Abstract
Rationale
Stimulant medications for Attention-Deficit/Hyperactivity Disorder (ADHD) in adolescents remains controversial with respect to later development of cocaine abuse. Past research demonstrated that adolescent methylphenidate treatment increased several aspects of cocaine self-administration during adulthood using the Spontaneously Hypertensive Rat (SHR) model of ADHD. Presently, we determined effects of the alternate stimulant medication, d-amphetamine, on cocaine self-administration.
Objectives
We tested the hypothesis that adolescent d-amphetamine would not increase cocaine self-administration in adult SHR, given that d-amphetamine has a different mechanism of action than methylphenidate.
Methods
A pharmacologically relevant dose of d-amphetamine (0.5 mg/kg) or vehicle was administered throughout adolescence to SHR and two control strains, Wistar-Kyoto (WKY) and Wistar (WIS). Three aspects of cocaine abuse vulnerability were assessed in adulthood after discontinuing adolescent treatments: acquisition rate and dose-related responding under fixed (FR) and progressive (PR) ratio schedules.
Results
Adult SHR acquired cocaine self-administration faster and self-administered more cocaine across multiple doses compared to WKY and WIS under FR and PR schedules, indicating SHR is a reliable animal model of comorbid ADHD and cocaine abuse. Relative to vehicle, SHR and WIS with adolescent d-amphetamine treatment self-administered less cocaine upon reaching acquisition criteria, and WIS additionally acquired cocaine self-administration more slowly and had downward shifts in FR and PR cocaine dose-response curves. WKY with adolescent d-amphetamine treatment acquired cocaine self-administration more quickly relative to vehicle.
Conclusions
In contrast to methylphenidate, adolescent d-amphetamine did not augment cocaine self-administration in SHR. Adolescent d-amphetamine treatment actually protected against cocaine abuse vulnerability in adult SHR and WIS.
Keywords: Adolescence, Attention deficit/hyperactivity disorder, Cocaine self-administration, d-Amphetamine, Spontaneously Hypertensive Rat
Introduction
Adolescents and adults with non-medicated ADHD have 2-3 times greater use of cocaine, other stimulants, tobacco, and marijuana compared to controls without ADHD (Lee et al. 2011). Over the years, a number of investigators have examined the relationships between stimulant medication for childhood ADHD and vulnerability to cocaine and other substance use disorders (SUD) during adolescence or adulthood. The first meta-analysis examining this question in ADHD patients concluded that stimulant medication in childhood is associated with reduced SUD risk during adolescence and young adulthood (Wilens et al. 2003). This stance regarding protective effects of stimulant medications has shifted over the years, with the most recent meta-analysis and another broadly conducted study in ADHD patients concluding that stimulant medication in childhood neither protects against nor increases later SUD risk (Humphreys et al. 2013; Molina et al. 2013). While this is good news, caution should be exercised in generalizing these findings beyond the initiation of medication during childhood. A concern may arise when stimulant treatment begins during adolescence, as the later the initiation in childhood, the greater the risk of developing SUD (Dalsgaard et al. 2014; Lambert et al. 1998; Manuzza et al. 2008; Steinhausen and Bisgaard, 2014). Evaluation of long-term effects of stimulant medications on SUD has been complicated by difficulty in controlling certain variables in human populations, such as presence of comorbid conduct disorder, precise age of treatment initiation, and duration of untreated symptoms (Steinhausen and Bisgaard, 2014). Moreover, clinical studies rarely distinguish between different stimulant medications in ADHD, which have different mechanisms of action and potentially different long-term neurochemical consequences. To more closely and systematically examine the relationships between adolescence stimulant medication and cocaine abuse vulnerability, we conduct preclinical studies using SHR, the most widely studied animal model of ADHD (Russell 2011). We employ Wistar-Kyoto (WKY) and Wistar (WIS) rats as inbred and outbred control strains, respectively. The WKY controls for the genetic homogeneity of the SHR (Russell 2011), but also has been used to model depressive, anxious, and autism-related phenotypes (Paré et al. 1997; Zhang-James et al. 2014). WIS is a common ancestor to both WKY and SHR and represents the genetic variance of a normative population.
SHR exhibit core behavioral and cognitive symptoms of ADHD, including hyperactivity, impulsivity, and inattention (Adriani et al. 2003; Bayless et al. 2015; Hill et al. 2012; Sagvolden et al. 2000; Somkuwar et al. 2016). SHR also model neurobiological abnormalities observed in ADHD, including greater striatal dopamine transporter density (Roessner et al. 2010; Silva et al. 2014). Notably, SHR have elevated cocaine self-administration (Baskin et al. 2015; Harvey et al. 2011; Jordan et al. 2014; 2016; Somkuwar et al. 2013b), consistent with the known comorbidity between ADHD and cocaine abuse (Lee et al. 2011). Moreover, treatment with a pharmacologically relevant dose of methylphenidate during adolescence further increases several aspects of cocaine self-administration in SHR during adulthood (see below) without altering cocaine self-administration behavior in WKY and WIS controls (Baskin et al. 2015; Harvey et al. 2011; Jordan et al. 2014). Mechanistically, methylphenidate inhibits dopamine and norepinephrine transporters (DAT and NET, respectively), reducing neurotransmitter uptake into nerve terminals (Sulzer et al. 2005). One potential mechanism by which adolescent methylphenidate may uniquely augment cocaine self-administration in SHR is by increasing DAT function in medial prefrontal cortex (mPFC; Somkuwar et al. 2013a).
Alternative medications for teenagers with ADHD may be needed given findings with methylphenidate in SHR. As non-stimulant medications are not as clinically efficacious as is methylphenidate for ADHD symptoms (Sibley et al. 2014), the current study determined whether or not the alternative stimulant medication d-amphetamine would increase cocaine self-administration behaviors in the SHR model of ADHD. We selected d-amphetamine because it is the active agent in Dexedrine®, Adderall®, and Vyvanse®, the three most commonly prescribed amphetamine formulations for ADHD worldwide (Heal et al. 2013). Notably, the d-isomer of amphetamine is more efficacious than the l-isomer in alleviating ADHD symptoms (Arnold et al. 1973) and in modulating dopamine activity (Patrick and Morowitz 1997). Although both d-amphetamine and methylphenidate increase extracellular dopamine and norepinephrine, these drugs act via different primary mechanisms. Unlike methylphenidate, d-amphetamine is a substrate at DAT and NET, reversing neurotransmitter transport from inside the neuron terminal to the extracellular space, in addition to being a substrate at serotonin and vesicular monoamine-2 transporters (Sulzer et al. 2005). Importantly, d-amphetamine decreases DAT function in nucleus accumbens of adult outbred rats (Ferris et al. 2015). Thus, we hypothesized that adolescent d-amphetamine would not increase cocaine self-administration behaviors in adulthood in the SHR model. Previously, we reported that d-amphetamine (0.5 mg/kg) had procognitive effects in adolescent SHR, and that after discontinuing treatment in adulthood, cocaine seeking and reactivity to cocaine cues were not increased under a second-order schedule of cocaine delivery and cue presentation (Jordan et al. 2016). In comparison, adolescent methylphenidate increased cocaine self-administration, but not reactivity to cocaine cues, under the same second-order schedule in adult SHR (Jordan et al. 2014). We also reported that in adult SHR, adolescent methylphenidate increased the rate of acquisition of cocaine self-administration and enhanced the reinforcing effects of cocaine studied under FR and PR schedules (Baskin et al. 2015; Harvey et al. 2011). These measures model the early phases of cocaine use and identify phenotypes that are most vulnerable to developing addiction or dependence (Piazza et al. 2000). The current study therefore investigated these important aspects of cocaine abuse vulnerability following treatment with a pharmacologically relevant dose of d-amphetamine.
Materials and methods
Subjects and adolescent drug treatments
Male WKY/Cr, WIS/Cr, and SHR/Cr rats (Charles River Laboratories, USA) arrived on postnatal day 25 (P25) and were individually housed in a temperature- (21–23°C) and light- (07:30 h on; 19:30 h off) controlled vivarium. To minimize potential litter effects, two cohorts of animals were procured 6 months apart; each cohort consisted of 6-10 rats/strain. Additionally, animals were randomly assigned to a treatment group upon arrival, with VEH and AMPH treatments alternating between rats within each cohort. From P28-P55, food was restricted to ~90% of a growth-adjusted free-feeding body weight specific for each strain to mimic the feeding schedule used in our prior comparator studies (Harvey et al. 2011; Jordan et al. 2014; 2016; Somkuwar et al. 2013b). Animals had free access to water, and food was unrestricted after P55. On P77, when animals began cocaine self-administration (see Experiment 1), body weights were as follows: VEH WKY 255 ± 5 g, AMPH WKY 254 ± 5 g, VEH WIS 356 ± 11 g, AMPH WIS 359 ± 8 g, VEH SHR 238 ± 3 g, and AMPH SHR 240 ± 6 g. All procedures were approved by the Boston University Institutional Animal Care and Use Committee and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th Edition).
