Abstract
Background
The handling and reporting of testicular tumours is difficult due to their rarity.
Design
A survey developed by the European Network of Uro-Pathology (ENUP) and sent to its members and experts to assess the evaluation of testicular germ cell tumours
Results
25 experts (E) and 225 ENUP members replied. Areas of disagreement included immaturity in teratomas, reported by 32% (E) but 68% (ENUP). Although the presence of rete testis invasion was widely reported, the distinction between pagetoid and stromal invasion was made by 96% (E) but only 63% (ENUP). Immunohistochemistry was used in more than 50% of cases by 68% (ENUP) and 12% (E). Staging revealed the greatest areas of disagreement. Invasion of the tunica vaginalis without vascular invasion was interpreted as T1 by 52% (E) and 67% (ENUP), but T2 by the remainder. Tumour invading the hilar adipose tissue adjacent to the epididymis without vascular invasion was interpreted as T1: 40% (E), 43% (ENUP), T2: 36% (E), 30% (ENUP) and T3: 24% (E), 27% (ENUP).
Conclusions
There is remarkable consensus in many areas of testicular pathology. Significant areas of disagreement included staging and reporting of histologic types, both of which have the potential to impact on therapy.
Keywords: testis, germ cell tumour, consensus, rete testis, staging, classification
Introduction
Testicular pathology creates many challenges for both expert and general histopathologists1, 2. Orchidectomies are relatively simple surgical procedures and therefore often performed by general or junior urologists in local hospitals where there is a lack of specialist genitourinary (GU) pathologists. For practicing pathologists, orchidectomy specimens pose two main problems. Firstly, these tumours are generally rare and only a handful may be encountered in a year, thus limiting the experience of the pathologist. The second problem is the huge range of testicular pathology. Within merely the germ cell tumours, the most commonly encountered testicular tumours, there is a protean range of morphology with many mimics and confounding patterns. This problem is magnified by the vast range of non-germ cell malignancies in the testicular parenchyma and spermatic cord. Some testicular tumour subtypes may be encountered only once in a career, if at all. Some are associated with rare clinical syndromes.
The handling and staging of testicular tumours, particularly germ cell tumours, may also be problematic. Both staging and typing may be affected by macroscopic examination. Although most germ cell tumours are now treated by surveillance3, the decision to give adjuvant therapy may be dependent on a number of clinic-pathological factors4. These include tumour stage, but there are a number of other predictive factors that have been suggested over the past 10 years not included in the current TNM terminology.
In some countries testicular pathology has been centralised, so that within the GU community there are certain designated experts who see a large volume of testicular tumours and therefore are more able to recognise the rarer variants. It has been shown that this subspecialisation may affect both typing and staging of tumours5, 6. There are numerous prospective and retrospective studies where pathology interpretation may be variable and greatly affect the results. There is a necessity of uniform pathology, not only to address the problems of correct diagnosis and treatment but also to address the consequences of pathology variability in clinical trials and avoid contamination of the literature with inaccurate prognostic factors. We therefore wished to examine the variability and conformity in practice among both experts and general GU pathologists. This would hopefully highlight areas of agreement, and also areas where practice is variable and consensus needed.
Materials and Methods
A survey was developed by the steering group of the European Network of Uro-Pathology (ENUP). This focused on macroscopy, microscopy and especially known controversies in staging. The survey was sent to all 661 ENUP members as well as selected international experts. Experts were invited because of their known publication record or volume of their testicular pathology practice. The expert survey was analysed separately from the ENUP survey. The survey questions are listed in Table 1.
Table 1.
Testis Questionnaire.
|
Results
Replies were received from 25 experts (E) and 225 ENUP members. Both groups had remarkably similar responses for many questions.
Demographics
The experts worked in 10 different countries: 13 in North America, 11 in Europe and one in New Zealand. ENUP members came from 22 European countries, the largest representation coming from the UK with 17% (38), Spain 14% (31), Italy 9% (21) and Germany 7% (16). This may at least partly reflect that most surveys are conducted in English.
Of the experts, 24 received more than 20 testis cases in consult or routine practice per year and 6 (24%) received more than 100 cases. 48% of the experts had more than half their cases as consults. Among ENUP members, 39% (87) saw fewer than 20 and 50% (113) saw between 20 and 50. 2% (4) of ENUP members saw more than half their cases as consults. For clarity, only percentages of each cohort will be reported for the remaining results.
