Opinion Statement
The last decade has seen considerable advances in the treatment of anxiety disorders in children and adolescents and a considerable expansion of the evidence base for psychopharmacologic in this population. The extant data suggest that, for fear-based anxiety disorders (e.g., generalized anxiety disorder, social phobia/social anxiety disorder, and separation anxiety disorder), selective serotonin reuptake inhibitors (SSRIs) and selective serotonin norepinephrine reuptake inhibitors (SSNRIs) are well tolerated and offer considerable benefit. However, the salutary effects of SSRIs and SSNRIs in pediatric anxiety disorders are consistently amplified by the addition of psychotherapy, particularly in individuals with social anxiety disorder. Additionally, several key demographic and clinical factors, including male sex, non-minority status, and better family functioning and younger age predict greater symptomatic improvement in youth with fear-based anxiety disorders. Thus, current data suggest that in addition to several forms of psychotherapy, including cognitive-behavioral therapy (CBT), SSRIs and SSNRIs are efficacious in the treatment of these conditions in youth and that CBT + an SSRI may be associated with greater improvement than would be expected with either treatment as monotherapy. Finally, given that some children and adolescents may exhibit partial response to current pharmacotherapies, benzodiazepines, anti-histamines and other agents may have adjunctive roles, despite a lack of data in terms of large, randomized controlled trials.
Keywords: antidepressant, anxiety disorders
INTRODUCTION
Fear and anxiety are prevalent during childhood and adolescence and thematically vary by age and gender in healthy children and adolescents [1]. However, for 15–20% of adolescents in the United States, [2,3] this anxiety is associated with significant impairment and crosses the diagnostic threshold of an “anxiety disorder”. Thus, anxiety disorders are prevalent among youth and are linked with an increased risk of suicidal ideation and suicide attempts [4]. In addition, fear-based anxiety disorders in youth are associated with significant morbidity[5], including comorbidity with depressive disorders [6]. Moreover, longitudinal studies suggest that anxiety disorders in childhood are associated with academic impairment and significant interferences in family, school, and social life [7–9]. Finally, youth with anxiety disorders are at increased risk for secondary anxiety disorders, depressive disorder, nicotine or alcohol dependence as well as other substance use disorders [8].
With current knowledge about the prevalence of pediatric anxiety disorders, early intervention is critical to decrease the likelihood of recurrent anxiety and other psychiatric disorders developing into adulthood. Additionally, studies have shown that youth and adults with anxiety disorders suffer from impairments in quality of life [10,11]. Consequently, data from multiple studies reveal the advantages of pharmacologic treatment for children and adolescents. Herein, we review recent psychopharmacologic studies of youth with anxiety disorders including generalized anxiety disorder (GAD), social phobia (SoP)/social anxiety disorder, panic disorder (PD), and mixed anxiety disorders. This review is limited to randomized, controlled trials of selective serotonin reuptake inhibitors (SSRIs), selective serotonin norepinephrine reuptake inhibitors (SSNRIs), benzodiazepines, and atypical anxiolytics.
PEDIATRIC GAD
Sertraline
In a 9 week randomized, controlled study, children ages 5–17 (n=22) diagnosed with GAD were treated with sertraline (n=11) or placebo (n=11).[12]
Sertraline was initiated at 25 mg/day during the first week and then dosed at 50 mg/day from week 2–9.
Hamilton Anxiety Scale for Anxiety (HAM-A) scores and the Clinical Global Impression (CGI) severity and improvement scale scores showed significant improvement in sertraline-treated patients, compared to those receiving placebo.
Adverse events included dry mouth, drowsiness, leg spasms, and restlessness.
Fluoxetine
In a 12 week randomized, controlled study of anxious youth (aged 7–17 years), fluoxetine (n=24) decreased anxiety compared to a placebo (n=22) [13].
Fluoxetine was initiated at 10 mg daily and titrated to a maximum dosage of 20 mg daily.
67% of fluoxetine patients (compared to 36% of those receiving placebo), had CGI-I scores ≤2.
Common side effects included nausea, abdominal pain, headaches, and drowsiness.
Duloxetine
One double-blind, placebo-controlled trial evaluated the efficacy of flexibly-dosed duloxetine in pediatric patients with a primary diagnosis of GAD (ages 7–17 years) with flexibly-dosed duloxetine over a 10 week period followed by 18 weeks of open-label duloxetine (30–120mg daily) [14].
Compared to patients treated with placebo (n=137), duloxetine-treated patients demonstrated reductions in anxiety symptom severity and had higher rates of remission.
