Abstract
Background
Recently, the number of reports on focal adhesion kinase (FAK) as a vital therapeutic target in solid carcinomas has increased; however, the prognostic role of FAK status remains poorly understood. This study aims to evaluate the prognostic effect of FAK by means of a meta-analysis.
Methods
We performed a systematic literature search in order to examine the correlation between expression of FAK and overall survival(OS). The hazard ratio (HR) of OS was used to measure survival. A random-effects model was used to pool study statistics. Sensitivity and publication bias analyses were also conducted.
Results
Thirty eligible studies involving 4702 patients were included. The median expression rate of FAK was 54%. Meta-analysis of the HRs demonstrated that high FAK expression was associated with worse OS (average HR = 2.073, 95%confidence interval[CI]:1.712–2.510, p = 0.000). Regarding cancer type, FAK was associated with worse OS in gastric cancer (HR = 2.646,95% CI:1.743–4.017, p = 0.000), hepatocellular carcinoma (HR = 1.788,95% CI:1.228–2.602, p = 0.002), ovarian cancer (HR = 1.815, 95% CI: 1.193–2.762, p = 0.005), endometrial cancer (HR = 4.149, 95% CI:2.832–6.079, p = 0.000), gliomas (HR = 2.650, 95% CI: 1.205–5.829, p = 0.015), and squamous cell carcinoma (HR = 1,696, 95% CI: 1.030–2.793, p = 0.038). No association was found between HR and disease staging according to our meta-regression analysis.
Conclusions
Our study shows that high expression of FAK is associated with a worse OS in patients with carcinomas, but the association between FAK and prognosis varies according to cancer type. The value of FAK status in clinical prognosis in cancer needs further research.
Introduction
Prognostic studies of cancer biomarkers are valuable, and allow a more accurate prediction of treatment response and prognosis, ultimately leading to a favorable therapeutic outcome. Focal adhesion kinase (FAK), an intracellular tyrosine kinase recruited to sites of integrin clustering or focal adhesions, is a multi-functional regulator of cell signaling within the tumor microenvironment [1–3]. FAK functions as a major mediator of signal transduction by cell surface receptors including integrins, growth factor, and cytokine receptors [1]. Therefore, FAK plays a crucial role in tumor carcinogenesis, especially in cell proliferation, apoptosis inhibition, angiogenesis, invasiveness, immunosuppression, and cell motility [4]. Dysregulation of FAK leads to the development of malignancies, including initiation of invasion, metastasis and neoangiogenesis [5–7]. These functional characteristics suggest that FAK may be also involved in promoting tumorigenesis and metastasis.
Recently, FAK has been proposed as a new candidate for molecular-based therapeutic approaches. However, the prognostic value of FAK overexpression across human solid carcinomas has yet to achieve a recognized consensus. Controversial results have been reported among the different types of cancer. High FAK expression in cancer samples has been evident in hepatocellular carcinoma [8], invasive breast carcinoma [9], gastric carcinoma [10], endometrial cancer [11], and ovarian carcinoma [12]. It has been demonstrated that the overexpression of FAK in these carcinomas is associated with a worse outcome. On the other hand, there are conflicting data demonstrating no prognostic value of FAK expression in node-negative breast cancer, colon carcinoma, and resectable pancreatic cancer[13–15]. In addition, overexpressed FAK was linked with poorer survival rates in esophageal and head and neck squamous cell carcinoma patients, although no statistical significance was established[16,17]. Thus, a systematic and comprehensive meta-analysis designed to explore the association of FAK overexpression with cancer prognosis is urgently required and will provide a useful reference for doctors and researchers working in this field.
Materials and Methods
Literature search
A search of Pubmed and Web of Science was performed for studies evaluating the expression of FAK and survival in tumors from January 1995 to April 2016. The following keywords were used in the search strategy: (FAK OR “focal adhesi* kinase”) AND (Carcinoma* OR neoplasm* OR cancer OR tumor) AND (prognosis OR prognostic OR survival). The results were limited to English language studies. Manual searches of reference articles from applicable studies were performed to identify articles that may have been missed by the computer-assisted search.
Study selection
Two investigators (Zeng and Li) reviewed all citation titles identified by the search strategy to generate a list of potentially relevant articles. The abstract of each study was then reviewed individually by the two investigators. If the applicability of a study could not be determined through the title or abstract alone, the full text was reviewed. The articles were independently screened for possible eligibility and any disagreements were resolved by conferring with a third investigator (Chen).
Study inclusion and exclusion criteria
The meta-analysis included studies that met the following standards: i) all patients were diagnosed with cancer via histopathology; ii) reported FAK expression level in patients and their prognoses; iii) original study; iv)inclusion of the most complete and newest study if duplicate articles were published; v) reported explicit methods for the detection of FAK expression in solid cancers; vi) FAK data presented in dichotomy; high or low. The exclusion criteria were as follows: i)studies investigating the relationship between co-expression of FAK and other factors and prognosis; ii) studies with no hazard ratios (HR) or 95% confidence intervals (CI), or data failed to provide a Kaplan-Meier(K-M) curve for HR and CI calculations; iii) abstracts were excluded due to insufficient data to evaluate the methodological quality of the trial and/or to carry out a meta-analysis; iv) non-eligible trials included ecological studies, case reports, reviews, editorials, and animal trials. If a study reported results from more than one method (i.e., immunohisto-chemistry [IHC], polymerase chain reaction[PCR] and fluorescence in situ hybridization[FISH]), for more than one well described patient group or with multiple cut off values, results of all analyses were included in the meta-analysis. This study was conducted in accordance with the PRISMA guidelines [18].
Data extraction
The following characteristics were extracted: name of the first author, publication year, country, sample size, age, sex, test method, cut-off value, tumor staging, follow up time, and HR estimation. If a study reported both the results of univariate and multivariate analysis, the latter was selected as it takes confounding factors into account. The primary outcome measure was overall survival (OS).
Quality assessment
For study methodological evaluation, three investigators (Zeng, Li, and Chen) read through each publication independently. There is no widely accepted standard for evaluating study quality, thus study quality was assessed and scored according to the REMARK guidelines and quality scale predefined form by De Graeff [19,20], which was adapted from McShane et al. (2005) and Hayes et al. (1996). Studies with a total score of 8 were considered to show optimal study quality, whereas a score of 0 indicated poor study quality. The three readers provided independent quality scores for comparison, then a mutual consensus was reached for each item.
Statistical analysis
The expression of FAK was judged “high” or “low” according to the cut-off value used in each study. The association between FAK and clinical outcomes was evaluated using the HR of low FAK expression level patients over high FAK level patients.
When described in original articles, HR values were obtained directly. If the data were not provided directly, the available data from K-M survival curves were interpreted through Getdata. Three independent authors read the curves to minimize reading variability. HR and 95% CI were calculated using the methods reported by Parmar [21].