Macroscopy
A summary of the results are displayed in Table 2. Notable differences of opinion in technique were noted. Ink was not used by 62% (ENUP) and 52% (E). Of those using ink, experts all used one colour. While 93% of ENUP members did the same, 6% used two colours and one member used 3 colours. Other significant differences were in methods of tumour measurement where some measured the tumour only in 1 dimension, while others used two or three measurements. Tumour blocks were taken by a variety of methods. A subjective method was used, correlating to tumour size, in 65% (ENUP) and 56% (E). The classical method of a fixed number of blocks per cm was used by 24% (ENUP) and 22% (E). Others used no fixed protocol and commented on their specific method. Some experts said they would take more blocks from a suspected seminoma than from a non-seminoma.
Table 2.
Macroscopic methods for testis examination of Experts (E) and ENUP members. Where the total number of respondents does not add up to 25 (Expert) or 225 (ENUP), the remainder chose not to supply an answer to the question.
| Experts %(N) | ENUP Members %(N) | |||||||
|---|---|---|---|---|---|---|---|---|
| Tissue received Fresh? | Yes | No | Yes | No | ||||
| 36% (9) | 64% (16) | 37% (82) | 63% (141) | |||||
| Frozen section performed? | All cases | Selected cases | Never | All cases | Selected cases | Never | ||
| 8%(2) | 68%(17) | 24%(6) | 8% (17) | 57% (127) | 35% (79) | |||
| Surgeons incise specimen? | Yes | Sometimes | No | Yes | Sometimes | No | ||
| 4% (1) | 42%(10) | 54%(13) | 14%(31) | 33%(71) | 53%(115) | |||
| Fresh tissue biobanked? | Yes | No | Yes | No | ||||
| 56%(14) | 44%(11) | 30% (68) | 70% (155) | |||||
| Cord margin Sampled? | Yes | No | Yes | No | ||||
| 96%(24) | 4%(1) | 98% (219) | 2% (5) | |||||
| Cord margin sampled before incision? | Yes | No | Yes | No | ||||
| 84%(21) | 16%(4) | 70% (154) | 30% (66) | |||||
| Do you measure cord length? | Yes | No | Yes | No | ||||
| 100%(25) | 0%(0) | 96% (211) | 4% (8) | |||||
| Do you measure testis size? | Yes | No | Yes | No | ||||
| 100%(25) | 0%(0) | 99% (220) | 1% (1) | |||||
| No of other cord blocks taken? | None | 1 | 2 or more | Only if abnormal | None | 1 | 2 or more | Only if abnormal |
| 0%(0) | 36%(9) | 48%(12) | 16%(4) | 1% (2) | 37%(82) | 7% (15) | ||
| Do you use ink? | Yes | No | Yes | No | ||||
| 48%(12) | 52%(13) | 38% (85) | 62% (138) | |||||
| If yes, how many colours | 1 | More than 1 | 1 | More than 1 | ||||
| 100%(12) | 0%(0) | 93% (80) | 7% (6) | |||||
| Do you measure tumour size? | Yes | No | Yes | No | ||||
| 100%(25) | 0%(0) | 99% (221) | 1% (1) | |||||
| How tumour Measured? | 1 dimension | 2 dimensions | 3 dimensions | 1 dimension | 2 dimension | 3 dimension | ||
| 20%(5) | 24%(6) | 56%(14) | 30%(51) | 29%(65) | 47%(104) | |||
| No of blocks taken? | Fixed number | Subjective decision | Fixed no per cm tumour | Fixed number | Subjective decision | Fixed no per cm tumour | ||
| 0%(0) | 56%(13) | 22%(5) | 2%(5) | 65%(143) | 24%(53) | |||
| Block from the rete taken? | Yes | No | Yes | No | ||||
| 100%(25) | 0%(0) | 98%(220) | 2%(4) | |||||
| Block taken of normal? | Yes | No | Yes | No | ||||
| 100%(25) | 0%(0) | 98%(218) | 2%(4) | |||||
| Comment on tunica vaginalis? | Yes | No | Yes | No | ||||
| 78%(18) | 22%(5) | 82%(184) | 17% (39) | |||||
Classification and microscopy
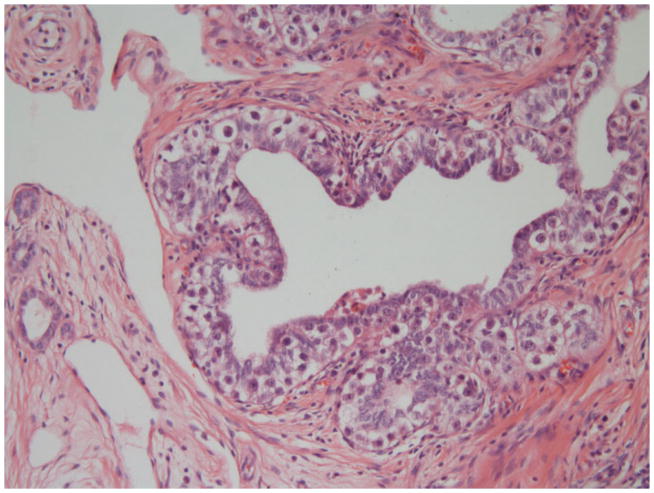
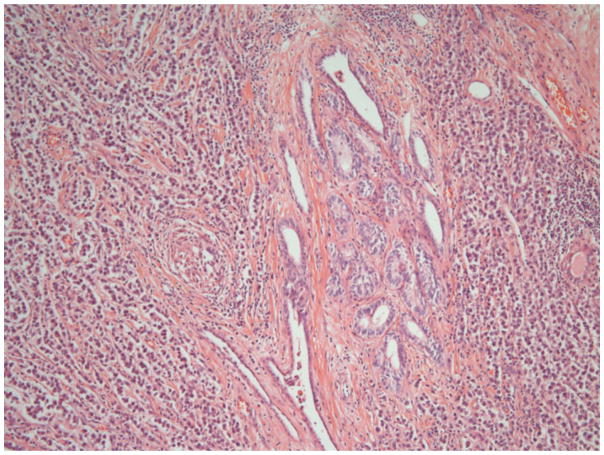