For adolescent treatment, we selected a dose of 0.5 mg/kg d-amphetamine hemisulfate salt (AMPH; Sigma-Aldrich, St. Louis, MO, USA), which was dissolved in sterile 0.9% saline and injected intraperitoneally (i.p.) in a volume of 2 ml/kg. This dose increases prefrontal cortex (PFC) catecholamines without inducing locomotor hyperactivity when administered to young animals (Heitjz et al. 2003; Solanto 2000; Tanda et al. 1997). The oral dose range (salt weights) prescribed to individuals with ADHD is 0.1 - 0.7 mg/kg/day (Wilkes et al. 2015). After administration of 0.5 mg/kg AMPH, plasma levels peak at 60 ng/ml (adult rats) and 85 ng/ml (juvenile rats) after i.p and subcutaneous injection, respectively (Heijtz et al. 2003; West et al. 1999), which corresponds to the range of plasma levels found in children with ADHD after oral AMPH treatment (64-84 ng/mL, Brown et al. 1979). A main difference is that the half-life of AMPH is shorter in rats (~1 h) than in children (6-7 h). The i.p. route of administration for AMPH was used due to low oral bioavailability in the rat (Beránková et al. 2007; Kuczenski and Segal, 2001). Sterile 0.9% saline injections (2 ml/kg) served as vehicle (VEH) control. Treatments were administered once daily, Monday-Friday during adolescence (P28-P55, Spear 2000). This schedule mimics the weekend medication “holiday” often elected by ADHD patients (Martins et al. 2004). AMPH treatment was discontinued on P56.
Experiment 1: Acquisition of cocaine self-administration
This experiment evaluated acquisition of cocaine self-administration under a FR schedule in adult WKY, WIS, and SHR treated during adolescence with AMPH or VEH. The operant chambers used for self-administration sessions and the procedures for catheter implantation on P67 and catheter maintenance were described previously (Jordan et al. 2016). On P77, rats (n = 8/strain and treatment group) were allowed to press the active lever (designated left or right, counterbalanced across animals) to earn a 0.3 mg/kg cocaine infusion under a FR1 schedule of reinforcement. The alternate lever was designated the inactive lever for which responses were recorded, but had no consequences, throughout the sessions. Cocaine hydrochloride (NIDA, Bethesda, MD, USA) was dissolved in sterile 0.9% saline containing 3 IU/ml heparin. The training dose was 0.3 mg/kg (a 0.8 mg/ml concentration infused at a rate of 1.8 ml/min for a duration of 1.2s/100 g body weight). The 0.3 mg/kg training dose was selected because it maintains moderate rates of responding under an FR1 schedule from which increases and decreases in responding can be detected (Harvey et al. 2011; Somkuwar et al. 2013). The concentration of cocaine was adjusted to keep infusion parameters constant across doses. Infusions coincided with extinguishing of the house light and illumination of the stimulus light above the active lever for a 20 s timeout period, during which additional cocaine infusions could not be earned with an active lever press. Animals did not receive any external inducements to lever press (i.e., no prior lever press training and no lever baiting). All sessions were 2 h and occurred once daily, Monday-Friday, during the light phase. The number of sessions required to reach the acquisition criterion was recorded. The acquisition criterion was defined as ≥ 20 infusions in 2 h for two consecutive sessions and a ratio of at least 2:1 active to inactive lever responses. These procedures are identical to those used to determine the effects of adolescent methylphenidate (Harvey et al. 2011) and the non-stimulant atomoxetine (Somkuwar et al. 2013b) on acquisition of cocaine self-administration in SHR, WKY and WIS. Animals transitioned to Experiment 2 immediately upon reaching the acquisition criterion in Experiment 1, which occurred between P79 and P102 in individual rats.
Experiment 2: Fixed ratio cocaine dose-response functions
Following Experiment 1, the same rats continued to self-administer 0.3 mg/kg cocaine under a FR1 20-s timeout schedule until responding and cocaine intake varied <15% for 5 consecutive 2 h sessions (Monday-Friday). The reinforcing effects of cocaine then were evaluated across a range of doses under this schedule in adult WKY, WIS, and SHR treated during adolescence with AMPH or VEH. This experiment used procedures identical to those determining the effects of adolescent methylphenidate (Harvey et al. 2011) and atomoxetine (Somkuwar et al. 2013b) on FR cocaine dose-response functions in SHR, WKY and WIS. Dose-response functions were generated by substituting various cocaine test doses (0.003, 0.01, 0.03, 0.1, and 1.0 mg/kg) in pseudo-random order, every Tuesday, Thursday, and Friday. Each test dose was evaluated in 2-3 sessions, or until no upward or downward trends in responding were observed. The 0.3 mg/kg dose was available on intervening days, and for 2-3 days after completion of the dose-response curve. One VEH- and one AMPH-treated SHR died prior to completing Experiment 2, resulting in n = 7-8/strain and treatment group. Animals transitioned to Experiment 3 immediately upon completing FR1 dose-response testing in Experiment 2, which occurred between P112 and P140 in individual rats.
Experiment 3: Progressive ratio cocaine dose-response functions
Following Experiment 2, the reinforcing effects of cocaine were evaluated across a range of doses under a PR schedule in the same rats treated during adolescence with AMPH or VEH and used in Experiments 1 and 2. This experiment used procedures identical to those determining the effects of adolescent methylphenidate (Harvey et al. 2011) and atomoxetine (Somkuwar et al. 2013b) on PR cocaine dose-response functions in SHR, WKY and WIS. The PR schedule involved a geometric increment in the number of responses required to earn each successive cocaine infusion (Loh and Roberts 1990). A 20-s timeout also was used. PR breakpoint was defined as the number of infusions earned as well as the last FR completed to earn a cocaine infusion. Sessions terminated when a rat failed to reach the next response requirement within 1 h of the previous infusion, or when 4.5 h elapsed, whichever occurred first. Rats self-administered 0.3 mg/kg cocaine under the PR schedule until performance varied <15% for 5 consecutive sessions (Monday-Friday). Cocaine test doses (0.01, 0.1, 0.3, and 1.0 mg/kg) were then substituted in descending order, with 2-3 consecutive sessions for each test dose. One VEH- and one AMPH-treated WKY and one AMPH-treated SHR died prior to completing Experiment 3, resulting in n = 6-8/strain and treatment group. Animals were euthanized upon completing PR dose-response testing in Experiment 3, which occurred between P140 and P165 for individual rats.
Data analysis
The primary dependent measures were the number of sessions required to spontaneously acquire cocaine self-administration to criterion levels (square root transformed prior to analysis due to non-normality) and the number of cocaine infusions earned. Additional dependent measures included active and inactive lever responses, and the last FR completed under the PR schedule. Although a FR1 schedule was used in Experiments 1 and 2, the number of cocaine infusions and active lever responses were analyzed as separate measures because the ratio of active responses to infusions was greater than 1:1. This effect was due to the 20-s timeout that followed each cocaine infusion, during which animals could respond on the active lever, but additional cocaine infusions could not be earned. Thus, active lever responses during the timeout period were analyzed as an additional dependent measure in Experiments 1 and 2. For Experiment 1, the number of infusions and lever responses reported in the results were taken from the session during which rats met the acquisition criterion and is referred to as the post-acquisition time point. For Experiment 3, it should be noted that the number of infusions is considered to be a better measure of breakpoint than last FR completed because it is a continuous rather than a discrete variable (Richardson and Roberts 1996). All dependent measures were analyzed using two-factor (strain × treatment) and three-factor (strain × treatment × dose) ANOVAs, with repeated measures for dose. For all significant main and interaction effects, the Tukey-HSD procedure was used for post-hoc pairwise comparisons. To better delineate treatment differences within each strain in cases where the treatment main effect was significant, but the strain × treatment or the strain × treatment × dose interaction was not, t-tests with Bonferroni correction were used for post-hoc pairwise comparisons of cell means. In addition, effect sizes (eta squared) were computed for significant ANOVA factors. As described by Cohen (1988), effect sizes can be characterized as small (η2 = 0.01), medium (η2 = 0.06), or large (η2 = 0.14). Accordingly, the majority of effect sizes in the current study were in the medium to large range.
Results
Experiment 1: Acquisition of cocaine self-administration
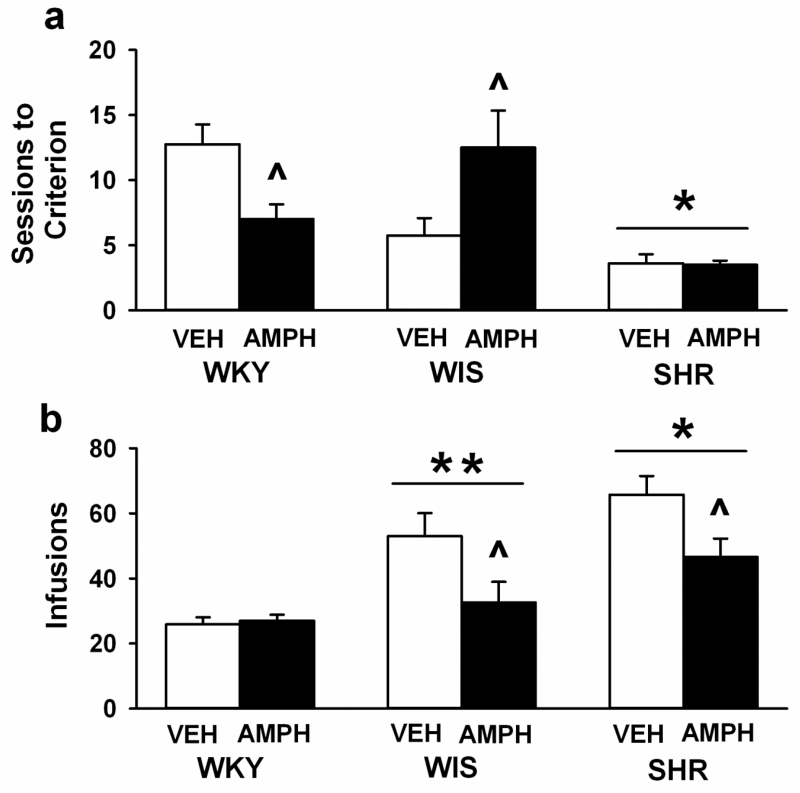
Individual rats met the acquisition criterion after a minimum of 2 and a maximum of 25 sessions. Analysis of the number of sessions required to reach the acquisition criterion (Figure 1a) identified strain differences [F (2, 42) = 14.7, p<0.001, η2= 0.4] and a strain × treatment interaction [F (2, 42) = 6.6, p<0.003, η2= 0.2]. The main effect of treatment did not reach statistical significance (p<0.5). Pairwise comparisons revealed that, overall, SHR acquired cocaine self-administration faster (fewer sessions) than WKY and WIS (ps<0.03). Moreover, both VEH- and AMPH-treated SHR acquired cocaine self-administration faster than VEH- and AMPH-treated WKY and WIS, respectively (ps<0.03). After adolescent AMPH treatment, acquisition was slower in adult WIS (p<0.004) and faster in adult WKY (p<0.04) compared to VEH in each strain. Adolescent AMPH treatment did not significantly alter the rate of acquisition compared to VEH in adult SHR.
