Abstract
The glomerular basement membrane (GBM) is an essential component of the glomerular filtration barrier. Heparan sulfate proteoglycans such as agrin are major components of the GBM, along with α345(IV) collagen, laminin-521 and nidogen. A loss of GBM heparan sulfate chains is associated with proteinuria in several glomerular diseases and may contribute to the underlying pathology. As the major determinants of the anionic charge of the GBM, heparan sulfate chains have been thought to impart charge selectivity to the glomerular filtration, a view challenged by the negligible albuminuria in mice that lack heparan sulfate in the GBM. Recent studies provide increasing evidence that heparan sulfate chains modulate local complement activation by recruiting complement regulatory protein factor H, the major inhibitor of the alternative pathway in plasma. Factor H selectively inactivates C3b bound to surfaces bearing host-specific polyanions such as heparan sulfate, thus limiting complement activation on self surfaces such as the GBM, which are not protected by cell-bound complement regulators. We discuss mechanisms whereby the acquired loss of GBM heparan sulfate can impair the local regulation of the alternative pathway, exacerbating complement activation and glomerular injury in immune-mediated kidney diseases such as membranous nephropathy and lupus nephritis.
Keywords: Glomerular basement membrane, heparan sulfate, complement, factor H, alternative pathway
1. Introduction
The glomerular basement membrane (GBM) is an amorphous layer of extracellular matrix separating glomerular endothelial cells and podocytes. Together, these components form the glomerular filtration barrier, which acts as a semi-permeable sieve allowing the relatively unimpeded flow of water and small solutes while restricting the passage of proteins such as albumin from blood into the primary urine. The GBM forms during the glomerular development though the fusion of the basement membranes of glomerular endothelial cells and podocytes, and both kinds of cells contribute components to the mature GBM [1, 2]. Reflecting adaptions to a specialized role in the glomerular filtration, the GBM is thicker (250–350 nm in humans) than other basement membranes and has a distinctive biochemical composition.
The major components of the mature GBM are α345(IV) collagen, laminin-521, nidogens, and heparan sulfate proteoglycans (HSPG), including agrin and perlecan [3]. Several GBM components have been identified as essential for normal glomerular structure and function, based on the phenotype of mutations associated with human disease. In Alport syndrome, mutations in COL4A3, COL4A4 or COL4A5 genes (encoding the α3, α4 and α5 chains of type IV collagen, respectively) impair the normal assembly of α345(IV) collagen molecules [4, 5], causing abnormal GBM deposition of α12(IV) collagen, GBM thickening and splitting, progressive proteinuria with onset in childhood, and eventually renal failure. In Pierson syndrome, mutations in LAMB2 gene (encoding laminin β2 chain) impair the GBM deposition of laminin-521 and cause congenital nephrotic syndrome [6].
More enigmatic are the roles of HSPG in the GBM under physiological and pathological conditions—the focus of this mini-review. An acquired loss of GBM heparan sulfate chains is associated with proteinuria in several human and experimental kidney diseases, providing circumstantial evidence that GBM HSPG are important for normal glomerular function and homeostasis. As the major determinants of the anionic charge of the GBM, heparan sulfate chains have been presumed important for the charge selectivity of the glomerular filtration, a view challenged by recent studies. An emerging role of heparan sulfate is the local regulation of complement activation in tissues, including in the kidneys [7–9]. Heparan sulfate chains function as so-called “self-associated molecular patterns” [10] recognized by complement regulatory proteins, which enable the complement system to discriminate between host and pathogens. We will discuss in detail how this self-recognition function may be compromised in kidney disease with detrimental results.
2. Heparan sulfate proteoglycans in the normal GBM
Heparan sulfate proteoglycans (HSPG) consists of one or more heparan sulfate chains attached to a core protein [11]. Heparan sulfate chains are linear polysaccharides consisting of 50–200 repeating disaccharide units. Their biosynthesis is initiated by the assembly of a tetra-saccharide structure (xylose-galactose-galactose-glucuronic acid) onto specific serine residues of the core protein, followed by the addition of one N-acetylglucosamine unit (catalyzed by ExtL3), which signals the heparan sulfate copolymerase complex Ext1/Ext2 to assemble the heparan sulfate chain by adding alternating glucuronic acid and N-acetylglucosamine units [12]. The chains are further modified by N-deacetylation/N-sulfation of the glucosamine residue (catalyzed by an N-deacetylase/N-sulfotransferase), epimerization of glucuronic to iduronic acid (catalyzed by a C5-epimerase), and addition of sulfate groups to the 2-O, 3-O and 6-O positions (catalyzed by various O-sulfotransferases). These modifications are incomplete and vary along the chain, creating a mosaic of high N-sulfated domains and high N-acetylated domains separated by transition zones with intermediary composition [13]. As a result, heparan sulfate chains exhibit considerable structural diversity within and among tissues. After biosynthesis, the structure of heparan sulfate can be further edited by enzymes such as the endoglucuronidase heparanase (discussed below), and Sulf-1 and Sulf-2, extracellular sulfatases that remove 6-O sulfate groups, which may fine-tune function and biological activity.
Within the kidney glomerulus, several types of HSPG are found with distinct distribution: syndecans and glypicans are associated with cell surfaces, while perlecan, agrin, and type XVIII collagen are components of basement membranes [14, 15]. Perlecan is the prominent HSPG in most basement membranes, though in the mature GBM it is confined to the subendothelial side [16]. It consists of a 467 kDa core protein comprising five domains, which bears three heparan sulfate chains within the amino-terminal domain I [17]. Perlecan is anchored in basement membranes by interactions with other constituents, binding to nidogen via its core protein and to laminin and collagen IV via its heparan sulfate chains [18]. Another HSPG often co-localized with perlecan is collagen XVIII [19], which is prominently expressed in the mesangial matrix and Bowman capsule basement membrane, but is a minor component of the normal GBM.
Agrin is the most abundant HSPG in the GBM [20]. It consists of a 212 kD core protein, to which two or three heparan sulfate chains are attached in the amino terminal half [21]. Full length agrin is restricted to the GBM while truncated isoforms lacking C-terminal epitopes are more broadly found in other kidney basement membranes, possibly reflecting alternative splicing or post-translational modifications [22]. Agrin may play a role in cell-matrix adhesion because its N-terminal end binds to the coiled coil domain of laminin γ1 chain while its C-terminal end binds to cell surface receptors such as integrins or α-dystroglycan [23–25]. The ultrastructural localization of agrin in the GBM has been recently determined by ultra-high resolution STORM imaging correlated with electron microscopy [26]. In the mouse GBM, agrin is localized in two layers and is oriented perpendicular or slightly oblique to the plane of the GBM, with its C-terminal end near adjacent cell membranes and its N-terminus toward the center of the GBM. In the human GBM, significantly more agrin (as detected with antibodies to its C-terminal domain) is present in a subepithelial layer, adjacent to podocytes, which may reflect differences in expression or alternatively spliced isoforms [26].
The phenotype of known mutations affecting HSPG core proteins in human disease affords no inferences about the role of HSPG in the GBM. Whereas missense mutations in the genes encoding agrin [27, 28] or perlecan [29, 30] do occur in congenital myasthenic syndrome and Schwartz-Jampel syndrome, respectively, no kidney dysfunction has been reported in these patients, presumably because these mutations have limited, localized effect on protein function. Nonetheless, as discussed in the next section, the expression of heparan sulfate in the GBM is altered in various kidney diseases, suggesting a functional role under pathologic conditions.
2. Alterations of glomerular heparan sulfate chains in kidney diseases
A reduction of GBM anionic sites (presumably heparan sulfate) in several human and experimental glomerulopathies has been first observed using non-specific cationic probes [31–33]. The distribution of heparan sulfate chains and HSPG core protein in normal kidneys and various glomerular diseases has been further determined by immunohistochemical studies using mAbs as more specific probes. A global or segmental loss of staining for heparan sulfate chains in the GBM, with preserved staining for the agrin core protein, occurs most prominently in lupus nephritis, membranous nephropathy, minimal change disease, and diabetic nephropathy [34]. It also occurs in some cases of focal glomerulosclerosis and crescentic glomerulonephritis, but not in IgA nephropathy or Alport syndrome [34]. A loss of GBM heparan sulfate has also been described in single cases of dense deposit disease/C3 glomerulopathy [35] and Denys-Drash syndrome [36].
