Abstract
Background and Purpose
Impairments in metabolic capacity and economy (O2cost) are hallmark characteristics of locomotor dysfunction following stroke. High-intensity (aerobic) training has been shown to improve peak oxygen consumption in this population, with fewer reports of changes in O2cost. However, inconsistent gains in walking function are observed, particularly in persons with subacute stroke, with minimal associations with gains in metabolic parameters. The purpose of this study was to evaluate changes in aerobic exercise performance in participants with subacute stroke following high-intensity, variable stepping training as compared to conventional therapy.
Methods
A secondary analysis was performed from a randomized controlled trial (RCT ) comparing high-intensity training to conventional interventions, and a pilot study that formed the basis for the RCT. Participants 1–6 months post-stroke received ≤40 sessions of high intensity variable stepping training (n=21) or conventional interventions (n=12). Assessments were performed at baseline (BSL), post-training and 2–3 month follow-up, and included changes in submaximal VO2 (VO2submax) and O2cost at fastest possible treadmill speeds and peak speeds at BSL testing.
Results
Significant improvements were observed in VO2submax with less consistent decreases in O2cost, although individual responses varied substantially. Combined changes in both VO2submax and VO2 at matched peak BSL speeds revealed stronger correlations to improvements in walking function as compared to either measure alone.
Conclusions
High-intensity stepping training may elicit significant improvements in VO2submax, while changes in both peak capacity and economy better reflect gains in walking function. Providing such training to improve locomotor and aerobic exercise performance may increase the efficiency of rehabilitation sessions. See Video Abstract available for more insights from the authors (see supplemental digital content).
Keywords: locomotion, rehabilitation, gait training, aerobic
Introduction
Approximately 70–80% of individuals with hemiparesis post-stroke walk without physical assistance1 although many ambulate at slow speeds and for limited distances.2 Impaired locomotion post-stroke is linked primarily to neuromuscular impairments, but is also associated with deficits in peak metabolic capacity3,4, defined as peak rate of oxygen consumption (VO2peak [ml O2/kg/min]), and decreased gait economy,2,5,6 defined as increased metabolic cost of walking per unit distance (O2cost; ml O2/kg/m). In individuals with stroke, VO2peak can be 13–74% lower than age- and gender-matched controls7, and the energy expenditure may be doubled during walking.8 These metabolic impairments are correlated to decreased mobility, and gains in metabolic capacity and economy may contribute to improved walking function.2,9
High-intensity (aerobic) exercise interventions are often utilized to target cardiopulmonary impairments to improve walking function post-stroke. Most aerobic training strategies utilize lower extremity ergometry,10–14 recumbent stepping,15,16 treadmill training2,9,17–19 or mixed interventions,20–23 with targeted heart rate (HR) ranges between 60–85% of maximum age-predicted HR. However, the resultant changes in metabolic and functional measures vary. Some studies9,24–26 demonstrate improved VO2peak while others reveal improvements (decreases) in gait economy post-stroke;2,5,24,27 the latter is accomplished by increasing walking speeds with small gains in VO2,6 decreasing VO2 at similar walking speeds,2,24,27 or both. Despite these improvements, recent studies suggest inconsistent improvements in walking function and small or negligible correlations between metabolic and locomotor changes.2,9,20
Reasons for the inconsistent findings across studies are not clear, but may be related to differences in study inclusion criteria or training strategies. In individuals with chronic (> 6 months) stroke9,17,19 consistent improvements in VO2peak are observed with training as compared to those with subacute stroke,14,15 although many of the former studies focus on treadmill training. However, in individuals with subacute stroke, many training strategies focus on non-stepping tasks10–12,16,20 with inconsistent locomotor improvements. Conversely, and with limited exceptions (e.g.22,28), most gait training strategies delivered early post-stroke do not focus on achieving higher (aerobic) intensities.
The present investigation represents a secondary analysis from two separate studies29–31 detailing the effects of a 10-week (≤ 40 sessions) high-intensity stepping training protocol on metabolic measures in individuals with subacute stroke. The published experimental interventions focused on providing practice of variable stepping tasks at high aerobic intensities (70–80% HR reserve). Gains in locomotor and non-locomotor measures have been described previously from both a randomized controlled trial (RCT) comparing this training strategy with conventional therapy,29 and in a pilot experimental study that served as the basis for the RCT.30,31 In the present analysis, we were specifically interested in changes in aerobic performance during graded treadmill testing and overground walking in individuals with subacute stroke at baseline (BSL), following training (POST) and at a 2–3 month follow-up (F/U) assessment. We hypothesized that variable stepping training performed at high aerobic intensities would lead to substantial gains in peak metabolic capacity and economy during stepping that would be associated with walking performance.
Methods
Changes in locomotor and metabolic function in individuals 1–6 months post stroke were assessed following an 8–10 week, experimental, high-intensity stepping training paradigm30,31 or conventional interventions.32,33 Data from both an assessor-blinded RCT comparing the two interventions,29 and a non-blinded, pilot experimental study30 that used nearly identical inclusion criteria are incorporated in this analysis.
Inclusion criteria for the combined sample consisted of: history of unilateral, supratentorial stroke 1–6 months previously; 18 to 75 years of age; ability to walk with at least moderate assistance (able to perform at least 50% of work to ambulate) up to walking without physical assistance but with braces and devices as necessary but ≤0.9 m/s overground at self-selected pace, ability to follow 3 step commands or Mini-Mental Status Exam > 22/30, and medical clearance to participate. In addition, metabolic data were available only for participants who were able to walk ≥ 0.1 m/s, or the minimum speed, on a motorized treadmill during graded exercise testing. Exclusion criteria consisted of: cardiovascular, respiratory or metabolic instability, inability to ambulate >45.7 m independently prior to stroke, history of other nervous system injury, and inability to adhere to study requirements. All procedures were approved by the Northwestern University Institutional Review Board and participants provided written informed consent.
