Abstract
Introduction and hypothesis
Many providers recommend concurrent estrogen therapy with pessary use to limit complications; however, limited data exist to support this practice. We hypothesized that vaginal estrogen supplementation decreases incidence of pessary-related complications and discontinuation.
Methods
We performed a retrospective cohort study of women who underwent a pessary fitting from 1 January 2007 through 1 September 2013 at one institution; participants were identified by billing code and were eligible if they were post-menopausal and had at least 3 months of pessary use and 6 months of follow-up. All tests were two sided, and P values < 0.05 were considered statistically significant.
Results
Data from 199 women were included; 134 used vaginal estrogen and 65 did not. Women who used vaginal estrogen had a longer median follow-up time (29.5 months) compared with women who did not (15.4 months) and were more likely to have at least one pessary check (98.5 % vs 86.2 %, P < 0.001). Those in the estrogen group were less likely to discontinue using their pessary (30.6 % vs 58.5 %, P < 0.001) and less likely to develop increased vaginal discharge than women who did not [hazard ratio (HR) 0.31, 95 % confidence interval (CI) 0.17–0.58]. Vaginal estrogen was not protective against erosions (HR 0.93, 95 % CI 0.54–1.6) or vaginal bleeding (HR 0.78, 95 % CI 0.36–1.7).
Conclusions
Women who used vaginal estrogen exhibited a higher incidence of continued pessary use and lower incidence of increased vaginal discharge than women who did not.
Keywords: Pessary, Prolapse, Vaginal estrogen
Introduction
Pelvic organ prolapse (POP) and stress urinary incontinence (SUI) are widespread conditions that can affect up to 41 % of women and increase in prevalence with age [1]. While there rarely is serious morbidity secondary to prolapse or SUI, these conditions can have a significant effect on a woman’s quality of life due to a protruding mass, pelvic pressure, dyspareunia, incontinence, and pain [2]. As the aging population continues to increase, focus will also need to increase on management options for these conditions. Current treatment options include expectant management, pelvic floor physical therapy, pessary use, and surgery.
The pessary is a simple, noninvasive, and inexpensive treatment option for patients with POP or SUI. A survey of members of the American Urogynecologic Society estimated that 77 % of urogynecologists use a pessary as a first-line treatment for POP [3]. Among patients using a pessary, 67–92 % report being satisfied, with nearly all symptoms from POP resolving within 2 months of use in one study [4]. Rarely, there are serious side effects associated with pessary use, such as fistula development, fecal impaction, or hydronephrosis. These serious complications are almost always due to neglected pessary care. More typical complications include vaginal discharge, foul smell, bleeding, pain, and erosions [2]. Rates of vaginal erosions among pessary users vary in the literature from 3 to 24 % [5–7].
Vaginal atrophy in menopause may predispose women to these more typical complications associated with pessary use. Without estrogen replacement, the vaginal epithelium in post-menopausal women changes in architecture leading to dryness, dyspareunia, discomfort, and pruritus [8]. Multiple studies have demonstrated that vaginal estrogen use results in maturation of vaginal epithelium with improvement in vaginal dryness and atrophy [9, 10]. Currently, the majority of providers recommend concurrent estrogen therapy with pessary use to limit complications; however, limited data exist to support this practice [2]. A retrospective medical record review identified that systemic and local hormone replacement therapy aide in successful pessary fitting and, based on expert opinion, vaginal erosions are routinely treated with vaginal estrogen [2, 6, 11]. However, there is a lack of data evaluating the concurrent use of vaginal estrogen and the development of complications.
The primary objective of our study was to examine the effect of vaginal estrogen supplementation on the incidence of erosions, other pessary-related complications, and discontinuation among women with POP and SUI who used a pessary for at least 3 months. We hypothesized that vaginal estrogen supplementation decreases the incidence of pessary-related complications and discontinuation.
Materials and methods
We performed a retrospective cohort study of women who had a pessary fitting from 1 January 2007 through 1 September 2013 at our institution. The Mount Auburn Hospital institutional review board approved the study protocol. We identified women using the current procedural terminology (CPT) billing code 57160, a five-digit numeric code used to describe medical, surgical, and diagnostic procedures or services. Women were eligible if they were postmenopausal. We further restricted participants to women with at least 3 months of pessary use to allow sufficient time for erosion development, which was the primary outcome of interest. All women were followed for a minimum of 6 months after the start of their pessary use. This was a sample of convenience.