Fig. 1.
Mean (± SEM) number of sessions required to reach criterion for acquisition of cocaine self-administration (a), and number of post-acquisition cocaine infusions (b). Experiments were performed in adult WKY, WIS, and SHR following vehicle (VEH) or d-amphetamine (AMPH) treatment during adolescence. Group sizes are n=8 per strain and treatment group. * p<0.05 compared to WKY and WIS overall (main effect of strain). ** p<0.05 compared to WKY overall (main effect of strain). ^ p<0.05 compared to VEH in the same strain.
Analysis of the number of cocaine infusions earned at the post-acquisition time point (Figure 1b) revealed strain [F (2, 42) = 16.4, p<0.001, η2=0.4] and treatment [F (2, 42) = 9.1, p<0.004, η2= 0.1] differences. The strain × treatment interaction did not reach statistical significance (p<0.08). Pairwise comparisons revealed that, overall, SHR earned a greater number of cocaine infusions than WKY and WIS (p<0.001 and 0.03, respectively) and that WIS earned a greater number of infusions than WKY (p<0.008). Moreover, there were fewer cocaine infusions post-acquisition after adolescent AMPH than VEH treatment (p<0.005). This effect of adolescent AMPH was detected only in SHR and WIS (ps< 0.016).
Analysis of total active lever responses at the post-acquisition time point (Table 1) revealed strain [F (2, 42) = 10.5, p<0.001, η2= 0.3] and treatment [F (1, 42) = 6.2, p<0.01, η2= 0.08] differences. The strain × treatment interaction did not reach statistical significance (p<0.08). Pairwise comparisons revealed that, overall, SHR made a greater number of active lever responses than WKY and WIS (ps<0.001 and 0.01, respectively). Moreover, there were fewer active lever responses after adolescent AMPH than VEH treatment (p<0.01). This effect of adolescent AMPH was detected only in SHR (p< 0.016). Analysis of timeout responses on the active lever at the post-acquisition time point (Table 1) also revealed a strain difference [F (2, 42) = 5.4, p<0.008, η2=0.2], but there was no significant influence of treatment, either as a main effect (p<0.09) or as an interaction with strain (p<0.11). Pairwise comparisons revealed that, overall, SHR made more timeout responses on the active lever than WKY (p<0.009) or WIS (p<0.04). For total inactive lever responses at the post-acquisition time point, strain was the only significant factor [F (2, 42) = 3.8, p<0.03, η2= 0.2]. Pairwise comparisons revealed that SHR made a greater number of inactive lever responses than WKY (p<0.05) and that WIS did not differ significantly from either SHR or WKY (Table 1). When inactive lever responses were analyzed as a percentage of all responses, no significant strain or treatment differences were revealed (Table 1).
Table 1.
Lever responses at the post-acquisition time point.
| Strain | Treatment | Active Lever | Inactive Lever | ||
|---|---|---|---|---|---|
| Total Responses | Timeout Responses | Total Responses | % of All Responses | ||
| WKY | VEH | 28.3 (± 2.4) | 2.4 (± 1.1) | 2.1 (± 0.5) | 7.5% (± 1.8) |
| AMPH | 31.4 (± 1.5) | 4.4 (± 1.3) | 4.0 (± 1.1) | 13.3% (± 3.9) | |
| WIS | VEH | 67.4 (± 13.1) | 14.4 (± 8.3) | 3.3 (± 0.9) | 7.4% (± 3.1) |
| AMPH | 38.9 (± 9.4) | 8 (± 2.8) | 3.6 (± 1.6) | 11.7% (± 5) | |
| SHR | VEH | 125.0 (± 29.5) * | 59.3 (± 24.9)* | 11.5 (± 5.2) ** | 11.7% (± 4.5) |
| AMPH | 63.4 (± 8.6) * ^ | 16.8 (± 4.9)* | 9.3 (± 4.7) ** | 12.0% (± 4.8) | |
Mean (± SEM) active and inactive lever responses. Inactive lever responses expressed as the percentage of all responses were calculated for each animal and then averaged across the group. Group sizes are n=8 per strain and treatment group.
p<0.05 compared to WKY and WIS overall (main effect of strain).
p<0.05 compared to WKY overall (main effect of strain).
p<0.05 compared to VEH in the same strain.
Experiment 2: Cocaine dose-response functions under the fixed ratio schedule
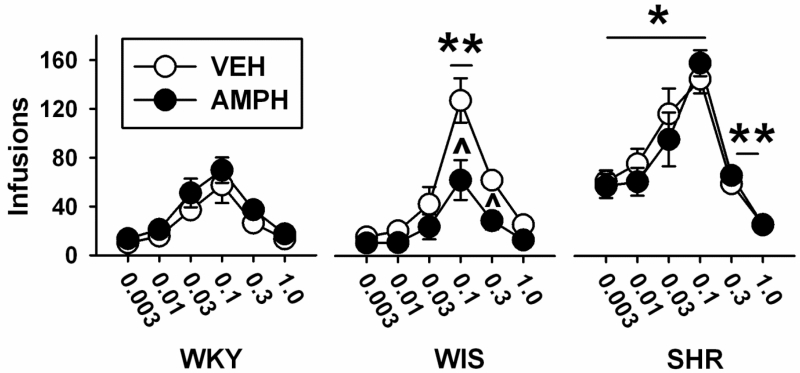
Analysis of the number of cocaine infusions during dose-response testing under FR1 (Figure 2) identified differences in strain [F (2, 40) = 36.3, p<0.001, η2=0.6] and dose [F (5, 200) = 87, p<0.001, η2= 0.6], as well as strain × treatment [F (2, 40) = 3.9, p<0.03, η2=0.06], strain × dose [F (10, 200) = 8, p<0.001, η2=0.1], and strain × treatment × dose [F (10, 200) = 2.5, p<0.007, η2=0.03] interactions. The main effect of treatment (p<0.21) and the treatment × dose interaction (p<0.84) did not reach statistical significance. Based on pairwise comparisons, SHR earned a greater number of infusions at 0.003, 0.01, 0.03 and 0.1 mg/kg cocaine than WKY and WIS (ps<0.001), and a greater number of infusions at 0.3 mg/kg cocaine than WKY (p<0.006). WIS earned a greater number of infusions at 0.1 mg/kg cocaine than WKY (p<0.004). After adolescent AMPH treatment, adult WIS earned fewer infusions at 0.1 and 0.3 mg/kg cocaine compared to VEH (p<0.001 and 0.01, respectively). Adolescent AMPH treatment did not significantly alter the number of infusions earned compared to VEH at any cocaine dose in adult SHR or WKY.
Fig. 2.
Cocaine dose-response functions based on the mean ± SEM number of infusions earned under a fixed ratio 1 (FR1) schedule of reinforcement. Experiments were performed in adult WKY, WIS, and SHR following vehicle (VEH) or d-amphetamine (AMPH) treatment during adolescence. Group sizes are n=8 for VEH and AMPH-treated WKY and WIS, and n=7 for VEH and AMPH-treated SHR. * p<0.05 compared to WKY and WIS overall (mai effect of strain). ** p<0.05 compared to WKY overall (main effect of strain). ^ p<0.05 compared to VEH in the same strain.