Randomization and Intervention
From the experimental pilot study,30 data from 9/12 subjects with subacute stroke are included, with 3 excluded due to inability to ambulate on the treadmill at 0.1 m/s (n=2) or equipment failure (n=1) at BSL. From the RCT,29 data from 24/33 subjects are included (n=12 experimental, n=12 control), with 8 others unable to walk 0.1 m/s at BSL for 2 minutes. For one additional experimental subject, an assessor could not be blinded for any outcome, and their data were excluded from all analysis. There were no additional drop-outs in the RCT or pilot studies. Data from both studies used similar inclusion criteria and training protocols, and are included to demonstrate consistency of outcomes. All participants wore validated accelerometers (StepWatch, Modus, Inc) on their paretic ankle throughout the study duration to evaluate stepping activity both during and outside of therapy sessions.
The goal of experimental training was to provide continuous stepping practice in multiple, variable environments while maintaining the cardiovascular training zone of 70–80% of HR reserve or ratings of perceived exertion (RPE) of 15–17 on the 6–20 Borg scale.34 Heart rate was monitored during training using pulse-oximeters. For individuals on β-blockers, the target HR training zone was lowered 10 bpm.35 Participants were provided up to 40 1-hr training sessions over 10 weeks. During each session, subjects performed up to 40 minutes of stepping with rest breaks as needed. The first 5–10 sessions consisted of forward treadmill training with limited body weight support provided, and with body weight support reduced as quickly as possible. The primary focus was on increasing treadmill speed to reach the targeted aerobic intensities as quickly as possible (i.e., speed-dependent treadmill training). A safety harness was used during treadmill training as a safety precaution only, with no weight support provided. Remaining sessions included 25% forward treadmill walking, 25% variable walking on the treadmill (skill-dependent treadmill training), 25% overground training, and 25% stair climbing. Skill-dependent treadmill training was performed by applying perturbations to challenge postural stability, propulsion, and limb swing, and included walking in multiple directions, over inclines and obstacles, and/or with weighted vests and leg weights with limited handrail use as tolerated.36 Perturbations were applied such that 2–5 different tasks were randomly alternated and repeated within 10 minute periods. Overground training focused on fast walking speeds or variable tasks as described above, with use of a gait belt or overhead mobile or rail suspension system for safety. Stair climbing was performed over static or rotating stairs (Stairmaster, Vancouver, WA) using reciprocal gait patterns with progression to higher speeds and reduced hand rail use. If specific tasks were not practiced during individual sessions, subsequent sessions focused on missed tasks. Blood pressure was monitored throughout training; subjects were not trained if resting blood pressure was greater than 220/110 mmHg at rest, and training was discontinued each session if blood pressure surpassed 240/110mmHg.37 Step activity monitors allowed estimation of the total amount of stepping practice during sessions and comparisons between training groups.
The goal of conventional (control) training was to provide standard physical therapy interventions, consistent with typical clinical practice observed during treatment of patients post-stroke. Participants who received conventional training (n=12) continued with clinical physical therapy as possible without influence from research personnel. Details of the types and amounts of therapeutic activities were extracted from medical records as possible, with stepping activities also recorded during sessions. The number of conventional (control) therapy sessions was supplemented by research staff in an effort to achieve 40 sessions over 10 weeks, similar to experimental therapy. Interventions during supplemented sessions focused on practice of multiple therapeutic tasks consistent with published reports of conventional therapy activities.32 The amounts and types of activities derived from published data included (mean repetitions [confidence intervals]): active lower extremity exercises (75 [58–93]); passive/stretching exercises (12 [9–16]); transfers (11[9–13]); balance activities (27 [19–35]); gait (357 [296–418]), and stairs (3 episodes [2–4]). The targeted amount of stepping activity delivered during supplemental sessions was augmented based on a separate observational study that provided larger amounts of stepping practice (i.e., 800–900 steps/session).2 Stepping practice occurred both on the treadmill and overground/stairs without limitations on cueing and feedback. Intensity of stepping was targeted at 30–40% of their HR reserve, consistent with observational data of HR ranges during therapy in subacute stroke.33 The treating physical therapist progressed participants with devices and bracing as appropriate. Of the conventional interventions, approximately 62% were supplemented by research staff, with detailed records of activities available in 70% of all therapies. Additional records from participants performing clinical physical therapy at other rehabilitation centers were often not accessible, or the types and amounts of activities were not well described. In addition, stepping activities was recorded in approximately 76% of all sessions (detailed below and in Table 1).
Table 1.
Demographic Characteristics
| Experimental | Control | Pilot | |
|---|---|---|---|
| Demographic | |||
| Gender, m/f | 9/3 | 8/4 | 6/3 |
| Age, years | 55±12 | 61±10 | 53±14 |
| Duration post-stroke, days | 108±57 | 89±40 | 118±59 |
| Type, hemorrhagic/ischemic | 4/6 | 1/11 | 3/6 |
| Lesion side, right/left | 7/5 | 9/3 | 6/3 |
| Assistive Device at BSL, y/n | 10/2 | 9/3 | 9/0 |
| Training Outcomes | |||
| Sessions, n | 34±9.7 | 34±8.2 | 37±7.6 |
| Steps/session | 2641±727 | 1043±465 | 2993±637 |
| Average peak HR reserve (%) | 73±8.5 | 57±8.6 | 73±12 |
| Adverse Events | |||
| Falls without injury (n) | 6 | 8 | 2 |
| Hypertension/angina (n) | 1 | 0 | 3 |
| Seizure (n) | 0 | 1 | 0 |
| Joint/back pain (n) | 3 | 1 | 2 |
| Skin breakdown (n) | 3 | 3 | 0 |
Unable to determine stroke type from medical records for 2 experimental subjects; P Value represents significance level of independent t-test between experimental and control training outcomes
Measures
Metabolic testing was performed at BSL POST, with a 2–3 month F/U during a graded-intensity exercise test on a motorized treadmill and during a 6 minute walk test (6MWT). Cardiopulmonary data were collected on a breath-by-breath basis during either testing using a portable indirect calorimeter (CosMed USA Inc, Chicago, Illinois) calibrated prior to each assessment. Data were collected for 2 minutes during quiet sitting (resting VO2). For graded exercise testing, participants walked on a treadmill with unilateral handrail use if needed, and an overhead safety harness in case of loss of balance (i.e., no weight support). Testing began at 0.1m/s for 2 minutes, with speeds increased by 0.1m/s every 2 minutes. Heart rate (HR) was evaluated using a pulsoximeter (Masimo, Irvine, CA) and HR and RPE were recorded every 2 minutes. Testing ended when a participant could no longer take steps, stated they could not continue, or if HR reached 85% of age predicted maximum. Testing HRs were limited to 85% predicted maximum consistent with ACSM guidelines without previous stress testing.38 Given the HR limitations and motor impairments of participants, graded exercise testing was considered a submaximal exercise assessment. For determination of O2cost during the 6MWT, participants walked overground for 6 min at their self-selected pace to minimize fall risk, with evaluation of cardiopulmonary measures as described above. Two participants (1 experimental RCT, 1 control RCT) who were able to walk at the minimum treadmill speeds could not perform the entire 6MWT without physical assistance, and their data for O2cost is excluded. Additional walking measures included self-selected speeds (SSS) and fastest-possible speeds (FS) performed over an instrumented walking platform (Equitest, Inc, Chalfont, PA) and averaged over 2 trials.
