Abstract
Iron and copper salts have complex olfactory and gustatory properties including a metallic flavor component that is decreased by nasal occlusion. To examine the sensory properties of ferrous sulfate and copper sulfate, a trained descriptive panel evaluated these compounds at equal molarity and perceived equal intensity with and without nasal occlusion. Ferrous sulfate exhibited a metallic taste and metallic aftertaste and copper sulfate exhibited a more pronounced metallic aftertaste. Metallic sensations were decreased by nasal occlusion, which in the absence of any orthonasal metallic smell, implies that the sensations were retronasally perceived volatiles in the nose open condition. Ferrous sulfate showed a larger effect of nasal occlusion. A second experiment isolated ferrous sulfate solutions from oral contact via a plastic barrier. In comparison to a condition in which oral contact was allowed, intensity ratings were decreased. This result is consistent with the hypothesis that rinses with solutions of metal salts, particularly ferrous sulfate, generate volatile lipid oxidation products in the mouth that are perceived retronasally as metallic flavors.
Keywords: Descriptive analysis, nasal occlusion, retronasal smell, metallic taste
1. Introduction
The nature and interpretation of metallic taste sensations remains a matter whose origin, status and mechanisms are poorly understood. “Metallic” was once considered (by Wundt in the 1880s) to be one of the basic or primary taste sensations (Bartoshuk, 1978). In the Japanese clinical literature on electrogustometry, metallic is the most frequent word evoked by anodal stimulation of the tongue (Tomiyama, Tomita & Okuda, 1971). Tomita, Ikeda and Okuda (1986) concluded from an analysis of area and quality from electrical stimulation that metallic taste was a unique quality “different from the four primary tastes (p. 11).” However, other researchers have been reluctant to give this word status as a legitimate taste quality descriptor (e.g. Adjukovic, 1984, 1990) and it appears only four times in Bujas’s extensive review of the electric taste literature (Bujas, 1971). Recent work has shown that metallic sensations are evoked both by rinses with metal salts and from electrical tongue stimulation. The former are attenuated by nasal occlusion (suggesting a retronasal smell component) but electrically induced metallic sensations are not (Lawless, Schlake, Smythe, Lim, Yang, Chapman & Bolton, 2004; Lawless, Stevens, Chapman & Kurtz, 2005) suggesting at least two distinct mechanisms for evoking this class of sensations.
Rinses with solutions of ferrous sulfate are particularly effective at evoking a metallic sensation, which is decreased or nearly eliminated when the nose is occluded (Hettinger, Myers & Frank, 1990; Lawless et al., 2004, Lawless et al., 2005). Murphy and Cain (1980) demonstrated that retronasal smells are effectively eliminated by closing the nose during tasting, and thus nasal occlusion has become a standard practice to study the contribution of retronasal smell to apparent taste sensations. However, this manipulation also blocks orthonasal smell, so the route is not clear unless orthonasally perceived odors are not present in the samples. Orthonasally, there is no metallic odor over the headspace of solutions of ferrous sulfate until very high concentrations are reached (Lawless et al., 2004; Lim & Lawless, 2005; Lubran, Lawless, Lavin & Acree, 2005). This suggests that the nasal occlusion is preventing a metallic smell, originating in the mouth, from reaching olfactory receptors retronasally. To a lesser extent, copper salts may also evoke metallic taste responses, although they are more complex in their sensory properties including bitter, metallic, sour and/or salty tastes and astringency (Cuppett, Duncan & Dietrich, 2006; Lawless et al., 2005; Zacarias, Yanez, Araya, Oraka, Olivares & Uauy, 2001).
An extensive specification of the sensory profile of divalent salts using a descriptive panel was conducted by Yang and Lawless (2005). Of ten divalent salts investigated, iron compounds (FeCl2, FeGluconate and FeSO4) were the most pronounced in metallic taste and aftertaste. FeSO4 at 0.05 M elicited several sensatons, including metallic aftertaste and taste, followed by bitter taste, astringent aftertaste, astringency, sourness, umami sensation, spicy sensation, bitter aftertaste and umami aftertaste. However, this study did not include a nasal occlusion condition to examine which of these sensations might be due to smell. Recently, a nasal occlusion effect was seen for copper thresholds (Epke & Lawless, 2007), with both compounds showing increased thresholds when retronasal sensations were eliminated. The purpose of Experiment 1 was to duplicate the methodology of Yang et al. which involved a trained descriptive panel, and include a nasal occlusion condition.
