethylated MGMT promoters (5/27 patients with an unmethylated MGMT promoter; 18.5%). Eleven patients out of an evaluable 38 (29%) had a methylated MGMT promoter, which conferred a significant survival advantage (28.3 versus 14.8 months; p=0.025 using Log-rank test; data not shown).
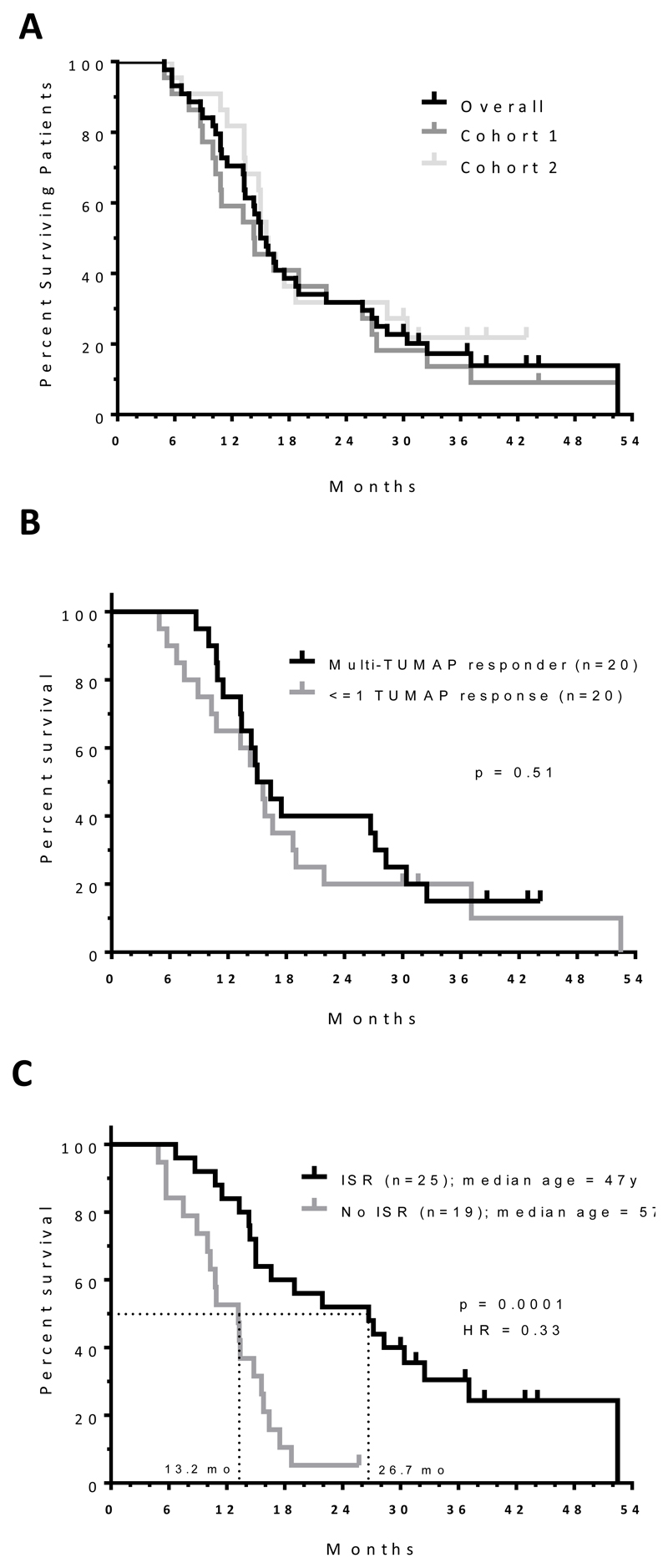
As of the cutoff date (18-Feb-15), median OS for the study was 15.3 months (Fig. 4A) with no significant differences between the cohorts or those patients that responded to multiple TUMAPs compared to those that did not respond or to one TUMAP only (Fig. 4B). Interestingly, patients experiencing one or more ISRs had a significantly improved (p = 0.0001; hazard ratio 0.33) median OS of 26.7 months compared to 13.2 months for those that did not (Fig. 4C). The median age of patients in the ISR group was significantly lower than that of the non-ISR (47 versus 57 years respectively; p = 0.023 by Mann Whitney test). Imaging parameters in patients displaying ISRs showed no significant difference. However in Cohort 2 ISR was associated with lower Ktrans (p <0.05), vp (p<0.01), ve (p<0.05) and rate constant Kep (p<0.05) values at baseline.