Data Analysis
Peak metabolic capacity during submaximal testing (VO2submax) was defined as the average VO2 during the last 30 seconds of the fastest speed achieved, and O2cost was calculated by dividing average VO2 by peak speeds. Metabolic data at POST and F/U was also analyzed at the peak speed achieved during BSL testing (i.e., “matched” speeds). Data at these matched speeds (VO2match) were compared separately to estimate the contribution of improvements in O2cost (i.e., decreased VO2) at BSL speeds with training. Secondary measures included minute ventilation (VE, mL/min), HR (beats/min), respiratory exchange ratio (RER=VCO2/VO2), and the Oxygen Uptake Efficiency Slope (OUES),39 an index of cardiopulmonary reserve that estimates O2 extraction and peripheral utilization. The OUES provides the ability to assess aerobic capacity without the need for maximal effort during testing,39,40 and can be utilized in individuals who may not be able to achieve maximal exertion.41 The OUES was calculated as the slope of the log [VE/VO2] for each individual, and performed if data were collected at ≥ 2 speeds (necessary for slope calculation).40 During the 6MWT, VO2 was averaged over the last 3 minutes of walking, and O2cost determined by dividing VO2 by average speed (m/min).
Statistical Analysis
Outcomes were assessed for normality using Kolmogorov-Smirnov with only O2cost not normally distributed, although normalization (square-root) revealed similar changes to non-normalized data and the latter is presented. Primary measures included changes in VO2submax and O2cost at the highest treadmill speeds at each test, the OUES, and VO2match (i.e., VO2 achieved during POST or F/U testing at peak BSL speeds). For the RCT, two-way repeated measures analysis of variance (ANOVA) was used to compare measures with main effects of groups (experimental vs control) and time (BSL, POST, F/U), but specific interest in the groupXtime interactions. Significance was adjusted to correct for multiple ANOVAs (i.e., α=0.0125). For the pilot study, separate repeated measures ANOVAs were performed.
Secondary measures included VE, percentage of predicted HRmax achieved, RPE, and RER. Separate ANOVAs were performed for comparison of changes between groups at each assessment at peak treadmill speeds (i.e., BSL, POST, F/U), and at speeds matched to peak BSL (BSL, POST-match, F/U-match). Repeated measures ANOVA were also calculated for testing during 6MWT, with emphasis on O2cost and VO2 during the last 3 minutes of testing. Separate statistical calculations were made for pilot study. Pearson’s correlation analyses and multiple linear regressions were used to evaluate the relationship between selected metabolic and clinical measures at BSL and POST assessments. For regressions, we were interested in the relative contributions of changes in VO2submax and VO2match on locomotor outcomes (peak speed and walking speeds overground). Residuals were checked for normality and collinearity was monitored, with variance inflation factors <3.0 considered acceptable. Because of the exploratory nature of the correlation analysis, Bonferroni corrections were not performed. SPSS (Version 21) and Stat View (SAS Institute Inc. Version 5.0.1) were used for all analyses.
Results
Demographic data from 33 participants who completed the study (RCT: 12 experimental versus 12 control; pilot: 9 experimental) are presented in Table 1, with no differences between groups. Training characteristics are also presented, indicating no differences in average number of training sessions, but large differences in stepping amount and intensity. In the RCT, significant differences were observed for mean steps/session (p < 0.01) and mean peak HR/session documented in records (p< 0.01).
Similar stepping amounts and intensities were observed in the pilot study as in the experimental RCT group. Adverse events are also listed in Table 1, with non-significant differences across groups. Slightly higher rates of hypertension were observed the experimental groups, while small increases in fall events were observed in the control group. Baseline and changes in clinical locomotor measures have been presented previously.29,30 Data included from the RCT29 indicate significant differences in gains between training groups at POST for SSS (experimental: Δ0.31±0.23 m/s; control: Δ0.10±0.10 m/s), FS (Δ0.40±0.39 versus Δ0.12 ±0.16 m/s), and 6MWT (141±119 versus 29±28 m) with all differences maintained at F/U. Similar gains following experimental training were observed in the pilot study (e.g., SSS: Δ0.34±0.23 m/s; FS: Δ0.47±0.23 m/s; 6MWT: Δ132±93 m).30
Cardiopulmonary outcomes during treadmill testing
At BSL, graded treadmill testing was terminated in 25/33 (76%) participants prior to reaching 85% maximum predicted HR secondary to gait instability or subjective termination. All data were similar between groups at BSL in the RCT, although specific measures were altered following training. Peak BSL speeds were not different between RCT training groups, with greater gains (groupXtime interaction; p < 0.001) observed at POST and F/U. Changes in the pilot study were also significant and slightly larger than those observed in the RCT experimental groups. Assessment of metabolic parameters revealed variable improvements as depicted in Figure1A–B in 2 participants who performed experimental training. In Fig 1A, one subject demonstrated gains in VO2submax with little changes in VO2match, whereas the subject in Fig 1B demonstrated no increase in VO2submax, but large decreases in VO2match.
Figure 1.