CGI-S improved 54% for patients treated with duloxetine compared to 35% for the placebo control group, (p< .001); PARS: 50% for patients treated with duloxetine, and 34% for placebo control group; Children’s Global Assessment Scale (CGAS): 37% for patients treated with duloxetine and 24% for placebo control group [14].
Duloxetine-associated adverse events included increased in heart rate and blood pressure, and weight loss. Suicidal ideation—in terms of Columbia Suicide Severity Scores [15] did not differ between duloxetine and placebo-treated patients during the double-blind portion of the study.
Buspirone
The safety and effectiveness of the 5-HT1A partial agonist, buspirone, has been evaluated in 2 randomized, controlled, 6 week, trials of children and adolescents with GAD (N=559). However, the efficacy data from these studies are unpublished.
PEDIATRIC SOCIAL ANXIETY DISORDER/SOCIAL PHOBIA
Venlafaxine ER
A 16 week randomized, placebo-controlled trial with Venlafaxine ER included 293 diagnosed social anxiety disorder patients ages 8–17 (Venlafaxine ER: n=137). Dosing was initiated at 37.5 mg and then increased depending on the weight of the patient (2.6mg/kg–3.0mg/kg) [16].
Venlafaxine showed a 56% improvement on the CGI-I scale (95% confidence interval [CI], 47%–64%) compared to 37% improvement with placebo (95% CI, 29%–45%).
Adverse events for venlafaxine include asthenia, anorexia, nausea, weight loss, and suicidal ideation.
Paroxetine
Wagner and colleagues [17] treated children with social anxiety disorder, aged 8–17 years, with flexibly dosed paroxetine over the course of a 16-week, multi-center, parallel group trial. Paroxetine was initiated at 10 mg/day, and then flexibly dosed to a maximum of 50 mg/day.
Patients receiving paroxetine exhibited a greater response rate (CGI-I: 77.6% of the paroxetine-treated vs. 33.8% of placebo-treated patients, p<0.001) and, in this sample, paroxetine was well tolerated.
However, decreased appetite, vomiting and insomnia were observed among the sample. Four paroxetine-treated patients (compared to zero placebo-treated patients) experienced emotional lability and suicidal ideation.
Tandospirone
The 5-HT1A receptor partial agonist, tandospirone, which is commonly utilized anxiolytic in Asia, has been evaluated in an eight week open-label parallel controlled trial in youth with social anxiety disorder (N=71) ages 14–21 [26].
Tandospirone was flexibly dosed and titrated to 20–60 mg/day. An active comparator, sertraline, was initiated at 25 mg for 1 week 1 and then titrated to 200 mg/day.
48.6% of tandospirone-treated patients were improved in terms of CGI-I scores compared to 55.6% of patients receiving sertraline. HAM-A scores significantly improved in both treatment groups (p<0.0001). Social Phobia Inventory scores also showed significant improvements over baseline in both treatments (p<0.0001). These pilot studies raise the possibility that tandospirone is not inferior to sertraline in the treatment for SAD in youth. However, it is of interest that buspirone, also a 5-HT1A partial agonist did not differ from placebo in terms of anxiety-related symptoms in pediatric patients with GAD (GSK, 2015)
The most common adverse events for tandospirone were drowsiness, fatigue, and decreased appetite [26]
Clonazepam
In a double-blind, placebo-controlled trial, the benzodiazepine, clonazepam, decreased anxiety symptoms in youth with social anxiety disorder and/or GAD (aged: 7–13 years, N=15), but no differences were detected between placebo and clonazepam in terms of CGI-I scores.
MIXED ANXIETY DISORDERS
Sertraline
The Child/Adolescent Anxiety Multimodal Study (CAMS) was a 12 week randomized, controlled study with youth patients ages 7–17 (n=488) diagnosed with separation anxiety disorder, GAD, or social anxiety disorder [12].
The trial divided the children into four study groups: sertraline (N=133), cognitive behavioral therapy (N=139), combination therapy (N=140), and placebo (N=76).
Sertraline was initiated at 25 mg/day and titrated to 200 mg/day. More than half of sertraline patients (54.9%) exhibited improvement based on CGI-I scores while 80.7% of youth treated with cognitive based therapy (CBT) +sertraline met response criteria for (p<0.001) (compared to 59.7% in patients receiving CBT (p<0.001). All active treatments were statistically superior to placebo [12,18].