In our practice, after a woman was fitted for a pessary, she was instructed to return to see a provider in 2 weeks. After this visit, she was instructed to follow-up every 3 months for pessary checks with a provider if she was not able to remove of felt uncomfortable removing her pessary at home. If the woman was managing her pessary at home, she was instructed to follow-up every 6–12 months.
Each medical record was reviewed for demographic information, relevant comorbidities and medical history, vaginal estrogen use, pessary care, dates of erosions, vaginal bleeding, increased vaginal discharge, and date of and reason for pessary discontinuation. Any information that could not be obtained from the medical record was recorded as missing. Increased vaginal discharge was defined as either patient report of increase in vaginal discharge or findings of moderate leukorrhea on physical exam at the time of pessary removal or if the provider listed vaginitis or leukorrhea as a diagnosis in the medical record. Our primary exposure of interest was estrogen use, which was defined as any form of vaginal estrogen including topical creams, suppositories, or rings. This was dichotomized into women who did and did not use estrogen. The practice of our providers is to recommend vaginal estrogen creams or suppositories to be used two to three times per week.
Our primary outcome was development of erosions as defined by the provider’s documentation of an erosion in the patient medical record; secondary outcomes included discontinuation of pessary use, reason for discontinuation, increased vaginal discharge, and vaginal bleeding. Outcomes could only be assessed among women who had at least one follow-up visit to have the pessary checked.
Data are presented as median and interquartile range (IQR) or proportion. Consistent with reporting guidelines, tests of statistical significance were not conducted for the descriptive characteristics; thus, P values are not presented in Table 1 [12]. Categorical outcome variables were compared using a chi-square or Fisher’s exact test, and continuous outcome variables were compared using a Wilcoxon rank-sum test. We plotted Kaplan–Meier survival curves and compared them with the log-rank test. Hazard ratios (HR) and 95 % confidence intervals (CI) were calculated using the Cox proportional hazards model. Variables were selected as potential confounders if they were believed to influence both estrogen use and risk of erosion; potential confounders were not identified based on statistical criterion [13]. Potential confounders were assessed using univariate analysis and were considered for addition in the final model if they changed the HR by >10 %. Data were analyzed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). All tests were two sided, and P values <0.05 were considered statistically significant.
Table 1.