Analysis of total active lever responses during dose-response testing under FR1 (Table 2) identified differences in strain [F (2, 40) = 30.1, p<0.001, η2=0.6] and dose [F (5, 200) = 50.3, p<0.001, η2=0.5], as well as a strain × dose interaction [F (10, 200) = 7.1, p<0.001, η2=0.1]. Pairwise comparisons revealed that SHR made a greater number of active lever responses than WKY and WIS for 0.003, 0.01, 0.03 and 0.1 mg/kg cocaine (ps<0.001). The treatment main effect (p<0.09), and the strain × treatment (p<0.07) and strain × treatment × dose (p<0.09) interactions did not reach statistical significance, though WIS appeared to make fewer active lever responses for 0.1 and 0.3 mg/kg cocaine after adolescent AMPH than VEH (see below). Analysis of active lever responses during the timeout period (Table 2) also revealed strain [F (2, 40) = 13.4, p<0.001, η2=0.4] and dose [F (5, 200) = 18.9, p<0.001, η2=0.3] differences, as well as a strain × dose interaction [F (10, 200) = 3.8, p<0.001, η2=0.1]. There was no significant influence of treatment, either as a main effect (p<0.18) or as an interaction with strain and/or dose (ps<0.43-0.99). Pairwise comparisons revealed that SHR made more timeout responses on the active lever than WKY and WIS for 0.003, 0.01, 0.03, and 0.1 mg/kg cocaine (ps<0.04, except vs. WIS at 0.003 where p<0.07). WIS also made more timeout responses on the active lever than WKY for 0.1 mg/kg cocaine (p<0.002). Analysis of total inactive lever responses during dose-response testing under FR1 (Table 2) revealed only a strain difference [F (2, 40) = 4.3, p<0.02, η2=0.03]. Pairwise comparisons revealed that SHR made a greater number of inactive lever responses than WKY (p<0.02), and that WIS did not differ significantly from either SHR or WKY. When inactive lever responses were analyzed as a percentage of all responses, no significant strain, treatment or dose differences were revealed (Table 2).
Table 2.
Lever responses for fixed ratio dose-response functions.
| Group | Cocaine Dose (mg/kg) |
Active Lever | Inactive Lever | ||
|---|---|---|---|---|---|
| Total Responses | Timeout Responses | Total Responses | % of All Responses | ||
| WKY VEH |
0.003 | 12.6 (± 2) | 2.5 (± 0.6) | 9.9 (± 2.8) | 41.6% (± 5.6%) |
| 0.01 | 21.8 (± 4.2) | 6.1 (± 1.9) | 10.2 (± 2.9) | 31.4% (± 4.3%) | |
| 0.03 | 49.2 (± 6.7) | 12.1 (± 2.3) | 15.7 (± 3.3) | 24.0% (± 2.8%) | |
| 0.1 | 74.1 (± 20.8) | 16.5 (± 6.3) | 9.6 (± 3) | 15.6% (± 4.8%) | |
| 0.3 | 31.6 (± 2.3) | 5.1 (± 1.6) | 4.6 (± 1.5) | 11.7% (± 3.3%) | |
| 1.0 | 15.5 (± 2.3) | 2.1 (± 0.5) | 4.2 (± 1.1) | 20.6% (± 5.2%) | |
|
| |||||
| WKY AMPH |
0.003 | 18.4 (± 3.6) | 4.7 (± 1.6) | 10.8 (± 2.3) | 41.3% (± 5.8%) |
| 0.01 | 27.9 (± 6.3) | 6.6 (± 2.3) | 12.1 (± 4.4) | 36.6% (± 7%) | |
| 0.03 | 66.8 (± 15.6) | 15.6 (± 4.2) | 18.1 (± 7.2) | 25.9% (± 6%) | |
| 0.1 | 97.9 (± 18.7) | 28 (± 10) | 11.9 (± 3.9) | 14.4% (± 3.7%) | |
| 0.3 | 43 (± 3.3) | 5.7 (± 1.5) | 4.4 (± 1.5) | 11.1% (± 3.8%) | |
| 1.0 | 19.9 (± 2.1) | 2.4 (± 1.1) | 3.5 (± 1.1) | 16.0% (± 4.3%) | |
|
| |||||
| WIS VEH |
0.003 | 22.9 (± 5.2) | 7.2 (± 2.7) | 23.0 (± 4.2) | 56.9% (± 6%) |
| 0.01 | 29.1 (± 7.9) | 8.4 (± 3.2) | 21.1 (± 5.1) | 52.8% (± 6.5%) | |
| 0.03 | 66.4 (± 26.5) | 23.9 (± 12.4) | 30.6 (± 11.5) | 38.6% (± 9.6%) | |
| 0.1 | 208.5 (± 45.4) | 80.9 (± 32.1)** | 31.6 (± 21.1) | 15.6% (± 7.3%) | |
| 0.3 | 95.5 (± 28.3) | 33.4 (± 25.5) | 23.9 (± 13.6) | 17.4% (± 8.3%) | |
| 1.0 | 54.7 (± 23.7) | 29 (± 21.3) | 10.9 (± 8) | 17.0% (± 7.2%) | |
|
| |||||
| WIS AMPH |
0.003 | 15.8 (± 3.6) | 5.1 (± 1.8) | 8.2 (± 2.9) | 34.1% (± 6.3%) |
| 0.01 | 17.3 (± 3.9) | 6.3 (± 2.1) | 13.8 (±3.3) | 50.9% (± 7.9%) | |
| 0.03 | 40.1 (± 16.9) | 16.1 (± 6.9) | 20.4 (± 7.6) | 42.7% (± 5.5%) | |
| 0.1 | 89.3 (± 31.3) ^ | 57.5 (23.8)** | 16.1 (± 8.2) | 22.6% (± 6.5%) | |
| 0.3 | 35.8 (± 9.1) ^ | 6.5 (± 2.1) | 27.9 (± 23.9) | 25.2% (± 9.4%) | |
| 1.0 | 15.5 (± 3.6) | 2.4 (± 1.1) | 19.9 (± 7.2) | 48.0% (± 7.9%) | |
|
| |||||
| SHR VEH |
0.003 | 106.6 (± 11.8) * | 45.3 (± 9.2)** | 40.1 (± 8.9) ** | 39.4% (± 8.3%) |
| 0.01 | 128.5 (± 20.6) * | 52.7 (± 13.8)* | 47.3 (± 12.8) ** | 37.8% (± 7.4%) | |
| 0.03 | 197.5 (± 29.1) * | 81 (± 12.9)* | 40.0 (± 8.5) ** | 28.4% (± 7.9%) | |
| 0.1 | 275.1 (± 53.2) * | 130.2 (47)* | 183.6 (± 164.9) ** | 23.3% (± 11.4%) | |
| 0.3 | 83.9 (± 9.2) | 24.7 (± 7.2) | 64.5 (± 46.4) ** | 27.6% (± 12.2%) | |
| 1.0 | 36.6 (± 3.7) | 10.7 (± 2.1) | 97.1 (± 44.1) ** | 45.5% (± 16.2%) | |
|
| |||||
| SHR AMPH |
0.003 | 87.1 (± 12.5) * | 29.6 (± 4.3)** | 26.1 (± 6.8) ** | 32.0% (± 5.4%) |
| 0.01 | 96.4 (± 13.6) * | 35.5 (± 4.2)* | 26.2 (± 6.3) ** | 32.1% (± 8.3%) | |
| 0.03 | 143.4 (± 37.1) * | 47.7 (± 12.6)* | 21.9 (± 5.4) ** | 21.7% (± 5%) | |
| 0.1 | 275.4 (± 48.2) * | 117.2 (± 41.3)* | 56.8 (± 46.2) ** | 15.5% (± 9.1%) | |
| 0.3 | 81.3 (± 7.2) | 15.2 (± 3.9) | 8.0 (± 3.5) ** | 9.9% (± 3.8%) | |
| 1.0 | 37.2 (± 7.7) | 11.6 (± 6.5) | 98.0 (± 93.5) ** | 25.2% (± 12.9%) | |
Mean (± SEM) active and inactive lever responses. Inactive lever responses expressed as the percentage of all responses were calculated for each animal, and then averaged across the group. Group sizes are n=8 for VEH and AMPH-treated WKY and WIS, and n=7 for VEH and AMPH-treated SHR.
p<0.05 compared to WKY and WIS overall (strain × dose interaction).
p<0.05 compared to WKY overall (strain × dose interaction).
p<0.05 compared to VEH in the same strain.
It is possible that the greater amount of cocaine self-administered by SHR relative to WKY and WIS may have impacted the results of FR dose-response testing. To rule this out, we performed analysis of covariance (ANCOVA) on infusions and active lever responses, with baseline cocaine intake at the acquisition criterion as the covariate. The significant ANOVA factors reported above for each measure remained significant in the ANCOVA (ps<0.001-0.045), indicating that baseline cocaine intake does not account for the strain, treatment and dose differences under FR dose-response testing. An additional feature revealed by the ANCOVA was a significant strain × treatment × dose interaction for active lever responses [F (10, 195) = 2.0, p<0.032]. Confirming previous trends in the data, further analysis with pairwise comparisons indicated that when baseline cocaine intake was taken into consideration, adult WIS treated with adolescent AMPH made fewer active lever responses maintained by 0.1 (p<0.001) and 0.3 (p<0.05) mg/kg cocaine compared to VEH-treated WIS.