A summary of the results is displayed in Table 3. Nearly all respondents from both groups classified by WHO 2004. Significant differences included recording of immaturity in teratoma which was reported by 68% (ENUP) but only 32% of experts. Respondents were asked whether they made any assessment of ‘differentiation’ in seminomas. Some authors have previously reported mitotic rates or so called ‘anaplastic’ seminomas and we wished to investigate whether pathologists reported these features.7–9. This was done by 12% (ENUP) and 28% (E) though methods were very variable including mitotic counts, and nuclear morphology. Reporting the type of tumour involved in vascular invasion was reported by 54% (ENUP) and 56% (E). Also, there were differences on whether blood vessel invasion should be distinguished from lymphatic invasion. This was reported by 41% (ENUP) and 28% (E). Invasion of the rete testis was reported by 94% (ENUP) and 96% (E), though when asked whether any distinction was made between pagetoid and stromal invasion of the rete (Figures 1a and b), this was done by 63% (ENUP) and 96% (E).
TABLE 3.
Differences in microscopic assessments of testicular tumours between experts and ENUP members. Where the total number of respondents does not add up to 25 (Expert) or 225 (ENUP), the remainder chose not to supply an answer to the question.
| Experts % (N) | ENUP %(N) | |||||||
|---|---|---|---|---|---|---|---|---|
| Classification system used | WHO2004 | BTTP | Earlier WHO | Both BTTP and WHO | WHO2004 | BTTP | Earlier WHO | Both BTTP and WHO |
| 96%(24) | 0%(0) | 0%(0) | 4%(1) | 86%(192) | 1%(1)) | 2%(5 | 11%(25) | |
| % of tumour types reported? | Yes | No | Only EC | Yes | No | Only EC | ||
| 92%(23) | 4%(1) | 4%(1) | 77%(173) | 20%(45) | 2%(5) | |||
| Immaturity reported in teratoma? | Yes | No | Yes | No | ||||
| 32%(8) | 68%(17) | 68%(150) | 32%(71) | |||||
| PNET reported? | Yes | No | Yes | No | ||||
| 88%(22) | 12%(3) | 84%(186) | 16%(35) | |||||
| TNM stage always reported | Yes | No | Yes | No | ||||
| 96%(24) | 4%(1) | 95%(207) | 5%(10) | |||||
| Vascular invasion(VI) reported? | Yes | No | Yes | No | ||||
| 100%(25) | 0%(0) | 99%(221) | 1%(2) | |||||
| Tumour type in VI reported? | Yes | No | Yes | No | ||||
| 56%(14) | 44%(11) | 54%(120) | 46%(102) | |||||
| Lymphatics and blood vessels distinguished? | Yes | No | Yes | No | ||||
| 28%(7) | 72%(18) | 41%(93) | 59%(131) | |||||
| Stage of blood vessel invasion in cord but no stromal invasion | T2 | T3 | T2 | T3 | ||||
| 79%(19) | 21%(5) | 78%(169) | 22%(47) | |||||
| Rete invasion reported? | Yes | No | Yes | No | ||||
| 96%(24) | 4%(1) | 94%(208) | 6%(14) | |||||
| Distinction between pagetoid/stromal rete invasion? | Yes | No | Yes | No | ||||
| 96%(24) | 4%(1) | 63%(139) | 37%(83) | |||||
| Assessments of differentiation made in seminoma? | Yes | No | Yes | No | ||||
| 28%(7) | 72%(18) | 12%(26) | 88%(195) | |||||
| IGCNU reported? | Yes | No | Yes | No | ||||
| 92%(23) | 8%(2) | 95%(207) | 5%(10) | |||||
| Stage of tumour invading inner serosal lining of testis with no vascular invasion | T1 | T2 | T1 | T2 | ||||
| 52%(13) | 48%(12) | 67%(146) | 33%(72) | |||||
| Stage of tumour invading epididymis with no vascular invasion? | T1 | T2 | T3 | T1 | T2 | T3 | ||
| 88%(22) | 12%(3) | 0%(0) | 91%(195) | 7%(15) | 1%(3) | |||
| Stage of tumour invading hilar fatty tissue with no vascular invasion | T1 | T2 | T3 | T1 | T2 | T3 | ||
| 40%(10) | 36%(9) | 24%(6) | 43%(90) | 30%(63) | 27%(56) | |||
| Stage of tumour deposit in upper cord with separate tumour in testis? | T2 with soft tissue deposit | T3 | T2 with soft tissue deposit | T3 | ||||
| 28%(7) | 68%(17) | 23%(50) | 75%(162) | |||||
Figure 1.