Demographics
| All women (n = 199) | Vaginal estrogen (n = 134) | No vaginal estrogen (n = 65) | |
|---|---|---|---|
| Age | 76.0 (66.0–84.0) | 76.5 (66.0–86.0) | 75.0 (67.0–82.0) |
| Body mass index | 25.7 (23.4–28.7) | 25.8 (23.2–28.5) | 25.7 (23.7–30.1) |
| Race | |||
| White | 187 (94.0) | 128 (95.5) | 59 (90.8) |
| Black | 2 (1.0) | 1 (0.7) | 1 (1.5) |
| Asian | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 5 (2.5) | 3 (2.2) | 2 (3.1) |
| Unknown | 5 (2.5) | 2 (1.5) | 3 (4.6) |
| Ethnicity | |||
| Hispanic | 2 (1.0) | 0 (0.0) | 2 (3.1) |
| Not Hispanic | 192 (96.5) | 132 (98.5) | 60 (92.3) |
| Unknown | 5 (2.5) | 2 (1.5) | 3 (4.6) |
| Marital status | |||
| Single | 11 (5.5) | 9 (6.7) | 2 (3.1) |
| Married/partner | 88 (44.2) | 59 (44.0) | 29 (44.6) |
| Divorced/separated | 23 (11.6) | 12 (9.0) | 11 (16.9) |
| Widowed | 48 (24.1) | 33 (24.6) | 15 (23.1) |
| Unknown | 29 (14.6) | 21 (15.7) | 8 (12.3) |
| Smoking status | |||
| Current smoker | 7 (3.5) | 6 (4.5) | 1 (1.5) |
| Former smoker | 61 (30.7) | 41 (30.6) | 20 (30.8) |
| Never smoker | 130 (65.3) | 86 (64.2) | 44 (67.7) |
| Unknown | 1 (0.5) | 1 (0.7) | 0 (0.0) |
| Follow-up (months) | 23.1 (8.7–46.3) | 29.5 (11.0–55.4) | 15.4 (5.5–29.4) |
| Diabetes | |||
| Yes | 22 (11.1) | 16 (11.9) | 6 (9.2) |
| No | 177 (88.9) | 188 (88.1) | 59 (90.8) |
| Hysterectomy | |||
| Yes | 58 (29.1) | 42 (31.3) | 16 (24.6) |
| No | 141 (70.9) | 92 (68.7) | 49 (75.4) |
| Bilateral salphingo-oophorectomy | |||
| Yes | 28 (14.1) | 22 (16.4) | 6 (9.2) |
| No | 171 (85.9) | 112 (83.6) | 59 (90.8) |
| Prolapse stage | |||
| 0 | 5 (2.5) | 3 (2.2) | 2 (3.1) |
| 1 | 16 (8.0) | 12 (9.0) | 4 (6.2) |
| 2 | 88 (44.2) | 64 (47.8) | 24 (36.9) |
| 3 | 73 (36.7) | 45 (33.6) | 28 (43.1) |
| 4 | 11 (5.5) | 6 (4.5) | 5 (7.7) |
| Missing | 6 (3.0) | 4 (3.0) | 2 (3.1) |
Data are presented as median (interquartile range) or n (%)
Results
Of the 404 women identified by CPT code, 205 were excluded. Of the 205 excluded women, 19 could not be fitted with a pessary, 82 were premenopausal, 83 had a pessary for <3 months, and 22 did not have 6 months of follow-up. Thus, 199 women were included in the analysis. Approximately twice as many women used vaginal estrogen (67.3 %) than did not (32.7 %). The two groups of women had similar demographic characteristics, including body mass index, race, ethnicity, marital status, smoking history, diabetes, past hysterectomy or bilateral salpingo-oophorectomy, and prolapse stage. The length of follow-up was greater among women who used vaginal estrogen (29.5) months, than women who did not (15.4 months) (Table 1). Rings with support were the most common type used overall (52.8 %), and more women who used vaginal estrogen used rings with support (58.2 %) compared with women who did not use (41.5 %, P = 0.03). No significant differences were noted between groups with regard to other pessary types. (Table 2)
