Abstract
A common reaction from anyone confronted with allergy is the question: what prevents universal allergy? We will discuss recent findings in the mouse system that have provided us with clues on why allergy is not more common. We will also address one crucial aspect of atopic allergy in humans, which is absent in most mouse model systems, an IgG/IgE ratio <10. We consider the typical mouse IgE response to be more closely related to the “modified TH2” response in humans. We will discuss the similarities and differences between the IgE and IgG4 response to allergens and an update on the IgG4 B cell, partly derived from studies on eosinophilic esophagitis and IgG4-related diseases.
Keywords: Atopy, IgG4-RD, Eosinophilic esophagitis, B cell, Germinal center, Plasma cell, TH2
Introduction
The main topic of this paper is an update of our “Current perspectives” paper [1], in which we already discussed the exciting mouse data based on IgE reporter mice [2] that indicated the presence of IgE+ B cells in germinal centers. In our paper [1] we stressed the gap between the mouse data and the atopic immune response in humans. Since that paper [2], an important correction was published [3••], in which the authors convincingly show that the IgE reporter system used in their 2012 paper [2] is not completely specific. They conclude that some of the IgE-positive cells were IgG- rather than IgE-producing cells. Four subsequent follow-up papers are also relevant for the interpretation of the mouse IgE reporter system data [4••, 5••, 6•, 7]. In our discussion of these studies, we will focus on aspects that are of particular interest in relation to our views on human atopy and on the relation between IgE and IgG4. For a more extensive and balanced discussion, see for example [8•]. Our second topic is the IgG4 aspect of the atopic condition: why IgE and IgG4 have much in common and yet are strikingly different. We will discuss how all the recent information on eosinophilic esophagitis (EoE) and the hyper-IgG4 syndrome (IgG4-related disease, IgG4-RD) fits as well as contrasts with our newly acquired information on the origins and fates of the IgE- and IgG4-switched B cells.
Our conceptual framework on the atopic IgE response is based on five assumptions [9] (Table 1). Furthermore, we want to point out some textual restrictions used in this paper. (1) “Allergy” is “IgE-mediated sensitization”. (2) An allergen is an antigen that commonly sensitizes. (3) When we mention “IgG1,” we refer to the human isotype. Mouse IgG1 is a different type of antibody, in some ways (particularly in its TH2 dependency) more similar to human IgG4 than to human IgG1. (4) Our focus is on IgE and IgG4 B cells in relation to the IgE immune response to allergens. We will avoid discussions on the relation between antibodies and clinical symptoms.
Table 1.
Hypothetical framework for the human IgE response
| • Allergens come in different flavors, characterized by their tendency to induce not only IgE antibodies but also IgG antibodies. For some allergens, the ratio of allergen-specific IgG to allergen-specific IgE (the sIgG/sIgE ratio) is low (often undetectable) in subjects without sIgE. The prototypic examples are the traditional atopic allergens from pollen and mites. For other allergens, the ratio is >100, and more often than not, IgG is found without demonstrable sIgE (“conventional antigens”). Prototypic examples aremilk and egg proteins, cat allergen, proteins from stinging insects, and toxoids from tetanus and diphtheria. The IgE responses in most animal models are also in this category. It is not clear which factors are responsible for these two distinct patterns of antibody formation. It may be related to dose, route of exposure, etc. Because the latter type of immune response is associated with IgG4 antibodies, indicative of a TH2 response, it has been described as a “modified TH2 response”, i.e., a TH2 response without IgE [10]. |
| • The age of onset for sensitization to atopic allergens is predominant in infancy, whereas late onset sensitization in non-atopic subjects is linked to the modified TH2 response. |
| • The atopic IgE response (i.e., an immune response with a low IgG/IgE ratio) is the most relevant from the allergy point of view. Because of its very modest IgG contribution, we assume that the immune response is infrequent andminimalist, i.e., without much involvement of GCs, and thus little SHM and memory B cell formation. |
| • Because of this minimal GC activity, IgE-switched B cells do not expand much, do hardly ever become classical memory cells, and are not so strictly subjected to negative selection. |
| • The IgE repertoire tends to diversify rapidly (“broad epitope spreading”). This reflects the immunological feed-forward activity of the IgE-allergen interaction: the presence of sIgE facilitates the recruitment of additional IgE B cells with different specificities. This results not only in IgE antibodies to different epitopes on the same allergen molecule, but also to epitopes on unrelated allergens that happen to be present at the same time and place. |
The canonical first step to IgE (or IgG4) is triggered by antigen exposure. A complex interplay of soluble and cellular factors determines the outcome of the interaction between antigen and the B cell receptor on the naïve B cell. Since our focus is on the B cell, we will not discuss the major influence of prior recruitment and activation of antigen-specific T cells by dendritic cells and other antigen-presenting cells and the subsequent activation of TFH. We will also ignore for the moment the diverse panel of inhibitors (cells and extracellular factors) that may modify the outcome of the interaction between the antigen and the B cell. For recent reviews on this topic, see for example [11–13].