Estimates of HRs were weighted and pooled using the Mantel-Haenszel random-effects model. The heterogeneity of results between studies was assessed using I2 statistics, with increasing heterogeneity implying less utility in generalization across studies. The χ2-test P-value<0.10 or I2 values >50% were suggestive of substantial heterogeneity. A sensitivity analysis was conducted to evaluate sources of heterogeneity both in the overall pooled estimate as well as within subgroups. Subgroup analysis was investigated with respect to cancer, ethnicity, assay method, HR estimate, type of tumor, sample size, and study quality score. Meta-regression analysis was conducted in an attempt to establish the relationship between HR and disease stage. To test the robustness of the HR estimates, a sensitivity analysis was conducted by individually excluding studies and analyzing the effects on the remaining studies. In addition, the presence of publication bias which was assessed using the Begg’s test. All analyses were carried out using Stata 12.0. All P-values were two-sided and the significance level was defined as 0.05.
Results
Literature search
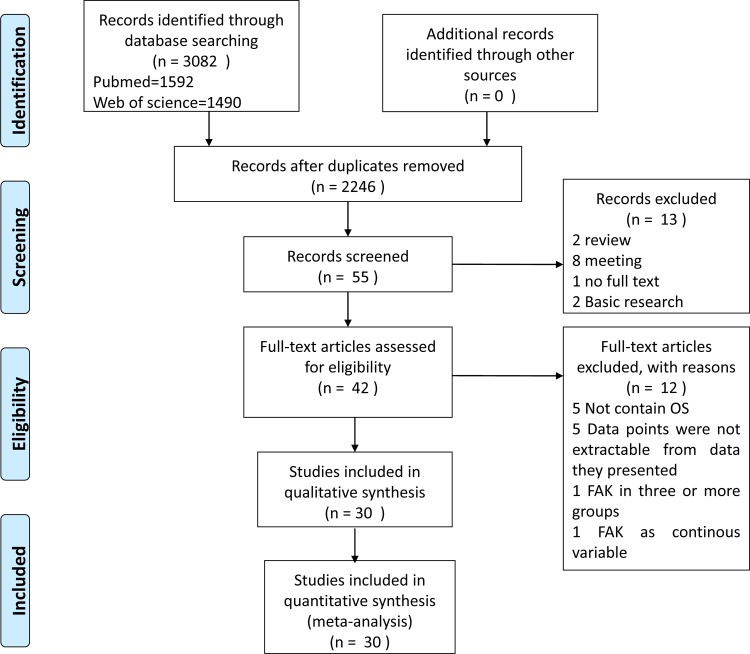
The computer-assisted search yielded 2,246 unique published titles. After an initial review, 55 titles were considered to be potentially appropriate. The abstracts of each of these papers was reviewed, after which 13 studies were excluded. The full text was not available for one article [22]. The full text of the remaining 42 citations was then carefully read, and twelve articles were subsequently excluded. Of the excluded articles, five employed an alternative survival endpoint instead of OS [23–27], five had no data available[8,13–14,28–30], one divided the FAK level into three or more groups [30], and one article treated FAK scores as a continuous variable[31]. A total of 30 studies were thus ultimately included in our meta-analysis (Fig 1) [10,15,16,32–58].
Fig 1. Selection of studies included in the analysis.
Characteristics of included studies
The principal characteristics of the selected studies are summarized in Table 1. From the 30 studies included, a total of 4702 patients were analyzed. Chen et al.[33] stratified the data into a training and validation set, presenting the two groups separately. The outcome data of two studies were presented independently according to the method of ascertaining FAK expression in the same patient population, and these datasets were analyzed separately. Of these, Park et al. [47] evaluated FAK protein expression in both the cytoplasm and the cell membrane, and the datasets were interpreted individually. Three studies presented FAK expression distinctly according FAK status and these datasets were treated separately [32,41,43]. Specifically, Giaginis et al. [10] stratified outcome data into two histological types of human gastric cancer, of which the subgroup of diffuse-type carcinomas was excluded since the relevant effect estimates could not be obtained.
Table 1. Characteristics of studies reporting FAK expression and outcomes.
| Ref | Type of cancer | country | Age, median(range) | Male/fe-male | advanced stage(%) | FAK+ tumor(%) | HR(95%CI) |
|---|---|---|---|---|---|---|---|
| Albasri 2014 | CRC | U.K | 72(45–80) | 257/192 | 43.3% | 44.0% | 1.129(0.763–2.12) |
| Dy 2014 | NSCLC | USA | 68(46–86) | 75/82 | 0% | 56.7% | 0.79(0.53–1.16) * |
| Zhang 2014 | GC | China | 64(30–91) | 428/173 | 56.4% | 54.1% | 2.463(1.119–5.405) |
| Gao 2014 | HCC | China | NR | 146/14 | NR | 51.3% | 1.613(1.035–2.514) |
| Chen 2013(training) | GC | China | NR | 61/21 | 67.1% | 52.4% | 7.76(3.76–16.0) |
| Chen 2013(validation) | GC | China | NR | 296/89 | 51.7% | 64.7% | 1.70(1.23–2.34) |
| Ji 2013 | NSCLC | China | 58.1#(31–78) | 97/56 | 42.6% | 38.6% | 2.22 (1.43–3.44) * |
| Zhou 2013(FAK) | Endometrial carcinoma | USA | 63.2#(24–91) | 0/202 | 42.6% | 46.0% | 3.79(2.01–7.13) * |
| Zhou 2013(pFAK) | Endometrial carcinoma | USA | 63.2#(24–91) | 0/202 | 42.6% | 64.9% | 4.37(2.71–7.06) * |
| Garouniatis 2013 | CRC | Greece | 69#(36–90) | 96/87 | 38.8% | 31.7% | 2.517(1.468–4.315) |
| Kim 2012 | CRC | Korea | 62.2#(28–84) | 136/84 | 48.0% | 61.1% | 1.382(0.813–2.349) |
| Qayyum 2012 | Renal cancer | U.K | 60(41–80) | 32/25 | 36.8% | 36.8% | 3.35(1.40–7.98) |
| Theocharis 2012 | Tonque SCC | Greece | 60(33–94) | 25/23 | NR | 50% | 2.431(0.747–6.698) |
| Fan 2011 | Ovarian cancer | China | NR | 0/60 | 100% | 73.3% | 3.922(1.126–13.664) |
| Yom 2011(FISH) | Breast cancer | Korea | NR | 0/240 | 36.3% | 13.8% | 5.393(2.185–13.308) |
| Yom 2011(IHC) | Breast cancer | Korea | NR | 0/240 | 36.3% | 29.2% | 0.964(0.383–2.425) |
| Park 2010(IHC cyto) | GC | Korea | NR | 322/122 | 36.5% | 84.2% | 4.57(2.20–9.48) |
| Park 2010(IHC mem) | GC | Korea | NR | 322/122 | 36.5% | 80.0% | 3.17(1.73–5.80) |
| Park 2010(FISH) | GC | Korea | NR | 281/103 | 32.8% | 8.9% | 1.71(1.32–2.23) |