Table 2.
Pessary use and discontinuation
| All women (n = 199) | Vaginal estrogen (n = 134) | No vaginalestrogen (n = 65) | P value* | |
|---|---|---|---|---|
| Type of pessary | ||||
| Ring | 12 (6.0) | 6 (4.5) | 6 (9.2) | 0.21 |
| Gellhorn | 53 (26.6) | 36 (26.9) | 17 (26.2) | 0.92 |
| Incontinence ring | 4 (2.0) | 3 (2.2) | 1 (1.5) | 1.0 |
| Ring with support | 105 (52.8) | 78 (58.2) | 27 (41.5) | 0.03 |
| Incontinence dish | 15 (7.5) | 7 (5.2) | 8 (12.3) | 0.09 |
| Other | 10 (5.0) | 4 (3.0) | 6 (9.2) | 0.08 |
| At least one pessary check | <0.001 | |||
| Yes | 188 (94.5) | 132 (98.5) | 56 (86.2) | |
| No | 11 (5.5) | 2 (1.5) | 9 (13.8) | |
| Number of pessary checks** | 6.0 (3.0–13.0) | 8.0 (4.0–15.0) | 4.0 (2.0–7.5) | <0.001 |
| Patient taught self-care | 0.29 | |||
| Yes | 63 (31.7) | 39 (29.1) | 24 (36.9) | |
| No | 132 (66.3) | 91 (67.9) | 41 (63.1) | |
| Other | 3 (1.5) | 3 (2.2) | 0 (0.0) | |
| Missing | 1 (0.5) | 1 (0.8) | 0 (0.0) | |
| Discontinued | <0.001 | |||
| Yes | 79 (39.7) | 41 (30.6) | 38 (58.5) | |
| No | 120 (60.3) | 93 (69.4) | 27 (41.5) | |
| Time to discontinuation (months)*** | 12.0 (5.5–36.4) | 13.2 (5.7–50.9) | 10.9 (4.6–24.0) | 0.33 |
| Reason for discontinuation*** | ||||
| Pain | 8 (10.1) | 1 (2.4) | 7 (18.4) | 0.03 |
| Bleeding/erosions | 17 (21.5) | 10 (24.4) | 7 (18.4) | 0.52 |
| Unable to fit/falls out | 4 (5.1) | 2 (4.9) | 2 (5.3) | 1.0 |
| Prefers surgery | 32 (40.5) | 16 (39.0) | 16 (42.1) | 0.78 |
| Other | 18 (22.8) | 12 (29.3) | 6 (15.8) | 0.15 |
Data are presented as median (interquartile range) or n (%)
P compares with and without vaginal estrogen
Only among women who had at least one pessary check
Only among women who discontinued their pessary
Women who used vaginal estrogen were less likely to discontinue their pessary than those who did not use (30.6 % vs 58.5 %, P < 0.001). Seven (18.4 %) women who did not indicated pain as the primary reason for pessary discontinuation compared with only one woman who in the estrogen use group (2.4 %, P = 0.03). Median time to pessary discontinuation was similar between the groups (P = 0.33). The most common reason for discontinuation in both groups was a desire for surgical correction of the prolapse. Although most (94.5 %) women returned to have their pessary checked at least once, women who used vaginal estrogen were more likely to return for an evaluation (P < 0.001). Among those who returned, women who used estrogen also had more follow-up visits than those who did not (P < 0.001). (Table 2). In both groups, approximately one third of women were able to remove and care for their pessary at home. We did not record the frequency of pessary removal among women who managed their pessaries at home.
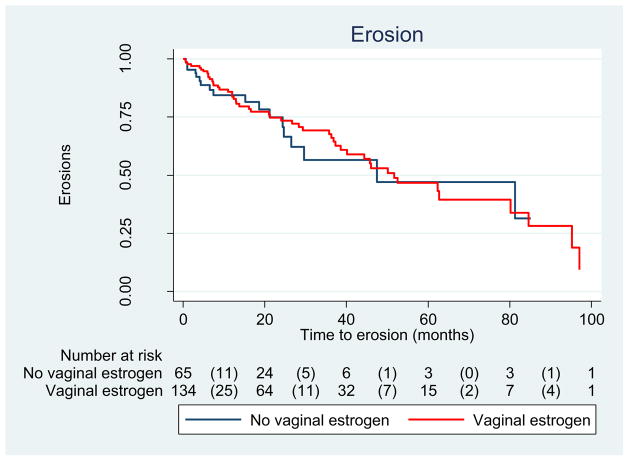
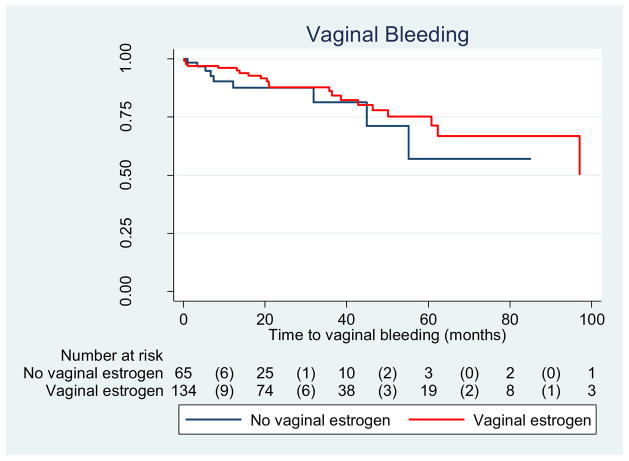
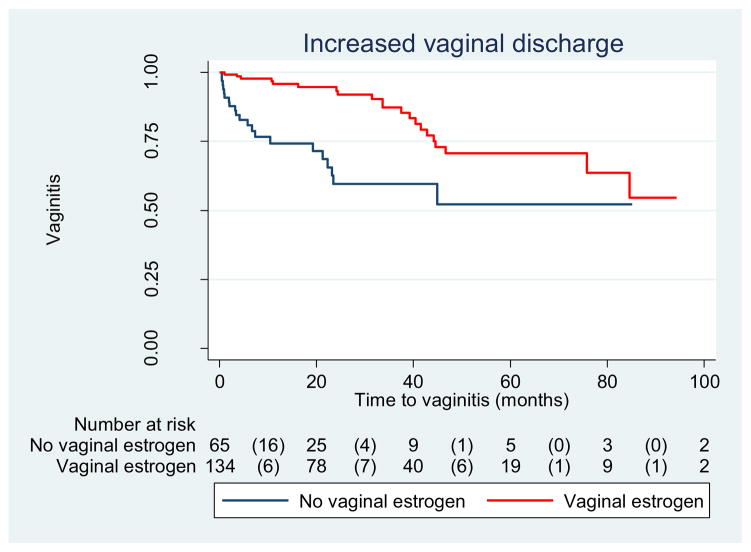