Figure 1a. Rete testis showing pagetoid invasion by intra tubular germ cell neoplasia, unclassified
Figure 1b. Rete testis, showing widespread invasion of the interstitium by seminoma.
TNM staging
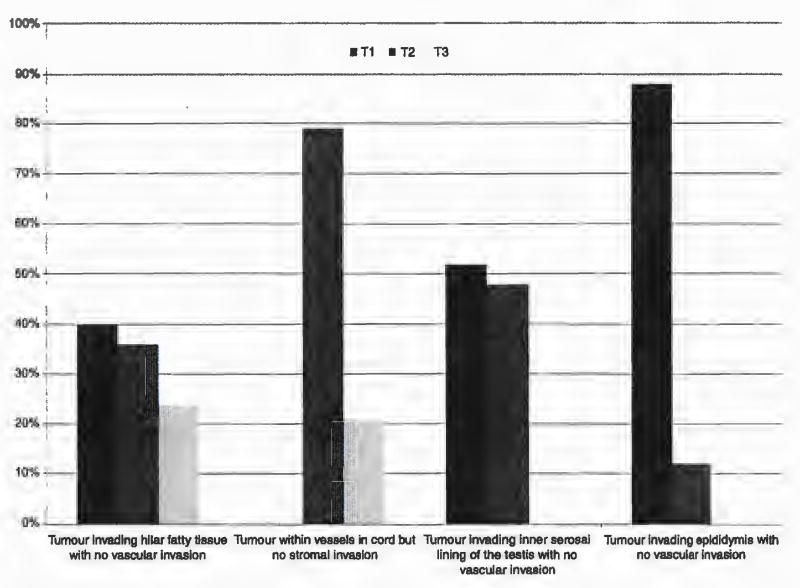
The detailed usage of TNM classification is presented in Table 3 and the expert differences in Figure 2. Major differences included the staging of tumour invading the inner serosal lining of the testis but not the outer layer with no vascular invasion. This would be staged as T1 by 67% (ENUP) and 52% (E) and T2 by the remainder. Tumour invading the hilar fatty tissue adjacent to the epididymis with no vascular invasion would be staged T1 by 43% (ENUP) and 40% (E), T2 by 30% (ENUP) and 36% (E) and T3 by 27% (ENUP) and 24% (E). A tumour deposit in the upper cord with a separate tumour in the testis associated with vascular invasion would be staged as T2 with a soft tissue deposit by 23% (ENUP) and 28% (E) and T3 by 75% (ENUP) and 68% (E). The remainder made comments that they would stage as a metastasis.
Figure 2.
Variation in tumour-node-metastasis (TNM) staging in particular scenarios by the expert panel.
Immunochemistry and genetics
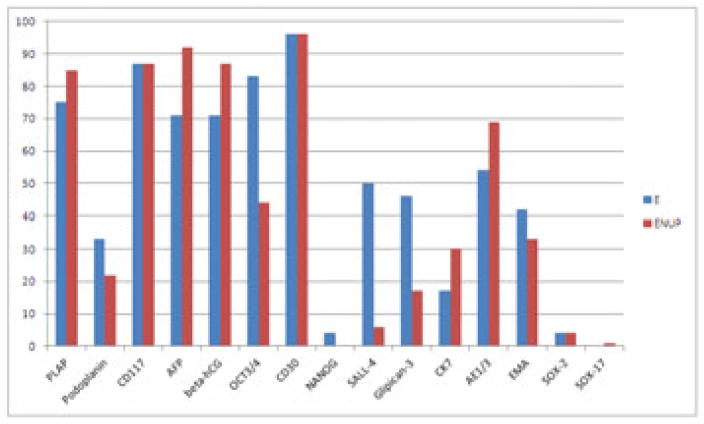
Use of immunochemistry is presented in Table 4 and specific antibodies used presented in Figure 3. Isochromosome 12p assessment was requested on challenging cases by 7% (ENUP) and 48% (E).