Figure 4. Overall survival from date of surgery for different patient sub-sets.
A) Median OS was 15.3 months for all patients (n = 44), 14.4 months for patients in Cohort 1 (n =22) and 15.7 months for patients in Cohort 2 (n = 22). There was no significant difference between each of the cohorts (p = 0.63, Log-rank test); one patient was lost for follow up in Cohort 2 and excluded from survival analysis. B) Relationship between survival and TUMAP response. Only patients that were immune evaluable were included in the analysis. Log-rank test was used to calculate significance between the two different patient populations. C) Relationship between overall survival and injection site reaction. One patient was lost to survival follow up and is excluded from the analysis. Log-rank test was used to calculate significance and hazard ratio. Median age of patients in the ISR group was significantly lower than that of the non-ISR (47 versus 57 years respectively; p = 0.023 by Mann Whitney test).
Abbreviations: HR, hazard ratio; ISR, injection site reaction; TUMAP, tumor associated peptide; y, years.
Discussion
In the majority of treated GBM patients, IMA950 produced antigen specific peripheral CD8+ T-cell immune responses to the TUMAPs contained within the vaccine, with a relatively benign drug related toxicity profile comprising mainly minor injection site reactions. The two cohort study design was used to help define the most biologically effective and clinically feasible administration schedule of IMA950 for subsequent development as determined by the level of vaccine induced TUMAP specific immune responses for each schedule. However, it does not allow direct comparison of clinical efficacy between cohorts since recruitment was not randomized nor was the trial prospectively powered to make such a comparison. Both cohorts presented challenges that had the potential to interfere with successful vaccination and the mounting of a measurable TUMAP specific immune response. In Cohort 1, there was a risk that CRT could be immunosuppressive (18, 19) and interfere with the induction and maintenance of TUMAP specific CD8+ T cells. Whereas in Cohort 2 there was the possibility that following completion of CRT, patient lymphocyte counts would be depleted and have lost the ability to mount a detectable immune response to IMA950. Indeed, immune data showed that Cohort 1 patients had a decreased detection rate of vaccine induced TUMAP responses at later time points (Fig. 2), suggesting that CRT may interfere with the induction and maintenance of antigen specific CD8+ T cells. The greater number and improved durability of TUMAP responses in Cohort 2 suggests that lymphocyte depletion caused by CRT is either insufficient to hinder induction of antigen specific CD8+ T cells or can be recovered sufficiently rapidly to support their expansion.
Treg are a potent immunosuppressive cell population (20) that may interfere with the immunogenicity of cancer vaccines (21). Given this, an additional key biological endpoint of this study was to explore the effect of pre-treatment Treg levels on the immunogenicity of IMA950. There was no correlation between pre-treatment Treg levels (relative to the overall lymphocyte population) and the number of vaccine induced TUMAP responses for the overall group of immune evaluable study patients. This result is similar to previous reports in other GBM vaccine studies (22, 23). There was a significant increase in the Treg levels at the start of vaccinations in Cohort 2 compared to Cohort 1, likely indicating a relative increase of Treg compared to other lymphocyte subpopulations as a result of the preceding CRT (24). The importance of this finding is unclear given that there were more vaccine-induced immune responses in Cohort 2.
The overall number of immune evaluable patients responding to multiple TUMAPs in this study (50%) exceeded that demonstrated for other similar vaccine products (13) such as IMA901, which had a multi-TUMAP response rate of 26%. In contrast to that found with IMA901, there was no apparent correlation between the number of TUMAP responses and improved survival (Fig. 4B). However, there are key differences between this study and that of IMA901. IMA901 comprises different TUMAPs, selected specifically for the treatment of renal cell carcinoma (RCC) patients and the IMA901 study was conducted in the absence of potentially confounding standard of care therapy. Low dose cyclophosphamide (shown to decrease the number and function of Treg (25, 26)) was also used alongside GM-CSF to further enhance immune response potential. In addition, RCC is known to be an immune-responsive tumor type (27), whereas immunotherapy for GBM is still in its infancy. Indeed, cancer vaccine immunotherapy strategies for GBM patients require considerable refinement due to the challenges posed by immune resistance and suppression in this tumor type (28). Multiple immunosuppressive mechanisms are likely to be important in GBM including, enhanced secretion of immunosuppressive factors after exposure to standard therapy (29), induction of tumor infiltrating lymphocytes and Treg activity (30), as well as immune checkpoint pathways such as PD-1/PD-L1 and CTLA-4 (31, 32).