Experiment 3: Cocaine dose-response functions under the progressive ratio schedule
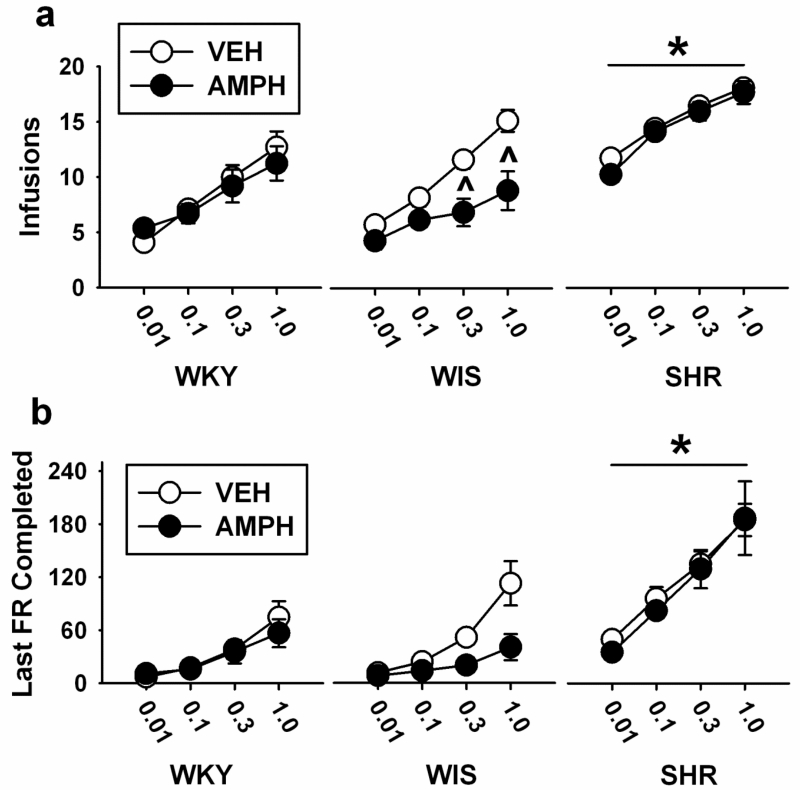
Analysis of the primary measure of PR breakpoint (number of infusions) over a range of cocaine doses (Figure 3a) revealed differences in strain [F (2, 37) = 43.6, p<0.001, η2=0.6], treatment [F (1, 37) = 5.8, p<0.02, η2=0.04], and dose [F (3, 111) = 120.4, p<0.001, η2=0.7], as well as treatment × dose [F (3, 111) = 3.4, p<0.02, η2=0.02] and strain × treatment × dose [F (6, 111) = 2.2, p<0.05, η2=0.03] interactions. The strain × treatment (p<0.07) and strain × dose (p=0.69) interactions did not reach statistical significance. Pairwise comparisons revealed that, overall, SHR had higher breakpoints (more infusions) than WKY and WIS across all cocaine doses (ps<0.001). Pairwise comparisons for treatment revealed that breakpoints were lower (fewer infusions) after adolescent AMPH than VEH at 0.3 and 1.0 mg/kg cocaine (p<0.05 and 0.01, respectively). When treatment was analyzed by dose and strain, breakpoints at 0.3 and 1.0 mg/kg cocaine were lower after adolescent AMPH than VEH only in adult WIS (p<0.003 and 0.001, respectively). Compared to VEH, adolescent AMPH did not significantly alter breakpoint at any cocaine dose in adult WKY or SHR.
Fig. 3.
Cocaine dose-response functions based on the mean ± SEM number of infusions earned (a) and last FR completed (b) under a progressive ratio (PR) schedule of reinforcement. Experiments were performed in adult WKY, WIS, and SHR following vehicle (VEH) or d-amphetamine (AMPH) treatment during adolescence. Group sizes are n=8 for VEH and AMPH-treated WIS, n=7 for VEH and AMPH-treated WKY and VEH-treated SHR, and n=6 for AMPH-treated SHR. * p<0.05 compared to WKY and WIS overall (main effect of strain). ^ p<0.05 compared to VEH in the same strain.
Analyses of the secondary measure of PR breakpoint (last FR completed) over a range of cocaine doses (Figure 3b) revealed differences in strain [F (2, 37) = 42.6, p<0.001, η2=0.6] and dose [F (3, 111) = 71.6, p<0.001, η2=0.6], as well as a strain × dose interaction [F (6, 111) = 6.5, p<0.001, η2=0.1]. The main effect of treatment (p<0.08) and the strain × treatment (p<0.37), treatment × dose (p<0.24), and strain × treatment × dose (p<0.17) interactions did not reach statistical significance. Pairwise comparisons revealed that, overall, SHR had higher breakpoints (larger last FR completed) than WKY and WIS across all cocaine doses (ps<0.001, except for 0.01 mg/kg where ps<0.05).
Analysis of total active lever responses under the PR schedule (Table 3) revealed differences in strain [F (2,37) = 39, p<0.001, η2=0.6] and dose [F (3, 111) = 62.7, p<0.001, η2=0.5] as well as a strain × dose interaction [F (6, 111) = 6.3, p<0.001, η2=0.1]. The main effect of treatment (p<0.09) and the strain × treatment (p<0.36), treatment × dose (p<0.23), and strain × treatment × dose (p<0.16) interactions did not reach statistical significance. Pairwise comparisons revealed that, overall, SHR made more active lever responses than WKY and WIS (all dose ps<0.001, except for 0.01 mg/kg where ps<0.06). Analysis of total inactive lever responses under the PR schedule (Table 3) revealed only a main effect of cocaine dose [F (3, 111) = 2.6, p<0.05, η2=0.06], although pairwise comparisons did not detect any significant between-dose differences. Additionally, when inactive lever responses were analyzed as a percentage of all responses, there were no significant strain, treatment or dose differences revealed (Table 3).
Table 3.
Lever responses for progressive ratio dose-response functions.
| Group | Cocaine Dose (mg/kg) |
Active Lever | Inactive Lever | |
|---|---|---|---|---|
| Total Responses | Total Responses | % of All Responses | ||
| WKY VEH |
0.01 | 25.4 (± 10.4) | 8.5 (± 5.3) | 20.8% (± 4.3%) |
| 0.1 | 78.8 (± 15.8) | 7.1 (± 3.1) | 8.2% (± 2.5%) | |
| 0.3 | 189.1 (± 60.3) | 9.9 (± 4.3) | 6.3% (± 2.7%) | |
| 1.0 | 413.6 (± 111.2) | 17.6 (± 7.6) | 5.1% (± 1.9%) | |
|
| ||||
| WKY AMPH |
0.01 | 46.6 (± 13.4) | 5.7 (± 0.6) | 17.3% (± 5.3%) |
| 0.1 | 67.4 (± 16.4) | 3.5 (± 1.3) | 7.7% (± 4.2%) | |
| 0.3 | 182.7 (± 76.1) | 4.9 (± 2.1) | 4.8% (± 2.3%) | |
| 1.0 | 297.5 (± 94.9) | 5.8 (± 2.7) | 5.8% (± 4.3%) | |
|
| ||||
| WIS VEH |
0.01 | 51.5 (± 11) | 16.1 (± 2.6) | 26.9% (± 5.6%) |
| 0.1 | 122 (± 37.4) | 23.8 (± 3) | 20.8% (± 3.2%) | |
| 0.3 | 299.4 (± 62.6) | 47.8 (± 13) | 15.3% (± 3.3%) | |
| 1.0 | 696.1 (± 177.3) | 84.4 (± 18.5) | 13.5% (± 3.6%) | |
|
| ||||
| WIS AMPH |
0.01 | 30.3 (± 11.9) | 6.4 (± 1.9) | 18.3% (± 5.5%) |
| 0.1 | 56.3 (± 18.1) | 12.4 (± 4.6) | 17.8% (± 5.1%) | |
| 0.3 | 99.1 (± 40.1) | 21.3 (± 9.9) | 13.8% (± 4.3%) | |
| 1.0 | 219.3 (± 88.5) | 47.4 (± 26.1) | 10.5% (± 3.9%) | |
|
| ||||
| SHR VEH |
0.01 | 296.5 (± 35.9) | 39.2 (± 12.6) | 10.9% (± 3.7%) |
| 0.1 | 557.7 (± 95.8) * | 77 (± 26.8) | 12.1% (± 4.2%) | |
| 0.3 | 826.4 (± 93.1) * | 127.7 (± 48.1) | 13.1% (± 5.2%) | |
| 1.0 | 1115 (± 101) * | 448.8 (± 342) | 17.8% (± 9.8%) | |
|
| ||||
| SHR AMPH |
0.01 | 195.5 (± 18.7) | 31.9 (± 13.5) | 13.1% (± 4.8%) |
| 0.1 | 476.6 (± 55) * | 27.2 (± 11.9) | 5.6% (± 2.2%) | |
| 0.3 | 789 (± 168) * | 35.7 (± 10.7) | 4.7% (± 1.6%) | |
| 1.0 | 1140 (± 271) * | 66.1 (± 45.9) | 3.8% (± 1.6%) | |
Mean (± SEM) active and inactive lever responses. Inactive lever responses expressed as the percentage of all responses were calculated for each animal, and then averaged across the group. Group sizes are n=8 for VEH and AMPH-treated WIS, n=7 for VEH and AMPH-treated WKY and VEH-treated SHR, and n=6 for AMPH-treated SHR.
p<0.05 compared WKY and WIS overall (strain × dose interaction).
To ascertain whether the greater amount of cocaine self-administered by SHR relative to WKY and WIS at baseline could have impacted the results of PR dose-response testing, we performed ANCOVA on infusions, last FR completed, and active lever responses. The significant ANOVA factors reported above for each measure remained significant in the ANCOVA (ps<0.001-0.019), indicating that baseline cocaine intake does not account for the strain, treatment and dose differences under PR dose-response testing.