Table 4.
Frequency of use of Immunochemistry for testicular tumours.
| Use of immunochemistry in the testis | ENUP N (%) | Experts N (%) |
|---|---|---|
| Every case | 78 (35%) | 0 (0%) |
| 50–100% of cases | 75 (33%) | 3 (12%) |
| 10–50% of cases | 54 (24%) | 9 (36%) |
| Less than 10% of cases | 17 (8%) | 13 (52%) |
| Never | 0 (0%) | 0 (0%) |
Figure 3.
Frequency of use of different immunochemical antibodies by ENUP members and experts. E=Experts ENUP=European network of Uro-Pathology.
Discusssion
This survey highlights a surprising degree of concordance between urological pathologists whether or not they have a specialist interest in testicular pathology. Although some protocols are evidence based, others appear to have evolved with very little evidence base, however logic has dictated certain approaches which are widely adopted. Pathologists are also guided by expert opinion and by cancer guidelines which have been published by national pathological associations10..
Macroscopic protocols are broadly similar. However opinion was split on the use of ink. While only one colour is used as a maximum by all the expert groups, two or more colours are used by some ENUP members. This routine appears to be unnecessary for total orchidectomy specimens. The use of ink is necessary on partial orchidectomy specimens where it is the only way that margin positivity can be demonstrated due to the lack of an anatomic boundary. The taking of a cord block before incising the testis was widespread. However some pathologists receive their testes already incised by the surgeon. The logic of this is to ensure formalin fixation, as suboptimal fixation can compromise tissue typing. The taking of the cord block prior to excision derives from a sole paper and serves to avoid contamination of the margin, and a false positive report of vascular invasion11. It should be noted, however, that it is not possible to protect testicular parenchyma from contamination of vessels by free floating tumour cells in a similar way.
A majority of pathologists take tumour blocks in a subjective manner, but claim that the number of blocks correlates with tumour size. It was commented by some experts that they sample probable seminomas more widely to look for non-seminomatous transformation. Tumour size is most commonly measured in three diameters, but by a minority in two dimensions. The maximal tumour dimension has been shown in many studies to be prognostically significant, particularly in seminoma. One pooled analysis of data from 4 cohort studies of 638 patients showed that if tumours were greater than 4cm in maximum diameter there was a two-fold risk of recurrence and the importance of tumour size is strongly supported by more recent studies. 4, 12–14. It would appear logical that it is important to give as a minimum the maximal diameter. Blocking of the rete testis, normal parenchyma and examination of the tunics appears now to be very widely accepted. Rete testis invasion has been shown in some studies to be an important prognostic factor4, 15, though this may be a surrogate for tumour size and has been disputed by other studies16 Including recent large series which did not however include pathological review 12.
While nearly all respondents report on rete testis invasion, 37% of ENUP members did not distinguish between pagetoid invasion, which is probably a phenomenon related to spread of intratubular germ cell neoplasia into the rete, and true interstitial invasion. This shows the possible dangers of relying on extraction of pooled pathological data from multiple centres without central review, which is often done in clinical studies.
Reporting vascular invasion was virtually universal in the survey and has an excellent evidence base, particularly for non seminomas17–22. The evidence that vascular invasion is an important prognostic factor in seminoma is much however less certain 4, 12, 23 . There were general disagreements in whether lymphatics should be reliably distinguished from small venules. One trial has suggested that this is an important distinction24, but older and more recent work suggests that such distinctions are not possible on H and E25, 26. There was also disagreement on whether the type of tumour present within vessels should be reported. The is no evidence to support this. In pure seminomas this should be self apparent, and the vast majority of non seminoma cases show vascular invasion by the embryonal carcinoma component.
Classification by the WHO 2004 now appears widespread. The British Testicular Panel (BTTP) devised a classification in the 1960s27 and is still used by a few UK based pathologists though is not recommended in the latest Royal College of Pathologists dataset10.
77% of ENUP and 92% of the experts reported the percentages of all elements of a germ cell tumour. There is considerable work supporting that the percentage of embryonal carcinoma is prognostically significant. A surveillance study of 373 men with stage 1 non seminomatous germ cell tumours showed that the percentage of embryonal carcinoma predicted relapse28. This has been supported by numerous other smaller studies18, 29, 30. More recent cohort studies, one on 1,226 patients22, have supported this.