| Yuan 2010 | HCC | China | 48.5#(13–72) | 47/3 | 54.0% | 50% | 1.3(0.13–12.61) * |
| Chatzizacharias 2010 | PDAC | Greece | NR | NR | 27.7% | 35.4% | 0.97(0.59–1.59) * |
| Ding 2010(FAK) | Gliomas | China | NR | 61/35 | 81.3% | 89.6% | 3.25(0.8–13.15) * |
| Ding 2010(pFAK) | Gliomas | China | NR | 61/35 | 81.3% | 50.0% | 2.41(0.93–6.27) * |
| Hayashi 2010 | EBD carcinoma | Japan | 66.1#(NR) | 54/22 | 18.4% | 77.6% | 2.239(0.894–5.603) |
| Giaginis 2009 | GC | Greece | NR | 22/8 | 63.3% | 56.7% | 0.93(0.2–4.32) * |
| Wang 2009 | Lung adnocarcinoma | China | 60.1#(NR) | 42/35 | 34.3% | 87.8% | 3.28(1.335–8.064) |
| Sun 2007 | HCC | China | NR | 78/94 | NR | 57.1% | 2.42(0.59–9.87) |
| Furuyama 2006 | Pancreatic cancer | Japan | 64.3#(45–79) | 31/19 | 46% | 48.0% | 0.69 (0.18–7.28) * |
| Ohta 2006 | ICC | Japan | 60.3#(33–75) | 38/18 | 83.9% | 28.6% | 1.62(0.47–5.52) |
| Sood 2004 | Ovarian cancer | USA | 59.3#(34–81) | 0/79 | 81.0% | 68.4% | 1.93 (0.51–7.28) * |
| Itoh 2004 | HCC | Japan | NR | 47/17 | 23.4% | 28.1% | 3.05(1.16–7.99) |
| Miyazaki 2003 | ESCC | Japan | 61#(NR) | 77/14 | 42.9% | 59.3% | 1.04 (0.17–6.19) * |
| Jan 2009 | HCC | Taiwan | 60(34–80) | 39/16 | 42% | 61.8% | 1.02 (0.14–7.37) * |
| Li 2012 | Laryngeal SCC | China | 51(37–84) | 81/5 | 73.3% | 73.3% | 1.611(0.84–2.73) |
| Aust 2014(FAK) | Ovarian cancer | Austria | 57.6# | 0/179 | 96% | 92.2% | 1.10(0.45–2.70) |
| Aust 2014(pFAK) | Ovarian cancer | Austria | 57.6# | 0/179 | 96% | 36.9% | 1.85(1.04–3.23) |
| Li 2015 | Ovarian cancer | China | 50.5#(NR) | 0/50 | 83.9% | 72.0% | 2.52(0.12–52.68)* |
CRC, colorectal cancer; NSCLC, non-small-cell lung cancer; GC, gastric cancer; HCC, hepatocellular carcinoma; OSCCs, oral squamous cell carcinomas; SCC, squamous cell carcinoma;SCLC, small-cell lung carcinoma; EBD, extrahepatic bile duct; ICC, intrahepatic cholangiocarcinoma; ESCC, oesophageal squamous cell carcinoma; PDAC, pancreatic ductal adenocarcinoma; cyto: cytoplasmic; mem: membranous; NR, not report; HR, hazard ratio; CI, confidence interval
# mean
*survival curve.
All of the studies were retrospective in design. Sample sizes ranged from 30 to 601 (median, 96). Eighteen of the 30 studies included patients with both early and advanced disease (stage I–IV) [10,15,16,33,34–38,44,46,47,51–54,56–58]. However, none of the studies analyzed the OS according to stage. Five studies included patients with stage I–III disease [32,40,42,43,48,55], two studies included patients with stage II–IV disease [41,43], and one study each included patients with stage I disease [45] and stage III–IV disease [49]. Stages of cancer were not reported in three studies [34,39,50]. The mean percentage of patients with advanced stages of disease was 52.0% (range, 0–100%). Five studies evaluated hepatocellular carcinoma [34,35,39,46,56], Four studies each evaluated gastric cancer [10,33,37,47] and ovarian cancer [36,41,49,58], three studies each evaluated lung cancer [40,45,55], and colorectal cancer[52–54], two studies each evaluated pancreatic cancer [15,44], and cholangiocarcinoma [38,42], and one study each evaluated breast cancer [48], gliomas [49], endometrial carcinoma [32], esophageal squamous cell carcinoma [16], renal cancer [51], tongue squamous cell carcinoma [50], and laryngeal squamous cell carcinoma [57]. Twenty studies were performed in Asian populations, while the remaining 10 studies were conducted in Western populations.
Follow-up time was 42 months (range, 0.1–192.2 months). Eighteen studies had readily available HR and 95% CI data, while the remaining 12 studies presented neither HR nor 95% CI, which were consequently estimated from the available K-M curves. The 30 included studies had a mean quality score of 3 (range, from 1–6).
Evaluation and expression of FAK
The rate of high FAK status varied from 8.9–92.2% (median, 54%) (Table 1). The rate of high FAK expression was 57% (range, 28.1–92.2%) in studies using IHC and 14% (range, 8.9–51.3%) in studies using other methods. A description of the antibodies and cut off values of overexpression used is given in S1 Table. Various antibodies were used for the evaluation of FAK expression. Among the 34 datasets that employed IHC, the FAK expression level was evaluated according to the intensity of staining, percentage of stained cells and method applied. Marked heterogeneity was observed in studies evaluating FAK expression by IHC between thresholds used to dichotomize FAK status. Among the three datasets which assigned FAK expression at the gene level, FISH was used in two datasets[47,48], and PCR was used in the remaining one [34].
Association of FAK with survival
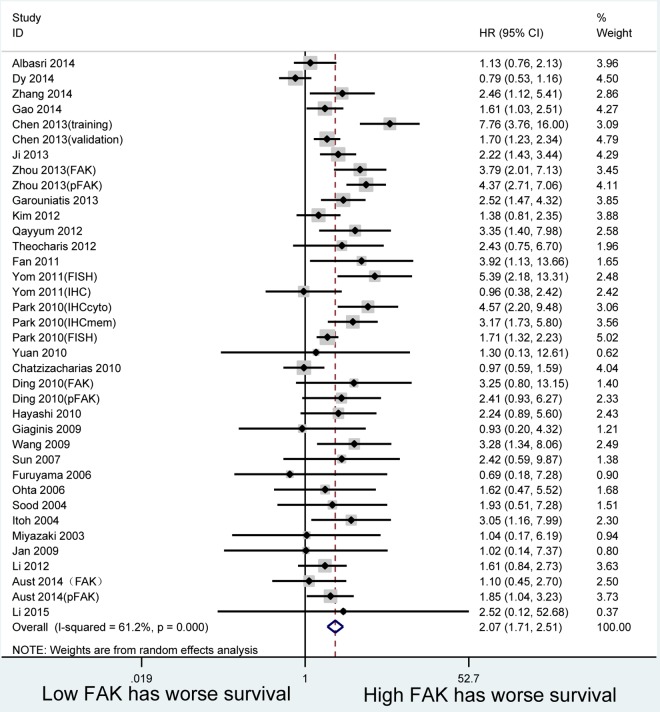
The combined HR for the 30 studies included in the analysis was 2.073 (95% CI:1.712–2.510, p = 0.000), indicating that FAK overexpression is associated with worse survival among patients. However, a significant inter-study heterogeneity effect model (I2 = 61.2%, p = 0.000) was indicated for the prognostic effect (Fig 2).