The most common adverse events for sertraline include headache, gastric distress, insomnia, as well as a few reported psychiatric symptoms [12].
Fluvoxamine
An eight week randomized, controlled trial of children, ages 6–17 (N=128), who met criteria for social anxiety disorder, separation anxiety disorder, or generalized anxiety disorder has examined the efficacy of fluvoxamine. In this study, fluvoxamine was titrated by 50 mg each week to a maximum of 300 mg/day for adolescents and 250 mg/day for children <12 years of age [19].
The fluvoxamine-treated patients exhibited a 76% improvement based on CGI-I scores compared to only 29% of the placebo group (p<0.001). Additionally, cumulative response rates on the CGI-I scores significantly increased over time raising the possibility that an adequate fluvoxamine trial may be >6 weeks.
Abdominal discomfort and increased motor activity were the two statistically significant adverse events[19]. Although, it is noteworthy that fluvoxamine was associated with a reduction in somatic symptoms in [20].
Fluoxetine
Birmaher and colleagues (2003) studied the treatment of fluoxetine (20 mg/day) on anxious youth (N=74) diagnosed with GAD, SAD, and/or social anxiety disorder (ages 7–17) in a randomized, controlled trial.
Patients treated with fluoxetine (N=37) exhibited improvement by showing 61% improvement on the CGI-I scale compared to 35% of the placebo group [13].
Side effects included abdominal pain, nausea, drowsiness, and headaches [13].
A 9-week open-label pilot study involving 16 patients (ages 9–18) with mixed anxiety disorders examined the efficacy of fluoxetine. In this study, fluoxetine was initiated at 5 mg/day and then increased weekly, with a maximum of 40 mg for children less than 12 years, and 80 mg for adolescents [21].
Fluoxetine-treated youth exhibited clinically significant improvement on fluoxetine from evaluating CGAS and CGI-S scores [21].
The most common side effects include drowsiness, insomnia, decreased appetite, nausea, abdominal pain, and being easily excited [21]
This preliminary study suggests the efficacy of fluoxetine as a treatment for mixed anxiety disorders [21].
Alprazolam
In a double-blind, placebo-controlled study of youth with GAD, (mean age: 12.6 years, N=30), alprazolam did not result in statistically significant improvement compared to placebo in terms of global improvement [24].
Side effects of benzodiazepines in youth include irritability, drowsiness, and oppositional behavior’ [25] as well as dry mouth and sedation [24].
Guanfacine (extended release)
A recent double-blind, placebo controlled was completed in which 83 patients with GAD, SAD and/or social anxiety disorder were randomized 3:1 to an extended release formulation of the α2A receptor agonist guanfacine (GXR) [Strawn et al, 2016].
Adverse events including headache, somnolence/fatigue, abdominal pain, and dizziness, were consistent with the known side-effect profile of GXR; treatment-emergent suicidality did not differ between groups. [Strawn et al, 2016].
At endpoint/early termination, improvement in PARS score was observed in both groups and 6 (31.6%) and 32 (54.2%) patients on placebo or GXR, respectively, had CGI-I scores <2. However, the study was underpowered to detect significant changes in efficacy [Strawn et al, 2016].
PEDIATRIC PANIC DISORDER
Paroxetine
A chart review of child and adolescent patients with panic disorder (N=18) retrospectively examined the effectiveness and safety of paroxetine. Treatment duration was variable [22].
The mean initial dosage was 8.9 mg/day and the maximum dosage was 40 mg day.
83.3% of the patients responded in terms of CGI-S score (baseline-to-endpoint p<0.0001).
Side effects included nausea, tension, headache, sedation, insomnia, agitation, palpitations, and diarrhea.
Paroxetine was tolerated in this retrospective sample [22], although subsequent prospective studies of this medication in anxious youth have raised concerns related to tolerability and safety [23].