The degree of immaturity in a teratoma has been shown to have little impact on disease natural history. Interestingly 68% of ENUP still reported on this while only 32% of experts would do so: an example of a practice where less is done by the experts because of insight that it has little prognostic importance.
The most important differences were in tumour staging by TNM, and here there was a lack of consistency despite over 95% of respondents using it. This is reflected in a very poor evidence base, where apart from vascular invasion in non-seminomas, the TNM staging appears to be largely unhelpful. Tunica vaginalis invasion is staged as T2 in the current TNM classification, though in fact there is no evidence for this in the literature, despite being an anatomical boundary. The experts and ENUP members were broadly split on how to interpret tumour involvement of the inner serosal lining part of the tunica vaginalis. Although anatomically both serosal linings represent the tunica vaginalis, there is a reluctance of pathologists to upstage to T2 in these circumstances, possibly because some believe that the inner serosal lining represents the tunica albiginea.
There was even further disagreement on the staging of hilar fat involvement, without vascular invasion: split fairly evenly between T1, T2 and T3. Recent papers have suggested that this is a poor prognostic factor, but many are reluctant to stage as T331.
The variation seen in this survey suggests a number of consequences. Firstly, the variation in reporting TNM stage and rete testis invasion will affect any analysis of prognostic factors in germ cell tumours especially in pooled data. Any reliance on extracting the data from pathology reports should be treated with extreme caution as it may be unreliable.
Immunochemistry was used in far fewer cases among the expert group, reflecting the familiarity of those pathologists with these tumours. However there were significant differences in the markers that were utilised between both groups (Figure 3). Notably, OCT3/432 was used far more among experts than amongst ENUP members. It has been shown to be both sensitive and specific for embryonal carcinoma and seminoma, and widely promulgated in many articles2, 33. A recent expert opinion paper from ISUP may promote changes in some practices34. The lack of its use in many departments may reflect budget restraints or caution in developing new tests. The same holds true for SALL-435, 36 and Glipican-337, 38.
Finally, molecular testing for isochromosome 12p may be helpful in rare and challenging cases, for instance to distinguish dermoid cysts from mature teratomas.39. It was used by half the expert group, it only 7% of the ENUP group, again probably reflecting a lack of local availability and molecular expertise.
In conclusion we have shown that while some areas of testicular pathology show remarkable degrees of consensus, others show great variability which may lead to differences in the power of prognostic information provided and even effect treatment decisions. International agreement and consensus statements may lessen the variability in these areas.
Acknowledgments
DB, EC, ALB, FA, MV and LE devised the survey. DB wrote the manuscript. All authors participated in the survey and commented and refined the survey results and commented on the conclusions. We gratefully acknowledge the other experts who took part in the survey: Phillippe Camparo, Jeffrey Theaker, Isabell Sesterhenn and Grete Krag Jacobsen.
DB gratefully acknowledges the support of Orchid.
Footnotes
Conflict of Interest Statement. No conflicts of interest are declared.
References
- 1.Ulbright TM. The most common, clinically significant misdiagnoses in testicular tumor pathology, and how to avoid them. Adv Anat Pathol. 2008;15:18–27. doi: 10.1097/PAP.0b013e318159475d. [DOI] [PubMed] [Google Scholar]
- 2.Rajab R, Berney DM. Ten testicular trapdoors. Histopathology. 2008;53:728–39. doi: 10.1111/j.1365-2559.2008.03001.x. [DOI] [PubMed] [Google Scholar]
- 3.Powles T. Stage I nonseminomatous germ cell tumor of the testis: more questions than answers? Hematol Oncol Clin North Am. 25:517–27. viii. doi: 10.1016/j.hoc.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Warde P, Specht L, Horwich A, et al. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol. 2002;20:4448–52. doi: 10.1200/JCO.2002.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Lee AH, Mead GM, Theaker JM. The value of central histopathological review of testicular tumours before treatment. BJU Int. 1999;84:75–8. doi: 10.1046/j.1464-410x.1999.00048.x. [DOI] [PubMed] [Google Scholar]
- 6.Delaney RJ, Sayers CD, Walker MA, Mead GM, Theaker JM. The continued value of central histopathological review of testicular tumours. Histopathology. 2005;47:166–9. doi: 10.1111/j.1365-2559.2005.02207.x. [DOI] [PubMed] [Google Scholar]
- 7.Tickoo SK, Hutchinson B, Bacik J, et al. Testicular seminoma: a clinicopathologic and immunohistochemical study of 105 cases with special reference to seminomas with atypical features. Int J Surg Pathol. 2002;10:23–32. doi: 10.1177/106689690201000105. [DOI] [PubMed] [Google Scholar]
- 8.von Hochstetter AR. Mitotic count in seminomas--an unreliable criterion for distinguishing between classical and anaplastic types. Virchows Arch A Pathol Anat Histol. 1981;390:63–9. doi: 10.1007/BF00443897. [DOI] [PubMed] [Google Scholar]
- 9.Stein ME, Zidan J, Charas T, Drumea K, Ben-Yosef R. Anaplastic variant of classical seminoma of the testis: northern Israel oncology center experience and brief review of literature. Rambam Maimonides medical journal. 2014;5:e0006. doi: 10.5041/RMMJ.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berney DT, Verril JC. RC Pathologists, editor. Dataset for the histological reporting of testicular neoplasms. (3) 2014 http://www.rcpath.org/Resources/RCPath/Migrated%20Resources/Documents/G/G046_TestisDatasetMay14.pdf.