The aim of administering adjuvant(s) alongside therapeutic vaccines is to attempt to augment immune response and overcome immune suppression by either: moving the immune response toward Th1 or Th2 immunity, activating innate immunity or to serve as a local repository for prolonged antigen release and protection from degradation. In this study we utilized GM-CSF as an adjuvant based on the principle that it should enhance effective priming of T-cell responses (33, 34) and the fact that it had been successfully applied in late stage clinical trials (35). There is evidence to suggest that in some circumstances at least, GM-CSF may not significantly enhance immune responses and may even be detrimental (36). Even so, an earlier meta-analysis of published trials suggests that low-dose GM-CSF (40-80 μg for 1-5 days) given s.c. or i.d. at the site of vaccination enhances the cellular immune response, while high-dose, systemic treatment (>=100 μg) does not increase the efficacy of a peptide vaccine due to expansion of immune-inhibiting MDSCs (10). Based on this evidence, we opted for a fixed dose of 75 μg GM-CSF given i.d. prior to vaccination with IMA950. In light of the relatively low magnitude and transient immune responses, enhancement of the vaccination regimen, including selection of the most effective adjuvant partner(s), is necessary; for example by using alternate or additional adjuvants such as locally applied poly-ICLC (37), imiquimod (38) or systemically administering CD40 ligand (39) or cyclophosphamide (40). Combining cancer vaccines such as IMA950 with immune checkpoint inhibitors such as anti-PD1/PD-L1 or anti-CTLA4 antibodies should also be expected to enhance anti-tumor immune responses. This is based on the rationale that overcoming local immune suppression and T cell anergy by checkpoint blockade can be limited by the specificity/size of the pre-existing T cell population and the fact that some tumors are relatively non-immunogenic. Indeed, preclinical and clinical data is beginning to emerge demonstrating that the anti-tumor activity of immune checkpoint blockade can be enhanced by vaccination (41, 42).
The observation that patients experiencing one or more ISRs had improved survival and were generally of younger age, suggest that ISR may be a prognostic marker for a patient population with an inherently healthier immune system (43). This is supported by the significantly different imaging features in Cohort 2 patients experiencing ISRs whose tumors showed less vascularity and reduced angiogenesis associated vascular permeability. Although this was an unplanned and retrospective analysis, a contribution of the vaccine to patient survival for those with a more vigorous immune system cannot be ruled out and could be investigated in future randomized studies that might include a non-specific immunogen. In addition, methylation of MGMT promoter conferred a survival advantage for GBM patients, as previously reported (44).
A key factor that will need to be considered during the future development of IMA950 and therapeutic cancer vaccines more generally is the need to continue vaccination even after the disease appears to be progressing. Unlike conventional cancer chemotherapy, the effect of cancer immunotherapies is not directly on the disease but rather on the immune system which leads to a cellular immune response followed by tumoricidal biological activity and potentially improved patient survival (45). This can lead to non-typical patient survival curves and misinterpretation of study results. Given this, chronic vaccination beyond disease progression, and potentially during subsequent therapy, will need to be carefully planned as part of future positioning alongside other therapy for the treatment of GBM.
IMA-HBV-001 was also included in the IMA950 vaccine to act as a positive control in cases where no vaccine-induced T cell responses to TUMAPs from “self” antigens are observed. There was a trend (albeit not reaching statistical significance) for patients mounting an immune response toward IMA-HBV-001 also to respond to one or more TUMAP, supporting its use as a general immunogenicity marker. However these findings also suggest that IMA-HBV-001 has limited use as an independent control peptide for association analysis.