PHARMACOLOGIC TREATMENT OF ANXIETY DISORDERS IN ADOLESCENTS
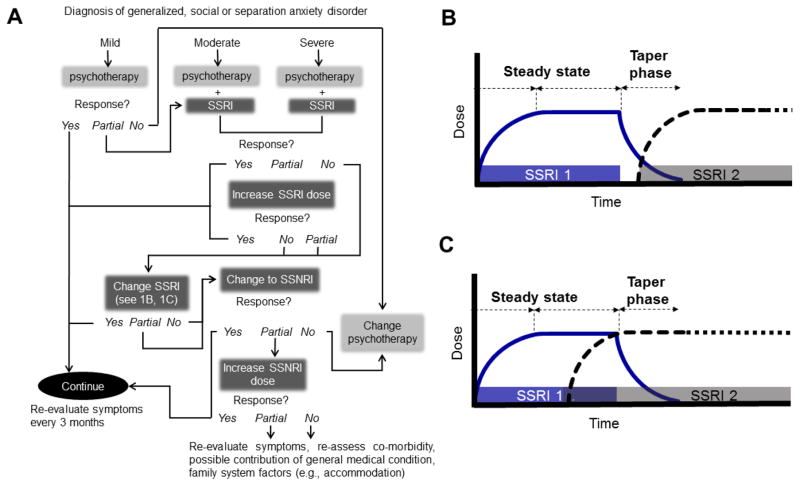
Current data suggest that improved outcomes with psychotherapy in pediatric anxiety disorders and, while there are no randomized clinical trials related to a severity-based treatment algorithm, expert opinion and American Academy of Child & Adolescent Psychiatry Practice Parameters [27,28] suggests that pharmacotherapy is reserved for patients with moderate to severe anxiety (Figure 1A). In such cases, an SSRI is considered a first-line psychopharmacologic intervention, although a switch to another SSRI or SSNRIs may be considered for patients who have persistent anxiety symptoms despite titration of the SSRI and optimization of psychotherapy (Figure 1A).
Figure 1. Treatment algorithm for pediatric anxiety disorders.
In 1A, blue boxes represent pharmacological interventions. Beneath each intervention is a “Response” box that continues to recommend other options if a certain treatment shows partial, or no improvement. Additionally, two cross-titration strategies for antidepressants are illustrated in 1B and 1C. SSRI, selective serotonin reuptake inhibitor; SSNRI, selective serotonin norepinephrine reuptake inhibitor.
Additionally, there has been considerable discussion with regard to the cross-titration of antidepressants in pediatric patients with anxiety disorders. Two approaches are generally utilized in clinical practice, although there is debate related to the “overlap” of the two medications. While starting the second medication after the withdrawal of the first medication (Figure 1B) may be advantageous in situations wherein there are significant side effects, this may result in a rapid loss of the any anxiolytic benefit, if any benefit had been conferred by the first antidepressant. By contrast, titrating the second antidepressant while maintaining the first antidepressant dose represents a second approach (Figure 1C). In general, and in the absence of significant side effects or when partial response is present, an overlap in administration of the two medications is preferred as illustrated in Figure 1C.
PREDICTORS OF PHARMACOLOGIC TREATMENT RESPONSE IN PEDIATRIC PATIENTS WITH ANXIETY
Demographic factors including male sex, non-minority status, and better family functioning predict improved treatment response in pediatric anxiety disorders. Additionally, being older, having a first-degree relative with an anxiety disorder also predict poorer outcomes [13,12,29,30].
Importantly, specific anxiety disorders and comorbidities influence treatment response and remission and in this regard, a primary diagnosis of social anxiety disorder is associated with poorer treatment responses and remission rates [13,29,31], with one study nothing pharmacotherapy (e.g., sertraline) as a requisite for any response, even when CBT is present.
Comorbid internalizing disorder with a pediatric triad anxiety disorder decreases the likelihood of remission [29].
Several studies reveal anxiety severity to be an important predictor of treatment outcome, with patients having more severe anxiety at baseline to have poorer outcomes [13,29,31,32].
Conclusions
Accumulating data suggest that antidepressants—including SSRIs and SSNRIs are effective for the treatment of pediatric patients with anxiety disorders and are well tolerated [28,33,34]. Though, the salutary effects of SSRIs and SSNRIs in pediatric anxiety disorders are consistently amplified by the addition of psychotherapy, particularly in individuals with social anxiety disorder. Thus, current data and practice parameters from the American Academy of Child & Adolescent Psychiatry recommend multimodal treatments for youth with GAD, social anxiety disorder, and separation anxiety disorders. However, treatment approaches are constantly evolving and definitive answers related to treatment duration, the role of adjunctive treatments in partial responders, and the potential utility of pharmacogenomic testing are urgently needed.
Abbreviations
- SSRI
selective serotonin reuptake inhibitor
- SAD
separation anxiety disorder
- SoP
social phobia
- GAD
generalized anxiety disorder
Footnotes
Human and Animal Rights and Informed Consent
This article contains references to published studies with human subjects performed—in party—by Jeffrey R. Strawn, MD. These clinical trials were conducted in accordance with ethical guidelines and all studies on which Dr. Strawn was an author or investigator were reviewed an approved by appropriate institutional review boards.