- 11.Nazeer T, Ro JY, Kee KH, Ayala AG. Spermatic cord contamination in testicular cancer. Mod Pathol. 1996;9:762–6. [PubMed] [Google Scholar]
- 12.Chung P, Daugaard G, Tyldesley S, et al. Evaluation of a prognostic model for risk of relapse in stage I seminoma surveillance. Cancer medicine. 2014 doi: 10.1002/cam4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aparicio J, Maroto P, Garcia Del Muro X, Sanchez-Munoz A, Guma J, Margeli M, et al. Prognostic factors for relapse in stage I seminoma: a new nomogram derived from three consecutive, risk-adapted studies from the Spanish Germ Cell Cancer Group (SGCCG) Ann Oncol. 2014;25:2173–8. doi: 10.1093/annonc/mdu437. [DOI] [PubMed] [Google Scholar]
- 14.Mortensen MS, Lauritsen J, Gundgaard MG, et al. A Nationwide Cohort Study of Stage I Seminoma Patients Followed on a Surveillance Program. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Kamba T, Kamoto T, Okubo K, et al. Outcome of different post-orchiectomy management for stage I seminoma: Japanese multi-institutional study including 425 patients. Int J Urol. 17:980–7. doi: 10.1111/j.1442-2042.2010.02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogt AP, Chen Z, Osunkoya AO. Rete testis invasion by malignant germ cell tumor and/or intratubular germ cell neoplasia: what is the significance of this finding? Hum Pathol. 41:1339–44. doi: 10.1016/j.humpath.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Fossa SD, Jacobsen AB, Aass N, et al. How safe is surveillance in patients with histologically low-risk non-seminomatous testicular cancer in a geographically extended country with limited computerised tomographic resources? Br J Cancer. 1994;70:1156–60. doi: 10.1038/bjc.1994.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunphy CH, Ayala AG, Swanson DA, Ro JY, Logothetis C. Clinical stage I nonseminomatous and mixed germ cell tumors of the testis. A clinicopathologic study of 93 patients on a surveillance protocol after orchiectomy alone. Cancer. 1988;62:1202–6. doi: 10.1002/1097-0142(19880915)62:6<1202::aid-cncr2820620627>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 19.Colls BM, Harvey VJ, Skelton L, et al. Late results of surveillance of clinical stage I nonseminoma germ cell testicular tumours: 17 years' experience in a national study in New Zealand. BJU Int. 1999;83:76–82. doi: 10.1046/j.1464-410x.1999.00869.x. [DOI] [PubMed] [Google Scholar]
- 20.Alexandre J, Fizazi K, Mahe C, et al. Stage I non-seminomatous germ-cell tumours of the testis: identification of a subgroup of patients with a very low risk of relapse. Eur J Cancer. 2001;37:576–82. doi: 10.1016/s0959-8049(00)00442-1. [DOI] [PubMed] [Google Scholar]
- 21.Wishnow KI, Johnson DE, Swanson DA, et al. Identifying patients with low-risk clinical stage I nonseminomatous testicular tumors who should be treated by surveillance. Urology. 1989;34:339–43. doi: 10.1016/0090-4295(89)90436-6. [DOI] [PubMed] [Google Scholar]
- 22.Daugaard G, Gundgaard MG, Mortensen MS, et al. Surveillance for Stage I Nonseminoma Testicular Cancer: Outcomes and Long-Term Follow-Up in a Population-Based Cohort. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.53.5831. [DOI] [PubMed] [Google Scholar]
- 23.Kamba T, Kamoto T, Okubo K, et al. Outcome of different post-orchiectomy management for stage I seminoma: Japanese multi-institutional study including 425 patients. Int J Urol. 2010;17:980–7. doi: 10.1111/j.1442-2042.2010.02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedman LS, Parkinson MC, Jones WG, et al. Histopathology in the prediction of relapse of patients with stage I testicular teratoma treated by orchidectomy alone. Lancet. 1987;2:294–8. doi: 10.1016/s0140-6736(87)90889-0. [DOI] [PubMed] [Google Scholar]