Discussion
Strain differences in cocaine abuse vulnerability
Adult SHR acquired cocaine self-administration faster and, at the post-acquisition time point, earned more cocaine infusions and made more active lever responses than adult WKY and WIS control strains. SHR also earned more infusions and made more active lever responses during cocaine dose-response testing under FR and PR schedules relative to WKY and WIS, indicating that cocaine functioned as a reinforcer to a greater degree in SHR. These findings replicate our prior studies using SHR (Baskin et al. 2015; Harvey et al. 2011; Jordan et al. 2014; Somkuwar et al. 2013b), and are consistent with increased cocaine abuse vulnerability reported in adolescents and adults with ADHD (Lee et al. 2011).
In the current study, the ratio of active lever responses to cocaine infusions under the FR1 schedule was greater than 1:1 in all strains due to the 20-s timeout period that followed each cocaine infusion. During the timeout periods, animals could respond on the active lever, but additional cocaine infusions could not be earned. Increased active lever responding during the timeout period could represent a loss of stimulus control or a generalized activity-enhancing effect (Spealman and Goldberg 1978), particularly in SHR who made significantly more timeout responses on the active lever than WKY and WIS. The greater number of inactive lever responses under the FR 1 schedule in SHR compared to WKY and/or WIS also suggests a potential loss of stimulus control in SHR. However, when inactive responses were analyzed as a percentage of total lever responses, no significant strain, treatment or dose differences were observed, suggesting stimulus control was relatively intact in SHR. Importantly, the timeout period was signaled by illumination of a stimulus light above the active lever. Thus, the increase in active lever responses in SHR during the timeout period may represent increased cocaine-seeking behavior and reflect a greater degree of reactivity to cocaine-paired cues, rather than loss of stimulus control. Previously, SHR were shown to be more reactive to cocaine-paired cues than WKY or WIS strains under a second-order schedule of cocaine delivery (Jordan et al. 2014; 2016). To the best of our knowledge, no studies have examined cocaine cue reactivity in ADHD patients, but our findings in SHR suggest that this may be an important clinical question to pursue for understanding the association of ADHD with heightened cocaine abuse vulnerability (Lee et al. 2011).
One criticism against using WKY as a control for the SHR model of ADHD has been that WKY are hypoactive relative to other strains (Ferguson and Cada, 2003). Indeed, we found that WKY display less open field activity than SHR and WIS (Somkuwar et al. 2016). However, in the current work assessing cocaine abuse vulnerability, VEH-treated WKY and WIS did not significantly differ on any measure of cocaine self-administration, with the exception of differences in cocaine intake at the post-acquisition time point and at the 0.1 mg/kg cocaine dose under the FR1 schedule. These results oppose the possibility that WKY hypoactivity influenced our interpretation concerning cocaine abuse vulnerability in SHR. Nonetheless, differences in WKY and WIS activity levels emphasize the importance of concurrent evaluation of both WKY and an outbred control strain when evaluating the SHR as a model of ADHD.
Effects of adolescent d-amphetamine treatment on cocaine abuse vulnerability: potential mechanisms
As hypothesized, adolescent AMPH treatment did not increase cocaine self-administration behaviors in SHR. Adolescent AMPH was protective to varying degrees in SHR and WIS. In adult SHR, adolescent AMPH reduced post-acquisition cocaine intake, but there were no significant differences in the rate at which VEH- and AMPH-treated SHR acquired cocaine self-administration. This lack of a treatment finding for the rate of acquisition of cocaine self-administration in SHR may represent a floor effect, as a minimum of 2 days were required to reach acquisition criterion. However, subsequent testing showed that AMPH treatment did not significantly alter cocaine intake under FR and PR schedules in SHR, suggesting that adolescent AMPH treatment does not further increase cocaine self-administration in this strain. We previously showed that adolescent AMPH treatment reduced cue-induced reinstatement of cocaine-seeking responses during the first of seven test sessions in adult SHR (Jordan et al. 2016). Effects of adolescent treatment with the non-stimulant ADHD medication, atomoxetine (a selective NET inhibitor; Bymaster et al. 2002), were similar to AMPH in adult SHR, except that cocaine-seeking responses were reduced across the seven reinstatement test sessions (Jordan et al. 2014). These findings contrast with the effects of adolescent methylphenidate treatment, which augmented the rate of acquisition of cocaine self-administration, the reinforcing effects of cocaine under FR and PR schedules, and cocaine intake under a second-order schedule of reinforcement in adult SHR (Baskin et al. 2015; Harvey et al. 2011; Jordan et al. 2014).
One reason why adolescent AMPH and methylphenidate treatment may differ in their effects on cocaine self-administration is through different long-term effects on DAT. Unlike methylphenidate, AMPH can be taken up into the cell through monoamine transporters, where it inhibits VMAT-2 and also binds to the intracellular trace amine-associated receptor 1 (TAAR1), which is co-localized with DAT (Lindemann et al. 2008; Xie et al. 2007). Activation of TAAR1 by AMPH reduces cell surface expression of DAT, and may decrease DAT function (Miller, 2011; Xie and Miller, 2009). Therefore, whereas adolescent methylphenidate treatment increases DAT function in mPFC of SHR (Somkuwar et al. 2013a), adolescent AMPH treatment may reduce DAT expression and function. Although effects of adolescent AMPH on mPFC DAT in SHR are not yet confirmed, acute AMPH infusions reduce DAT function and expression in the nucleus accumbens of adult outbred rats (Ferris et al. 2015). It is also worth noting that adolescent atomoxetine treatment did not increase DAT function in mPFC of adult SHR, but rather reduced DAT function in orbitofrontal cortex of adult SHR (Somkuwar et al. 2013b). A decrease in DAT function may lead to slower clearance of dopamine and higher basal dopaminergic tone in the synapse (Zahniser and Sorkin, 2004). When cocaine is self-administered under these conditions, post-synaptic responses to phasically released dopamine could therefore be reduced (Grace, 2001), leading to a diminished reinforcing effect of cocaine. Notably, neither our previous work (Harvey et al., 2011; Somkuwar et al., 2013) nor work by other laboratories (e.g., Womersley et al. 2011) have demonstrated differences in DAT function or expression in SHR vs. WKY or WIS control in other brain regions, such as the striatum. Although long-term changes in DAT function following adolescent ADHD medication have not yet been documented outside mPFC or orbitofrontal cortex (OFC), these regions are among the primary loci of dysfunction in ADHD as well as in substance use disorders (e.g., Cubillo et al. 2011; Hester and Garavan, 2004). Medication-induced changes in these regions may have important implications for the long-term course of these disorders.
Surprisingly, adolescent AMPH treatment had robust protective effects on cocaine self-administration in adult WIS. In this outbred control strain, adolescent AMPH not only reduced cocaine intake at the acquisition criterion, but also slowed the rate to acquire cocaine self-administration and produced downward shifts in cocaine dose-response curves for cocaine intake under FR and PR schedules in adulthood. Notably, these effects were observed months after discontinuation of AMPH treatment. Downward shifts reflect a reduced vulnerability to the reinforcing value of cocaine (Piazza et al. 2000). In a previous report, adolescent methylphenidate slowed the rate of acquisition of cocaine self-administration in adult WIS, but did not significantly alter cocaine dose-response functions under FR and PR schedules in adult WIS (Harvey et al. 2011). Interestingly, adolescent methylphenidate decreased the affinity of DAT for dopamine in OFC of adult WIS, but did not alter DAT function in mPFC in this strain (Somkuwar et al. 2013b). If adolescent AMPH treatment reduces DAT function, this may contribute to the drug’s protective effects against cocaine self-administration in adult WIS.
In contrast to the beneficial effects of adolescent AMPH on cocaine self-administration in SHR and WIS, this treatment increased the rate of acquisition of cocaine self-administration in adult WKY, similar to adolescent atomoxetine in adult WKY (Somkuwar et al. 2013b). Conversely, adolescent methylphenidate did not alter the rate of acquisition of cocaine self-administration in adult WKY (Harvey et al. 2011). It is worth noting that WKY exhibit a number of behavioral and neurochemical abnormalities that may represent a depressive/anxiety-prone phenotype (De La Garza and Mahoney, 2004; Pardon et al. 2002; Paré et al. 1997; Scholl et al. 2010), including decreased DAT density in the nucleus accumbens (Jiao et al. 2003). Consistent with the proposed role of DAT in cocaine abuse vulnerability, adolescent atomoxetine treatment increased, whereas adolescent methylphenidate decreased, DAT function in OFC of adult WKY (Somkuwar et al. 2013a, b). If the effects on DAT resulting from adolescent AMPH resemble those of atomoxetine in WKY, AMPH may produce increases in DAT function in OFC, and thereby, potentially augment the reinforcing value of cocaine in this strain to increase the rate of acquisition of cocaine self-administration.