Fig 2. Forest plots.
Hazard ratios for each study are represented by the squares, the size of square represents the weight of the study in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval(CI). All statistical tests were two-sided.
To explore the study heterogeneity, we performed stratified analyses across a number of key study characteristics and clinical factors (Table 2). In the subgroup analysis based on tumor types, the negative prognostic role of high FAK expression was observed in gastric cancer (HR = 2.646, 95% CI:1.743–4.017, p = 0.000), hepatocellular carcinoma (HR = 1.788, 95% CI:1.228–2.602, p = 0.002), ovarian cancer (HR = 1.815, 95% CI: 1.193–2.762, p = 0.005), endometrial cancer (HR = 4.149, 95% CI: 2.832–6.079, p = 0.000), gliomas (HR = 2.650, 95% CI:1.205–5.829, p = 0.015), and SCC (HR = 1.696, 95% CI:1.030–2.793, p = 0.038). However, there was no association between FAK overexpression and survival in colorectal cancer (HR = 1.569, 95% CI:0.981–2.509, p = 0.060), lung cancer (HR = 1.694, 95% CI: 0.719–3.993, p = 0.228), breast cancer (HR = 2.286, 95% CI: 0.423–12.357, p = 0.337), bile duct cancer (HR = 1.995, 95% CI: 0.956–4.164, p = 0.066), and pancreatic cancer (HR = 0.948, 95% CI: 0.587–1.530, p = 0.827). For subgroup analyses based on method, ethnicity, HR estimate method, sample size, and FAK status quality score, all results suggested that FAK overexpression had a poor impact on survival.
Table 2. Subgroup analysis of main outcome in vary cancer types.
| subgroup | Datasets | pts | HR | 95%CI | P for subgroup difference | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2 | P value | ||||||
| Overall survival | 37 | 6247 | 2.073 | 1.712–2.510 | 61.2% | 0.000 | |
| Ethnicity | |||||||
| Asian | 25 | 4404 | 2.221 | 1.823–2.707 | 0.000 | 39.9% | 0.022 |
| Non-Asian | 12 | 1843 | 1.803 | 1.207–2.694 | 0.004 | 77.8% | 0.000 |
| Method | |||||||
| protein | 34 | 5463 | 2.069 | 1.668–2.566 | 0.000 | 61.8% | |
| Gene | 3 | 784 | 2.065 | 1.281–3.326 | 0.003 | 66.9% | 0.049 |
| HR estimate | |||||||
| Direct | 23 | 4871 | 2.174 | 1.782–2.654 | 0.000 | 51.7% | 0.002 |
| Indirect | 14 | 1376 | 1.760 | 1.127–2.749 | 0.013 | 71.5% | 0.000 |
| Tumor type | |||||||
| Gastric | 7 | 2370 | 2.646 | 1.743–4.017 | 0.000 | 74.9% | 0.001 |
| Liver | 5 | 378 | 1.788 | 1.228–2.602 | 0.002 | 0.0% | 0.746 |
| Ovarian | 5 | 547 | 1.815 | 1.193–2.762 | 0.005 | 0.0% | 0.605 |
| Endometrium | 2 | 404 | 4.149 | 2.832–6.079 | 0.000 | 0.0% | 0.725 |
| Gliomas | 2 | 192 | 2.650 | 1.205–5.829 | 0.015 | 0.0% | 0.729 |
| SCC | 3 | 225 | 1.696 | 1.030–2.793 | 0.038 | 0.0% | 0.695 |
| Sample size | |||||||
| >100 | 18 | 4790 | 2.042 | 1.606–2.598 | 0.000 | 72.9% | 0.000 |
| ≤100 | 19 | 1457 | 2.137 | 1.540–2.964 | 0.000 | 39.3% | 0.041 |
| FAK status | |||||||
| FAK | 30 | 5126 | 2.074 | 1.685–2.553 | 0.000 | 56.6% | |
| pFAK | 7 | 1121 | 2.108 | 1.262–3.521 | 0.004 | 76.8% | 0.000 |
| Quality score | |||||||
| >3 | 12 | 1747 | 1.947 | 1.398–2.710 | 0.000 | 47.0% | 0.031 |
| ≤3 | 25 | 4500 | 2.136 | 1.680–2.717 | 0.000 | 67.1% | 0.000 |
Pts, patients;SCC, squamous cell carcinomas.
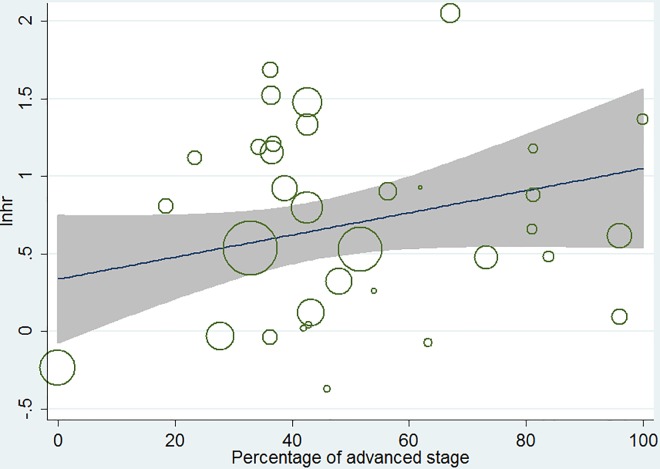
A meta-regression analysis demonstrated that there was no relationship between HR and the disease stage of patients (p = 0.69, Fig 3).
Fig 3. Results of meta-regression.
Ln(HR)-ln(percentage of advanced stage).
Sensitivity analyses
A sensitivity analysis was conducted to test the robustness of the HR estimates by removing studies individually and analyzing the effects on the remaining studies. The result showed that no individual study lay outside the 95% CI of the overall HR estimate.