If a retronasal smell is being evoked from rinses with metal salts, the question arises as to its origin. One possibility is a rapid lipid oxidation reaction in the oral cavity. Metal salts, especially those of iron and copper are effective catalysts for lipid oxidation (Bodyfelt, Tobias & Trout, 1988). Lipid oxidation products have been extensively studied in the food chemistry literature, and many of them are described as metallic in character (Swoboda & Peers, 1977; Guth & Grosch, 1990; Heiler & Scheiberle, 1997). If lipid oxidation is playing a part, it seems likely that contact with saliva or oral tissues would be required for the reaction to take place, as lipids, like metal salts, are nonvolatile. Pierce and Halpern (1996) used a simple isolation device consisting of a film canister cap to place solutions in the mouth to eliminate oral contact and study the effects of orthonasal and retronasal smell without oral contact. In a second experiment, we studied the effects of isolating ferrous sulfate solutions from oral contact using a plastic cap similar to the device of Pierce and Halpern. If oral contact is necessary for the lipid oxidation reaction to occur, this manipulation should reduce the sensation of metallic “taste.”
The first objective of this study was to profile FeSO4 and CuSO4 at equal molarities and perceived equal intensities of “metallic taste” with and without nasal occlusion using a descriptive analysis panel. A primary interest was the effect of nasal occlusion which condition was omitted from our previous descriptive analysis of divalent salts (Yang et al., 2005). The second objective was to examine the effect of isolating the oral stimulus from contact with saliva and oral tissues.
2. Experiment 1: Sensations from iron and copper sulfate with and without nasal occlusion
2.1 Materials and methods
2.1.1 Subjects
Twenty one healthy subjects from the Cornell University community in Ithaca, NY volunteered to complete a questionnaire concerning availability and general health and participated in two screening sessions. Fifteen were asked to continue into training based on their performance in the screening tests. Of these fifteen, twelve completed training and participated in the formal evaluation sessions with the two test compounds. All twelve were non-smokers, had no reported anosmias, had at least some college education and were fluent English speakers. Four subjects were male; ages were from 20–61 with a mean of 35. Panelists received compensation for training and testing. Informed consent was given before the test. The research protocol was approved by the University Committee on Human Subjects.
2.1.2 Stimuli
The iron and copper compounds used in this study were 0.001 and 0.015M ferrous sulfate (FeSO4 • 7H20, Mallinckrodt Baker, Inc., Phillipsburg, NJ), and 0.001M cupric sulfate (CuSO4 • 5H20, EMD Pharmaceuticals, Durham, NC). Concentrations of iron and copper sulfate were chosen to produce approximately equal overall perceived intensity, as found in preliminary testing (Yang & Lawless, 2005) as well as to allow comparison at equal molarities. All stimuli were reagent grade and were prepared by dissolving the compounds in deionized water. Solutions were prepared daily to prevent by-products from oxidation (Wong, 1989). During screening, the following compounds were given for identification: sucrose (2% w/v), NaCl (0.2% w/v), citric acid (0.07% w/v), quinine HCl (0.0036% w/v), monosodium glutamate (0.1% w/v), aluminum ammonium sulfate (0.09% w/v) and capsaicin (2ppm). The solutions, mean volume 20ml, were presented in 2 oz odorless plastic cups (Solo 2 oz Plastic Cups, Solo Cup Company. Urbana, IL) labeled with three-digit random numbers at constant room temperature (approximately 21 °C). Stimuli during training were sipped and expectorated, except for the capsaicin solution which was swabbed on the upper lip with a cotton swab, and a clean copper penny which was simply tasted and expectorated. The following odorants were given for identification during screening: diacetyl (buttery), benzaldehyde (almond or cherry), anise (licorice), lavender, vanilla extract and 1-octen-3-one (mushroom, earthy, metallic). Odorants were presented on fragrance testing strips kept in screw cap amber jars. They were evaluated by unscrewing the caps, sniffing the air within the jar and replacing the cap. Ferrous gluconate was chosen by the panel to serve as a reference for metallic flavor.