The decisive step on the path to IgE/IgG4 production is the class switch. It results in the production by the B cell of an immunoglobulin isotype other than IgM or IgD. Particularly for IgE it is important to appreciate that indirect (secondary) switching is possible, but only to an isotype encoded by DNA that is more downstream than the current isotype. This means that an IgG4-switched B cell can switch to IgE. A switch from IgE to IgG4 is not possible because the DNA encoding for IgG4 is upstream of the DNA encoding for IgE and is deleted during the class switch to IgE. The major impact of the discovery of IL-4 and its requirement for the IgE isotype switch was that it seemed to provide the explanation for the low level of IgE production and the uncommonness of allergy. This explanation seemed even more likely upon the discovery of many factors that could prevent the switch to IgE. It took many years before it was generally appreciated that the IgE-switched B cell is strikingly different from the IgG-switched B cells. Compared to the IgG4-switched B cell, the chances for survival are much poorer for the IgE-switched B cell. An explanation for this poor post-switch survival was provided by the low level of expression of the membrane form of IgE [14, 15], which prevents the B cell from receiving signals needed to acquire the life expectancy of a B memory cell. The full story has not yet been unraveled, but the recent mouse data indicate that it is more complex (see below).
Update on the Major Conclusions of the IgE-Reporter-Mouse Studies: the Intrinsic and Extrinsic Problems of the IgE-Switched B Cell
Localization of the First Detectable IgE-Switched B Cells in Lymph Nodes
The lymph nodes are the traditional site to study the initial antigen-driven B cell response. Other sites, particularly mucosal tissue, are potentially more relevant for human allergy. We will discuss this later. It is now well-established that IgE expressing B cells are present in the germinal center (GC), but very transiently and only in the dark zone, as claimed already by Kelly et al. [16], but initially not observed by others [17]. The technical problems with the identification of IgE-switched B cells by IgE immunostaining (false-positives and false-negatives) are very real and still not completely solved. One factor explaining the virtual absence of IgE-switched B cells in the light zone is their lack of expression of CXCR5 and thus a lack of responsiveness to the relevant chemotactic stimulus [8•].
Cell Fate of the IgE-Switched B Cell Part 1: Premature Death
In all model systems studied, the number of IgE-positive B cells in the GC drops fast. The difference with the kinetics of IgG is interesting. During the early GC phase, the increase is similar for IgE as for IgG, i.e., the ratio IgG/IgE B cell remains constant. In the second week, however, the ratio increases due to a decrease in IgE B cells. The two obvious explanations for the loss of IgE B cells are death and emigration. Both happen. For all GC B cells, Fas-mediated apoptosis is a potentially important factor, given the high expression of Fas on GC B cells. This is assumed to be crucial for affinity maturation in the light zone, which depends on antigen-dependent rescue of B cells with a high-affinity B cell receptor (BCR) [11–13] and removal of self-reactive B cells generated during somatic hypermutation (SHM). BCR-mediated signals might be needed to receive survival signals from TFH cells and possibly other GC cells.
In addition, IgE-switched B cells are found to have a high intrinsic apoptotic tendency that does not depend on FAS expression and is not influenced by contact between antigen and the BCR. This is true not only for IgE+ GC B cells but also for non-lymphoid cells transfected with the membrane form of IgE. It has been suggested that this effect may be related to some unique interaction of the intracellular tail of membrane-anchored IgE (mIgE) [18], possibly the anti-apoptotic protein Hax1 [19]. The mIgE intracellular tail could be a sink for Hax1 [6•].