Several animal models of kidney disease recapitulate this loss of GBM heparan sulfate, which is associated with the disease severity. In MRL/lpr mice, a model of lupus nephritis, the heparan sulfate staining in glomerular capillary loops almost completely disappears in mice with prolonged albuminuria but is unaltered in non-albuminuric mice [37]. In active Heymann nephritis, a rat model of membranous nephropathy, the loss of GBM heparan sulfate correlates with complement deposition and albuminuria [38]. A relative decrease of glomerular heparan sulfate also occurs in rats with streptozotocin-induced diabetic nephropathy [39] and in rats with adriamycin nephropathy, a model of minimal change disease [40, 41].
The loss of heparan sulfate chains from the GBM may occur through several mechanisms, including decreased synthesis, increased degradation, or masking by cationic proteins [42]. Heparanase, a β(1–4)-endoglucuronidase, is the only mammalian enzyme that degrades heparan sulfate chains [43]. Heparanase is secreted as a latent 65 kD proenzyme, which is proteolytically processed by cathepsin L to produce the active enzyme, a heterodimer comprising 50 kDa and 8 kDa fragments [44]. Active heparanase trims and shortens heparan sulfate chains by hydrolyzing internal glycosidic bonds at specific sites, releasing fragments of 5–7 kDa. Heparanase recognizes specific patterns of O-sulfation as substrate, but its specificity is further modulated by saccharide structures around the cleavage site [45]. The enzyme activity requires acidic conditions (pH 5–6); at physiological pH heparanase binds to heparan sulfate without cleaving it and can mediate cell-matrix adhesion [46, 47]. Heparanase is not expressed in most normal tissues, but is upregulated in malignant cells, as well as in angiogenesis and inflammation. The loss of GBM heparan sulfate in human kidney diseases is often associated with increased glomerular staining for heparanase, reflecting an upregulation of its expression in glomerular endothelial cells and/or podocytes. Thus far, an increase of glomerular heparanase has been described in membranous nephropathy, IgA nephropathy, minimal change disease [48], overt diabetic nephropathy [49], and dense deposit disease [35].
A causal role for heparanase in the development of proteinuria is supported by studies in animal models. In passive Heymann nephritis, glomerular heparanase increases in the proteinuric phase secondary to complement activation, and treatment with polyclonal anti-heparanase antibodies results in a three-fold reduction in proteinuria [50]. In the same model, treatment with PI-88, a highly sulfated oligosaccharide inhibitor of heparanase, prevents the loss of GBM heparan sulfate and reduces proteinuria two-fold [51]. In accelerated nephrotoxic anti-GBM nephritis, another model in which glomerular heparanase is upregulated, anti-heparanase antibodies reduce proteinuria 5-fold [52]. In mouse models of diabetic nephropathy, heparanase inhibitor SST0001 decreases albuminuria and renal damage, while diabetic heparanase knockout mice are protected from nephropathy [53]. In experimental glomerulonephritis induced by anti-GBM antibodies or lipopolysaccharide, increased heparanase expression is associated with the loss of heparan sulfate and proteinuria; heparanase-null mice show preserved glomerular heparan sulfate expression, reduced albuminuria, and less renal damage [54]. Notwithstanding biological effects of heparanase independent of its enzyme activity, it seems likely that the ablation of heparanase function ameliorates proteinuria in experimental kidney disease at least in part by reducing the loss of heparan sulfate in the GBM.
A loss of GBM heparan sulfate staining may also be due to masking by cationic proteins, autoantibodies, or immune complexes. Neutrophil cationic proteins have been localized in glomeruli in human lupus nephritis in association with the loss of GBM anionic charges [55]. A subset of cationic anti-DNA antibodies from lupus sera also bind to heparan sulfate [56]. Since lupus autoantibodies complexed to nucleosomal antigens inhibit in vitro binding of anti-heparan sulfate mAbs to heparan sulfate chains, masking by IgG immune deposits has been proposed to explain the loss of GBM heparan sulfate staining associated with proteinuria in MRL/lpr mice, a model of lupus nephritis [37]. In a later study, masking by IgG deposits has been found to alter the expression of specific GBM heparan sulfate domains both in human and murine lupus [57]. In secondary membranous nephropathy, subepithelial immune complexes may form when antibodies target “planted antigens”, i.e. cationic proteins which are extrinsic to the glomerulus but bind to anionic sites in the GBM, such as cationic bovine serum albumin [58–60]. It is thus possible that cationic planted antigens may bind to and mask GBM heparan sulfate chains in some cases of membranous nephropathy. More studies are needed to clarify the functional consequences of the actual or apparent loss of GBM heparan sulfate chains in kidney diseases.
Besides enzymatic cleavage or masking, the structure of GBM heparan sulfate may also be altered in kidney disease as a result of changes in its biosynthesis or editing, which would likely affect functionality. Thus, disease-associated changes in the activity of sulfotransferases or sulfatases may alter the pattern of sulfation, impacting not only the overall anionic charge but also heparan sulfate interactions with protein ligands.
3. GBM heparan sulfate chains and the charge selectivity of glomerular filtration
How the loss of GBM heparan sulfate chains is causally related to proteinuria remains incompletely understood. A direct role for heparan sulfate chains in the glomerular filtration has been initially proposed. Glomerular filtration barrier exhibits both size selectivity and charge selectivity, as established by classic studies with molecular tracers [61]. Anionic sites in the GBM, visualized by electron microscopy with cationic probes (e.g. polyethylenimine) in lamina rara interna and externa [62], have been thought to confer charge selectivity to the glomerular filtration, restricting the passage of negatively charged macromolecules such as albumin. The loss of these GBM anionic sites after treatment with heparanase demonstrates that the negative charge of the GBM is mainly imparted by heparan sulfate chains [63]. Consistent with a role for heparan sulfate in charge selectivity, permeability of GBM to native (anionic) ferritin is increased after enzymatic removal of GBM heparan sulfate with heparitinase [64]. In a rat model, acute selective proteinuria is immediately induced after the injection of a monoclonal antibody against heparan sulfate chains, which binds in vivo to the GBM [65].
Notwithstanding these findings, the results of more recent studies ablating GBM heparan sulfate in vivo challenge the concept that GBM anionic sites establish a charge selective barrier. Hspg2(Δ3/Δ3) mice that lack heparan sulfate on perlecan have been generated by deleting perlecan exon 3, which removes all three heparan sulfate attachment sites in perlecan domain I. These mice have normal glomerular ultrastructure and do not show any proteinuria under physiological conditions, but are more susceptible than wild type mice to proteinuria induced by albumin overload [66, 67]. The overall GBM anionic charge was not significantly reduced in these mice, implying that perlecan heparan sulfate chains are not a major contributor to GBM anionic sites. By contrast, podocyte-specific agrin-null mice show an extensive loss of GBM heparan sulfate chains and a profound reduction in GBM anionic sites, yet they do not develop albuminuria even when challenged with albumin overload [68]. Extending these findings, double knockout mice lacking both perlecan heparan sulfate and podocyte agrin also have normal podocytes and glomerular architecture, with no proteinuria [69]. More extensive loss of glomerular heparan sulfate chains has been induced by podocyte-specific ablation of Ext-1 (a key enzyme in heparan sulfate biosynthesis), which halts the assembly of heparan sulfate on all core proteins secreted by podocytes, including agrin. Podocyte Ext-1 knockout mice have fewer glomerular anionic sites and develop podocyte abnormalities such as foot process effacement as early as one month after birth, yet they also show only a slight increase in proteinuria, not statistically significant [70]. The ultrastructural abnormalities in this model are likely due to the absence of cell surface HSPG, which podocyte use for adhesion to the GBM [71]. Transgenic mice over-expressing heparanase also exhibit almost complete absence of heparan sulfate in the GBM, associated with relatively mild proteinuria [49, 72]. Also, intravenous injection of heparitinase in rats causes in vivo degradation of heparan sulfate in the GBM but no overt proteinuria [73].