Conflict of Interest
Farah S. Hussain and Eric T. Dobson declare that they have no conflict of interest.
Jeffrey R. Strawn reports grants and other from National Institute of Mental Health, grants from Shire, grants from Eli Lilly, grants from Forest Research Institute, grants from Lundbeck, grants from Edgemont, other from Neuronetics,royalties from Springer Publishing, material support from Assurex, outside the submitted work.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Strauss CC, Frame CL, Forehand R. Psychosocial impairment associated with anxiety in children. J Clin Child Psychol. 1987;16:235–9. [Google Scholar]
- 2.Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–9. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC, Avenevoli S, Costello EJ, Georgiades K, Green JG, Gruber MJ, et al. Prevalence, Persistence, and Sociodemographic Correlates of DSM-IV Disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69(4):372–80. doi: 10.1001/archgenpsychiatry.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, et al. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry. 2013;70(3):300–10. doi: 10.1001/2013.jamapsychiatry.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 6.Brady EU, Kendall PC. Psychol Bull. US: American Psychological Association; 1992. Comorbidity of anxiety and depression in children and adolescents; pp. 244–55. [DOI] [PubMed] [Google Scholar]
- 7.Ialongo N, Edelsohn G, Werthamer-Larsson L, Crockett L, Kellam S. The significance of self-reported anxious symptoms in first grade children: prediction to anxious symptoms and adaptive functioning in fifth grade. J Child Psychol Psychiatry. 1995;36(3):427–37. doi: 10.1111/j.1469-7610.1995.tb01300.x. [DOI] [PubMed] [Google Scholar]
- 8.Woodward LJ, Fergusson DM. Life course outcomes of young people with anxiety disorders in adolescence. J Am Acad Child Adolesc Psychiatry. 2001;40(9):1086–93. doi: 10.1097/00004583-200109000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Langley AK, Bergman RL, McCracken J, Piacentini JC. Impairment in childhood anxiety disorders: preliminary examination of the child anxiety impact scale-parent version. J Child Adolesc Psychopharmacol. 2004;14(1):105–14. doi: 10.1089/104454604773840544. [DOI] [PubMed] [Google Scholar]
- 10.Bastiaansen D, Koot HM, Ferdinand RF, Verhulst FC. Quality of life in children with psychiatric disorders: self-, parent, and clinician report. J Am Acad Child Adolesc Psychiatry. 2004;43(2):221–30. doi: 10.1097/00004583-200402000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Rapaport MH, Clary C, Fayyad R, Endicott J. Quality-of-life impairment in depressive and anxiety disorders. Am J Psychiatry. 2005;162:1171–8. doi: 10.1176/appi.ajp.162.6.1171. [DOI] [PubMed] [Google Scholar]
- 12.Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–66. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birmaher B, Axelson Da, Monk K, Kalas C, Clark DB, Ehmann M, et al. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(4):415–23. doi: 10.1097/01.CHI.0000037049.04952.9F. [DOI] [PubMed] [Google Scholar]
- 14*.Strawn JR, Prakash A, Zhang Q, Pangallo BA, Stroud CE, Cai N, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283–93. doi: 10.1016/j.jaac.2015.01.008. This study represents a recent double-blind placebo-controlled study of youth with GAD and summarizes the data that was considered as part of the recent FDA approval of duloxetine for pediatric patients with GAD. [DOI] [PubMed] [Google Scholar]
- 15.Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–77. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.March JS, Entusah AR, Rynn M, Albano AM, Tourian KA. A Randomized controlled trial of venlafaxine ER versus placebo in pediatric social anxiety disorder. Biol Psychiatry. 2007;62:1149–54. doi: 10.1016/j.biopsych.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 17.Wagner KD, Berard R, Stein MB, Wetherhold E, Carpenter DJ, Perera P, et al. A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry. 2004;61:1153–62. doi: 10.1001/archpsyc.61.11.1153. [DOI] [PubMed] [Google Scholar]