Cell Fate of the IgE-Switched B Cell Part 2: Premature Differentiation to a Plasma Cell
During a GC reaction, IgG memory B cells are generated during the first 2 weeks. In contrast, the generation of long-lived IgG plasma cells (LLPCs) requires several more weeks [7]. This situation has been established to be strikingly different for IgE B cells, which started to acquire a plasma cell phenotype already at the second week. One sign is the appearance of the characteristic plasma cell transcription factor Blimp-1 [3, 5••]. Equally striking is the up-regulation of BCR expression [4••, 5••]. The causes and possible consequences of this finding are still unresolved. The longevity of the plasma cell depends on finding a survival niche, preferably in the bone marrow. Unexpectedly, eosinophils have an important role in the maintenance of the PC survival niche [20•]. Factors that may be relevant for the LLPC fate in humans but have not been described in mice are the uniquely large membrane-proximal extracellular domain (MPED) of the human IgE BCR [18, 19, 21] and the downregulation of CD19, which has been shown to promote PC survival [22].
Is the IgE B Memory Cell Relevant for IgE Antibody Persistence?
One reason why this topic is debated relatively extensively is its relation with antibody affinity. Both memory formation and affinity maturation are supposed to depend on extensive GC activity. Since IgE-switched B cells spend so little time in GCs, IgE memory might be expected to be minimal and result at best in the production of IgE antibodies of low affinity. However, this would be true only for IgE B cells directly derived from low-affinity IgM antibodies. It has been known for some time that an alternative, indirect route to IgE B cells exists. This involves an IgM B cell switching to an IgG B cell, which subsequently (after affinity maturation) switches to IgE. Since IgE antibodies with high affinity were found in all mouse models, the indirect route to IgE-switched B cells was suggested to be a major source of IgE memory cells. Our interpretations of the currently available data are as follows: (1) true IgE memory (via direct switching) exists but is rare; (2) IgE B cell memory derived via one or more intermediate non-IgE stages is relatively common in experimental models with high antigen doses; (3) a substantial fraction of IgE in plasma is derived from IgE-producing plasma cells without any B memory cell precursor; (4) IgE B memory cells are rare in blood; and (5) persistence of plasma IgE is largely due to IgE-producing long-lived plasma cells (IgE LLPCs).
Somatic Hypermutation in IgE-Switched B Cells
While switching to IgE via the direct route leaves little time for SHM, some SHM does occur. A relevant question is: does this SHM lead to affinity maturation if these B cells avoid the light zone? The definite answer is not available yet, but the mouse data indicate that affinity maturation in IgE produced via direct switching is less effective compared to IgG, but not absent.
IgG4, the Other TH2-Dependent Antibody
In the human immune response to allergens, two isotypes are most prominent: IgE and IgG4; most if not all allergens will induce substantial amounts of IgG4 antibodies. The prototypic human models are the immune responses to honey bee venom and food proteins. The similarity of the allergen specificity pattern of IgE and IgG4 is due to their common dependence on IL-4 as switching factor. However, the details of the IgE and IgG4 immune reactions are quite different. Upon natural exposure, IgE antibodies appear usually well before substantial IgG4 production is observed. Even in a TH2-dominated immune response, the initial IgG subclass is usually IgG1 rather than IgG4. Only upon frequent exposure the plasma IgG4 level rises and IgG4 becomes the dominating antibody [23].
At the time of our initial model [9], we were not able to actually measure IgG4 B cells in blood. Our hypothesis was that the IgG4-switched B cell was a memory cell that was reluctant to develop into a plasma cell, which behavior was in sharp contrast to that of the IgE-switched B cell, which develops rapidly into a plasmablast or plasma cell. When we obtained monoclonal anti-IgG4 antibodies that were suitable for the analysis of IgG4-switched B cells [24], we did not observe the relatively high numbers of IgG4 B cells that we expected, but numbers that were close to the IgG4/IgG total protein ratio in plasma (Table 2). This indicated that the chance to become an IgG-secreting plasma cell was the same for IgG4 as for IgG1. From this observation, we concluded that the late appearance of IgG4 antibodies reflected an initially low prevalence of switching to IgG4 even when sufficient TH2 help was provided (based on the appearance of IgE antibodies). This suggested the need for additional switching factors. Because the appearance of IgG4 was dependent on chronic antigenic stimulation, which is known to evoke immune tolerance, a dependency on IL-10, possibly in combination with other tolerance-associated cytokines such as TGF-β, may provide an alternative explanation for the late appearance of IgG4.
Table 2.