These findings have been taken to imply that neither agrin nor heparan sulfate chains of the GBM are essential for the normal function of the glomerular filtration barrier under physiological conditions [74]. One should keep in mind, though, that some albumin from the primary urine can be recycled by tubular transcytosis [75], which may obscure an increase in glomerular permeability to albumin that by itself is insufficient to overwhelm the capacity of the tubular uptake system. Importantly, the evidence currently available is insufficient to exclude a functional role for GBM heparan sulfate under various pathologic conditions. New investigations of the renal phenotype of mice lacking GBM heparan sulfate in the setting of kidney disease are needed to address this gap in knowledge.
4. The role of GBM heparan sulfate chains in local complement regulation
Heparan sulfate glycosaminoglycans have multifarious biological functions [76, 77]. Their ability to modulate complement activation through interactions with complement regulatory proteins is gaining increased recognition [7, 9]. In the setting of the GBM, complement regulation by heparan sulfate is particularly relevant, given that complement activation mediates glomerular injury in numerous kidney diseases. As a key component of the innate immune system, complement is important for host defense against pathogens and also for the clearance of immune complexes and cell debris. However, excessive activation or insufficient regulation of complement causes tissue injury in many pathological conditions. To better appreciate how GBM heparan sulfate can modulate the activity of complement system, it is helpful to briefly outline how complement is activated and regulated.
4.1. Overview of the complement activation and regulation
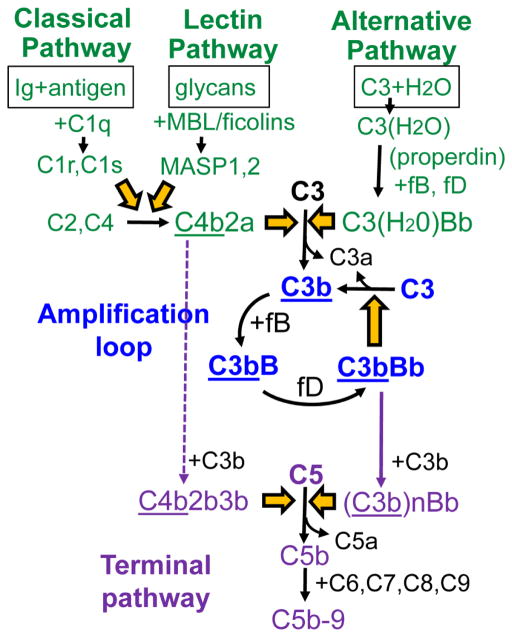
Complement activation is initiated by three pathways converging toward the generation of C3 convertases (Figure 1). The classical pathway is initiated when its recognition molecule, C1q, binds to immune complexes containing IgM or certain IgG subclasses (IgG1 and IgG3), activating serine proteases C1r and C1s, which cleave C4 and C2. The lectin pathway is initiated when its recognition molecules, mannan-binding lectin (MBL) or ficolins, bind to patterns of carbohydrates present on pathogens or damaged self, activating MBL-associated serine proteases, which also cleave C2 and C4. C4b attaches covalently to the target and forms C4b2b, the C3 convertase of the classical and lectin pathways. Unlike the classical and lectin pathways, the alternative pathway is always active at low level. Slow spontaneous hydrolysis of the thioester bond of C3 generates C3(H2O), which in the presence of factors B and D produces C3(H2O)Bb, the initial C3 convertase of the alternative pathway.
Figure 1. Overview of the complement activation cascade.
Complement activation is initiated by three pathways (green), converging toward activation of C3, which is further amplified in a positive feed-back loop (blue). The terminal complement pathway (purple) is activated upon the formation of C5 convertases that cleave C5. The underlined components attach covalently to target.
All C3 convertases cleave C3 into C3a and C3b. C3b undergoes a conformational change that unmasks a highly reactive thioester group, which attaches covalently to hydroxyl or amino groups on nearby targets. The fate of surface-bound C3b depends on the nature of the surface. On self surfaces, C3b is typically inactivated by protease factor I in the presence of cofactors; C3b is cleaved initially to iC3b and then to C3d (which remain surface-bound) and C3c (which is released). On pathogen surfaces, surface-bound C3b binds factor B, which is cleaved by factor D to form C3bBb, the major C3 convertase of the alternative pathway. C3bBb cleaves additional C3 molecules, generating more surface-bound C3b. Through this positive feedback loop, complement activation is amplified exponentially, regardless of how C3b is produced initially [78]. C3bBb is unstable and decays when Bb dissociates irreversibly. Properdin is a positive regulator of the alternative pathway which binds to and stabilizes surface-bound C3bBb, significantly extending its half-life [79]. In addition, properdin tethered to surfaces (e.g. via C3b) can provide a nidus for C3bBb convertase assembly, directing alternative pathway activation [80, 81]. Of note, heparan sulfate on proximal tubular epithelial cells binds properdin and thus acts as a docking platform for alternative pathway activation in proteinuric kidney disease [82, 83]. A similar role for GBM heparan sulfate in properdin binding has not been investigated.
Addition of C3b to existing C3 convertases alters their substrate specificity, generating C5 convertases that cleave C5 into C5a and C5b, thus activating the terminal complement pathway. C5b sequentially binds C6, C7, C8 and C9, forming C5b-9. Effector molecules produced by complement activation include anaphylatoxins (C3a, C5a) which recruit and activate inflammatory cells, opsonins (C3b, iC3b) that bind to target surfaces and promote phagocytosis, and the membrane attack complex (C5b-9), which lyses cells. While these effectors are potent weapons against harmful pathogens, they can also mediate considerable damage to the filtration barrier when inadvertently deployed against glomerular structures.
To avoid damage to host, complement activation is tightly regulated by cell-associated and fluid-phase complement regulatory proteins. Cells express several membrane-bound regulators that inactivate the C3/C5 convertases of all pathways through decay acceleration or as cofactors for the cleavage of C3b and C4b by factor I. These include decay accelerating factor (CD55), membrane cofactor protein (CD46), and complement receptor 1 (CR1, CD35). In addition, cells are protected by membrane-bound CD59, which inhibits the formation of C5b-9. Membrane-anchored complement regulators act in the immediate proximity of cell surfaces, but not in the GBM, which requires plasma regulators for protection against complement.
4.2. Factor H recognizes heparan sulfate to inhibit complement on host surfaces
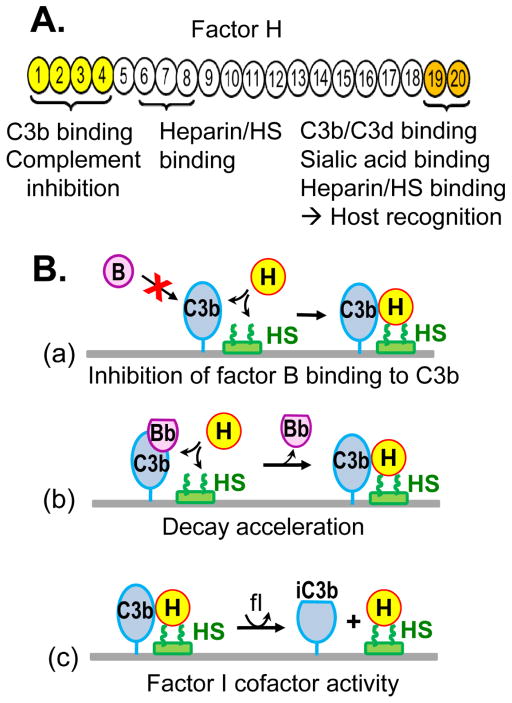
A relatively abundant plasma glycoprotein, factor H is the major regulator of the alternative pathway in the fluid phase and on surfaces carrying certain polyanions such as heparan sulfate. Factor H has a modular structure and is composed of 20 domains named short consensus repeats (SCR) which fold independently and harbor distinct activities [84]. Factor H inhibits the alternative pathway by several mechanisms (Figure 2): it competes with factor B for binding to C3b, it accelerates the decay of the amplification convertase C3bBb, and it serves as cofactor for factor I, catalyzing the proteolytic inactivation of C3b to iC3b [85, 86]. All complement regulatory activities of factor H map to its N-terminal domains SCR1-4, which contain a major binding site for C3b [87]. SCR1-4 of factor H is sufficient to inhibit the activation of the alternative pathway in the fluid phase. The C-terminal domains SCR19-20 of factor H, which contain a distinct binding site for C3b and C3d, are additionally required to inhibit the alternative pathway activation on self surfaces.