2.1.3 Screening and training
Candidates performed four screening exercises in two sessions: taste identification and a scaling exercise in the first session and odor identification and description of a complex unfamiliar product in the second session. The scaling exercise involved estimation of proportions of different geometric figures that were shaded (Meilgaard et al., 1999). The unfamiliar complex product was honey chrysanthemum tea. Scores were assigned based on the number of descriptor words generated for this product. The 15 subjects with the highest total scores in identification, scaling and description were invited for training.
Training involved 12 one-hour sessions for sample evaluation and vocabulary development. During the first session subjects were given a short introduction to descriptive analysis as a sensory method. Test samples were given for evaluation and descriptors were generated. The list of descriptors was circulated and reference standards were provided. After the third training session the subjects decided to use aqueous references since the products they were evaluating were in liquid form. After the final list of attributes was agreed upon, the subjects decided on an intensity rating for each standard. The subjects determined four levels of each reference standard denoted 2, 5, 10 and 15 on a scale of 0 to 15. The final test ballot included the following attributes: sweet, sour, salty, bitter, metallic and capsaicin tastes, astringency, and sweet, salty, sour, bitter, metallic and astringent aftertastes.
2.1.4 Test sessions
Subjects participated in six testing sessions with at least 24 hours between sessions. Compounds were evaluated with the nose open and closed, and all samples and nasal conditions were evaluated in duplicate. All six test sessions were conducted in the sensory evaluation facility in the Department of Food Science, Cornell University. Tests were conducted under red light to mask any visual cues. Data were collected using Compusense five (version 4.4.8. Compusense, Inc., Guelph, Ontario, Canada). Each session was used for testing two of the test solutions and nasal conditions. The samples were assigned random three-digit codes, and the testing order of the compounds and nasal occlusion condition was randomized and counterbalanced over subjects. Subjects were requested not to eat or drink anything for one hour preceding each session. Each experimental session started with subjects rinsing their mouths twice with deionized water. Stimuli were sipped, held in the mouth as attributes were rated and then expectorated. Panelists then rinsed with deionized water and a 0.05 M sucrose solution, which was found to be helpful in reducing persistent astringency (Zacarias et al., 2001) and again with deionized water. A mandatory five-minute delay was programmed into the ballot sequence. Unsalted crackers were also available for palate cleansing. In the nasal occlusion condition, subjects wore Spirometrics Spiro Nose clips (Spiro No. 2110; Spirometrics Medical Equipment, Grey, ME). Intensity judgments were made on continuous line scales, coded from 0 to 15. The lower anchor labels were “not very (sweet, salty, sour, bitter, astringent, metallic, peppery, tingly) at all” and the upper end anchors were “very (sweet, salty, sour, bitter, astringent, metallic, peppery, tingly)”.
2.1.5 Data analysis
Analysis of variance (ANOVA) was performed using SYSTAT® 5.5 at an alpha level of 0.01, to adjust for the large number (91 total) of effects examined. There were three main effects: compound, nose condition and replication and all interactions were retained in the model. Panelists were treated as a random variable. Comparisons across product pairs were tested with Least Significant Difference (LSD) and paired t-tests.
2.2 Results and Discussion
Nasal occlusion reduced the metallic taste for both of the iron concentrations but not copper (interaction F(2,22) = 6.04, p = 0.008) as shown in Figure 1. Nasal occlusion reduced metallic aftertaste for all three compounds (main effect, F(1,11) = 17.6, p < 0.001). Iron exhibited metallic taste and aftertaste and copper exhibits more metallic aftertaste than metallic taste. The mean metallic taste of 0.001 M CuSO4 and 0.001 M FeSO4 over all conditions was approximately equal (1.8 vs. 1.6).
Figure 1.