Successful development of effective therapeutic vaccines for cancer has proven to be particularly challenging. In the context of GBM, the most advanced therapeutic vaccine approach was that of rindopepimut (CDX-110) which consists a single 14-mer peptide derived from epidermal growth factor receptor variant III deletion mutation (EGFRvIII) (46). Results from a Phase II single arm study of rindopepimut, given to newly diagnosed EGFRvIII+ GBM patients post-CRT in combination with adjuvant TMZ, demonstrated a median OS of 21.8 months, an increase in anti-EGFRvIII antibody titer and clearance of EGFRvIII from the majority of analyzed post-treatment tumors (47). Even so, the resulting pivotal, double-blind, randomized, Phase III trial using the same schedule and setting was terminated at a planned interim analysis due to emergent data indicating that the study would not reach statistical significance for the primary OS endpoint (48). It is currently unclear as to why the study failed to meet the primary endpoint, albeit a median OS of 21.1 months was reported for the placebo treated group (versus 20.4 months for vaccinated), well above the expected median of approximately 16 months, which may have confounded the data. A previous report suggests that GBM patients taking part in US based Phase II trials have significantly longer survival compared to historical data (49). The authors speculate that this may be due to the novel agent being tested or advances in standard of care. If the latter is correct, the apparent improvement in survival found in the Phase II rindopepimut study may have lead to an overly optimistic prediction of clinical benefit and subsequent failure of the Phase III trial. It is also possible that the reported loss of EGFRvIII from tumors during the vaccination period may have led to escape from immune surveillance, an issue that the IMA950 vaccine attempts to address by simultaneous targeting of 11 different antigens (TUMAPs). Nevertheless, even though the study reported here clearly met predefined immune response success criteria, further clinical optimization should precede transition of IMA950 into the next phase of clinical development. This should include selection of the most appropriate adjuvant(s) and gaining a deeper understanding of how best to combine IMA950 with other immunotherapies, such as immune checkpoint inhibitors, in order to maximize the magnitude of immune response, as well as gaining a better understanding as to the optimal position and schedule of the vaccine relative to the current standard of care.
Supplementary Material
Statement of Translational Relevance.
Survival rates for patients with glioblastoma (GBM) are abysmal, with median overall survival of approximately 15 months. Immunotherapy of GBM is a promising area of investigation, although challenges around identification of novel and immunogenic target antigens exist. IMA950 is a GBM specific vaccine comprising 11 tumor-associated peptides (TUMAPs) developed to address this challenge. We have performed a phase 1 safety and immunogenicity study in newly diagnosed GBM patients using IMA950 plus GM-CSF alongside standard of care chemo-radiotherapy. Our results demonstrate that IMA950 is well tolerated with 90% of patients having a CD8+ T-cell immune response to at least one TUMAP, with 50% responding to two or more TUMAPs. No effect of pre-treatment regulatory T-cell levels on IMA950 immunogenicity was found and steroids did not appear to affect immune responses to the TUMAPs. This data provides evidence to support further development and optimization of IMA950 together with other immunotherapies for GBM.
Acknowledgments
Funding: This work was supported by grant C222/A11422 from Cancer Research UK. This work was also managed and sponsored by the Cancer Research UK Centre for Drug Development. Immatics Biotechnologies provided pharmacodynamic assay support and supplied IMA950 for this study.
Footnotes
Conflicts of interest Statement: Norbert Hilf, Sarah Kutscher, Juha Lindner, Oliver Schoor and Harpreet Singh are current or past employees of Immatics Biotechnologies. Sarah Kutscher, Norbert Hilf, Oliver Schoor and Harpreet Singh have stock ownership interests in Immatics Biotechnologies. Sarah Kutscher, Norbert Hilf, Oliver Schoor, Harpreet Singh have intellectual property interests in Immatics Biotechnologies. Sarah Kutscher, Norbert Hilf, Oliver Schoor and Harpreet Singh have received either travel, accommodation or other expenses from Immatics Biotechnologies during the previous two years. Harpreet Singh has a leadership role at Immatics Biotechnologies. Other authors disclosed no conflicts of interest.
Presented in abstract form at the European Society for Medical Oncology 2012 Congress, Vienna, Austria, September 28 to October 2, 2012; and European Society for Medical Oncology 2014 Congress, Madrid, Spain, September 26 to 30, 2014.