- 18.Compton SN, Walkup JT, Albano AM, Piacentini JC, Birmaher B, Sherrill JT, et al. Child/Adolescent Anxiety Multimodal Study (CAMS): rationale, design, and methods. Child Adolesc Psychiatry Ment Heal. 2010;4:1. doi: 10.1186/1753-2000-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pine DS, Walkup JT, Labellarte MJ, Riddle MA, Greenhill L, Klein R, et al. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344:1279–85. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- 20.Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179–87. doi: 10.1097/01.chi.0000231974.43966.6e. [DOI] [PubMed] [Google Scholar]
- 21.Fairbanks JM, Pine DS, Tancer NK, Dummit ES, III, Kentgen LM, Martin J, et al. Open fluoxetine treatment of mixed anxiety disorders in children and adolescents. J Child Adolesc Psychopharmacol. 1997;7:17–29. doi: 10.1089/cap.1997.7.17. [DOI] [PubMed] [Google Scholar]
- 22.Masi G, Toni C, Mucci M, Millepiedi S, Mata B, Perugi G. Paroxetine in child and adolescent outpatients with panic disorder. J Child Adolesc Psychopharmacol. 2001;11(2):151–7. doi: 10.1089/104454601750284054. [DOI] [PubMed] [Google Scholar]
- 23.Marks DM, Park M-H, Ham B-J, Han C, Patkar AA, Masand PS, et al. Paroxetine: safety and tolerability issues. Informa UK Ltd; London, UK: 2008. [DOI] [PubMed] [Google Scholar]
- 24.Simeon JG, Ferguson HB, Knott V, Roberts N, Gauthier B, Dubois C, et al. Clinical, cognitive, and neurophysiological effects of alprazolam in children and adolescents with overanxious and avoidant disorders. J Am Acad Child Adolesc Psychiatry. 1992;31(1):29–33. doi: 10.1097/00004583-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Graae F, Milner J, Rizzotto L, Klein RG. Clonazepam in childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 1994;33(3):372–6. doi: 10.1097/00004583-199403000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Li C, Li W, Luo Y, Wang B, Zhang W, et al. Clinical evaluation of the efficacy and safety of tandospirone versus sertraline monotherapy for social anxiety disorder: a randomized open-label trial. Hum Psychopharmacol Clin Exp. 2013;28(6):594–9. doi: 10.1002/hup.2361. [DOI] [PubMed] [Google Scholar]
- 27.Connolly SD, Bernstein GA. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267–83. doi: 10.1097/01.chi.0000246070.23695.06. [DOI] [PubMed] [Google Scholar]
- 28.Wehry AM, Beesdo-Baum K, Hennelly MM, Connolly SD, Strawn JR. Assessment and treatment of anxiety disorders in children and adolescents. Curr Psychiatry Rep. 2015;17(7):52. doi: 10.1007/s11920-015-0591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ginsburg GS, Kendall PC, Sakolsky D, Compton SN, Piacentini J, Albano AM, et al. Remission after acute treatment in children and adolescents with anxiety disorders: findings from the CAMS. J Consult Clin Psychol. 2011;79:806–13. doi: 10.1037/a0025933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez A, Peris TS, Vreeland A, Kiff CJ, Kendall PC, Compton SN, et al. Parental Anxiety as a Predictor of medication and CBT response for anxious youth. Child Psychiatry Hum Dev. 2014 doi: 10.1007/s10578-014-0454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Compton SN, Peris TS, Almirall D, Birmaher B, Sherrill J, Kendall PC, et al. Predictors and moderators of treatment response in childhood anxiety disorders: results from the CAMS trial. J Consult Clin Psychol. 2014;71(3):310–8. doi: 10.1037/a0035458. This is a thorough review of clinical and demographic factors which influence treatment reponse in pediatric patients with anxiety disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masi G, Pfanner C, Mucci M, Berloffa S, Magazù A, Parolin G, et al. Pediatric social anxiety disorder: predictors of response to pharmacological treatment. J Child Adolesc Psychopharmacol. 2012;22(6):410–4. doi: 10.1089/cap.2012.0007. [DOI] [PubMed] [Google Scholar]
- 33**.Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149–57. doi: 10.1002/da.22329. This is currently the only meta-analysis of antidepressants in youth with non-OCD anxiety disorders and suggests a moderate effect size and a favorable tolerability profile in youth w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohatt J, Bennett SM, Walkup JT. Treatment of Separation, Generalized, and Social Anxiety Disorders in Youths. Am J Psychiatry. 2014;171(7):741–8. doi: 10.1176/appi.ajp.2014.13101337. [DOI] [PubMed] [Google Scholar]
- 35.Strawn J, Compton S, Robertson B, Albano A, Hamdani M, Rynn M. Guanfacine extended-release in pediatric anxiety disorders: a randomized, placebo-controlled trial. Annual Meeting of the Anxiety and Depression Association of America; Philadelphia, PA. 2016. [Google Scholar]