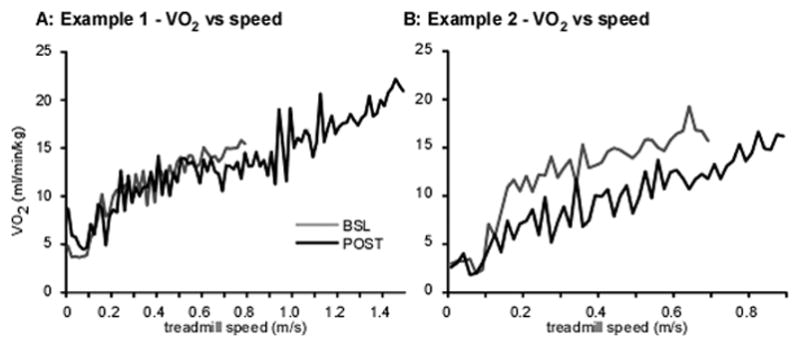
Two (A–B) single-participant examples of individual VO2 responses (averaged over 20 s) during graded treadmill testing at BSL and POST; A: participant demonstrated increased VO2submax with little change in VO2match (VO2 at POST during peak BSL speed), B: participant demonstrates decreased VO2 peak at higher treadmill speeds, and substantial decreases in VO2match at peak BSL speed.
Details regarding changes in primary metabolic measures from BSL to POST and F/U are given in Table 2. In the RCT, significant groupXtime interactions were observed for VO2submax favoring experimental training (p<0.01), with large differences at POST) which were sustained at F/U. Similarly, significant groupXtime interactions (p<0.01) were also observed for OUES. In contrast, there were no significant interactions for changes in VO2match at peak BSL speeds (p=0. 48) or for O2cost (p=0.29). Pilot data revealed similar gains in VO2submax as the experimental RCT groups. However, smaller improvements in OUES were not significant and changes in O2cost were larger.
Table 2.
Primary outcomes during graded treadmill assessments at peak speeds
| Experimental - RCT | Control | P-values | ||||||
|---|---|---|---|---|---|---|---|---|
| BSL | POST | F/U | BSL | POST | F/U | time | groupXtime | |
| VO2submax (ml/kg/min) | 15±5.2 | 21±9.1 | 20±6.3 | 14±3.6 | 15±3.6 | 15±3.4 | <0.01 | <0.01 |
| OUES (a.u.) | 1.5±0.67 | 2.2±0.67 | 2.0±0.59 | 1.7±0.47 | 1.7±0.43 | 1.7±0.58 | 0.02 | 0.01 |
| O2cost (ml/kg/m) | 0.48±0.15 | 0.33±0.07 | 0.34±0.11 | 0.65±0.91 | 0.61±0.91 | 0.37±0.15 | 0.02 | 0.29 |
| VO2match (ml/kg/min) | 15±5.2 | 14±5.0 | 13±4.5 | 14±3.6 | 13±2.8 | 13±3.5 | 0.07 | 0.48 |
| Experimental - Pilot | P -Values | |||||||
| BSL | POST | F/U | time | |||||
| VO2submax (ml/kg/min) | 12±2.3 | 16±4.5 | 17±3.6 | <0.01 | ||||
| OUES (a.u.) | 1.5±0.51 | 1.7±0.55 | 1.7±0.60 | 0.11 | ||||
| O2cost (ml/kg/m) | 0.50±0.32 | 0.25±0.03 | 0.27±0.06 | 0.01 | ||||
| VO2match (ml/kg/min) | 12±2.3 | 10±2.8 | 11±2.5 | 0.22 | ||||
Changes in secondary cardiopulmonary and subjective measures at both peak and matched speeds in the RCT and pilot study are provided in Table 3. In the RCT, groupXtime interactions at peak speeds approached significance for VE and RPE favoring experimental training. At matched peak BSL speeds, significant interactions were observed only for in RER favoring experimental training. Similar trends were observed in the pilot study as compared to experimental groups, including higher values of VE and lower RPEs at peak speeds, and lower RER values at matched speeds.
Table 3.
Secondary outcomes during graded treadmill assessments at peak and matched speeds
| Experimental | Control | P-values | ||||||
|---|---|---|---|---|---|---|---|---|
| Peak speed | BSL | POST | F/U | BSL | POST | F/U | time | group Xtime |
| HR (%max) | 72±14 | 75±14 | 73±12 | 77±12 | 77±10 | 73±13 | 0.07 | 0.69 |
| VE (L/min) | 44±15 | 59±24 | 57±18 | 48±5.3 | 52±21 | 50±15 | <0.01 | 0.02 |
| RER (a.u.) | 0.94±0.10 | 0.92±0.13 | 0.94±0.16 | 0.91±0.09 | 0.95±0.13 | 0.95±0.11 | 0.20 | 0.33 |
| RPE (a.u.) | 15±4.1 | 17±3.9 | 16±4.1 | 17±2.5 | 17±1.9 | 15±3.5 | 0.12 | 0.02 |
| Matched speed | ||||||||
| HR (%max) | 72±14 | 60±10 | 57±11 | 77±12 | 68±10 | 66±12 | <0.01 | 0.41 |
| VE (L/min) | 44±15 | 35±12 | 35±13 | 48±5.3 | 43±14 | 40±14 | <0.01 | 0.16 |
| RER (a.u.) | 0.94±0.10 | 0.82±0.10 | 0.84±0.12 | 0.91±0.09 | 0.91±0.13 | 0.92±0.15 | 0.01 | 0.01 |
| RPE (a.u.) | 15±4.1 | 16±3.0 | 15±3.1 | 17±2.5 | 14±3.2 | 14±2.4 | 0.02 | 0.49 |
| Pilot | P -Values | |||||||
| Peak speed | BSL | POST | F/U | time | ||||
| HR (%max) | 77±7.5 | 77±9.8 | 72±11 | 0.31 | ||||
| VE (L/min) | 43±19 | 58±24 | 62±24 | <0.01 | ||||
| RER (a.u.) | 0.88±0.15 | 0.86±0.12 | 0.97±0.15 | 0.13 | ||||
| RPE (a.u.) | 14±3.8 | 16±2.8 | 16±2.9 | 0.04 | ||||
| Matched speed | ||||||||
| HR (%max) | 77±7.5 | 61±6.5 | 62±6.6 | <0.01 | ||||
| VE (L/min) | 43±19 | 29±8.1 | 31±9.3 | <0.01 | ||||
| RER (a.u.) | 0.88±0.15 | 0.75±0.11 | 0.81±0.09 | 0.02 | ||||
| RPE (a.u.) | 14±3.8 | 9.9±2.9 | 9.7±4.1 | 0.19 | ||||
Cardiopulmonary outcomes during overground testing
Changes in metabolic parameters collected during the 6MWT were compared between groups (Table 2). While speeds during 6MWT were certainly different between groups, there were no significant groupXtime interactions for either VO2 or O2cost despite significant time effects for both variables.. Similar changes were observed in the pilot study.