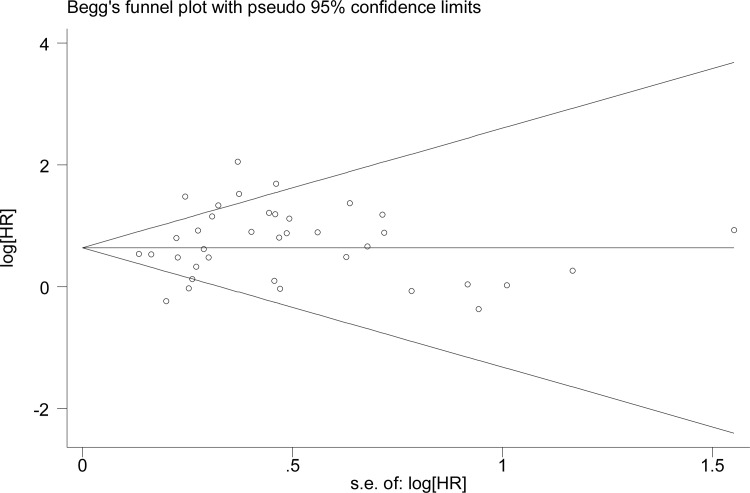
Publication bias
In terms of publication bias estimation, we observed symmetry according to the Begg’s test in all analyses (p = 1.000) (Fig 4), indicating no evidence of small study effects.
Fig 4. Begg’s funnel plot of FAK expression and overall survival (OS) in patients with solid tumor.
Discussion
FAK is a non-receptor tyrosine kinase that localizes to contact sites in focal adhesions, and holds a key position in the signal transduction network between cells and the extracellular matrix [1–3,59]. Over the years, numerous studies have shown a pivotal role of FAK in tumorigenesis and metastasis. In the present study, we observed that FAK expression was elevated in a broad range of somatic cancers, including astrocytic, breast, cervical, colorectal, endometrial, esophageal, gastric, head and neck, hepatocellular, laryngeal, lung, ovarian, pancreatic, prostate, lung, brain, skin, and thyroid cancers [8,15,16,30,60–70]. Furthermore, FAK overexpression was associated with aggressive human cancers. FAK can promote cancer invasion and metastasis [71]. In addition, this study confirmed that FAK activation, as determined by phosphospecific antibody recognition of the FAK tyrosine autophosphorylation site, increased with tumor progression. FAK is phosphorylated in response to clustering of integrins, cell spreading, or formation of focal adhesions [1]. At least six tyrosine residues (Y397, Y407, Y576, Y577, Y861, and Y925) have been identified as phosphorylation sites. IHC staining of activated (phosphorylated) receptors may be more informative than immunostaining of single markers regardless of their activation status. These above results suggest that high expression of both FAK and phosphorylation status may be a target for cancer therapeutics and may have an impact on survival.
A number of studies have shown that high FAK expression and activity are associated with not only malignancy [72,73], but also with poor prognosis [47]. FAK overexpression was positively correlated with lymph node and distal metastasis, as well as with a significant reduction in patient OS[12,47,48,74]. In this study, the primary results confirm that FAK expression is associated with poor prognosis based on pooled HR estimates. FAK was associated with worse OS in gastric cancer, hepatocellular carcinoma, ovarian cancer, endometrial cancer, gliomas, and squamous cell carcinoma. Interestingly, we also found overexpression of FAK was not associated with worse outcome in some types of solid cancers. This discrepancy may be partly explained by the small sample size in the individual studies. Although Begg’s test showed no evidence of publication bias and while sensitivity analyses further supported the robustness of the meta-analysis findings, it is essential for larger studies to enroll patients with specific tumor types in future studies. Besides FAK expression, its activation plays a critical role in tumor progression and prognosis. In some types of cancers, such as colorectal cancer, although the total expression of FAK was reported not to be associated with survival [14], the phosphorylation status was demonstrated to have an impact. In human colorectal cancer, nuclear expression of phosphorylated FAK is associated with poor prognosis. Albasri et al. [54] reported positive nuclear P-FAK expression was associated with shorter disease-specific survival in univariate (p = 0.005) and multivariate analysis (p = 0.016). In breast cancer, it was reported that increased FAK activity frequently correlates with metastatic disease and poor prognosis [75]. Differences in technique, IHC staining antibody and cut off values for positive protein expression may also have accounted for the observed heterogeneity. Overall, these results suggest that FAK may be an important marker for poor prognosis in a group of solid tumor patients.
The meta-regression analysis revealed no association between the overall HR and the percentage of advanced stage patients. This result supported that the prognostic value of FAK was independent from disease stage in solid tumors.
There are some limitations to this meta-analysis. First, the quality of individual studies was not always optimal. Second, the approach of extracting HRs from K-M curves could be a potential source of heterogeneity. Conversion of K-M curves could misestimate the variance of HRs, although the subgroup analysis did not indicate any major deviation. Thirdly, after cancer types were stratified, the sample size included in the meta-analysis was relatively small. The Gene Expression Omnibus(GEO) database includes a massive amount of gene chip data that profiles gene expression in many tumor types. The inclusion of GEO data would likely result in a more extensive data source and more realistic results. In the future, we will conduct a new study focusing on the GEO database.
In conclusion, through combining different study results, our meta-analysis provides evidence that FAK is associated with worse OS in diverse solid tumor types. In addition, high-quality studies should also be carried out to identify the potential role of FAK expression and phosphorylation status in solid tumors for clinical prognosis and treatment decision making.