The incidence of erosion was 36.6 % among women who used vaginal estrogen and 27.7 % among those who did not, yielding a crude HR of 0.93 (0.54–1.6). (Table 3) As shown in Fig. 1, the median time to erosion did not differ between groups. Similarly, the incidence of vaginal bleeding did not differ between groups, though it was an infrequent complication (16.4 % vs 13.8 %, P = 0.64) (Table 3, Fig. 2). In contrast, rates were different between groups regarding increased vaginal discharge; those who used estrogen were less likely to develop increased vaginal discharge (15.7 %) than those who did not (32.3 %), yielding a HR of 0.31 (95 % CI: 0.17–0.58). Adjusting for age, pessary type, smoking status, and diabetes did not appreciably alter the HR; thus, only crude results are presented (Table 3, Fig. 2)
Table 3.
Outcomes among women with at least 3 months of follow-up
| Vaginal estrogen (n = 134) | No vaginal estrogen (n = 65) | P value | Hazard ratio (95 % CI) | |
|---|---|---|---|---|
| Erosion | 0.21 | 0.96 (0.54–1.6) | ||
| Yes | 49 (36.6) | 18 (27.7) | ||
| Time to erosion (months) | 16.6 (7.3–40.1) | 11.3 (3.0–24.6) | 0.09 | |
| No | 85 (63.4) | 47 (72.3) | ||
| Vaginal bleeding | 0.64 | 0.78 (0.36–1.7) | ||
| Yes | 22 (16.4) | 9 (13.8) | ||
| Time to vaginal bleeding (months) | 21.0 (13.1–46.4) | 7.4 (5.4–31.9) | 0.22 | |
| No | 112 (83.6) | 56 (86.2) | ||
| Increased vaginal discharge | 0.007 | 0.31 (0.17–0.58) | ||
| Yes | 21 (15.7) | 21 (32.3) | ||
| Time to increased vaginal discharge (months) | 33.6 (16.2–42.8) | 4.1 (0.99–19.2) | 0.0004 | |
| No | 113 (84.3) | 44 (67.7) |
Data are presented as median (interquartile range) or n (%)
Fig. 1.
Erosions among women with pessary use (n = 199); log rank test P = 0.78
Fig. 2.
Increased vaginal discharge among women with pessary use (n = 199), log rank test, P = 0.0001
Discussion
In this study, we found no significant difference in the rate of erosions among pessary users who used vaginal estrogen compared with those who did not use vaginal estrogen (Fig. 3). Women in the vaginal estrogen group were noted to have longer and more frequent follow-up than those who did not using vaginal estrogen. This may be due to the need for women to refill their prescriptions for estrogen. In addition, women who were more agreeable to using vaginal estrogen may also be more compliant with a physician’s recommendations regarding follow-up appointments. With better follow-up evaluations, it is possible that we were better able to capture the vaginal erosions occurring in the estrogen group. At-home pessary care was not common in our study because our population was older (median 76 years of age). In addition, using estrogen may have enabled that group to continue use of their pessary for a longer period of time than those not using estrogen, resulting in erosions due to the longer duration of pessary.