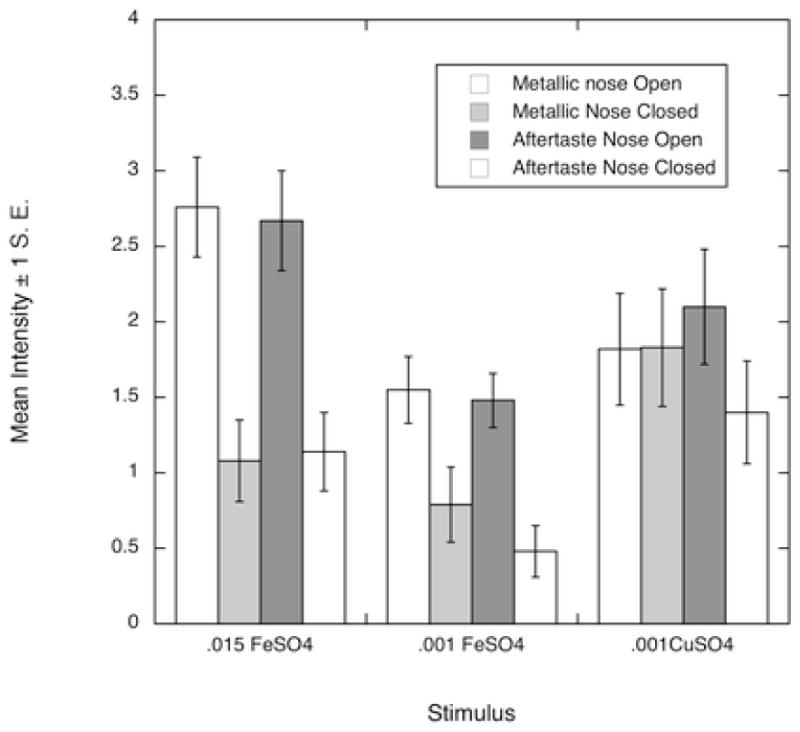
Mean metallic taste and metallic aftertaste intensity for the three compounds under two nasal conditions
Figure 2 shows differences in the other, non-metallic attributes. Copper sulfate exhibited a more prominent astringent mouthfeel (F (2,22) = 28.3, p < 0.001), astringent aftertaste (F(2,22) = 23.2, p < 0.001), bitter taste (F(2,22) = 60.8, p < 0.001), bitter aftertaste (F(2,22) = 60.4, p < 0.001) and sour taste (F(2,22) = 7.74, p = 0.003) than both of the iron concentrations. Astringency showed an overall replication effect which could be a result of a build up between stimuli (F(1,11) = 12.1, p = 0.005). Sweet taste and aftertaste, salty taste and aftertaste, capsaicin taste and sour aftertaste exhibited no significant differences among the compounds and between nasal conditions (data not shown).
Figure 2.
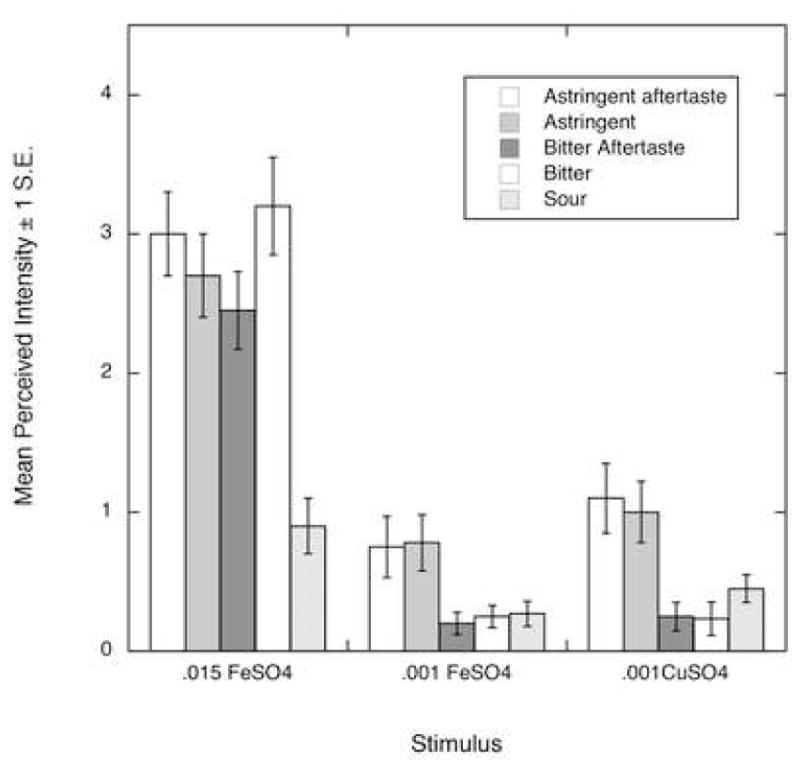
Mean rated intensity of sensory attributes other than metallic which showed differences among compounds, but no nasal occlusion effects.