- 25.Barsky SH, Baker A, Siegal GP, Togo S, Liotta LA. Use of anti-basement membrane antibodies to distinguish blood vessel capillaries from lymphatic capillaries. Am J Surg Pathol. 1983;7:667–77. doi: 10.1097/00000478-198310000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Van den Eynden GG, Van der Auwera I, Van Laere SJ, et al. Distinguishing blood and lymph vessel invasion in breast cancer: a prospective immunohistochemical study. Br J Cancer. 2006;94:1643–9. doi: 10.1038/sj.bjc.6603152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyubimov N. The Pathology of Testicular Tumours. Studies from the Testicular Tumour Panel and Registry of the Pathological Society of Great Britain and Ireland in Association with the British Empire Cancer Campaign for Research. Br J Urol. 1964;36:1–112. [PubMed] [Google Scholar]
- 28.Read G, Stenning SP, Cullen MH, et al. Medical Research Council prospective study of surveillance for stage I testicular teratoma. Medical Research Council Testicular Tumors Working Party. J Clin Oncol. 1992;10:1762–8. doi: 10.1200/JCO.1992.10.11.1762. [DOI] [PubMed] [Google Scholar]
- 29.Atsu N, Eskicorapci S, Uner A, et al. A novel surveillance protocol for stage I nonseminomatous germ cell testicular tumours. BJU Int. 2003;92:32–5. doi: 10.1046/j.1464-410x.2003.04270.x. [DOI] [PubMed] [Google Scholar]
- 30.Nicolai N, Pizzocaro G. A surveillance study of clinical stage I nonseminomatous germ cell tumors of the testis: 10-year followup. J Urol. 1995;154:1045–9. [PubMed] [Google Scholar]
- 31.Yilmaz A, Cheng T, Zhang J, Trpkov K. Testicular hilum and vascular invasion predict advanced clinical stage in nonseminomatous germ cell tumors. Mod Pathol. 26:579–86. doi: 10.1038/modpathol.2012.189. [DOI] [PubMed] [Google Scholar]
- 32.Looijenga LH, Stoop H, de Leeuw HP, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63:2244–50. [PubMed] [Google Scholar]
- 33.Emerson RE, Ulbright TM. The use of immunohistochemistry in the differential diagnosis of tumors of the testis and paratestis. Semin Diagn Pathol. 2005;22:33–50. doi: 10.1053/j.semdp.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Ulbright TM, Tickoo SK, Berney DM, Srigley JR Members of the IIiDUPG. Best Practices Recommendations in the Application of Immunohistochemistry in Testicular Tumors: Report From the International Society of Urological Pathology Consensus Conference. Am J Surg Pathol. 2014 doi: 10.1097/PAS.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 35.Cao D, Humphrey PA, Allan RW. SALL4 is a novel sensitive and specific marker for metastatic germ cell tumors, with particular utility in detection of metastatic yolk sac tumors. Cancer. 2009;115:2640–51. doi: 10.1002/cncr.24308. [DOI] [PubMed] [Google Scholar]
- 36.Cao D, Li J, Guo CC, Allan RW, Humphrey PA. SALL4 is a novel diagnostic marker for testicular germ cell tumors. Am J Surg Pathol. 2009;33:1065–77. doi: 10.1097/PAS.0b013e3181a13eef. [DOI] [PubMed] [Google Scholar]
- 37.Zynger DL, Dimov ND, Luan C, Teh BT, Yang XJ. Glypican 3: a novel marker in testicular germ cell tumors. Am J Surg Pathol. 2006;30:1570–5. doi: 10.1097/01.pas.0000213322.89670.48. [DOI] [PubMed] [Google Scholar]
- 38.Zynger DL, McCallum JC, Luan C, Chou PM, Yang XJ. Glypican 3 has a higher sensitivity than alpha-fetoprotein for testicular and ovarian yolk sac tumour: immunohistochemical investigation with analysis of histological growth patterns. Histopathology. 56:750–7. doi: 10.1111/j.1365-2559.2010.03553.x. [DOI] [PubMed] [Google Scholar]
- 39.Cheng L, Zhang S, MacLennan GT, et al. Interphase fluorescence in situ hybridization analysis of chromosome 12p abnormalities is useful for distinguishing epidermoid cysts of the testis from pure mature teratoma. Clin Cancer Res. 2006;12:5668–72. doi: 10.1158/1078-0432.CCR-06-0976. [DOI] [PubMed] [Google Scholar]