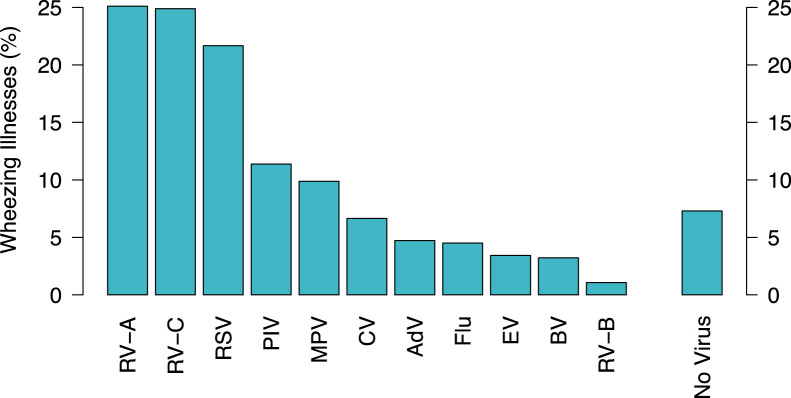To the Editor:
Viral respiratory wheezing illnesses are common in early childhood, and many children who wheeze subsequently develop asthma. In the Childhood Origins of ASThma (COAST) cohort, we previously reported that the development of rhinovirus (RV) wheezing illnesses in infancy and early childhood is the most robust predictor of subsequent asthma development in high-risk children at age 6 years, regardless of aeroallergen sensitization or other risk factors.1 However, a study in a similar high-risk birth cohort recently reported that the number of lower-respiratory episodes in the first 3 years of life, as opposed to a specific viral or bacterial etiology, was associated with asthma risk, and suggested that the specific microbiologic trigger is not important for the risk for later asthma development.2 This discrepancy in the literature is important to address as it may affect how risk for childhood asthma is ascertained; accurate identification of high-risk children may be key in asthma prevention. Therefore, we assessed the contribution of the etiology and frequency of wheezing illnesses on asthma risk in patients from ages 6 to 13 years in the COAST birth cohort study, which included children born to parents with either allergy or asthma. We hypothesized that the number of RV wheezing illnesses would be a more robust predictor of asthma development and persistence compared with the number of non-RV wheezing illnesses.
A total of 289 newborns were enrolled in the COAST study from November 1998 through May 2000 as previously described.3 Of these children, 259 were followed up prospectively to age 6 years and 217, to age 13 years. To qualify, at least 1 parent was required to have respiratory allergies (defined as 1 or more positive results on aeroallergen skin test) and/or a history of physician-diagnosed asthma. The Human Subjects Committee of the University of Wisconsin approved the study, and informed consent was obtained from the parents. Nasopharyngeal mucus samples were collected during scheduled clinic visits (2, 4, 6, 9, 12, 18, and 24 months) and during times of acute respiratory illness during the first 3 years of life.4 Nasal specimens were analyzed for respiratory viruses, including respiratory syncytial virus, RV, influenza types A and B, parainfluenza virus types 1-4, adenovirus, metapneumoviruses, coronaviruses (OC143, NL63, and 0229), bocaviruses, and enteroviruses, using cell culture and multiplex PCR (MultiCode-PLx respiratory virus assay; EraGen Biosciences, Madison, Wis).5 In addition, samples were tested for RV by seminested RT-PCR, using extraction procedures and primers that were optimized for RVs; specific RV type was determined on positive specimens using partial sequencing.6, 7 The relationship between the frequency of each virus-specific wheezing illness and the development of asthma was analyzed using generalized additive logistic regression models, which allow for the examination of nonlinear relationships between illness frequency and asthma risk.8
There were 478 wheezing illnesses documented in the first 3 years of life, with nasal specimens obtained in 466 (97%) of them. Respiratory viruses were detected in 93% (432/466) of wheezing illnesses. RV was detected in 49% (227/466) of wheezing illnesses (Fig 1 ).
Fig 1.
Respiratory viruses detected during wheezing illnesses in the first 3 years of life. respiratory syncytial virus (RSV); RV-A, -B, and -C; influenza types A and B (Flu); parainfluenza virus types 1-4 (PIV); adenovirus (AdV); enteroviruses (EV); OC43, NL63, and 229E (CV); metapneumoviruses (MPV), and bocaviruses (BV). The total is >100% because in some cases more than 1 virus was identified during a wheezing illness.