To the extent that the reduction with nasal occlusion implies the elimination of a retronasal smell, iron has a larger retronasal constituent than copper. Epke and Lawless (2007) found a larger difference between the nose open and nose closed condition of detection thresholds of ferrous sulfate than copper sulfate. This is parallel to the effect shown here but at very low levels. The consistent patter is one of a metallic flavor arising from stimulation with copper salt solutions, but one that is less potent than that which is evoked by equimolar solutions of iron salts.
3. Experiment 2: Perception of ferrous sulfate when isolated from oral contact
3.1 Materials and Methods
3.1.1 Subjects
Twenty two subjects (ages 18–55 years, eight male) were recruited from the Cornell University campus or the surrounding area. Subjects did not know the purpose of the study and received no prior training. Informed consent was obtained, and each subject received a token incentive at the end of the study. The protocol was approved by the University Committee on Human Subjects.
3.1.2 Stimuli
Deionized water and FeSO4 (0.003M and 0.012M) were used in the prescreening procedure. Deionized water, FeSO4 (0.006M), sucrose (10% w/v), and a citral solution were used as test stimuli. The citral solution was made by extracting the water phase of a 1% citral, 2% EtOH (v/v) mixture. However, due to the separation of residual (oily) citral from the aqueous phase, the true concentration of this stimulus was unknown, sensations were very weak, and it was dropped from the analysis. FeSO4 (0.003M) was also supplied as a reference, and spring water was used to rinse.
3.1.3 Procedure
Due to pilot work indicating large inter-individual variability in the perception of metallic flavors after ferrous sulfate rinses, subjects were evaluated as to their susceptibility to this sensation in a screening task, with the goal of identifying low and high responding subjects. In this session, subjects first rinsed with spring water. They were then presented with a triangle test containing two deionized water samples and one FeSO4 (0.003M) sample. If they got it correct, they were asked to identify what was different. If the subject provided a metallic descriptor (non-limiting list provided) they were labeled “normosmic.” If they got the test wrong or did not provide a metallic descriptor, they were given a 0.012M solution of FeSO4 to taste and then expectorate. They then ranked the intensity on a magnitude estimation scale with 10% (w/v) sucrose as the reference. If the subject ranked the FeSO4 as more intense, they were labeled normosmic, if they rank it as less intense they were labeled anosmic to FeSO4. Both normosmic and anosmic subjects participated in the next phase of the study. Subjects next completed a worksheet to explain the concept of magnitude estimation. The experimenter reviewed the answers with each subject to insure that they understood the scaling technique.
In the test sessions, subjects were given the three stimuli in each of the four different conditions in a randomized order. They rinsed with spring water before starting and after every sample, including references. At the beginning of the study, and every three samples after that, they were presented with FeSO4 (0.003M) and told to consider the intensity (not the taste) as the reference “10” for magnitude estimation rating. They then rated each sample with a magnitude and described it using verbal descriptors (the same non-limiting list was provided as in screening). The following conditions were used:
“Oral contact, Nose open” (OC/NO). 10 ml of the stimulus was presented in a 50 ml plastic cup. Subjects rinsed with the entire sample, expectorated, and then evaluated before rinsing.
“Oral contact, nose closed” (OC/NC). 10 ml of the stimulus was presented in a 50 ml plastic cup. Subjects put on a nose clip to prevent olfaction. Subjects rinsed with the entire sample, expectorated, and then evaluated before taking off the nose clip or rinsing.