Implications
In concurrence with observations of elevated cocaine use among individuals with ADHD (Lee et al. 2011), the current report demonstrates elevated cocaine self-administration behaviors in the SHR on measures that model the early phases of cocaine abuse, and which are thought to identify phenotypes that are most vulnerable to the transition to compulsive drug use and dependence (Piazza et al. 2000). Importantly, adolescent AMPH treatment did not augment cocaine self-administration behaviors in adult SHR. These results stand in contrast to the results observed following discontinuation of adolescent methylphenidate treatment in adult SHR (Harvey et al. 2011). Given the different long-term consequences of AMPH vs. methylphenidate in the SHR model of ADHD, it does not appear to be meaningful going forward to continue grouping stimulant drugs into a single medication variable in clinical studies seeking to elucidate relationships between ADHD, stimulant medications and SUD. Currently, 20% of teenage boys and 10% of teenage girls in the United States are diagnosed with ADHD (Visser et al. 2014). Approximately 20% of these teens receive a first-time ADHD diagnosis between ages 11–17, representing an estimated 700,000 individuals (National Survey of Children’s Health 2011/2012). The current study employed males only, which is a limitation because ADHD diagnosis in teenage girls also is escalating. In the future, it is imperative that the long-term impact of ADHD medications on SUD is determined in females as well as in males, particularly because women with ADHD may have a higher risk of substance use disorders than men with ADHD (Dalsgaard et al. 2014). Female SHR may represent an optimal preclinical model in which to investigate sex differences in the sensitivity and vulnerability to drug abuse (Bayless et al. 2015; Berger and Sagvolden 1998; Cailhol and Mormède, 1999; Vendruscolo et al. 2009).
The current work is the first to suggest that adolescent treatment with AMPH may prevent later cocaine abuse vulnerability in non-ADHD populations at risk for SUDs, as evidenced by findings in outbred WIS rats after discontinuing adolescent treatment. Interestingly, amphetamines show promise in the treatment of cocaine abuse. For example, in outbred rats with cocaine self-administration experience, concurrent AMPH produced downward shifts in cocaine dose-response functions (Chiodo et al. 2008; Zimmer et al. 2014), and reversed cocaine-induced deficits in DAT expression and function (Ferris et al. 2015). Similarly, in non-human primates, concurrent AMPH reduced cocaine self-administration under PR and second-order schedules of reinforcement, and increased choice for food over cocaine (Czoty et al. 2010; Negus 2003). In cocaine-abusing individuals, moderate doses of AMPH reduced cocaine intake and craving and improved treatment retention (Grabowski et al. 2001, Mooney et al. 2009). Amphetamines are effective also in reducing cocaine use and improving ADHD symptoms for adults with comorbid ADHD and cocaine use disorder (Levin et al. 2015). In contrast, methylphenidate is less effective in stimulant-dependent ADHD patients, possibly because of limited efficacy at DAT as a result of long-term stimulant abuse (Crunelle et al. 2013; Konstenius et al. 2010).
Preventative measures recently have received attention as potentially more effective ways to reduce the prevalence of cocaine use disorder (e.g., Stanis and Andersen, 2014). Early adolescence may represent an optimal period in which to implement preventative measures for SUD, as drug use before age 14 is associated with a four-fold greater risk of developing SUD (SAMHSA, 2006). The current findings suggest that adolescent AMPH treatment may be one viable measure for reducing future cocaine abuse risk in individuals with and without ADHD. However, caution is warranted given the differential effects of adolescent AMPH treatment in SHR and WIS vs. WKY during acquisition of cocaine self-administration. The long-term consequences of treatment with AMPH during adolescence could be favorable (SHR and WIS) or unfavorable (WKY) in individuals expressing different behavioral and neurochemical phenotypes.
Acknowledgements
The authors thank Laura Tabbaa, Sae-Mi Jeon, and Britahny Baskin for research assistance.
Funding: National Institutes of Health grant DA011716 and the Clara Mayo Memorial Fellowship at Boston University
Footnotes
Conflict of Interest: Chloe Jordan, Carley Lemay, Linda Dwoskin and Kathleen Kantak declare no conflicts of interest.
Compliance with Ethical Standards
Research Involving Animals: All procedures were approved by the Boston University Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health Guide For the Care and Use of Laboratory Animals (8th Edition).
References
- Adriani W, Caprioli A, Granstrem O, Carli M, Laviola G. The spontaneously hypertensive-rat as an animal model of ADHD: Evidence for impulsive and non-impulsive subpopulations. Neurosci Biobehav Rev. 2003;27:639–651. doi: 10.1016/j.neubiorev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Arnold LE, Kirilcuk V, Corson SA, Corson EOL. Levoamphetamine and dextroamphetamine: differential effect on aggression and hyperkinesis in children and dogs. Am J Psychiatry. 1973;130:165–170. doi: 10.1176/ajp.130.2.165. [DOI] [PubMed] [Google Scholar]
- Baskin BM, Dwoskin LP, Kantak KM. Methylphenidate treatment beyond adolescence maintains increased cocaine self-administration in the spontaneously hypertensive rat model of attention deficit/hyperactivity disorder. Pharm Biochem Behav. 2015;131:51–56. doi: 10.1016/j.pbb.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless DW, Perez MC, Daniel JM. Comparison of the validity of the use of the spontaneously hypertensive rat as a model of attention deficit hyperactivity disorder in males and females. Behav Brain Res. 2015;286:85–92. doi: 10.1016/j.bbr.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Beránková K, Szkutová M, Balíková M. Distribution profile of 2,5-dimethoxy-4-bromoamphetamine (DOB) in rats after oral and subcutaneous doses. Forensic Science International. 2007;170:94–99. doi: 10.1016/j.forsciint.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Berger DF, Sagvolden T. Sex differences in operant discrimination behaviour in an animal model of attention-deficit hyperactivity disorder. Behav Brain Res. 1998;94:73–82. doi: 10.1016/s0166-4328(97)00171-x. [DOI] [PubMed] [Google Scholar]
- Brown GL, Hunt RD, Ebert MH, Bunney WE, Jr, Kopin IJ. Plasma levels of d-amphetamine in hyperactive children: Serial behavior and motor responses. Psychopharmacology (Berl) 1979;62:133–140. doi: 10.1007/BF00427126. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Caihol S, Mormède P. Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. Brain Res. 1999;842:200–205. doi: 10.1016/s0006-8993(99)01742-4. [DOI] [PubMed] [Google Scholar]
- Chiodo KA, Läck CM, Roberts DC. Cocaine self-administration reinforced on a progressive ratio schedule decreases with continuous D-amphetamine treatment in rats. Psychopharmacology (Berl) 2008;200:465–473. doi: 10.1007/s00213-008-1222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Cubillo A, Halari R, Giampietro V, Taylor E, Rubia K. Fronto-striatal underactivation during interference inhibition and attention allocation in grown up children with attention deficit/hyperactivity disorder and persistent symptoms. Psychiatr Res. 2011;193:17–27. doi: 10.1016/j.pscychresns.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Crunelle CL, van den Brink W, Veltman DJ, van Emmerik-van Oortmerssen K, Dom G, Schoevers RA, Booij J. Low dopamine transporter occupancy by methylphenidate as a possible reason for reduced treatment effectiveness in ADHD patients with cocaine dependence. Eur Neuropsychopharm. 2013;23:1714–1723. doi: 10.1016/j.euroneuro.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Nader MA. Effects of chronic d-amphetamine administration on the reinforcing strength of cocaine in rhesus monkeys. Psychopharm (Berl) 2010;209:375–382. doi: 10.1007/s00213-010-1807-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH. ADHD, stimulant treatment in childhood and subsequent substance abuse in adulthood - a naturalistic long- term follow-up study. Addict Behav. 2014;39:325–328. doi: 10.1016/j.addbeh.2013.09.002. [DOI] [PubMed] [Google Scholar]
- De La Garza R, II, Mahoney JJ., III A distinct neurochemical profile in WKY rats at baseline and in response to acute stress: implications for animal models of anxiety and depression. Brain Res. 2004;1021:209–218. doi: 10.1016/j.brainres.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Cada AM. A longitudinal study of short- and long-term activity levels in male and female spontaneously hypertensive, Wistar-Kyoto, and Sprague-Dawley rats. Behav Neurosci. 2003;117:271–282. doi: 10.1037/0735-7044.117.2.271. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Rose JH, Siciliano CA, Sun H, Chen R, Jones SR. A single amphetamine infusion reverses deficits in dopamine nerve-terminal function caused by a history of cocaine self-administration. Neuropsychopharmacology. 2015;40:1826–1836. doi: 10.1038/npp.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: A double-blind randomized clinical trial. J Clin Psychopharm. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grace AA. Psychostimulant actions on dopamine and limbic system function: Relevance to the pathophysiology and treatment of ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. Oxford University Press; London, United Kingdom: 2001. pp. 134–157. [Google Scholar]
- Harvey RC, Sen S, Deaciuc A, Dwoskin LP, Kantak KM. Methylphenidate treatment in adolescent rats with an attention deficit/hyperactivity disorder phenotype: Cocaine addiction vulnerability and dopamine transporter function. Neuropsychopharmacology. 2011;36:837–847. doi: 10.1038/npp.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Gosden J, Nutt DJ. Amphetamine, past and present - a pharmacological and clinical perspective. J Psychopharm. 2013;27:479–496. doi: 10.1177/0269881113482532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijtz RD, Kolb B, Forssberg H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? European J Neurosci. 2003;18:3394–3399. doi: 10.1046/j.0953-816x.2003.03067.x. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: Evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JC, Herbst K, Sanabria F. Characterizing operant hyperactivity in the spontaneously hypertensive rat. Behav Brain Func. 2012;8:5. doi: 10.1186/1744-9081-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Eng T, Lee SS. Stimulant medication and substance use outcomes: A meta-analysis. JAMA Psychiatry. 2013;70:740–749. doi: 10.1001/jamapsychiatry.2013.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Pare WP, Tejani-Butt SM. Strain differences in the distribution of dopamine transporter sites in rat brain. Prog Neuropsychopharm Biol Psychiatry. 2003;27:913–919. doi: 10.1016/S0278-5846(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Jordan CJ, Harvey RC, Baskin BB, Dwoskin LP, Kantak KM. Cocaine-seeking behavior in a genetic model of attention-deficit/hyperactivity disorder following adolescent methylphenidate or atomoxetine treatments. Drug Alcohol Depend. 2014;140:25–32. doi: 10.1016/j.drugalcdep.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Taylor DM, Dwoskin LP, Kantak KM. Adolescent d-amphetamine treatment in a rodent model of ADHD: Pro-cognitive effects in adolescence without an impact on cocaine cue reactivity in adulthood. Behav Brain Res. 2016;297:165–179. doi: 10.1016/j.bbr.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstenius M, Jayaram-Lindström N, Beck O, Franck J. Sustained release methylphenidate for the treatment of ADHD in amphetamine abusers: A pilot study. Drug Alcohol Depend. 2010;108:130–133. doi: 10.1016/j.drugalcdep.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabilities. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clin Psych Rev. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, et al. Adolescent vs. adult-onset nicotine self-administration in male rats: Duration of effect and differential nicotinic receptor correlates. Neurotox Teratology. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharm Exper Therapeutics. 2008;324:948–956. doi: 10.1124/jpet.107.132647. [DOI] [PubMed] [Google Scholar]
- Loh EA, Roberts DCS. Break-points on a progressive ratio schedule reinforced by intravenous cocaine increase following depletion of forebrain serotonin. Psychopharmacology. 1990;101:262–266. doi: 10.1007/BF02244137. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Truong NL, Moulton JL, Roizen ER, Howell KH, et al. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: Prospective follow-up into adulthood. Am Journal Psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins S, Tramontina S, Polanczyk G, Eizirik M, Swanson JM, Rohde LA. Weekend holidays during methylphenidate use in ADHD children: A randomized clinical trial. J Child Adol Psychopharm. 2004;14:195–206. doi: 10.1089/1044546041649066. [DOI] [PubMed] [Google Scholar]
- Miller GM. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem. 2011;116:164–176. doi: 10.1111/j.1471-4159.2010.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Eugene Arnold L, Swanson JM, Pelham WE, Hechtman L, Hoza B, Epstein JN, Wigal T, Abikoff HB, Greenhill LL, Jensen PS, Wells KC, Vitiello B, Gibbons RD, Howard A, Houck PR, Hur K, Lu B, Marcus S. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry. 2013;52:250–63. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Survey of Children’s Health Database [Accessed 20 March 2016];2011/2012 Available at http://www.nschdata.org/browse/survey/results?q=2390&r=1&g=451.