Supporting Information
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Natural Science Foundation of Shanghai (Grant no. 14ZR1406600). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003; 116: 1409–1416. 10.1242/jcs.00373 . [DOI] [PubMed] [Google Scholar]
- 2.Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010; 123: 1007–1013. 10.1242/jcs.045112 . [DOI] [PubMed] [Google Scholar]
- 3.Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev.2009; 28: 35–49. 10.1007/s10555-008-9165-4 . [DOI] [PubMed] [Google Scholar]
- 4.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer.2014; 14: 598–610. 10.1038/nrc3792 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frisch SM, Schaller M, Cieply B. Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis. J Cell Sci. 2013; 126: 21–29. 10.1242/jcs.120907 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cance WG, Golubovskaya VM. Focal adhesion kinase versus p53: apoptosis or survival? Sci Signal. 2008; 1: e22 10.1126/stke.120pe22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilic D, Kovacic B, McDonagh S, Jin F, Baumbusch C, Gardner DG, et al. Focal adhesion kinase is required for blood vessel morphogenesis. Circ Res.2003; 92: 300–307. 10.1161/01.RES.0000055016.36679.23 . [DOI] [PubMed] [Google Scholar]
- 8.Fujii T, Koshikawa K, Nomoto S, Okochi O, Kaneko T, Inoue S, et al. Focal adhesion kinase is overexpressed in hepatocellular carcinoma and can be served as an independent prognostic factor. J Hepatol.2004; 41: 104–111. 10.1016/j.jhep.2004.03.029 . [DOI] [PubMed] [Google Scholar]
- 9.Lark AL, Livasy CA, Dressler L, Moore DT, Millikan RC, Geradis J, et al. High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype. Mod Pathol.2005; 18: 1289–1294. 10.1038/modpathol.3800424 . [DOI] [PubMed] [Google Scholar]
- 10.Giaginis CT, Vgenopoulou S, Tsourouflis GS, Politi EN, Kouraklis GP, Theocharis SE. Expression and clinical significance of focal adhesion kinase in the two distinct histological types, intestinal and diffuse, of human gastric adenocarcinoma. Pathol Oncol Res. 2009; 15: 173–181. 10.1007/s12253-008-9120-2 . [DOI] [PubMed] [Google Scholar]
- 11.Gabriel B, Hasenburg A, Waizenegger M, Orlowska-Volk M, Stickeler E, zue Hausen A. Expression of Focal Adhesion Kinase in Patients With Endometrial Cancer A Clinicopothologic Study. INTERNATIONAL JOURNAL OF GYNECOLOGICAL CANCER. 2009; 19: 1221–1225. 10.1111/IGC.0b013e3181b33c61 . [DOI] [PubMed] [Google Scholar]
- 12.Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM. Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am J Pathol. 2004; 165: 1087–1095. 10.1016/S0002-9440(10)63370-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz KJ, Grabellus F, Callies R, Otterbach F, Wohlschlaeger J, Levkau B. High expression of focal adhesion kinase (p125FAK) in node-negative breast cancer is related to overexpression of HER-2/neu and activated Akt kinase but does not predict outcome. Breast Cancer Res.2005; 7: R194–R203. 10.1186/bcr977 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theocharis SE, Kouraklis GP, Kakisis JD, Kanelli HG, Apostolakou FE, Karatzas GM, et al. Focal adhesion kinase expression is not a prognostic predictor in colon adenocarcinoma patients. Eur J Surg Oncol.2003; 29: 571–574. 10.1016/S0748-7983(03)00120-3 . [DOI] [PubMed] [Google Scholar]
- 15.Furuyama K, Doi R, Mori T, Toyoda E, Ito D, Kami K, et al. Clinical significance of focal adhesion kinase in resectable pancreatic cancer. World J Surg. 2006; 30: 219–226. 10.1007/s00268-005-0165-z . [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki T, Kato H, Nakajima M, Sohda M, Fukai Y, Masuda N, et al. FAK overexpression is correlated with tumour invasiveness and lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer. 2003; 89: 140–145. 10.1038/sj.bjc.6601050 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canel M, Secades P, Rodrigo JP, Cabanillas R, Herrero A, Suarez C, et al. Overexpression of focal adhesion kinase in head and neck squamous cell carcinoma is independent of fak gene copy number. Clin Cancer Res. 2006; 12: 3272–3279. 10.1158/1078-0432.CCR-05-1583 . [DOI] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med.2009; 151: W65–W94. . [DOI] [PubMed] [Google Scholar]
- 19.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst. 2005; 97: 1180–1184. 10.1093/jnci/dji237 . [DOI] [PubMed] [Google Scholar]
- 20.de Graeff P, Crijns AP, de Jong S, Boezen M, Post WJ, de Vries EG, et al. Modest effect of p53, EGFR and HER-2/neu on prognosis in epithelial ovarian cancer: a meta-analysis. Br J Cancer.2009; 101: 149–159. 10.1038/sj.bjc.6605112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med.1998; 17: 2815–2834. . [DOI] [PubMed] [Google Scholar]
- 22.Chen YJ, Lo KY, Tzen CY, Huang RD, Chang KH, Lai YL, et al. Relationship of bcl-2 and pp125(FAK) expression to outcome of radiation therapy in prostate carcinoma. JOURNAL OF TUMOR MARKER ONCOLOGY.1999; 14: 19–24. [Google Scholar]
- 23.Lai I, Chu P, Lin H, Liou J, Jan Y, Lee JC, et al. Phosphorylation of Focal Adhesion Kinase at Tyr397 in Gastric Carcinomas and its Clinical Significance. AMERICAN JOURNAL OF PATHOLOGY.2010; 177: 1629–1637. 10.2353/ajpath.2010.100172 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez Del Pulgar T, Cebrian A, Fernandez-Acenero MJ, Borrero-Palacios A, Del Puerto-Nevado L, Martinez-useros J, et al. Focal adhesion kinase: predictor of tumour reponse and risk factor for recurrence after neoadjuvant chemoradiation in rectal cancer. J Cell Mol Med. 2016; 20:1729–36. 10.1111/jcmm.12879 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skinner HD, Giri U, Yang L, Woo SH, Story MD, Pickering CR, et al. Proteomic Profiling Identifies PTK2/FAK as a Driver of Radioresistance in HPV-negative Head and Neck Cancer. Clin Cancer Res. 2016; [Epub ahead of print] 10.1158/1078-0432.CCR-15-2785 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B, Qi X, Li D, Feng M, Meng X, Fu S. Expression of pY397 FAK promotes the development of non-small cell lung cancer. oncol lett. 2016; 11: 979–983. 10.3892/ol.2015.3992 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omura G, Ando M, Saito Y, Kobayashi K, Yoshida M, Ebihara Y, et al. Association of the upregulated expression of focal adhesion kinase with poor prognosis and tumor dissemination in hypopharyngeal cancer. head Neck. 2016; 38:1164–9. 10.1002/hed.24176 . [DOI] [PubMed] [Google Scholar]