“No oral contact, nose open” (NOC/NO). 1 ml of the stimulus was presented on the center of a plastic lid (49mm outer diameter, 6mm outer lip/29mm inner diameter, 2mm inner lip). The lid was covered by a plastic cup to prevent smell perception before tasting. The subject put on the nose clip before removing the plastic cup. They then placed the lid on their tongue so that no liquid touched their mouth. Next, they inhaled through their mouth, closed their mouth, took off the nose clip, and exhaled through their nose. They removed the lid from their mouth without spilling any solution and evaluated the sample before rinsing. The experimenter was present at all times to guide them through the procedure.
“No oral contact, nose closed” (NOC/NC). The procedure was the same as the NOC/NO condition, except that the subject inhaled and exhaled through their mouth and evaluated the sample before removing the nose clip or rinsing.
3.2 Results and Discussion
Prescreening for FeSO4 responsiveness divided the subjects into two groups. There were no significant differences between groups on any measures and the data were combined for further analysis. None of the conditions (all p > .10) had any effect on the intensity of the water control sample (overall mean = 1.74 ± 0.4). Figure 3 shows the intensity ratings for ferrous sulfate and water. There was a significant interaction of nose condition with oral contact for ferrous sulfate (F(1,20) = 14.3, p < .01). Intensity ratings were significantly higher for the OC/NO condition than any other (Tukey HSD tests, p < .05). As expected, for sucrose, only oral contact had a significant effect (F(1,20) = 79.2, p < 0.001) on the intensity or the development of a taste (mean intensity of 9.5 ± 1.2 (S.E.) with oral contact and1.2 ± 0.8 without). The modal response for the condition with oral contact and the nose open was “metallic,” with astringent and drying sensations also reported. Since nasal occlusion reduced the intensity of ferrous sulfate, the data support the idea that metallic taste from FeSO4 could be a retronasal smell. The importance of oral contact for developing a metallic taste also supports the hypothesis that iron catalyzes lipid oxidation in the mouth producing metallic flavor.
Figure 3.
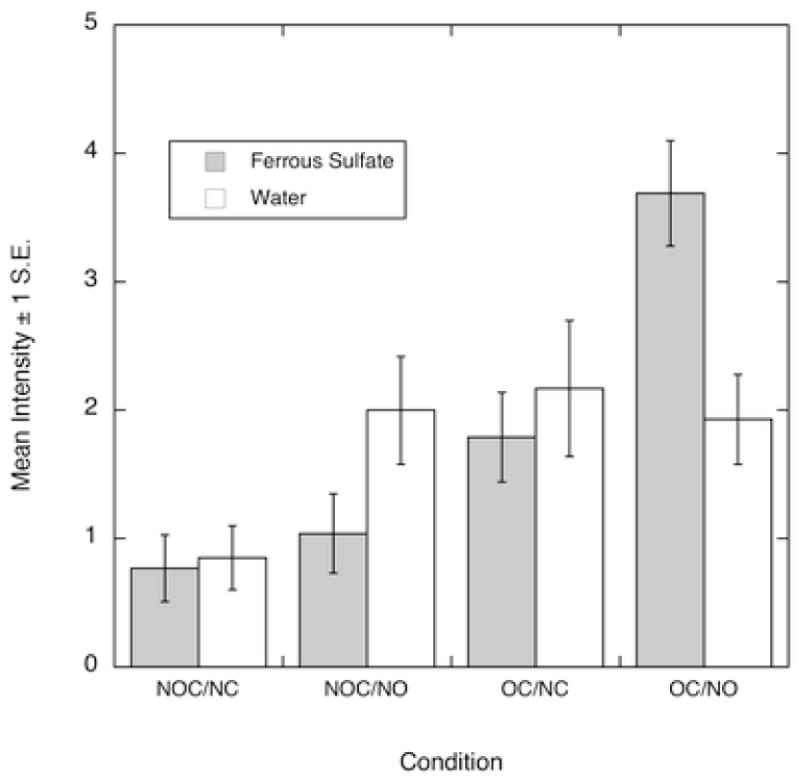
Mean rated intensity for water and ferrous sulfate stimuli in Experiment 2. Conditions: NOC/NC, no oral contact, nose closed; NOC/OC, no oral contact, nose open; OC/NC oral contact, nose closed; OC/NO, oral contact, nose open.