Figure 2. Factor H structure and function.
A. Domain organization of factor H and the location of functional sites. B. Factor H inhibits the alternative pathway by several mechanisms: (a) inhibiting the binding of factor B to C3b, (b) accelerating the decay of the C3bBb convertase, and (c) serving as cofactor for factor I (fI), which inactivates C3b to iC3b. The ability of factor H to inhibit complement amplification on self surfaces (depicted as horizontal lines) is contingent upon its recognition of host-specific polyanions, such as heparan sulfate (HS).
Factor H selectively inactivates C3b bound to surfaces carrying certain host-specific polyanions such as heparan sulfate or sialic acid as markers of self. Binding to polyanions is essential for the host recognition function of factor H. Two major heparin/glycosaminoglycan binding sites of factor H have been mapped to SCR6-8 and SCR19-20 [88–90]. The two sites recognize heparan sulfate chains in a tissue-specific manner, with SCR6-8 more important for host tissue recognition in the human eye, and SCR19-20 more important for factor H binding in the human kidney [91]. In addition, SCR20 binds to sialic acid structures in a specific linkage [92]. Dual recognition of C3b and polyanions enables factor H to discriminate between host surfaces and pathogens. Surface polyanions increase the affinity of factor H for surface-bound C3b, exposing the complement regulatory domains which inactivate C3b [93–95]. As a result, host surfaces bearing heparan sulfate (or sialic acid) effectively recruit factor H to inhibit the alternative pathway and are therefore complement non-activators [96–98]. In contrast, factor H has low affinity for C3b attached to surfaces lacking these polyanions (e.g. pathogens), which favors complement activation and amplification. Coupling of heparin to complement-activating particles such as Sepharose or zymosan inhibits their ability to activate the alternative pathway by promoting the interaction between factor H and particle-bound C3b [99]. Heparan sulfate from tissues (e.g. the eye Bruch’s membrane), has also been shown to inhibit the alternative pathway in functional assays [100].
4.3. Modulation of local complement activation by GBM heparan sulfate chains
The glomerulus is uniquely susceptible to complement-mediated injury caused by the dysregulation of the alternative pathway, as shown by mutations causing factor H dysfunction. Mutations that impair the complement regulatory function of factor H cause C3 glomerulopathy (dense deposit disease), characterized by dysregulation of the alternative pathway in the fluid phase, excessive consumption of C3 in plasma, and pathological deposition of C3 fragments in the GBM [101]. Impaired host recognition function of factor H due to mutations in SCR19-20 (or inhibitory autoantibodies to this region) causes atypical hemolytic uremic syndrome, in which the defective attachment of factor H to C3b and polyanions on host surfaces leads to glomerular endothelial cells injury, even though the alternative pathway regulation in plasma is normal [102–105]. Another putative mechanism that can locally dysregulate the host recognition function of factor H is the loss of its polyanion ligands, such as heparan sulfate chains, from self surfaces.
As an extracellular matrix, the GBM lacks intrinsic cell-anchored complement regulatory proteins, which protect adjacent glomerular endothelial cells and podocytes. To limit inadvertent complement activation, the GBM must recruit complement regulators from plasma—mainly factor H. Since factor H requires polyanions for effective complement inhibition on surfaces, GBM heparan sulfate chains can function as molecular markers of self, directing factor H activity. Accordingly, factor H binds to glomeruli via its C-terminal end (SCR19-20) in a manner that can be inhibited by heparin, suggesting an interaction with heparan sulfates [106].
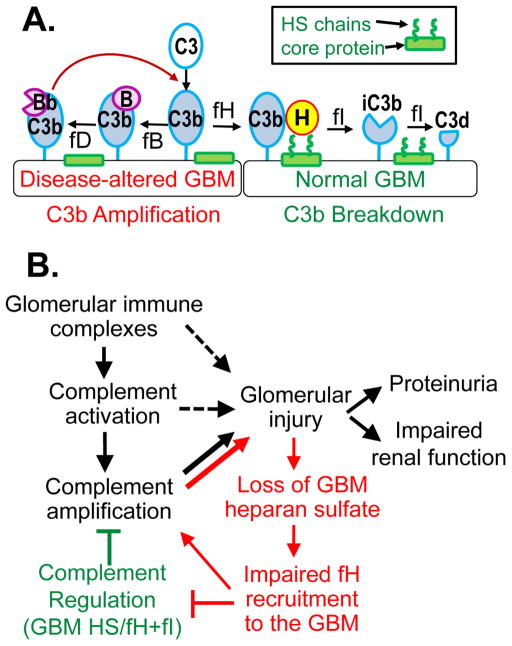
In disease states, the loss of GBM heparan sulfate chains may locally compromise the host recognition function of factor H [107]. In the absence of heparan sulfate chains, recruitment of factor H is impaired, which may lead to inadequate control of the complement amplification (Figure 3, A). In this regard, the altered GBM resembles non-self surfaces, like pathogens. The consequent dysregulation of the alternative pathway is maladaptive, particularly under pathologic conditions in which complement is activated by other mechanisms (Figure 3, B).
Figure 3. GBM heparan sulfate modulates local complement activation.
(A.) Heparan sulfate (HS), abundant in the normal GBM, helps recruit factor H to locally inactivate GBM-bound C3b (right). In the absence of GBM heparan sulfate, this regulation is ineffective, allowing local amplification of complement activation (left). (B.) In kidney diseases mediated by glomerular immune complexes, the acquired loss of GBM heparan sulfate chains impairs the recruitment of factor H and the local complement regulation, thereby creating a vicious cycle (shown in red), which amplifies complement activation and exacerbates glomerular injury.
Complement activation plays a major role in the pathogenesis of membranous nephropathy and lupus nephritis, diseases mediated by glomerular immune complexes and associated with a loss of GBM heparan sulfate. Although immune complexes mainly activate complement via the classical pathway, glomerular deposition of factor B and properdin also occurs in membranous nephropathy [108, 109], lupus nephritis [110], and anti-GBM antibody disease [111], indicative of the alternative pathway activation. These findings suggest insufficient complement regulation by factor H, which may be exacerbated by the loss of GBM heparan sulfate, as depicted in Figure 3. As glomerular immune complexes activate complement, C3b is being generated which attaches to nearby targets in the GBM. When GBM heparan sulfate are abundantly present, factor H binds with high affinity to GBM-bound C3b, which can be inactivated by factor I. As GBM heparan sulfate chains are lost, reduced binding of factor H to GBM-bound C3b allows the formation of the amplification convertase, C3bBb. Thus, the availability of GBM heparan sulfate modulates the balance between complement regulation and amplification. In particular, the amplification provided by the alternative pathway may be essential for pathogenic complement activation in primary membranous nephropathy [107]. This may explain the paradox that most patients show positive glomerular staining for C3 and C5b-9, even though subepithelial immune complexes contain predominantly IgG4 antibodies [112, 113], a subclass of IgG that does not bind C1q nor activates the classical pathway.
5. Concluding remarks
Structural and functional characterization of GBM heparan sulfate has been work in progress for decades. The emerging role of heparan sulfate in the local complement regulation is of interest because the kidney glomerulus is highly susceptible to complement-mediated injury. In particular, the GBM lacks protection by intrinsic complement regulators and must recruit factor H from plasma to control complement. The recognition of host-specific polyanions, such as heparan sulfate chains abundantly present in the normal GBM, is essential for the ability of factor H to effectively inactivate C3b bound to self-surfaces, ultimately preventing inadvertent amplification of complement activation. The loss of GBM heparan sulfate chains in kidney diseases such as membranous nephritis and lupus nephritis may compromise this host recognition function, exacerbating local complement activation and glomerular injury. More work is needed to fully characterize the interactions between GBM heparan sulfate and complement regulatory proteins, biochemically and functionally, in health and disease states. Further advances in our knowledge in this area may identify new therapeutic options.
Highlights.
Heparan sulfate chains attached to agrin core protein are abundantly present in the normal GBM but significantly decreased in several glomerular diseases.