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: Effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Pardon M-C, Gould GG, Garcia A, Phillips L, Cook MC, Miller A, et al. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: Implications for susceptibility to stress-related neuropsychiatric disorders. Neurosci. 2002;115:229–242. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Paré W, Kluczynski J. Differences in the stress response of Wistar-Kyoto (WKY) rats from different vendors. Physiology and Behavior. 1997;62:643–648. doi: 10.1016/s0031-9384(97)00191-1. [DOI] [PubMed] [Google Scholar]
- Patrick KS, Morowitz JS. Pharmacology of methylphenidate, amphetamine enantiomers and pemoline in Attention-Deficit Hyperactivity Disorder. Human Psychopharm. 1997;12:527–546. [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roessner V, Sagvolden T, DasBanerjee T, Middleton FA, Faraone SV, Walaas SI, et al. Methylphenidate normalizes elevated dopamine transporter densities in an animal model of the attention-deficit/hyperactivity disorder combined type, but not to the same extent in one of the attention-deficit/hyperactivity disorder inattentive type. Neurosci. 2010;167:1183–1191. doi: 10.1016/j.neuroscience.2010.02.073. [DOI] [PubMed] [Google Scholar]
- Russell VA. Overview of animal models of attention deficit hyperactivity disorder (ADHD) Curr Protoc Neurosci. 2011 doi: 10.1002/0471142301.ns0935s54. Chapter 9: Unit 9 35. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–39. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Scholl JL, Renner KJ, Forster GL, Tejani-Butt S. Central monoamine levels differ between rat strains used in studies of depressive behavior. Brain Res. 2010;1355:41–51. doi: 10.1016/j.brainres.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Kuriyan AB, Evans SW, Waxmonsky JG, Smith BH. Pharmacological and psychosocial treatments for adolescents with ADHD: An updated systematic review of the literature. Clin Psych Rev. 2014;34:218–232. doi: 10.1016/j.cpr.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Silva N, Szobot CM, Shih MC, Hoexter MQ, Anselmi CE, Pechansky F, Bressan RA, Rohde LA. Searching for a neurobiological basis for self-medication theory in ADHD comorbid with substance use disorders: an in vivo study of dopamine transporters using (99m)Tc-TRODAT-1 SPECT. Clin Nucl Med. 2014;39:e129–34. doi: 10.1097/RLU.0b013e31829f9119. [DOI] [PubMed] [Google Scholar]
- Solanto MV. Clinical psychopharmacology of AD/HD: Implications for animal models. Neurosci Biobehav Rev. 2000;24:27–30. doi: 10.1016/s0149-7634(99)00061-5. [DOI] [PubMed] [Google Scholar]
- Somkuwar SS, Darna M, Kantak KM, Dwoskin LP. Adolescence methylphenidate treatment in a rodent model of attention deficit/hyperactivity disorder: Dopamine transporter function and cellular distribution in adulthood. Biochem Pharmacology. 2013b;86:309–316. doi: 10.1016/j.bcp.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Jordan CJ, Kantak KM, Dwoskin LP. Adolescent atomoxetine treatment in a rodent model of ADHD: Effects on cocaine self-administration and dopamine transporters in frontostriatal regions. Neuropsychopharmacology. 2013a;38:2588–2597. doi: 10.1038/npp.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Kantak KM, Bardo MT, Dwoskin LP. Adolescent methylphenidate treatment differentially alters adult impulsivity and hyperactivity in the Spontaneously Hypertensive Rat model of ADHD. Pharmacol Biochem Behav. 2016;141:66–77. doi: 10.1016/j.pbb.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR. Drug self-administration by laboratory animals: control by schedules of reinforcement. Annu Rev Pharmacol Toxicol. 1978;18:313–339. doi: 10.1146/annurev.pa.18.040178.001525. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stanis JJ, Andersen SL. Reducing substance use during adolescence: a translational framework for prevention. Psychopharmacology (Berlin) 2014;231:1437–1453. doi: 10.1007/s00213-013-3393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhausen H-C, Bisgaard C. Substance use disorders in association with attention-deficit/hyperactivity disorder, co-morbid mental disorders, and medication in a nationwide sample. European Neuropsychopharmacology. 2014;24:232–241. doi: 10.1016/j.euroneuro.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2005 National Survey on Drug Use and Health: National findings. SAMHSA; Rockville, MD: 2006. Office of Applied Studies, NSDUH Series H-30, DHHS Publication No. SMA 06-4194. [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–33. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontiera FE, Frau R, Chiara GD. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. European J Neurosci. 1997;9:2077–2085. doi: 10.1111/j.1460-9568.1997.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Izidio GS, Takahashi RN. Drug reinforcement in a rat model of attention deficit/hyperactivity disorder - the Spontaneously Hypertensive Rat (SHR) Curr Drug Abuse Rev. 2009;2:177–183. doi: 10.2174/1874473710902020177. [DOI] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:34–46e2. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CH, Bonsall RW, Emery MS, Weiss JM. Rats selectively bred for high and low swim-test activity show differential responses to dopaminergic drugs. Psychopharmacology (Berlin) 1999;146:241–51. doi: 10.1007/s002130051113. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–85. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Wilkes MA, Spratt EG, Cobb SM. [Accessed 20 March 2016];Pediatric attention deficit hyperactivity disorder (ADHD) medication. 2015 Available at http://emedicine.medscape.com/article/912633-medication.
- Womersley JS, Hsieh JH, Kellaway LA, Gerhardt GA, Russell VA. Maternal separation affects dopamine transporter function in the Spontaneously Hypertensive Rat: An in vivo electrochemical study. Behav Brain Func. 2011;7:49. doi: 10.1186/1744-9081-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Miller GM. A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain. J Pharm Exper Therapeutics. 2009;330:316–325. doi: 10.1124/jpet.109.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Westmoreland SV, Bahn ME, Chen GL, Yang H, Vallender EJ, et al. Rhesus monkey trace amine-associated receptor 1 signaling: Enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro. J Pharm Exper Therapeutics. 2007;321:116–127. doi: 10.1124/jpet.106.116863. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Rapid regulation of the dopamine transporter: Role in stimulant addiction? Neuropharmacology. 2004;47:80–91. doi: 10.1016/j.neuropharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Zhang-James Y, Yang L, Middleton FA, Yang L, Patak J, Faraone SV. Autism-related behavioral phenotypes in an inbred rat substrain. Behav Brain Res. 2014;269:103–114. doi: 10.1016/j.bbr.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Chiodo KA, Roberts DC. Reduction of the reinforcing effectiveness of cocaine by continuous D-amphetamine treatment in rats: importance of active self-administration during treatment period. Psychopharmacology (Berlin) 2014;231:949–954. doi: 10.1007/s00213-013-3305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]