Fig. 3.
Time to vaginal bleed among women with pessary use (n = 199); log rank test P = 0.53
Women in our study population who used vaginal estrogen with their pessaries were less likely to discontinue its use or develop an increase in vaginal discharge than women who did not (Fig. 2). It is likely that vaginal estrogen helped restore the vaginal environment to a premenopausal state by decreasing the vaginal pH and increasing vaginal maturation index. These changes may have improved atrophic vaginitis [14] and, as a result, leukorrhea or discharge. Among women who discontinued pessary use, those who did not use vaginal estrogen were more likely to report pain as a reason for discontinuation, although the number of women in this group was small. The time to pessary discontinuation was similar between groups, which is likely because the most common reason for pessary discontinuation in both groups was desire for surgery, which would not be influenced by estrogen use. However, we cannot infer from our data if the reason for discontinuation and desire for surgery was due to ineffectiveness of the pessary vs another unidentified reason.
The literature on the effect of vaginal estrogen on pessary use and complications is limited [15]. A 2006 study by Hanson et al. demonstrated that local hormonal use can play a role in successful pessary fitting; however, it does not explore long-term complications and discontinuation rates when vaginal estrogen is used [6]. In their prospective study on pessary management, Wu et al. demonstrated no association between hormone replacement therapy and successful pessary fitting; however, their study did not specify whether hormones were used orally or locally. A difference may not have been noted if it was orally [7]. Postmenopausal women who use pessaries and do not use hormonal therapy have been shown to have higher rates of bothersome discharge and evidence of vaginitis on microscopy than similar women who do not use pessaries [16]. Similar to this study, Friedman et al. found that older age was associated with higher rates of continued pessary use; however, they found no difference between those who did or did not. Again, it is unclear whether these women used locally or systemically applied hormones [17].
Many women report significant concerns of using vaginal hormonal preparations, including safety and the risk of breast cancer [18]. Data on long-term results of vaginal estrogen use is limited; however, few contraindications are reported among postmenopausal women [19]. Unlike oral preparations, the incidence of adverse events with vaginal estrogen use compared with placebo has been reported to be equal [20, 21].
There are several strengths to this study. Both study arms were demographically similar and therefore comparable. Our practice uses an electronic prescription system; therefore, all prescriptions for vaginal estrogen use were documented in the electronic medical record. A thorough medical record abstraction was performed in an attempt to capture all potential confounders, such as prior hysterectomy, smoking status, and prolapse stage. Finally, both groups had a median follow-up of ~1 year.
Limitations of our study include its retrospective nature, thus limiting us to data documented in the medical record. In particular, we were unable to determine why women did not follow-up with their provider. Our cohort size was modest at 199. We also do not know if women were evaluated by a provider outside of our practice, leaving us unable to capture those complications. However, in our population, nearly all (94.5 %) women returned for at least one follow-up visit. In addition, we were unable to assess compliance with vaginal estrogen use. While we included women who used any type of vaginal estrogen, most studies concur that all formulations have similar efficacy and safety [22]. We also had information as to why some women decided to use vaginal estrogen and others did not and whether these factors contributed to complication rates. We found that more women in the estrogen group were using rings with support than those who did not use vaginal estrogen. However, this type of pessary was assessed in our analysis and did not appreciably alter our results. It was therefore not included in our final model. Finally, our study was conducted at a single institution with a relatively homogenous patient population, which may limit the generalizability of our findings.
Our results suggest that there is a benefit to concomitant use of vaginal estrogen use with pessaries among postmenopausal women. Additional larger, prospective studies are needed, however, to replicate our findings of longer pessary use and lower rates of vaginal discharge among women who use vaginal estrogen with their pessaries. The results of this study suggest that the current practice of using vaginal estrogen in conjunction with pessaries when possible appears to result in fewer minor complications and longer duration of pessary use.
Acknowledgments
Financial support Support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758) and financial contributions from Harvard University and its affiliated academic health care centers.