Genetic studies in mouse models challenge the long-held view that GBM heparan sulfate chains determine the charge selectivity of glomerular filtration.
Heparan sulfate chains modulate local complement activation by recruiting complement regulatory proteins, such as factor H, from plasma.
Factor H recognizes polyanions such as heparan sulfate as molecular markers of self to specifically inhibit complement activation on host surfaces.
The loss of GBM heparan sulfate in glomerular disease may impair complement regulation by factor H, exacerbating complement-mediated pathology.
Acknowledgments
Funding
This work was supported by the Norman S. Coplon Extramural Grant Program from Satellite Healthcare, a not-for-profit renal care provider, by the Meharry Translational Research Center grant U54 MD007593 from the National Institute on Minority Health and Health Disparities of the National Institutes of Health, and other funds from the Meharry Medical College.
Abbreviations
- SCR
short consensus repeats
- GBM
glomerular basement membrane
- HSPG
heparan sulfate proteoglycan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrahamson DR, Hudson BG, Stroganova L, Borza DB, St John PL. Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol. 2009;20(7):1471–9. doi: 10.1681/ASN.2008101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.St John PL, Abrahamson DR. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 2001;60(3):1037–46. doi: 10.1046/j.1523-1755.2001.0600031037.x. [DOI] [PubMed] [Google Scholar]
- 3.Miner JH. Glomerular basement membrane composition and the filtration barrier. Pediatr Nephrol. 2011;26(9):1413–7. doi: 10.1007/s00467-011-1785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;248(4960):1224–7. doi: 10.1126/science.2349482. [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, Verellen-Dumoulin C, Chan B, Schroder CH, Smeets HJ, et al. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nature genetics. 1994;8(1):77–81. doi: 10.1038/ng0994-77. [DOI] [PubMed] [Google Scholar]
- 6.Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dotsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13(21):2625–32. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- 7.Clark SJ, Bishop PN, Day AJ. The proteoglycan glycomatrix: a sugar microenvironment essential for complement regulation. Front Immunol. 2013;4:412. doi: 10.3389/fimmu.2013.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaferani A, Talsma D, Richter MK, Daha MR, Navis GJ, Seelen MA, van den Born J. Heparin/heparan sulphate interactions with complement--a possible target for reduction of renal function loss? Nephrol Dial Transplant. 2014;29(3):515–22. doi: 10.1093/ndt/gft243. [DOI] [PubMed] [Google Scholar]
- 9.Langford-Smith A, Day AJ, Bishop PN, Clark SJ. Complementing the Sugar Code: Role of GAGs and Sialic Acid in Complement Regulation. Front Immunol. 2015;6:25. doi: 10.3389/fimmu.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varki A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology. 2011;21(9):1121–4. doi: 10.1093/glycob/cwr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esko JD, Lindahl U. Molecular diversity of heparan sulfate. J Clin Invest. 2001;108(2):169–73. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–71. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 14.Rops AL, van der Vlag J, Lensen JF, Wijnhoven TJ, van den Heuvel LP, van Kuppevelt TH, Berden JH. Heparan sulfate proteoglycans in glomerular inflammation. Kidney Int. 2004;65(3):768–85. doi: 10.1111/j.1523-1755.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy KJ, Wassenhove-McCarthy DJ. The glomerular basement membrane as a model system to study the bioactivity of heparan sulfate glycosaminoglycans, Microscopy and microanalysis: the official journal of Microscopy Society of America, Microbeam Analysis Society. Microscopical Society of Canada. 2012;18(1):3–21. doi: 10.1017/S1431927611012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groffen AJ, Hop FW, Tryggvason K, Dijkman H, Assmann KJ, Veerkamp JH, Monnens LA, Van den Heuvel LP. Evidence for the existence of multiple heparan sulfate proteoglycans in the human glomerular basement membrane and mesangial matrix. Eur J Biochem. 1997;247(1):175–82. doi: 10.1111/j.1432-1033.1997.00175.x. [DOI] [PubMed] [Google Scholar]
- 17.Kallunki P, Tryggvason K. Human basement membrane heparan sulfate proteoglycan core protein: a 467-kD protein containing multiple domains resembling elements of the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J Cell Biol. 1992;116(2):559–71. doi: 10.1083/jcb.116.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1996;18(2):123–32. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 19.Halfter W, Dong S, Schurer B, Cole GJ. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J Biol Chem. 1998;273(39):25404–12. doi: 10.1074/jbc.273.39.25404. [DOI] [PubMed] [Google Scholar]
- 20.Groffen AJ, Ruegg MA, Dijkman H, van de Velden TJ, Buskens CA, van den Born J, Assmann KJ, Monnens LA, Veerkamp JH, van den Heuvel LP. Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1998;46(1):19–27. doi: 10.1177/002215549804600104. [DOI] [PubMed] [Google Scholar]
- 21.Groffen AJ, Buskens CA, van Kuppevelt TH, Veerkamp JH, Monnens LA, van den Heuvel LP. Primary structure and high expression of human agrin in basement membranes of adult lung and kidney. Eur J Biochem. 1998;254(1):123–8. doi: 10.1046/j.1432-1327.1998.2540123.x. [DOI] [PubMed] [Google Scholar]
- 22.Raats CJ, Bakker MA, Hoch W, Tamboer WP, Groffen AJ, van den Heuvel LP, Berden JH, van den Born J. Differential expression of agrin in renal basement membranes as revealed by domain-specific antibodies. The Journal of biological chemistry. 1998;273(28):17832–8. doi: 10.1074/jbc.273.28.17832. [DOI] [PubMed] [Google Scholar]
- 23.Martin PT, Sanes JR. Integrins mediate adhesion to agrin and modulate agrin signaling. Development. 1997;124(19):3909–17. doi: 10.1242/dev.124.19.3909. [DOI] [PubMed] [Google Scholar]
- 24.Gesemann M, Brancaccio A, Schumacher B, Ruegg MA. Agrin is a high-affinity binding protein of dystroglycan in non-muscle tissue. J Biol Chem. 1998;273(1):600–5. doi: 10.1074/jbc.273.1.600. [DOI] [PubMed] [Google Scholar]
- 25.Denzer AJ, Schulthess T, Fauser C, Schumacher B, Kammerer RA, Engel J, Ruegg MA. Electron microscopic structure of agrin and mapping of its binding site in laminin-1. EMBO J. 1998;17(2):335–43. doi: 10.1093/emboj/17.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suleiman H, Zhang L, Roth R, Heuser JE, Miner JH, Shaw AS, Dani A. Nanoscale protein architecture of the kidney glomerular basement membrane. Elife. 2013;2:e01149. doi: 10.7554/eLife.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huze C, Bauche S, Richard P, Chevessier F, Goillot E, Gaudon K, Ben Ammar A, Chaboud A, Grosjean I, Lecuyer HA, Bernard V, Rouche A, Alexandri N, Kuntzer T, Fardeau M, Fournier E, Brancaccio A, Ruegg MA, Koenig J, Eymard B, Schaeffer L, Hantai D. Identification of an agrin mutation that causes congenital myasthenia and affects synapse function. Am J Hum Genet. 2009;85(2):155–67. doi: 10.1016/j.ajhg.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maselli RA, Fernandez JM, Arredondo J, Navarro C, Ngo M, Beeson D, Cagney O, Williams DC, Wollmann RL, Yarov-Yarovoy V, Ferns MJ. LG2 agrin mutation causing severe congenital myasthenic syndrome mimics functional characteristics of non-neural (z-) agrin. Hum Genet. 2012;131(7):1123–35. doi: 10.1007/s00439-011-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicole S, Davoine CS, Topaloglu H, Cattolico L, Barral D, Beighton P, Hamida CB, Hammouda H, Cruaud C, White PS, Samson D, Urtizberea JA, Lehmann-Horn F, Weissenbach J, Hentati F, Fontaine B. Perlecan, the major proteoglycan of basement membranes, is altered in patients with Schwartz-Jampel syndrome (chondrodystrophic myotonia) Nature genetics. 2000;26(4):480–3. doi: 10.1038/82638. [DOI] [PubMed] [Google Scholar]