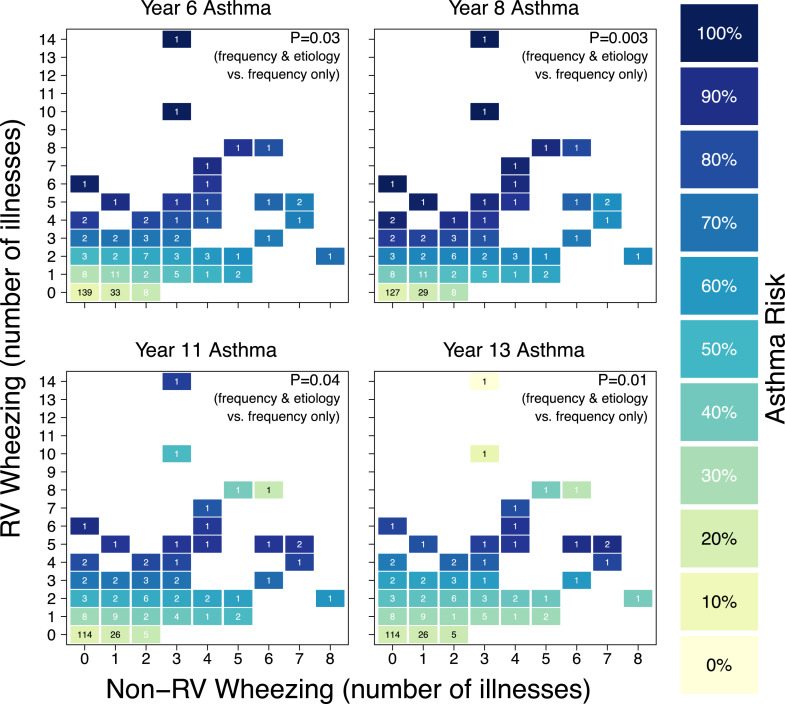
We next examined the impact of the frequency and viral etiology of wheezing illnesses during the first 3 years of life on subsequent asthma risk. Generalized additive models were fit under 2 scenarios: one that ignored etiology, with a single smooth term for the total number of wheezing illnesses, and one that included etiology, with smooth terms for the total numbers of RV wheezing illnesses and non-RV wheezing illnesses and their interactions. These 2 models were then compared using a likelihood ratio test. At every age, the model that included etiology was significantly more informative for asthma risk than was the model that ignored etiology (6 years, P = .03; 8 years, P = .003; 11 years, P = .04; 13 years, P = .01; Fig 2 ). Furthermore, asthma risk was higher with multiple RV wheezing illnesses compared with a single RV wheezing illness (2 or more RV wheezing illnesses compared with 1 RV wheezing illness at years 6, 8, 11, and 13; P = .003, .009, .009, and .11, respectively).
Fig 2.
Number of RV wheezing illnesses, number of non-RV wheezing illnesses, and asthma risk. The numbers in the squares represent the number of participants with each wheezing history. The colors of the squares represent asthma risk.
In this study, the number of RV wheezing episodes in early childhood was most closely associated with asthma risk. These findings support the concept that while the number of wheezing illnesses, irrespective of etiology, is important for asthma risk, the number of RV wheezing illnesses is the most robust asthma predictor. Therefore, both the number and the etiology of viral wheezing episodes in early life are important predictors of the risk for childhood asthma. Thus, the importance of pathogen-specific responses should not be underestimated when considering mechanisms driving disease, as infection with specific pathogens can lead to very different immune responses in the respiratory epithelium.9 Moreover, it is likely that both host factors and viral etiology contribute to the activation of immune pathways driving disease, which are important to identify in order to elucidate effective intervention strategies and/or to modify disease progression in each individual child.
One potential explanation for the discrepancy between these results and the recent report by Bonnelykke et al2 relates to differential frequency of viral detection in COAST (93%) compared with that in a similar Copenhagen Prospective Studies on Asthma in Childhood (COPSAC) high-risk cohort (65%). The detection of RV (49% vs 23%) accounts for the majority of the discrepancy. In our viral detection, we initially used a set of primers in seminested PCR that historically identified previously undetected strains of human RV now classified as RV-Cs.6 Moreover, our molecular typing assay was recently modified based on new sequence information to increase sensitivity and specificity of RV detection.7 We hypothesize that the molecular techniques utilized in our study more robustly detect RV, including novel RV-C types. Since RV-Cs are closely associated with lower respiratory infection and wheezing, incomplete RV-C detection could lead to an underestimate of how RV infections in early life are related to subsequent asthma.10 Additionally, the focus on wheezing episodes in COAST versus “troublesome lung symptoms” in COPSAC may also have contributed to the differences in study results.
In conclusion, the findings from our study suggest that viral etiology of early life wheezing and the number of episodes are important to subsequent asthma risk, and that the prevalence of RV wheezing illnesses is an important predictor of asthma development in high-risk children. Additional studies are needed to determine whether these same relationships hold true in unselected birth cohort studies.
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnelykke K., Vissing N.H., Sevelsted A., Johnston S.L., Bisgaard H. Association between respiratory infections in early life and later asthma is independent of virus type. J Allergy Clin Immunol. 2015;136:81–86.e4. doi: 10.1016/j.jaci.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemanske R.F. The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13(Suppl 15):38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 4.Lemanske R.F., Jr., Jackson D.J., Gangnon R.E., Evans M.D., Li Z., Shult P.A. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Lee W.M., Grindle K., Pappas T., Marshall D.J., Moser M.J., Beaty E.L. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007;45:2626–2634. doi: 10.1128/JCM.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee W.M., Kiesner C., Pappas T., Lee I., Grindle K., Jartti T. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochkov Y.A., Grindle K., Vang F., Evans M.D., Gern J.E. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol. 2014;52:2461–2471. doi: 10.1128/JCM.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hastie T., Tibshirani R. Generalized additive models for medical research. Stat Methods Med Res. 1995;4:187–196. doi: 10.1177/096228029500400302. [DOI] [PubMed] [Google Scholar]
- 9.Spann K.M., Baturcam E., Schagen J., Jones C., Straub C.P., Preston F.M. Viral and host factors determine innate immune responses in airway epithelial cells from children with wheeze and atopy. Thorax. 2014;69:918–925. doi: 10.1136/thoraxjnl-2013-204908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee W.M., Lemanske R.F., Jr., Evans M.D., Vang F., Pappas T., Gangnon R. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med. 2012;186:886–891. doi: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]