Associations between metabolic and locomotor outcomes
Correlation and regression analyses were performed between changes in walking function and metabolic measures from BSL to POST using all data, with selected examples depicted in Fig 2A–B. Significant low to moderate correlations were observed between ΔVO2submax and ΔSSS (r=0.47, p< 0.01), ΔFS (r=0.64, p < 0.01), and Δpeak treadmill speeds (r=0.66, p < 0.01, Fig. 2A), but not Δ6MWT (r=0.24, p =0.17). In contrast, ΔVO2match demonstrated low, negative correlations with all walking outcomes (r= −0.19 to −0.34); only the correlation with Δpeak treadmill speed was significant (p=0.04; Fig 2B). However, stepwise, multiple linear regression analysis revealed both ΔVO2submax and ΔVO2match contributed significantly to ΔSSS, ΔFS, and Δ peak treadmill speed:
Figure 2.
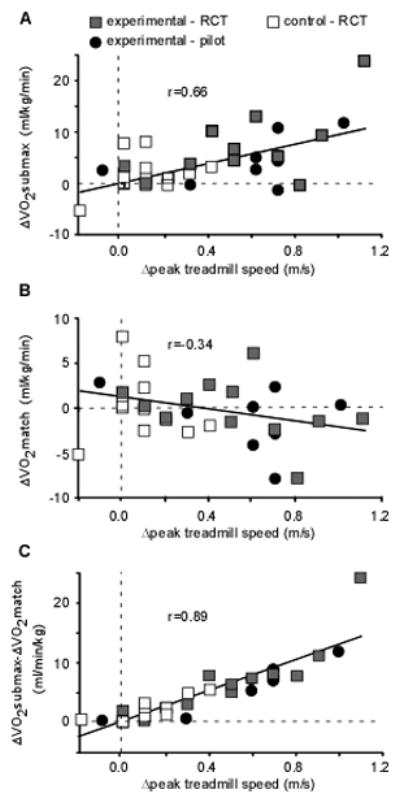
Relation between changes inVO2 at BSL and POST and peak treadmill speeds during graded treadmill testing. Changes (Δ) in peak treadmill speed were moderately correlated to changes in VO2submax (A) but not VO2match (B). Combined changes in ΔVO2submax and ΔVO2match were strongly correlated with Δ peak treadmill speed (C).
The resultant regression equations indicate similar β coefficients for ΔVO2submax and ΔVO2match, suggesting these variables may be additive. Accordingly, the association between the difference in ΔVO2submax and ΔVO2match at POST (i.e., ΔVO2submax − ΔVO2match) and changes in walking outcomes reveal very similar strength of associations as revealed in the regression analyses. This relation is demonstrated for Δ peak treadmill speed (r2=0.80; Fig 2C) but were also similar for ΔSSS (r2=0.45) and ΔFS (r2=0.49; all p<0.01). A smaller but significant relation was observed between combined metabolic changes (ΔVO2submax-ΔVO2match) and Δ6MWT was also observed (r2=0.26, p=0.002)
Secondary correlation analysis revealed no correlations between demographic and baseline functional characteristics with ΔVO2submax or ΔVO2match, with the exception of a low but significant negative correlation between ΔVO2match and BSL VO2submax (r=−0.41, p=0.04, i.e., those with larger baseline VO2submax demonstrated larger decreases in VO2match).
Discussion
The present study delineates changes in aerobic performance during treadmill walking following high intensity stepping training or conventional interventions in individuals with subacute stroke. Greater improvements in VO2submax were observed following experimental training, with smaller decreases in VO2match and O2cost that were not different from changes following conventional training. Nonetheless, regression analyses indicate a potential contribution of improvements in both VO2submax and VO2match to walking outcomes.
The improvements in VO2submax following experimental training were consistent with data from participants with chronic stroke following 3–6 months of aerobic treadmill exercise (for review, please see 26). The present study represents one of few performed in participants with subacute stroke that demonstrates substantial gains in both metabolic and locomotor function, and correlations between these changes. We believe these findings are due to the focus on locomotor activities as the primary intervention, and assessment of metabolic function with a locomotor task. Conversely, many training studies performed on participants early post-stroke provide interventions focused on practice of multiple tasks20,22 or on non-walking interventions (recumbent stepping15,16 or ergometry10–12,14), with metabolic assessments performed during other non-walking assessments. Minimal associations between metabolic and locomotor outcomes may be due to the lack of specificity of the metabolic testing protocol to trained activities or to walking assessments. In contrast, the present data are consistent with a recent study that examined the effects of body-weight supported treadmill training (BWSTT) on combined walking and aerobic outcomes early post-stroke.28 In their participant population (mean duration post-stroke < 30 days), similar (~30%) changes in VO2peak tested during treadmill stepping were observed, with significant improvements in walking outcomes. This latter trial is of additional interest as the authors focused on providing higher intensity of training, while previous BWSTT paradigms have not focused on aerobic capacity21. The present and previous studies suggest training that simultaneously focuses on stepping practice on a treadmill or over multiple variable environments may improve both walking and metabolic outcomes, and their associations.