- 30.Stum M, Davoine CS, Vicart S, Guillot-Noel L, Topaloglu H, Carod-Artal FJ, Kayserili H, Hentati F, Merlini L, Urtizberea JA, Hammouda el H, Quan PC, Fontaine B, Nicole S. Spectrum of HSPG2 (Perlecan) mutations in patients with Schwartz-Jampel syndrome. Hum Mutat. 2006;27(11):1082–91. doi: 10.1002/humu.20388. [DOI] [PubMed] [Google Scholar]
- 31.Vernier RL, Klein DJ, Sisson SP, Mahan JD, Oegema TR, Brown DM. Heparan sulfate--rich anionic sites in the human glomerular basement membrane. Decreased concentration in congenital nephrotic syndrome. N Engl J Med. 1983;309(17):1001–9. doi: 10.1056/NEJM198310273091701. [DOI] [PubMed] [Google Scholar]
- 32.Tomino Y, Yagame M, Eguchi K, Miyazaki M, Nomoto Y, Sakai H, Shirato I, Ito K. Detection of anionic sites and immunoglobulin A deposits in the glomerular capillary walls from patients with IgA nephropathy. J Clin Lab Anal. 1989;3(2):101–7. doi: 10.1002/jcla.1860030207. [DOI] [PubMed] [Google Scholar]
- 33.Okada K, Kawakami K, Miyao M, Oite T. Ultrastructural alterations of glomerular anionic sites in idiopathic membranous glomerulonephritis. Clinical nephrology. 1986;26(1):7–14. [PubMed] [Google Scholar]
- 34.van den Born J, van den Heuvel LP, Bakker MA, Veerkamp JH, Assmann KJ, Weening JJ, Berden JH. Distribution of GBM heparan sulfate proteoglycan core protein and side chains in human glomerular diseases. Kidney Int. 1993;43(2):454–63. doi: 10.1038/ki.1993.67. [DOI] [PubMed] [Google Scholar]
- 35.Smith RJ, Alexander J, Barlow PN, Botto M, Cassavant TL, Cook HT, de Cordoba SR, Hageman GS, Jokiranta TS, Kimberling WJ, Lambris JD, Lanning LD, Levidiotis V, Licht C, Lutz HU, Meri S, Pickering MC, Quigg RJ, Rops AL, Salant DJ, Sethi S, Thurman JM, Tully HF, Tully SP, van der Vlag J, Walker PD, Wurzner R, Zipfel PFG. Dense Deposit Disease Focus. New approaches to the treatment of dense deposit disease. J Am Soc Nephrol. 2007;18(9):2447–56. doi: 10.1681/ASN.2007030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Heuvel LP, Westenend PJ, van den Born J, Assmann KJ, Knoers N, Monnens LA. Aberrant proteoglycan composition of the glomerular basement membrane in a patient with Denys-Drash syndrome. Nephrol Dial Transplant. 1995;10(12):2205–11. doi: 10.1093/ndt/10.12.2205. [DOI] [PubMed] [Google Scholar]
- 37.van Bruggen MC, Kramers K, Hylkema MN, van den Born J, Bakker MA, Assmann KJ, Smeenk RJ, Berden JH. Decrease of heparan sulfate staining in the glomerular basement membrane in murine lupus nephritis. Am J Pathol. 1995;146(3):753–63. [PMC free article] [PubMed] [Google Scholar]
- 38.Raats CJ, Luca ME, Bakker MA, Van Der Wal A, Heeringa P, Van Goor H, Van Den Born J, De Heer E, Berden JH. Reduction in glomerular heparan sulfate correlates with complement deposition and albuminuria in active Heymann nephritis. J Am Soc Nephrol. 1999;10(8):1689–99. doi: 10.1681/ASN.V1081689. [DOI] [PubMed] [Google Scholar]
- 39.van den Born J, van Kraats AA, Bakker MA, Assmann KJ, van den Heuvel LP, Veerkamp JH, Berden JH. Selective proteinuria in diabetic nephropathy in the rat is associated with a relative decrease in glomerular basement membrane heparan sulphate. Diabetologia. 1995;38(2):161–72. doi: 10.1007/BF00400090. [DOI] [PubMed] [Google Scholar]
- 40.Wapstra FH, Navis GJ, van Goor H, van den Born J, Berden JH, de Jong PE, de Zeeuw D. ACE inhibition preserves heparan sulfate proteoglycans in the glomerular basement membrane of rats with established adriamycin nephropathy. Exp Nephrol. 2001;9(1):21–7. doi: 10.1159/000020704. [DOI] [PubMed] [Google Scholar]
- 41.Raats CJ, Bakker MA, van den Born J, Berden JH. Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J Biol Chem. 1997;272(42):26734–41. doi: 10.1074/jbc.272.42.26734. [DOI] [PubMed] [Google Scholar]
- 42.Raats CJ, Van Den Born J, Berden JH. Glomerular heparan sulfate alterations: mechanisms and relevance for proteinuria. Kidney Int. 2000;57(2):385–400. doi: 10.1046/j.1523-1755.2000.00858.x. [DOI] [PubMed] [Google Scholar]
- 43.Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, Spector L, Pecker I. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nature medicine. 1999;5(7):793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 44.Zetser A, Levy-Adam F, Kaplan V, Gingis-Velitski S, Bashenko Y, Schubert S, Flugelman MY, Vlodavsky I, Ilan N. Processing and activation of latent heparanase occurs in lysosomes. J Cell Sci. 2004;117(Pt 11):2249–58. doi: 10.1242/jcs.01068. [DOI] [PubMed] [Google Scholar]
- 45.Peterson SB, Liu J. Multi-faceted substrate specificity of heparanase. Matrix Biol. 2013;32(5):223–7. doi: 10.1016/j.matbio.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Gilat D, Hershkoviz R, Goldkorn I, Cahalon L, Korner G, Vlodavsky I, Lider O. Molecular behavior adapts to context: heparanase functions as an extracellular matrix-degrading enzyme or as a T cell adhesion molecule, depending on the local pH. J Exp Med. 1995;181(5):1929–34. doi: 10.1084/jem.181.5.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldshmidt O, Zcharia E, Cohen M, Aingorn H, Cohen I, Nadav L, Katz BZ, Geiger B, Vlodavsky I. Heparanase mediates cell adhesion independent of its enzymatic activity. FASEB J. 2003;17(9):1015–25. doi: 10.1096/fj.02-0773com. [DOI] [PubMed] [Google Scholar]
- 48.van den Hoven MJ, Rops AL, Vlodavsky I, Levidiotis V, Berden JH, van der Vlag J. Heparanase in glomerular diseases. Kidney Int. 2007;72(5):543–548. doi: 10.1038/sj.ki.5002337. [DOI] [PubMed] [Google Scholar]
- 49.van den Hoven MJ, Rops AL, Bakker MA, Aten J, Rutjes N, Roestenberg P, Goldschmeding R, Zcharia E, Vlodavsky I, van der Vlag J, Berden JH. Increased expression of heparanase in overt diabetic nephropathy. Kidney Int. 2006;70(12):2100–8. doi: 10.1038/sj.ki.5001985. [DOI] [PubMed] [Google Scholar]
- 50.Levidiotis V, Freeman C, Tikellis C, Cooper ME, Power DA. Heparanase is involved in the pathogenesis of proteinuria as a result of glomerulonephritis. J Am Soc Nephrol. 2004;15(1):68–78. doi: 10.1097/01.asn.0000103229.25389.40. [DOI] [PubMed] [Google Scholar]
- 51.Levidiotis V, Freeman C, Punler M, Martinello P, Creese B, Ferro V, van der Vlag J, Berden JH, Parish CR, Power DA. A synthetic heparanase inhibitor reduces proteinuria in passive Heymann nephritis. J Am Soc Nephrol. 2004;15(11):2882–92. doi: 10.1097/01.ASN.0000142426.55612.6D. [DOI] [PubMed] [Google Scholar]
- 52.Levidiotis V, Freeman C, Tikellis C, Cooper ME, Power DA. Heparanase inhibition reduces proteinuria in a model of accelerated anti-glomerular basement membrane antibody disease. Nephrology (Carlton) 2005;10(2):167–73. doi: 10.1111/j.1440-1797.2005.00388.x. [DOI] [PubMed] [Google Scholar]