- 28.Carlos De Vicente J, Rosado P, Lequerica-Fernandez P, Allonca E, Villallain L, Hernandez-Vallejo G. Focal adhesion kinase overexpression: Correlation with lymph node metastasis and shorter survival in oral squamous cell carcinoma. HEAD AND NECK-JOURNAL FOR THE SCIENCES AND SPECIALTIES OF THE HEAD AND NECK.2013; 35: 826–830. 10.1002/hed.23038 . [DOI] [PubMed] [Google Scholar]
- 29.Sheen-Chen S, Huang C, Chan Y, Tsai C, Chi S, Wu S, et al. An Evaluation of Focal Adhesion Kinase in Breast Cancer by Tissue Microarrays. ANTICANCER RESEARCH.2013; 33: 1169–1173. . [PubMed] [Google Scholar]
- 30.Gabriel B, Hausen AZ, Stickeler E, Dietz C, Gitsch G, Fischer DC, et al. Weak expression of focal adhesion kinase (pp125FAK) in patients with cervical cancer is associated with poor disease outcome. CLINICAL CANCER RESEARCH. 2006; 12: 2476–2483. 10.1158/1078-0432.CCR-05-1867 . [DOI] [PubMed] [Google Scholar]
- 31.Ocak S, Chen H, Callison C, Gonzalez AL, Massion PP. Expression of focal adhesion kinase in small-cell lung carcinoma. CANCER. 2012; 118: 1293–1301. 10.1002/cncr.26382 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J, Roh J, Bandyopadhyay S, Chen Z, Munkarah AR, Hussein Y, et al. Overexpression of enhancer of zeste homolog 2 (EZH2) and focal adhesion kinase (FAK) in high grade endometrial carcinoma. GYNECOLOGIC ONCOLOGY. 2013; 128: 344–348. 10.1016/j.ygyno.2012.07.128 . [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Xia X, Wang S, Wu X, Zhang J, Zhou Y, et al. High FAK combined with low JWA expression: clinical prognostic and predictive role for adjuvant fluorouracil-leucovorin-oxaliplatin treatment in resectable gastric cancer patients. JOURNAL OF GASTROENTEROLOGY. 2013; 48: 1034–1044. 10.1007/s00535-012-0724-7 . [DOI] [PubMed] [Google Scholar]
- 34.Gao S, Lin B, Yang Z, Zheng Z, Liu Z, Wu L, et al. Role of overexpression of MACC1 and/or FAK in predicting prognosis of hepatocellular carcinoma after liver transplantation. INTERNATIONAL JOURNAL OF MEDICAL SCIENCES. 2014; 11: 268–275. 10.7150/ijms.7769 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itoh S, Maeda T, Shimada M, Aishima S, Shirabe K, Tanaka S, et al. Role of expression of focal adhesion kinase in progression of hepatocellular carcinoma. CLINICAL CANCER RESEARCH. 2004; 10: 2812–2817. 10.1158/1078-0432 [DOI] [PubMed] [Google Scholar]
- 36.Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM, et al. Biological significance of focal adhesion kinase in ovarian cancer—Role in migration and invasion. AMERICAN JOURNAL OF PATHOLOGY. 2004; 165: 1087–1095. 10.1016/S0002-9440(10)63370-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, Wang H, Ma Y, Zhang J, He X, Ma J, et al. Overexpression of Nedd9 is a prognostic marker of human gastric cancer. MEDICAL ONCOLOGY. 2014; 31 10.1007/s12032-014-0033-5 . [DOI] [PubMed] [Google Scholar]
- 38.Ohta R, Yamashita Y, Taketomi A, Kitagawa D, Kuroda Y, Itoh S, et al. Reduced expression of focal adhesion kinase in intrahepatic cholangiocarcinoma is associated with poor tumor differentiation. Oncology. 2006; 71: 417–422. 10.1159/000107109 . [DOI] [PubMed] [Google Scholar]
- 39.Sun CK, Ng KT, Sun BS, Ho JWY, Lee TK, Nq I, et al. The significance of proline-rich tyrosine kinase2 (Pyk2) on hepatocellular carcinoma progression and recurrence. BRITISH JOURNAL OF CANCER. 2007; 97: 50–57. 10.1038/sj.bjc.6603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Yang R, Yue D, Zhang Z. Expression of FAK and PTEN in Bronchioloalveolar Carcinoma and Lung Adenocarcinoma. LUNG. 2009; 187: 104–109. 10.1007/s00408-008-9130-6 . [DOI] [PubMed] [Google Scholar]
- 41.Aust S, Auer K, Bachmayr-Heyda A, Denkert C, Sehouli J, Braicu I, et al. Ambivalent role of pFAK-Y397 in serous ovarian cancer—a study of the OVCAD consortium. Mol Cancer. 2014; 13: 67 10.1186/1476-4598-13-67 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayashi A, Aishima S, Inoue T, Nakata K, Morimatsu K, Nagai E, et al. Decreased expression of focal adhesion kinase is associated with a poor prognosis in extrahepatic bile duct carcinoma. HUMAN PATHOLOGY. 2010; 41: 859–866. 10.1016/j.humpath.2009.09.018 . [DOI] [PubMed] [Google Scholar]
- 43.Ding L, Sun X, You Y, Liu N, Fu Z. Expression of focal adhesion kinase and phosphorylated focal adhesion kinase in human gliomas is associated with unfavorable overall survival. TRANSLATIONAL RESEARCH. 2010; 156: 45–52. 10.1016/j.trsl.2010.05.001 . [DOI] [PubMed] [Google Scholar]
- 44.Chatzizacharias NA, Giaginis C, Zizi-Serbetzoglou D, Kouraklis GP, Karatzas G, Theocharis SE. Evaluation of the Clinical Significance of Focal Adhesion Kinase and Src Expression in Human Pancreatic Ductal Adenocarcinoma. PANCREAS. 2010; 39: 930–936. 10.1097/MPA.0b013e3181d7abcc . [DOI] [PubMed] [Google Scholar]
- 45.Dy GK, Ylagan L, Pokharel S, Miller A, Brese E, Bshara W, et al. The Prognostic Significance of Focal Adhesion Kinase Expression in Stage I Non-Small-Cell Lung Cancer. JOURNAL OF THORACIC ONCOLOGY. 2014; 9: 1278–1284. 10.1097/JTO.0000000000000248 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan Z, Zheng Q, Fan J, Ai K, Chen J, Huang XY. Expression and prognostic significance of focal adhesion kinase in hepatocellular carcinoma. JOURNAL OF CANCER RESEARCH AND CLINICAL ONCOLOGY. 2010; 136: 1489–1496. 10.1007/s00432-010-0806-y . [DOI] [PubMed] [Google Scholar]
- 47.Park JH, Lee BL, Yoon J, Kim J, Kim MA, Yang HK, et al. Focal adhesion kinase (FAK) gene amplification and its clinical implications in gastric cancer. Hum Pathol. 2010; 41: 1664–1673. 10.1016/j.humpath.2010.06.004 . [DOI] [PubMed] [Google Scholar]
- 48.Yom CK, Noh DY, Kim WH, Kim HS. Clinical significance of high focal adhesion kinase gene copy number and overexpression in invasive breast cancer. Breast Cancer Res Treat. 2011; 128: 647–655. 10.1007/s10549-010-1150-2 . [DOI] [PubMed] [Google Scholar]
- 49.Fan D, Shi H. Pilot Study: Alteration of Deleted in Liver Cancer1 and Phosphorylated Focal Adhesion Kinase Y397 Cytoplasmic Expression and the Prognostic Value in Advanced Epithelial Ovarian Carcinoma. INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES. 2011; 12: 8489–8501. 10.3390/ijms12128489 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theocharis S, Klijanienko J, Giaginis C, Alexandrou P, Patsouris E, Sastre-Garau X. FAK and Src expression in mobile tongue squamous cell carcinoma: associations with clinicopathological parameters and patients survival. JOURNAL OF CANCER RESEARCH AND CLINICAL ONCOLOGY. 2012; 138: 1369–1377. 10.1007/s00432-012-1215-1 . [DOI] [PubMed] [Google Scholar]