Footnotes
Presentations Poster presentation, International Continence Society Annual Meeting, Rio de Janeiro, Brazil, October 2014
Compliance with ethical standards
Conflict of Interest None
References
- 1.Hendrix SL, Clark A, Nygaard I, et al. Pelvic organ prolapse in the women’s health initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160–1166. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 2.Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369:1027–1038. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- 3.Cundiff GW, Weidner AC, Visco AG, et al. A survey of pessary use by members of the American urogynecologic society. Obstet Gynecol. 2000;95:931–935. doi: 10.1016/s0029-7844(00)00788-2. [DOI] [PubMed] [Google Scholar]
- 4.Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025–9. doi: 10.1016/j.ajog.2003.10.711. [DOI] [PubMed] [Google Scholar]
- 5.Powers K, Lazarou G, Wang A, et al. Pessary use in advanced pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:160–164. doi: 10.1007/s00192-005-1311-8. [DOI] [PubMed] [Google Scholar]
- 6.Hanson L-AM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:155–159. doi: 10.1007/s00192-005-1362-x. [DOI] [PubMed] [Google Scholar]
- 7.Wu V, Farrell SA, Baskett TF, Flowerdew G. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990–994. doi: 10.1016/s0029-7844(97)00481-x. [DOI] [PubMed] [Google Scholar]
- 8.Krause M, Wheeler TL, Snyder TE, Richter HE. Local effects of vaginally administered estrogen therapy: a review. J Pelvic Med Surg. 2009;15:105–114. doi: 10.1097/SPV.0b013e3181ab4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speroff L. Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms. Obstet Gynecol. 2003;102:823–834. doi: 10.1016/s0029-7844(03)00764-6. [DOI] [PubMed] [Google Scholar]
- 10.Notelovitz M, Funk S, Nanavati N, Mazzeo M. Estradiol absorption from vaginal tablets in postmenopausal women. Obstet Gynecol. 2002;99:556–562. doi: 10.1016/s0029-7844(01)01385-0. [DOI] [PubMed] [Google Scholar]
- 11.Oliver R, Thakar R, Sultan AH. The history and usage of the vaginal pessary: a review. Eur J Obstet Gynecol Reprod Biol. 2011;156:125–30. doi: 10.1016/j.ejogrb.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Vandenbroucke JP, Von Elm E, Altman DG, et al. Strengthening the reporting of observational epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18:805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 13.Hernán MA, Hernández-díaz S, Werler MM, Mitcheil AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 14.Cardozo L, Bachmann G, Mcclish D, et al. Meta-analysis of estrogen therapy in the management of urogenital atrophy in post-menopausal women: second report of the hormones and urogenital therapy committee. Obstet Gynecol. 1998;92:722–727. doi: 10.1016/s0029-7844(98)00175-6. [DOI] [PubMed] [Google Scholar]
- 15.Bugge C, Ej A, Gopinath D, Reid F. Pessaries (mechanical devices) for pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;2:CD004010. doi: 10.1002/14651858.CD004010.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins S, Beigi R, Mellen C, et al. The effect of pessaries on the vaginal microenvironment. Am J Obstet Gynecol. 2015;212:e1–6. doi: 10.1016/j.ajog.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Friedman S, Sandhu KS, Wang C, et al. Factors influencing long-term pessary use. Int Urogynecol J Pelvic Floor Dysfunct. 2010;21:673–678. doi: 10.1007/s00192-009-1080-x. [DOI] [PubMed] [Google Scholar]
- 18.Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal women’s VIews of treatment options for menopausal vaginal changEs) survey. J Sex Med. 2013;10:1790–1799. doi: 10.1111/jsm.12190. [DOI] [PubMed] [Google Scholar]
- 19.Lindahl SH. Reviewing the options for local estrogen treatment of vaginal atrophy. Int J Womens Health. 2014;6:307–312. doi: 10.2147/IJWH.S52555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahn DD, Ward RM, Sanses TV, et al. Vaginal estrogen use in postmenopausal women with pelvic floor disorders: systematic review and practice guidelines. Int Urogynecol J. 2014;26:3–13. doi: 10.1007/s00192-014-2554-z. [DOI] [PubMed] [Google Scholar]
- 21.Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;11:CD001500. doi: 10.1002/14651858.CD001500.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause. Obstet Gynecol. 2014;124:1147–1156. doi: 10.1097/AOG.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]