- 53.Gil N, Goldberg R, Neuman T, Garsen M, Zcharia E, Rubinstein AM, van Kuppevelt T, Meirovitz A, Pisano C, Li JP, van der Vlag J, Vlodavsky I, Elkin M. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes. 2012;61(1):208–16. doi: 10.2337/db11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garsen M, Benner M, Dijkman HB, van Kuppevelt TH, Li JP, Rabelink TJ, Vlodavsky I, Berden JH, Rops AL, Elkin M, van der Vlag J. Heparanase Is Essential for the Development of Acute Experimental Glomerulonephritis. Am J Pathol. 2016;186(4):805–15. doi: 10.1016/j.ajpath.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Camussi G, Tetta C, Segoloni G, Coda R, Vercellone A. Localization of neutrophil cationic proteins and loss of anionic charges in glomeruli of patients with systemic lupus erythematosus glomerulonephritis. Clin Immunol Immunopathol. 1982;24(3):299–314. doi: 10.1016/0090-1229(82)90001-0. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki N, Harada T, Mizushima Y, Sakane T. Possible pathogenic role of cationic anti-DNA autoantibodies in the development of nephritis in patients with systemic lupus erythematosus. J Immunol. 1993;151(2):1128–36. [PubMed] [Google Scholar]
- 57.Rops AL, van den Hoven MJ, Bakker MA, Lensen JF, Wijnhoven TJ, van den Heuvel LP, van Kuppevelt TH, van der Vlag J, Berden JH. Expression of glomerular heparan sulphate domains in murine and human lupus nephritis. Nephrol Dial Transplant. 2007;22(7):1891–902. doi: 10.1093/ndt/gfm194. [DOI] [PubMed] [Google Scholar]
- 58.Border WA, Ward HJ, Kamil ES, Cohen AH. Induction of membranous nephropathy in rabbits by administration of an exogenous cationic antigen. J Clin Invest. 1982;69(2):451–61. doi: 10.1172/JCI110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Debiec H, Lefeu F, Kemper MJ, Niaudet P, Deschenes G, Remuzzi G, Ulinski T, Ronco P. Early-childhood membranous nephropathy due to cationic bovine serum albumin. N Engl J Med. 2011;364(22):2101–10. doi: 10.1056/NEJMoa1013792. [DOI] [PubMed] [Google Scholar]
- 60.Chen JS, Chen A, Chang LC, Chang WS, Lee HS, Lin SH, Lin YF. Mouse model of membranous nephropathy induced by cationic bovine serum albumin: antigen dose-response relations and strain differences. Nephrol Dial Transplant. 2004;19(11):2721–8. doi: 10.1093/ndt/gfh419. [DOI] [PubMed] [Google Scholar]
- 61.Brenner BM, Hostetter TH, Humes HD. Molecular basis of proteinuria of glomerular origin. N Engl J Med. 1978;298(15):826–33. doi: 10.1056/NEJM197804132981507. [DOI] [PubMed] [Google Scholar]
- 62.Kanwar YS, Farquhar MG. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol. 1979;81(1):137–53. doi: 10.1083/jcb.81.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kanwar YS, Farquhar MG. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979;76(3):1303–7. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanwar YS, Linker A, Farquhar MG. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980;86(2):688–93. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van den Born J, van den Heuvel LP, Bakker MA, Veerkamp JH, Assmann KJ, Berden JH. A monoclonal antibody against GBM heparan sulfate induces an acute selective proteinuria in rats. Kidney Int. 1992;41(1):115–23. doi: 10.1038/ki.1992.15. [DOI] [PubMed] [Google Scholar]
- 66.Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, Fukai N, Olsen BR, Tryggvason K, Soininen R. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003;22(2):236–45. doi: 10.1093/emboj/cdg019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morita H, Yoshimura A, Inui K, Ideura T, Watanabe H, Wang L, Soininen R, Tryggvason K. Heparan sulfate of perlecan is involved in glomerular filtration. J Am Soc Nephrol. 2005;16(6):1703–10. doi: 10.1681/ASN.2004050387. [DOI] [PubMed] [Google Scholar]
- 68.Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, Berden JH, Moeller MJ, Holzman LB, Burgess RW, Miner JH. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol. 2007;171(1):139–52. doi: 10.2353/ajpath.2007.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldberg S, Harvey SJ, Cunningham J, Tryggvason K, Miner JH. Glomerular filtration is normal in the absence of both agrin and perlecan-heparan sulfate from the glomerular basement membrane. Nephrol Dial Transplant. 2009;24(7):2044–51. doi: 10.1093/ndt/gfn758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen S, Wassenhove-McCarthy DJ, Yamaguchi Y, Holzman LB, van Kuppevelt TH, Jenniskens GJ, Wijnhoven TJ, Woods AC, McCarthy KJ. Loss of heparan sulfate glycosaminoglycan assembly in podocytes does not lead to proteinuria. Kidney Int. 2008;74(3):289–99. doi: 10.1038/ki.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen S, Wassenhove-McCarthy D, Yamaguchi Y, Holzman L, van Kuppevelt TH, Orr AW, Funk S, Woods A, McCarthy K. Podocytes require the engagement of cell surface heparan sulfate proteoglycans for adhesion to extracellular matrices. Kidney Int. 2010;78(11):1088–99. doi: 10.1038/ki.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zcharia E, Metzger S, Chajek-Shaul T, Aingorn H, Elkin M, Friedmann Y, Weinstein T, Li JP, Lindahl U, Vlodavsky I. Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. FASEB J. 2004;18(2):252–63. doi: 10.1096/fj.03-0572com. [DOI] [PubMed] [Google Scholar]
- 73.Wijnhoven TJ, Lensen JF, Wismans RG, Lamrani M, Monnens LA, Wevers RA, Rops AL, van der Vlag J, Berden JH, van den Heuvel LP, van Kuppevelt TH. In vivo degradation of heparan sulfates in the glomerular basement membrane does not result in proteinuria. J Am Soc Nephrol. 2007;18(3):823–32. doi: 10.1681/ASN.2006070692. [DOI] [PubMed] [Google Scholar]
- 74.Harvey SJ, Miner JH. Revisiting the glomerular charge barrier in the molecular era. Current opinion in nephrology and hypertension. 2008;17(4):393–8. doi: 10.1097/MNH.0b013e32830464de. [DOI] [PubMed] [Google Scholar]
- 75.Tenten V, Menzel S, Kunter U, Sicking EM, van Roeyen CR, Sanden SK, Kaldenbach M, Boor P, Fuss A, Uhlig S, Lanzmich R, Willemsen B, Dijkman H, Grepl M, Wild K, Kriz W, Smeets B, Floege J, Moeller MJ. Albumin is recycled from the primary urine by tubular transcytosis. J Am Soc Nephrol. 2013;24(12):1966–80. doi: 10.1681/ASN.2013010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446(7139):1030–7. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 77.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–52. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 78.Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol. 2004;138(3):439–46. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hourcade DE. The role of properdin in the assembly of the alternative pathway C3 convertases of complement. The Journal of biological chemistry. 2006;281(4):2128–32. doi: 10.1074/jbc.M508928200. [DOI] [PubMed] [Google Scholar]
- 80.Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol. 2007;179(4):2600–8. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- 81.Lesher AM, Nilsson B, Song WC. Properdin in complement activation and tissue injury. Molecular immunology. 2013;56(3):191–8. doi: 10.1016/j.molimm.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zaferani A, Vives RR, van der Pol P, Navis GJ, Daha MR, van Kooten C, Lortat-Jacob H, Seelen MA, van den Born J. Factor h and properdin recognize different epitopes on renal tubular epithelial heparan sulfate. J Biol Chem. 2012;287(37):31471–81. doi: 10.1074/jbc.M112.380386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zaferani A, Vives RR, van der Pol P, Hakvoort JJ, Navis GJ, van Goor H, Daha MR, Lortat-Jacob H, Seelen MA, van den Born J. Identification of tubular heparan sulfate as a docking platform for the alternative complement component properdin in proteinuric renal disease. J Biol Chem. 2011;286(7):5359–67. doi: 10.1074/jbc.M110.167825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Makou E, Herbert AP, Barlow PN. Functional anatomy of complement factor H. Biochemistry. 2013;52(23):3949–62. doi: 10.1021/bi4003452. [DOI] [PubMed] [Google Scholar]