4. General Discussion
Nasal occlusion lowered the metallic taste and aftertaste ratings of the two iron concentrations but the copper metallic taste and aftertaste ratings were largely uninfluenced by nasal occlusion, at the concentrations used in this study. Figure 1 shows a larger magnitude of difference between the nose open and closed condition of the two iron concentrations for both metallic taste and aftertaste than that for copper. These results may provide insight into the time in the tasting process that the greatest degree of metallic flavor component is detected for each compound. For iron it appears to develop immediately after the stimulus is introduced to the oral cavity and iron exhibits a pronounced reduction in metallic flavor with nasal occlusion. For copper, the metallic sensation appears to develop after the stimulus has been in the mouth for a time and it shows a smaller nasal occlusion effect.
Lim and Lawless (2005) reported that solutions of ferrous sulfate had no smell when the headspace over the solutions was sniffed (orthonasal smell was absent) and suggested that the olfactory stimulus from tasted ferrous sulfate arises from rapidly generated lipid oxidation products in the mouth. If so, oral contact may be required for the retronasal component of iron to be developed, as implied by the results of Experiment 2. A parallel has recently been shown with metal to skin contact. Glindemann, Dietrich, Staerk and Kuschk (2006) found the metallic odor arising after iron contacts the skin is a result of the skin converting the iron metal to form reactive Fe2+ ions that are oxidized to Fe3+ ions while simultaneously reducing skin lipid peroxides to carbonyl compounds that are perceived as metallic odor. The current study is consistent with these findings for iron and also raises the possibility of more slowly generated lipid oxidation reaction for copper compounds. Iron and copper have long been known as lipid oxidation catalysts in milk, and copper sulfate added to milk is recommended to produce a reference standard for oxidized flavor (Bodyfelt et al., 1988).
The quantity of copper required to catalyze milkfat oxidation varies from sample to sample (Bodyfelt et al., 1988) and dairy scientists routinely speak in terms of the “susceptibility” of different milk samples to oxidative defects. One might expect some variability in human oral constituents to likewise lead to individual variability in metallic flavor perception. However, the attempt to classify persons in Experiment 2 in terms of their reactivity to metallic flavors did not show a consistent effect.
In conclusion, at the levels studied here, iron exhibited more metallic taste and a persistent metallic aftertaste, while copper exhibited a metallic taste and a more pronounced metallic aftertaste. Iron showed a larger reduction with nasal occlusion, and copper exhibited more bitter and astringent properties than iron. The results seen here using a descriptive analysis panel parallel those previously seen with untrained subjects in psychophysical studies (Lawless et al., 2004). Oral contact appears necessary for the perception of metallic flavor following rinses with ferrous sulfate. Note that some metallic sensations persisted after nasal occlusion, leaving open the possibility that there are other oral sensations giving rise to metallic perception and these may be either gustatory or trigeminal or possibly some combination.
Acknowledgments
Supported by NIH DC006223 to HTL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajdukovic D. The relationship between electrode areas and sensory qualities in electrical human tongue stimulation. Acta Oto-Laryngolologica. 1984;98:153–157. doi: 10.3109/00016488409107548. [DOI] [PubMed] [Google Scholar]
- Ajdukovic D. Electrical taste stimulus: Current intensity or current density. Chemical Senses. 1990;15:341– 347. [Google Scholar]
- Bartoshuk LM. History of taste research. In: Carterette EC, Friedman MP, editors. Handbook of Perception. IVA, Tasting and Smelling. Academic Press; New York: 1978. pp. 2–18. [Google Scholar]