References
- 1.Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nature clinical practice Neurology. 2006;2:494–503. doi: 10.1038/ncpneuro0289. quiz 1 p following 16. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro-oncology. 2012;14(Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobes M, Khurana VG, Shadbolt B, Jain S, Smith SF, Smee R, et al. Increasing incidence of glioblastoma multiforme and meningioma, and decreasing incidence of Schwannoma (2000-2008): Findings of a multicenter Australian study. Surgical neurology international. 2011;2:176. doi: 10.4103/2152-7806.90696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson DR. Rising incidence of glioblastoma and meningioma in the United States: Projections through 2050. ASCO Meeting Abstracts. 2012;30:2065. [Google Scholar]
- 6.Dutoit V, Herold-Mende C, Hilf N, Schoor O, Beckhove P, Bucher J, et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain : a journal of neurology. 2012;135:1042–54. doi: 10.1093/brain/aws042. [DOI] [PubMed] [Google Scholar]
- 7.Widenmeyer M, Griesemann H, Stevanovic S, Feyerabend S, Klein R, Attig S, et al. Promiscuous survivin peptide induces robust CD4+ T-cell responses in the majority of vaccinated cancer patients. International journal of cancer Journal international du cancer. 2012;131:140–9. doi: 10.1002/ijc.26365. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Li A, Glas M, Lal B, Ying M, Sang Y, et al. c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9951–6. doi: 10.1073/pnas.1016912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nature reviews Cancer. 2008;8:351–60. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 10.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2007;18:226–32. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–80. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 12.Chudley L, McCann KJ, Coleman A, Cazaly AM, Bidmon N, Britten CM, et al. Harmonisation of short-term in vitro culture for the expansion of antigen-specific CD8(+) T cells with detection by ELISPOT and HLA-multimer staining. Cancer immunology, immunotherapy : CII. 2014;63:1199–211. doi: 10.1007/s00262-014-1593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nature medicine. 2012;18:1254–61. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 14.Britten CM, Janetzki S, Ben-Porat L, Clay TM, Kalos M, Maecker H, et al. Harmonization guidelines for HLA-peptide multimer assays derived from results of a large scale international proficiency panel of the Cancer Vaccine Consortium. Cancer immunology, immunotherapy : CII. 2009;58:1701–13. doi: 10.1007/s00262-009-0681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. The Journal of experimental medicine. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. The Lancet Oncology. 2015;16:e534–42. doi: 10.1016/S1470-2045(15)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winney B, Boumertit A, Day T, Davison D, Echeta C, Evseeva I, et al. People of the British Isles: preliminary analysis of genotypes and surnames in a UK-control population. European journal of human genetics : EJHG. 2012;20:203–10. doi: 10.1038/ejhg.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kempuraj D, Devi RS, Madhappan B, Conti P, Nazer MY, Christodoulou S, et al. T lymphocyte subsets and immunoglobulins in intracranial tumor patients before and after treatment, and based on histological type of tumors. International journal of immunopathology and pharmacology. 2004;17:57–64. doi: 10.1177/039463200401700108. [DOI] [PubMed] [Google Scholar]
- 19.Kocher M, Kunze S, Eich HT, Semrau R, Muller RP. Efficacy and toxicity of postoperative temozolomide radiochemotherapy in malignant glioma. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al] 2005;181:157–63. doi: 10.1007/s00066-005-1314-x. [DOI] [PubMed] [Google Scholar]
- 20.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nature reviews Immunology. 2011;11:119–30. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butt AQ, Mills KH. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene. 2014;33:4623–31. doi: 10.1038/onc.2013.432. [DOI] [PubMed] [Google Scholar]
- 22.Chiba Y, Hashimoto N, Tsuboi A, Oka Y, Murao A, Kinoshita M, et al. Effects of concomitant temozolomide and radiation therapies on WT1-specific T-cells in malignant glioma. Japanese journal of clinical oncology. 2010;40:395–403. doi: 10.1093/jjco/hyp196. [DOI] [PubMed] [Google Scholar]