- 85.Jozsi M, Zipfel PF. Factor H family proteins and human diseases. Trends in immunology. 2008;29(8):380–7. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 86.Rodriguez de Cordoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sanchez-Corral P. The human complement factor H: functional roles, genetic variations and disease associations. Molecular immunology. 2004;41(4):355–67. doi: 10.1016/j.molimm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 87.Gordon DL, Kaufman RM, Blackmore TK, Kwong J, Lublin DM. Identification of complement regulatory domains in human factor H. J Immunol. 1995;155(1):348–56. [PubMed] [Google Scholar]
- 88.Schmidt CQ, Herbert AP, Kavanagh D, Gandy C, Fenton CJ, Blaum BS, Lyon M, Uhrin D, Barlow PN. A new map of glycosaminoglycan and C3b binding sites on factor H. J Immunol. 2008;181(4):2610–9. doi: 10.4049/jimmunol.181.4.2610. [DOI] [PubMed] [Google Scholar]
- 89.Blackmore TK, Hellwage J, Sadlon TA, Higgs N, Zipfel PF, Ward HM, Gordon DL. Identification of the second heparin-binding domain in human complement factor H. J Immunol. 1998;160(7):3342–8. [PubMed] [Google Scholar]
- 90.Blackmore TK, Sadlon TA, Ward HM, Lublin DM, Gordon DL. Identification of a heparin binding domain in the seventh short consensus repeat of complement factor H. J Immunol. 1996;157(12):5422–7. [PubMed] [Google Scholar]
- 91.Langford-Smith A, Keenan TD, Clark SJ, Bishop PN, Day AJ. The role of complement in age-related macular degeneration: heparan sulphate, a ZIP code for complement factor H? J Innate Immun. 2014;6(4):407–16. doi: 10.1159/000356513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blaum BS, Hannan JP, Herbert AP, Kavanagh D, Uhrin D, Stehle T. Structural basis for sialic acid-mediated self-recognition by complement factor H. Nature chemical biology. 2015;11(1):77–82. doi: 10.1038/nchembio.1696. [DOI] [PubMed] [Google Scholar]
- 93.Pangburn MK. Host recognition and target differentiation by factor H, a regulator of the alternative pathway of complement. Immunopharmacology. 2000;49(1–2):149–57. doi: 10.1016/s0162-3109(00)80300-8. [DOI] [PubMed] [Google Scholar]
- 94.Meri S, Pangburn MK. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proc Natl Acad Sci U S A. 1990;87(10):3982–6. doi: 10.1073/pnas.87.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kajander T, Lehtinen MJ, Hyvarinen S, Bhattacharjee A, Leung E, Isenman DE, Meri S, Goldman A, Jokiranta TS. Dual interaction of factor H with C3d and glycosaminoglycans in host-nonhost discrimination by complement. Proc Natl Acad Sci U S A. 2011;108(7):2897–902. doi: 10.1073/pnas.1017087108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jozsi M, Oppermann M, Lambris JD, Zipfel PF. The C-terminus of complement factor H is essential for host cell protection. Molecular immunology. 2007;44(10):2697–706. doi: 10.1016/j.molimm.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jozsi M, Manuelian T, Heinen S, Oppermann M, Zipfel PF. Attachment of the soluble complement regulator factor H to cell and tissue surfaces: relevance for pathology. Histology and histopathology. 2004;19(1):251–8. doi: 10.14670/HH-19.251. [DOI] [PubMed] [Google Scholar]
- 98.Ferreira VP, Herbert AP, Hocking HG, Barlow PN, Pangburn MK. Critical role of the C-terminal domains of factor H in regulating complement activation at cell surfaces. J Immunol. 2006;177(9):6308–16. doi: 10.4049/jimmunol.177.9.6308. [DOI] [PubMed] [Google Scholar]
- 99.Kazatchkine MD, Fearon DT, Silbert JE, Austen KF. Surface-associated heparin inhibits zymosan-induced activation of the human alternative complement pathway by augmenting the regulatory action of the control proteins on particle-bound C3b. J Exp Med. 1979;150(5):1202–15. doi: 10.1084/jem.150.5.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kelly U, Yu L, Kumar P, Ding JD, Jiang H, Hageman GS, Arshavsky VY, Frank MM, Hauser MA, Rickman CB. Heparan sulfate, including that in Bruch’s membrane, inhibits the complement alternative pathway: implications for age-related macular degeneration. J Immunol. 2010;185(9):5486–94. doi: 10.4049/jimmunol.0903596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Servais A, Noel LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey MA, Macher MA, Zuber J, Karras A, Provot F, Moulin B, Grunfeld JP, Niaudet P, Lesavre P, Fremeaux-Bacchi V. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82(4):454–64. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 102.Jozsi M, Heinen S, Hartmann A, Ostrowicz CW, Halbich S, Richter H, Kunert A, Licht C, Saunders RE, Perkins SJ, Zipfel PF, Skerka C. Factor H and atypical hemolytic uremic syndrome: mutations in the C-terminus cause structural changes and defective recognition functions. J Am Soc Nephrol. 2006;17(1):170–7. doi: 10.1681/ASN.2005080868. [DOI] [PubMed] [Google Scholar]
- 103.Manuelian T, Hellwage J, Meri S, Caprioli J, Noris M, Heinen S, Jozsi M, Neumann HP, Remuzzi G, Zipfel PF. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest. 2003;111(8):1181–90. doi: 10.1172/JCI16651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dragon-Durey MA, Loirat C, Cloarec S, Macher MA, Blouin J, Nivet H, Weiss L, Fridman WH, Fremeaux-Bacchi V. Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16(2):555–63. doi: 10.1681/ASN.2004050380. [DOI] [PubMed] [Google Scholar]
- 105.Jozsi M, Strobel S, Dahse HM, Liu WS, Hoyer PF, Oppermann M, Skerka C, Zipfel PF. Anti factor H autoantibodies block C-terminal recognition function of factor H in hemolytic uremic syndrome. Blood. 2007;110(5):1516–8. doi: 10.1182/blood-2007-02-071472. [DOI] [PubMed] [Google Scholar]
- 106.Clark SJ, Ridge LA, Herbert AP, Hakobyan S, Mulloy B, Lennon R, Wurzner R, Morgan BP, Uhrin D, Bishop PN, Day AJ. Tissue-specific host recognition by complement factor H is mediated by differential activities of its glycosaminoglycan-binding regions. J Immunol. 2013;190(5):2049–57. doi: 10.4049/jimmunol.1201751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Borza DB. Alternative Pathway Dysregulation and the Conundrum of Complement Activation by IgG4 Immune Complexes in Membranous Nephropathy. Front Immunol. 2016;7:157. doi: 10.3389/fimmu.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Segawa Y, Hisano S, Matsushita M, Fujita T, Hirose S, Takeshita M, Iwasaki H. IgG subclasses and complement pathway in segmental and global membranous nephropathy. Pediatr Nephrol. 2010;25(6):1091–9. doi: 10.1007/s00467-009-1439-8. [DOI] [PubMed] [Google Scholar]
- 109.Bally S, Debiec H, Ponard D, Dijoud F, Rendu J, Faure J, Ronco P, Dumestre-Perard C. Phospholipase A2 Receptor-Related Membranous Nephropathy and Mannan-Binding Lectin Deficiency. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2015101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sato N, Ohsawa I, Nagamachi S, Ishii M, Kusaba G, Inoshita H, Toki A, Horikoshi S, Ohi H, Matsushita M, Tomino Y. Significance of glomerular activation of the alternative pathway and lectin pathway in lupus nephritis. Lupus. 2011;20(13):1378–86. doi: 10.1177/0961203311415561. [DOI] [PubMed] [Google Scholar]
- 111.Ma R, Cui Z, Hu SY, Jia XY, Yang R, Zheng X, Ao J, Liu G, Liao YH, Zhao MH. The alternative pathway of complement activation may be involved in the renal damage of human anti-glomerular basement membrane disease. PloS one. 2014;9(3):e91250. doi: 10.1371/journal.pone.0091250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tomas NM, Beck LH, Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RA, Lambeau G. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371(24):2277–87. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]