- Bodyfelt FW, Tobias J, Trout GM. The sensory evaluation of dairy products. New York: Van Nostrand Reinhold; 1988. [Google Scholar]
- Bujas Z. Electrical Taste. In: Beidler L, editor. Handbook of Sensory Physiology, Vol. 4 Chemical Senses, Part 2: Taste. Springer-Verlag; Berlin: 1971. pp. 180–199. [Google Scholar]
- Cuppett JD, Duncan SE, Dietrich AM. Evaluation of Copper Speciation and Water Quality Factors That Affect Aqueous Copper Tasting Response. Chemical Senses. 2006;31(7):689–697. doi: 10.1093/chemse/bjl010. [DOI] [PubMed] [Google Scholar]
- Epke EM, Lawless HT. Retronasal smell and detection thresholds of iron, copper and sodium salts. Physiology and Behavior. 2007;92:487– 491. doi: 10.1016/j.physbeh.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glindermann D, Dietrich A, Staerk H, Kuschk P. The Two Odors of Iron when Touched or Pickled: (Skin) Carbonyl Compounds and Organophosphines. Angewandte Chemie International Edition. 2006;45:7006–7009. doi: 10.1002/anie.200602100. [DOI] [PubMed] [Google Scholar]
- Guth H, Grosch W. Comparison of stored soya-bean and rapeseed oils by aroma extract dilution analysis. Lebensmittel Wissenschaft und Technologie. 1990;23:59–65. [Google Scholar]
- Heiler C, Schieberle P. Model studies on the precursors and formation of metallic smelling (E,Z)-2,6-Nonadienol during the manufacture and storage of buttermilk. International Dairy Journal. 1997;7:667–674. [Google Scholar]
- Hettinger TP, Myers WE, Frank ME. Role of olfaction in perception of non-traditional ‘taste’ stimuli. Chemical Senses. 1990;15(6):755–760. [Google Scholar]
- Lawless HT, Schlake S, Smythe J, Lim J, Yang H, Chapman K, Bolton B. Metallic Taste and Retronasal Smell. Chemical Senses. 2004;29(1):25–33. doi: 10.1093/chemse/bjh003. [DOI] [PubMed] [Google Scholar]
- Lawless HT, Stevens DA, Chapman KW, Kurtz A. Metallic Taste from Electrical and Chemical Stimulation. Chemical Senses. 2005;30(3):185–194. doi: 10.1093/chemse/bji014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Lawless HT. Oral sensations from iron and copper sulfate. Physiology & Behavior. 2005;85(3):308–313. doi: 10.1016/j.physbeh.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Lubran MB, Lawless HT, Lavin E, Acree TE. Identification of Metallic-Smelling 1-Octen-3-one and 1-Nonen-3-one from Solutions of Ferrous Sulfate. Journal of Agricultural and Food Chemistry. 2005;53(21):8325–8327. doi: 10.1021/jf0511594. [DOI] [PubMed] [Google Scholar]
- Meilgaard M, Civille GV, Carr BT. Sensory evaluation techniques. Boca Raton, Fla: CRC Press; 1999. [Google Scholar]
- Murphy C, Cain WS. Taste and olfaction: Independence vs. interaction. Physiology and Behavior. 1980;24:601– 605. doi: 10.1016/0031-9384(80)90257-7. [DOI] [PubMed] [Google Scholar]
- Pierce J, Halpern BP. Orthonasal and retronasal odorant identification based upon vapor phase input from common substances. Chemical Senses. 1996;21:529– 543. doi: 10.1093/chemse/21.5.529. [DOI] [PubMed] [Google Scholar]
- Swoboda PAT, Peers KE. Metallic odor caused by vinyl ketones formed in the oxidation of butterfat. The identification of octa-1, cis-5-dien-3-one. Journal of the Science of Food and Agriculture. 1977;28:1019–1024. [Google Scholar]
- Tomita H, Ikeda M, Okuda Y. Basis and practice of clinical taste examinations. Auris Nasus Larynx (Tokyo) 1986;13(suppl):S1– S15. doi: 10.1016/s0385-8146(86)80029-3. [DOI] [PubMed] [Google Scholar]
- Tomiyama H, Tomita H, Okuda Y. Normal value of the electric taste. Japanese Journal of Otology (Tokyo) 1971;74:58–65. [Google Scholar]
- Wong DWS. Mechanism and theory in food chemistry. New York, NY: Van Nostrand Reinhodl; 1989. [Google Scholar]
- Yang HH, Lawless HT. Descriptive Analysis of Divalent Salts. Journal of Sensory Studies. 2005;20(2):97–113. doi: 10.1111/j.1745-459X.2005.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacarias I, Yanez CG, Araya M, Oraka C, Olivares M, Uauy R. Determination of the taste threshold of copper in water. Chemical Senses. 2001;26(1):85–89. doi: 10.1093/chemse/26.1.85. [DOI] [PubMed] [Google Scholar]