- 23.Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro-oncology. 2011;13:324–33. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadul CE, Fisher JL, Gui J, Hampton TH, Cote AL, Ernstoff MS. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro-oncology. 2011;13:393–400. doi: 10.1093/neuonc/noq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer immunology, immunotherapy : CII. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 27.Inamoto T, Azuma H. Immunotherapy of genitourinary malignancies. Journal of oncology. 2012;2012:397267. doi: 10.1155/2012/397267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro-oncology. 2015;(Suppl 7):17. vii9–vii14. doi: 10.1093/neuonc/nov151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Authier A, Farrand KJ, Broadley KW, Ancelet LR, Hunn MK, Stone S, et al. Enhanced immunosuppression by therapy-exposed glioblastoma multiforme tumor cells. International journal of cancer Journal international du cancer. 2015;136:2566–78. doi: 10.1002/ijc.29309. [DOI] [PubMed] [Google Scholar]
- 30.Wainwright DA, Dey M, Chang A, Lesniak MS. Targeting Tregs in Malignant Brain Cancer: Overcoming IDO. Frontiers in immunology. 2013;4:116. doi: 10.3389/fimmu.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro-oncology. 2015;17:1064–75. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, Tobias A, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:5290–301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahlers JD, Dunlop N, Alling DW, Nara PL, Berzofsky JA. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs: granulocyte-macrophage colony-stimulating factor and TNF-alpha synergize with IL-12 to enhance induction of cytotoxic T lymphocytes. J Immunol. 1997;158:3947–58. [PubMed] [Google Scholar]
- 35.Hege KM, Jooss K, Pardoll D. GM-CSF gene-modifed cancer cell immunotherapies: of mice and men. International reviews of immunology. 2006;25:321–52. doi: 10.1080/08830180600992498. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman HL, Ruby CE, Hughes T, Slingluff CL., Jr Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. Journal for immunotherapy of cancer. 2014;2:11. doi: 10.1186/2051-1426-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martins KA, Bavari S, Salazar AM. Vaccine adjuvant uses of poly-IC and derivatives. Expert review of vaccines. 2015;14:447–59. doi: 10.1586/14760584.2015.966085. [DOI] [PubMed] [Google Scholar]
- 38.Fehres CM, Bruijns SC, van Beelen AJ, Kalay H, Ambrosini M, Hooijberg E, et al. Topical rather than intradermal application of the TLR7 ligand imiquimod leads to human dermal dendritic cell maturation and CD8+ T-cell cross-priming. European journal of immunology. 2014;44:2415–24. doi: 10.1002/eji.201344094. [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, Termini JM, Kanagavelu S, Stone GW. Design of vaccine adjuvants incorporating TNF superfamily ligands and TNF superfamily molecular mimics. Immunologic research. 2013;57:303–10. doi: 10.1007/s12026-013-8443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madondo MT, Quinn M, Plebanski M. Low dose cyclophosphamide: Mechanisms of T cell modulation. Cancer treatment reviews. 2015 doi: 10.1016/j.ctrv.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Fu J, Malm IJ, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer research. 2014;74:4042–52. doi: 10.1158/0008-5472.CAN-13-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–9. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aruga A, Takeshita N, Kotera Y, Okuyama R, Matsushita N, Ohta T, et al. Long-term Vaccination with Multiple Peptides Derived from Cancer-Testis Antigens Can Maintain a Specific T-cell Response and Achieve Disease Stability in Advanced Biliary Tract Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:2224–31. doi: 10.1158/1078-0432.CCR-12-3592. [DOI] [PubMed] [Google Scholar]
- 44.Fukushima T, Takeshima H, Kataoka H. Anti-glioma therapy with temozolomide and status of the DNA-repair gene MGMT. Anticancer research. 2009;29:4845–54. [PubMed] [Google Scholar]
- 45.Hoos A. Evolution of end points for cancer immunotherapy trials. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(Suppl 8):viii47–52. doi: 10.1093/annonc/mds263. [DOI] [PubMed] [Google Scholar]
- 46.Del Vecchio CA, Wong AJ. Rindopepimut, a 14-mer injectable peptide vaccine against EGFRvIII for the potential treatment of glioblastoma multiforme. Current opinion in molecular therapeutics. 2010;12:741–54. [PubMed] [Google Scholar]
- 47.Schuster J, Lai RK, Recht LD, Reardon DA, Paleologos NA, Groves MD, et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro-oncology. 2015;17:854–61. doi: 10.1093/neuonc/nou348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Data Safety and Monitoring Board Recommends Celldex’s Phase 3 Study of RINTEGA® (rindopepimut) in Newly Diagnosed Glioblastoma be Discontinued as it is Unlikely to Meet Primary Overall Survival Endpoint in Patients with Minimal Residual Disease. [cited 2016 01 April];2016 Available from: http://ir.celldex.com/releasedetail.cfm?ReleaseID=959021.
- 49.Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:2443–9. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.