- 51.Qayyum T, McArdle PA, Lamb GW, Jordan F, Orange C, Seywright M, et al. Expression and prognostic significance of Src family members in renal clear cell carcinoma. BRITISH JOURNAL OF CANCER.2012; 107: 856–863. 10.1038/bjc.2012.314 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim YN, Choi JE, Bae JS, Jang KY, Chung MJ, Moon WS, et al. Expression of Cortactin and Focal Adhesion Kinase in Colorectal Adenocarcinoma: Correlation with Clinicopathologic Parameters and Their Prognostic Implication. KOREAN JOURNAL OF PATHOLOGY. 2012; 46: 454–462. 10.4132/KoreanJPathol.2012.46.5.454 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garouniatis A, Zizi-Sermpetzoglou A, Rizos S, Kostakis A, Nikiteas N, Papavassiliou AG. FAK, CD44v6, c-Met and EGFR in colorectal cancer parameters: tumour progression, metastasis, patient survival and receptor crosstalk. INTERNATIONAL JOURNAL OF COLORECTAL DISEASE. 2013; 28: 9–18. 10.1007/s00384-012-1520-9 . [DOI] [PubMed] [Google Scholar]
- 54.Albasri A, Fadhil W, Scholefield JH, Durrant LG, Ilyas M. Nuclear Expression of Phosphorylated Focal Adhesion Kinase Is Associated with Poor Prognosis in Human Colorectal Cancer. ANTICANCER RESEARCH. 2014; 34: 3969–3974. . [PubMed] [Google Scholar]
- 55.Ji H, Pang D, Fu S, Jin Y, Yao L, Qi JP, et al. Overexpression of focal adhesion kinase correlates with increased lymph node metastasis and poor prognosis in non-small-cell lung cancer. JOURNAL OF CANCER RESEARCH AND CLINICAL ONCOLOGY.2013; 139: 429–435. 10.1007/s00432-012-1342-8 . [DOI] [PubMed] [Google Scholar]
- 56.Jan YJ, Ko BS, Hsu C, Chang TC, Chen SC, Wang J, et al. Overexpressed focal adhesion kinase predicts a higher incidence of extrahepatic metastasis and worse survival in hepatocellular carcinoma. Hum Pathol. 2009; 40: 1384–1390. 10.1016/j.humpath.2009.03.006 . [DOI] [PubMed] [Google Scholar]
- 57.Li DW, Sun YJ, Sun ZF, Dong P. Involvement of focal adhesion kinase in cellular proliferation, apoptosis and prognosis of laryngeal squamous cell carcinoma. J Laryngol Otol. 2012; 126: 1127–1133. 10.1017/S0022215112001971 . [DOI] [PubMed] [Google Scholar]
- 58.Li M, Hong LI, Liao M, Guo G. expression and clinical significance of focal adhesion kinase and adrenomedullin in epithelial ovarian cancer. Oncol Lett. 2015; 10:1003–1007. 10.3892/ol.2015.3278 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nat Rev Mol Cell Biol. 2010; 11: 802–814. 10.1038/nrm2996 . [DOI] [PubMed] [Google Scholar]
- 60.Jones G, Machado J, Tolnay M, Merlo A. PTEN-independent induction of caspase-mediated cell death and reduced invasion by the focal adhesion targeting domain (FAT) in human astrocytic brain tumors which highly express focal adhesion kinase (FAK). CANCER RESEARCH. 2001; 61: 5688–5691. . [PubMed] [Google Scholar]
- 61.Weiner TM, Liu ET, Craven RJ, Cance WG. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993; 342: 1024–1025. . [DOI] [PubMed] [Google Scholar]
- 62.Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, et al. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995; 55: 2752–2755. . [PubMed] [Google Scholar]
- 63.Livasy CA, Moore D, Cance WG, Lininger RA. Focal adhesion kinase overexpression in endometrial neoplasia. Appl Immunohistochem Mol Morphol. 2004; 12: 342–345. . [DOI] [PubMed] [Google Scholar]
- 64.Su JM, Gui L, Zhou YP, Zha XL. Expression of focal adhesion kinase and alpha5 and beta1 integrins in carcinomas and its clinical significance. World J Gastroenterol. 2002; 8: 613–618. 10.3748/wjg.v8.i4.613 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Canel M, Secades P, Rodrigo JP, Cabanillas R, Herrero A, Suarez C, et al. Overexpression of focal adhesion kinase in head and neck squamous cell carcinoma is independent of fak gene copy number. CLINICAL CANCER RESEARCH. 2006; 12: 3272–3279. 10.1158/1078-0432.CCR-05-1583 . [DOI] [PubMed] [Google Scholar]
- 66.Rodrigo JP, Alvarez-Alija G, Menendez ST, Mancebo G, Allonca E, Garcia-Carracedo D, et al. Cortactin and focal adhesion kinase as predictors of cancer risk in patients with laryngeal premalignancy. Cancer Prev Res (Phila). 2011; 4: 1333–1341. 10.1158/1940-6207.CAPR-10-0338 . [DOI] [PubMed] [Google Scholar]
- 67.Carelli S, Zadra G, Vaira V, Falleni M, Bottiglieri L, Nosotti M, et al. Up-regulation of focal adhesion kinase in non-small cell lung cancer. Lung Cancer. 2006; 53: 263–271. 10.1016/j.lungcan.2006.06.001 . [DOI] [PubMed] [Google Scholar]
- 68.Judson PL, He X, Cance WG, Van Le L. Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer. 1999; 86: 1551–1556. . [DOI] [PubMed] [Google Scholar]
- 69.Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer. 1996; 68: 164–171. . [DOI] [PubMed] [Google Scholar]
- 70.Kim SJ, Park JW, Yoon JS, Mok JO, Kim YJ, Park HK, et al. Increased expression of focal adhesion kinase in thyroid cancer: immunohistochemical study. J Korean Med Sci. 2004; 19: 710–715. 10.3346/jkms.2004.19.5.710 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tai YL, Chen LC, Shen TL. Emerging roles of focal adhesion kinase in cancer. Biomed Res Int. 2015; 10.1155/2015/690690 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oktay MH, Oktay K, Hamele-Bena D, Buyuk A, Koss LG. Focal adhesion kinase as a marker of malignant phenotype in breast and cervical carcinomas. Hum Pathol. 2003; 34: 240–245. 10.1053/hupa.2003.40 . [DOI] [PubMed] [Google Scholar]
- 73.Madan R, Smolkin MB, Cocker R, Fayyad R, Oktay MH. Focal adhesion proteins as markers of malignant transformation and prognostic indicators in breast carcinoma. Hum Pathol. 2006; 37: 9–15. 10.1016/j.humpath.2005.09.024 . [DOI] [PubMed] [Google Scholar]
- 74.Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, Giancotti FG. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J Clin Invest. 2009; 119: 252–266. 10.1172/JCI37160 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo M, Guan J. Focal adhesion kinase: A prominent determinant in breast cancer initiation, progression and metastasis. CANCER LETTERS. 2010; 289: 127–139. 10.1016/j.canlet.2009.07.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.