Consistent with previous data, however, changes in VO2submax and O2cost at fastest or at matched speeds (VO2match) observed here demonstrated moderate42 or low correlations9 to gains in walking function. Regression analyses and combined changes in VO2submax and VO2match24,27 indicate much stronger associations, however, and accounts for some of the variability between subjects (Fig. 1). This combined measure reflecting changes in both peak metabolic capacity and economy may be helpful in future studies investigating altered metabolic demands with exercise training. Why participants demonstrate gains in metabolic capacity or economy or both is of interest, however. Increasing gait speed during treadmill stepping requires greater oxygen uptake by active musculature43–45 and readily accounts for the observed changes in VO2submax. Walking at higher speeds with smaller changes in VO2 could account for improvements in O2cost, particularly if the individuals post-stroke do not walk at their most economical speeds.5 The changes in VO2 at matched speeds are of particular interest, and the negative correlation with BSL VO2submax suggests initial walking strategies in many participants were metabolically inefficient but improved with training. Mechanisms underlying those changes with training are not clear. Long-standing data suggest muscle co-activation decreases during motor learning,46 although few studies have indicated changes in muscle timing occurs in persons post-stroke47,48 (see however 49). More recent studies in participants with neurological impairment8,50 indicate mechanical factors, such improved walking symmetry50 or alterations in center of mass movement8 may improve gait economy. Investigation of these factors is beyond the scope of the present study, although warrants further investigation.
A related interesting finding was the lack of significant differences in O2cost between groups during treadmill or overground testing. The increase in VO2 during 6MWT testing may have minimized differences in O2cost, and this measure may not be as sensitive to change in persons with subacute stroke with substantial impairments. Further, the correlations of changes in metabolic measures with gains in 6MWT were the lowest across walking measures. The combined findings may also suggest changes in 6MWT may not be reflective of changes in aerobic endurance or capacity.
Limitations of the present study include the lack of blinding of assessors and a control group for the pilot study. However, changes in locomotor and metabolic outcomes were consistent between the pilot study and the RCT, and preliminary findings corroborate the consistency of metabolic and locomotor changes. Further, the control group was exposed to both regular clinical therapy and more standardized interventions delivered by research staff during supplemental sessions. The activities performed during these clinical sessions were not accessible or well documented, and the tasks performed are not entirely clear. Such treatment is often typical of “usual care” delivered in other training studies, where the amounts and types of activities are often not accounted for. In the present study, we attempted provide some estimate of activities by monitoring stepping activity during sessions, although alternative strategies may be needed to more precisely document clinical practice patterns performed in remote settings. Another limitation is the estimation of VO2peak during treadmill testing, which was limited by motor impairments in most participants or by HR limitations. As such, VO2submax assessed during the graded treadmill testing is not a definitive measure of individual’s maximal aerobic capacity, although this is difficult to avoid in persons with substantial gait impairments post-stroke. The use of OEUS was calculated to provide a surrogate measure of aerobic function, and helps substantiate the hypothesis that differences in peak VO2 were likely.
Conclusions
Training strategies to improve locomotor and metabolic performance using task-specific practice at high aerobic intensities are fairly well-established in persons who are neurologically intact and persons with chronic neurological injury who are higher functioning. However these strategies are not well-developed, or in many cases have not been tested, in persons early post-stroke.51 Simultaneously focusing on both specificity and intensity of training activities, as performed in this and previous selected studies may contribute to changes in neuromuscular, cardiopulmonary and metabolic systems to facilitate gains in both mobility and health outcomes, thereby improving the efficiency of rehabilitation sessions.
Supplementary Material
Supplemental digital content 1: Video abstract
Table 4.
Metabolic and gait parameters during over ground walking
| Experimental | Control | P -Values | ||||||
|---|---|---|---|---|---|---|---|---|
| BSL | POST | F/U | BSL | POST | F/U | time | groupXtime | |
| Gait speed (m/s) | 0.35±0.18 | 0.76±0.36 | 0.78±0.38 | 0.47±0.28 | 0.58±0.25 | 0.63±0.29 | <0.01 | <0.01 |
| O2cost (ml/kg/m) | 0.65±0.29 | 0.40±0.19 | 0.37±0.21 | 0.77±0.61 | 0.54±0.44 | 0.46±0.22 | <0.01 | 0.46 |
| VO2 (ml/kg/min) | 11±3.8 | 15±4.9 | 14±4.3 | 11±2.5 | 12±3.9 | 13±3.4 | <0.01 | 0.82 |
| Pilot | P -Values | |||||||
| BSL | POST | F/U | time | |||||
| Gait speed (m/s) | 0.36±0.19 | 0.72±0.42 | 0.70±0.40 | <0.01 | ||||
| O2cost (ml/kg/m) | 0.65±0.38 | 0.40±0.27 | 0.42±0.27 | <0.01 | ||||
| VO2 (ml/kg/min) | 11.3±3.1 | 13±4.7 | 13±4.4 | 0.17 | ||||
Acknowledgments
Funding Source: Funding for the study was provided by National Institute on Disability and Rehabilitation Research grants H133B031127 and H133B140012, and the Bullock Foundation.
Funding for the study was provided by National Institute on Disability and Rehabilitation Research grants H133B031127 and H133B140012, and the Bullock Foundation.
Footnotes
Conflicts of Interest: none
Previous presentation/publication: Parts of this work were presented previously at the2013 Combined Sections Meeting, of the APTA, in San Diego, CA,
References
- 1.Gray CS, French JM, Bates D, Cartlidge NE, James OF, Venables G. Motor recovery following acute stroke. Age and ageing. 1990;19(3):179–184. doi: 10.1093/ageing/19.3.179. [DOI] [PubMed] [Google Scholar]
- 2.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke; a journal of cerebral circulation. 2010;41(1):129–135. doi: 10.1161/STROKEAHA.109.563247. [DOI] [PubMed] [Google Scholar]
- 3.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Archives of physical medicine and rehabilitation. 2005;86(8):1552–1556. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Patterson SL, Forrester LW, Rodgers MM, et al. Determinants of walking function after stroke: differences by deficit severity. Archives of physical medicine and rehabilitation. 2007;88(1):115–119. doi: 10.1016/j.apmr.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Reisman DS, Binder-MacLeod S, Farquhar WB. Changes in metabolic cost of transport following locomotor training poststroke. Topics in stroke rehabilitation. 2013;20(2):161–170. doi: 10.1310/tsr2002-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reisman DS, Rudolph KS, Farquhar WB. Influence of speed on walking economy poststroke. Neurorehabilitation and neural repair. 2009;23(6):529–534. doi: 10.1177/1545968308328732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith AC, Saunders DH, Mead G. Cardiorespiratory fitness after stroke: a systematic review. International journal of stroke : official journal of the International Stroke Society. 2012;7(6):499–510. doi: 10.1111/j.1747-4949.2012.00791.x. [DOI] [PubMed] [Google Scholar]
- 8.Stoquart G, Detrembleur C, Lejeune TM. The reasons why stroke patients expend so much energy to walk slowly. Gait & posture. 2012;36(3):409–413. doi: 10.1016/j.gaitpost.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke; a journal of cerebral circulation. 2005;36(10):2206–2211. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 10.Katz-Leurer M, Sender I, Keren O, Dvir Z. The influence of early cycling training on balance in stroke patients at the subacute stage. Results of a preliminary trial. Clinical rehabilitation. 2006;20(5):398–405. doi: 10.1191/0269215505cr960oa. [DOI] [PubMed] [Google Scholar]
- 11.Katz-Leurer M, Shochina M, Carmeli E, Friedlander Y. The influence of early aerobic training on the functional capacity in patients with cerebrovascular accident at the subacute stage. Archives of physical medicine and rehabilitation. 2003;84(11):1609–1614. doi: 10.1053/s0003-9993(03)00344-7. [DOI] [PubMed] [Google Scholar]
- 12.Katz-Leurer M, Carmeli E, Shochina M. The effect of early aerobic training on independence six months post stroke. Clinical rehabilitation. 2003;17(7):735–741. doi: 10.1191/0269215503cr671oa. [DOI] [PubMed] [Google Scholar]
- 13.Bateman A, Culpan FJ, Pickering AD, Powell JH, Scott OM, Greenwood RJ. The effect of aerobic training on rehabilitation outcomes after recent severe brain injury: a randomized controlled evaluation. Archives of physical medicine and rehabilitation. 2001;82(2):174–182. doi: 10.1053/apmr.2001.19744. [DOI] [PubMed] [Google Scholar]
- 14.Tang A, Sibley KM, Thomas SG, et al. Effects of an aerobic exercise program on aerobic capacity, spatiotemporal gait parameters, and functional capacity in subacute stroke. Neurorehabilitation and neural repair. 2009;23(4):398–406. doi: 10.1177/1545968308326426. [DOI] [PubMed] [Google Scholar]
- 15.Billinger SA, Mattlage AE, Ashenden AL, Lentz AA, Harter G, Rippee MA. Aerobic exercise in subacute stroke improves cardiovascular health and physical performance. Journal of neurologic physical therapy : JNPT. 2012;36(4):159–165. doi: 10.1097/NPT.0b013e318274d082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattlage AE, Ashenden AL, Lentz AA, Rippee MA, Billinger SA. Submaximal and peak cardiorespiratory response after moderate-high intensity exercise training in subacute stroke. Cardiopulmonary physical therapy journal. 2013;24(3):14–20. [PMC free article] [PubMed] [Google Scholar]
- 17.Luft AR, Macko RF, Forrester LW, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke; a journal of cerebral circulation. 2008;39(12):3341–3350. doi: 10.1161/STROKEAHA.108.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macko RF, Ivey FM, Forrester LW. Task-oriented aerobic exercise in chronic hemiparetic stroke: training protocols and treatment effects. Topics in stroke rehabilitation. 2005;12(1):45–57. doi: 10.1310/PJQN-KAN9-TTVY-HYQH. [DOI] [PubMed] [Google Scholar]
- 19.Globas C, Becker C, Cerny J, et al. Chronic stroke survivors benefit from high-intensity aerobic treadmill exercise: a randomized control trial. Neurorehabilitation and neural repair. 2012;26(1):85–95. doi: 10.1177/1545968311418675. [DOI] [PubMed] [Google Scholar]
- 20.Duncan P, Studenski S, Richards L, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke; a journal of cerebral circulation. 2003;34(9):2173–2180. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 21.Duncan PW, Sullivan KJ, Behrman AL, et al. Body-weight-supported treadmill rehabilitation after stroke. The New England journal of medicine. 2011;364(21):2026–2036. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eich HJ, Mach H, Werner C, Hesse S. Aerobic treadmill plus Bobath walking training improves walking in subacute stroke: a randomized controlled trial. Clinical rehabilitation. 2004;18(6):640–651. doi: 10.1191/0269215504cr779oa. [DOI] [PubMed] [Google Scholar]
- 23.Pang MY, Eng JJ. Determinants of improvement in walking capacity among individuals with chronic stroke following a multi-dimensional exercise program. Journal of rehabilitation medicine. 2008;40(4):284–290. doi: 10.2340/16501977-0166. [DOI] [PubMed] [Google Scholar]
- 24.Macko RF, Smith GV, Dobrovolny CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Archives of physical medicine and rehabilitation. 2001;82(7):879–884. doi: 10.1053/apmr.2001.23853. [DOI] [PubMed] [Google Scholar]
- 25.Gjellesvik TI, Brurok B, Hoff J, Torhaug T, Helgerud J. Effect of high aerobic intensity interval treadmill walking in people with chronic stroke: a pilot study with one year follow-up. Topics in stroke rehabilitation. 2012;19(4):353–360. doi: 10.1310/tsr1904-353. [DOI] [PubMed] [Google Scholar]
- 26.Pang MY, Charlesworth SA, Lau RW, Chung RC. Using aerobic exercise to improve health outcomes and quality of life in stroke: evidence-based exercise prescription recommendations. Cerebrovascular diseases. 2013;35(1):7–22. doi: 10.1159/000346075. [DOI] [PubMed] [Google Scholar]
- 27.Macko RF, DeSouza CA, Tretter LD, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients. A preliminary report. Stroke; a journal of cerebral circulation. 1997;28(2):326–330. doi: 10.1161/01.str.28.2.326. [DOI] [PubMed] [Google Scholar]
- 28.Mackay-Lyons M, McDonald A, Matheson J, Eskes G, Klus MA. Dual effects of body-weight supported treadmill training on cardiovascular fitness and walking ability early after stroke: a randomized controlled trial. Neurorehabilitation and neural repair. 2013;27(7):644–653. doi: 10.1177/1545968313484809. [DOI] [PubMed] [Google Scholar]
- 29.Hornby TG, Holleran CL, Hennessy PW, Leddy AL, Connolly M, Woodward J, Mahtani G, Lovell L, Roth EJ. Variable Intensive Early Walking post-Stroke (VIEWS): A Randomized Controlled Trial. Neurorehabilitation and neural repair. 2015 doi: 10.1177/1545968315604396. in press. [DOI] [PubMed] [Google Scholar]
- 30.Holleran CL, Straube DD, Kinnaird CR, Leddy AL, Hornby TG. Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehabilitation and neural repair. 2014;28(7):643–651. doi: 10.1177/1545968314521001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Straube DD, Holleran CL, Kinnaird CR, Leddy AL, Hennessy PW, Hornby TG. Effects of dynamic stepping training on nonlocomotor tasks in individuals poststroke. Physical therapy. 2014;94(7):921–933. doi: 10.2522/ptj.20130544. [DOI] [PubMed] [Google Scholar]
- 32.Lang CE, MacDonald JR, Gnip C. Counting repetitions: an observational study of outpatient therapy for people with hemiparesis post-stroke. Journal of neurologic physical therapy : JNPT. 2007;31(1):3–10. doi: 10.1097/01.npt.0000260568.31746.34. [DOI] [PubMed] [Google Scholar]
- 33.MacKay-Lyons MJ, Makrides L. Cardiovascular stress during a contemporary stroke rehabilitation program: is the intensity adequate to induce a training effect? Archives of physical medicine and rehabilitation. 2002;83(10):1378–1383. doi: 10.1053/apmr.2002.35089. [DOI] [PubMed] [Google Scholar]
- 34.Borg GA. Psychophysical bases of perceived exertion. Medicine and science in sports and exercise. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 35.Cohen-Solal A, Baleynaud S, Laperche T, Sebag C, Gourgon R. Cardiopulmonary response during exercise of a beta 1-selective beta-blocker (atenolol) and a calcium-channel blocker (diltiazem) in untrained subjects with hypertension. J Cardiovasc Pharmacol. 1993;22(1):33–38. doi: 10.1097/00005344-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Holleran CL, Rodriguez KS, Echauz A, Leech KA, Hornby TG. Potential contributions of training intensity on locomotor performance in individuals with chronic stroke. Journal of neurologic physical therapy : JNPT. 2015;39(2):95–102. doi: 10.1097/NPT.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 37.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Medicine and science in sports and exercise. 2004;36(3):533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 38.Johnson eEP. American College of Sports Medicine: Guidelines for Exercise Testing and Prescription. 6. Philadelphia: Lippincott, Williams & Wilkins; 2000. [Google Scholar]
- 39.Baba R, Nagashima M, Goto M, et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. Journal of the American College of Cardiology. 1996;28(6):1567–1572. doi: 10.1016/s0735-1097(96)00412-3. [DOI] [PubMed] [Google Scholar]
- 40.Hollenberg M, Tager IB. Oxygen uptake efficiency slope: an index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. Journal of the American College of Cardiology. 2000;36(1):194–201. doi: 10.1016/s0735-1097(00)00691-4. [DOI] [PubMed] [Google Scholar]
- 41.Van Laethem C, Bartunek J, Goethals M, Nellens P, Andries E, Vanderheyden M. Oxygen uptake efficiency slope, a new submaximal parameter in evaluating exercise capacity in chronic heart failure patients. American heart journal. 2005;149(1):175–180. doi: 10.1016/j.ahj.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Pohl PS, Perera S, Duncan PW, Maletsky R, Whitman R, Studenski S. Gains in distance walking in a 3-month follow-up poststroke: what changes? Neurorehabilitation and neural repair. 2004;18(1):30–36. doi: 10.1177/0888439003260494. [DOI] [PubMed] [Google Scholar]
- 43.Grabowski A, Farley CT, Kram R. Independent metabolic costs of supporting body weight and accelerating body mass during walking. J Appl Physiol. 2005;98(2):579–583. doi: 10.1152/japplphysiol.00734.2004. [DOI] [PubMed] [Google Scholar]
- 44.Gottschall J, Kram R. Energy cost and muscular activity required for propulsion during walking. J Appl Physiol. 2003;94(5):1766–1772. doi: 10.1152/japplphysiol.00670.2002. [DOI] [PubMed] [Google Scholar]
- 45.Gottschall J, Kram R. Energy cost and muscular activity required for leg swing during walking. J Appl Physiol. 2005;99(1):23–20. doi: 10.1152/japplphysiol.01190.2004. [DOI] [PubMed] [Google Scholar]
- 46.Enoka RM. Neural adaptations with chronic physical activity. Journal of biomechanics. 1997;30(5):447–455. doi: 10.1016/s0021-9290(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 47.Kautz SA, Duncan PW, Perera S, Neptune RR, Studenski SA. Coordination of hemiparetic locomotion after stroke rehabilitation. Neurorehabilitation and neural repair. 2005;19(3):250–258. doi: 10.1177/1545968305279279. [DOI] [PubMed] [Google Scholar]
- 48.Den Otter AR, Geurts AC, Mulder T, Duysens J. Gait recovery is not associated with changes in the temporal patterning of muscle activity during treadmill walking in patients with post-stroke hemiparesis. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2006;117(1):4–15. doi: 10.1016/j.clinph.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 49.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. Journal of neurophysiology. 2010;103(2):844–857. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS. Walking Speed and Step Length Asymmetry Modify the Energy Cost of Walking After Stroke. Neurorehabilitation and neural repair. 2014 doi: 10.1177/1545968314552528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katch VL, McArdle WD, Katch FI. Essentials of Exericse Physiology. Baltimore, MD: Lippincott Williams & Williams; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content 1: Video abstract