Information on IgG4, IgG4-RD, and IgG4 B cells
| • Allergens may induce an IgG4-dominated response, either as outcome of the “modified TH2 response”, or during sIT. |
| • IgG4 antibodies are associated with prolonged exposure to antigens, including food antigens (egg, milk) and biologics (FVIII, adalimumab). |
| • IgG4 is often associated with “tolerance”, due to its weak capacities to activate effector cells or complement. However, its generally high affinity makes it a good blocking antibody, which may impair clinical efficacy as part of an anti-drug antibody response. Furthermore, IgG4 autoantibodies may block the activity of endogenous targets resulting in pathogenesis, for instance, skin blistering diseases caused by antibodies to desmogleins. |
| • Both IgE and IgG4 are associated with a TH2 response driven by IL-4. IL-10 and regulatory T cells have been implicated as discriminating factors in favor of IgG4. |
| • In IgG4-RD, besides an elevated serum IgG4, other “TH2/Treg” features often found include elevated levels of IL-4 and IL-10 in affected tissues and an elevated level of circulating IgE. To date, a specific antigen driving the massive B cell proliferation/differentiation has not been found. |
| • IgG4+ B cells can be detected in PB; their frequency is proportional to the serum IgG4 concentration in healthy individuals and IgG4-RD patients. In healthy donors, IgG4+ B cells express relatively more CD23 compared to IgG1+ B cells. Culturing regulatory B cells (i.e., IL-10 producing B cells) in vitro can result in substantial IgG4 production. |
| • Rituximab induces a profound drop in serum IgG4 and IgE levels in IgG4-RD patients, suggesting that both isotypes are produced in part by short-lived antibody-secreting cells. This might reflect seasonal fluctuations in specific IgG4 and IgE levels to, e.g., grass pollen. |
Another difference with IgE is the lower persistence of IgG4 antibody levels, for example, following allergen immunotherapy. One possible explanation is that IgG4-producing plasma cells are produced late in the immune response and thus are less successful than IgE in finding a survival niche in the bone marrow.
The Hyper-IgG4 Syndrome (IgG4-Related Disease)
Another explanation for the lower persistence of IgG4 antibody titers is suggested by the recent data on the hyper-IgG4 syndrome, now usually referred to as IgG4-related disease (IgG4-RD). This is a serious disease caused by massive expansion of polyclonal, non-malign IgG4-switched B cells/plasma cells, causing tissue malfunction, for example, in the pancreas (this major member of the IgG4-RD family used to be called “autoimmune pancreatitis”, but no autoimmune reactivity has convincingly shown to be involved) [25, 26]. Due to some unidentified local disturbance in the pancreas or other affected tissue, IgG4-switched B cells invade the tissue and subsequently expand and differentiate to form a tumor-like plasma cells mass. The unexpected localization of this mass in the body suggests that IgG4-switched B cells may have an aberrant homing profile, possibly related to fibrosing conditions associated with the production of TGFβ or other cytokines. Based on the often fast drop in IgG4 levels following B cell depletion therapy (rituximab), the life span of the plasma cells in these unusual niches seems to be much shorter than in the bone marrow. Because the response to the therapy is incomplete, some plasma cells are likely to be more persistent, possibly because of heterogeneity of the niches in providing long-term support for survival.
IgE and IgG4 in Eosinophilic Esophagitis
EoE is a novel form of food allergy where the symptoms, as well as the low or very low IgE ab titers and the lack of response to omalizumab therapy, argue against a direct role for IgE antibodies in the disease. Several studies have suggested that cow's milk proteins play a major role. Indeed 60–65 % of children achieve a histological remission with avoidance of cow's milk proteins alone [27]. On the other hand, confusing results have come from assays of IgE to specific cow's milk proteins using Immuno Solid-phase Allergen Chip (ISAC) for IgE [28]. The results were negative, and a trial of diet based on ISAC results had to be stopped [29]. By contrast, assays of IgE to Bos d 4, Bos d 5, and Bos d 8 using immunoCAP gave positive results in ~40 % of the sera [30]. We have recently reported that patients with low or very low titers of IgE to milk can respond well to milk protein avoidance [31]. The argument has changed with recent evidence that these subjects often have high or very high titer IgG4 ab levels to the same cow's milk proteins [32]. It is likely that the high IgG4 antibody levels prevent sIgE binding by competition for the low amount of allergen that is available in a microarray and thus provide an explanation for the negative results of sIgE measurements based on a microarray platform. These IgG4 antibodies may perhaps reveal the milk proteins that are relevant to the pathology, but it is equally possible that a minor allergen that somehow fails to induce a protective IgG4 response is the culprit. Our IgG4 results do not provide compelling evidence that IgG4 plays a role in the pathogenesis. It seems more likely that the disease is driven by T cells that home to the esophagus and that also enhance IgG4 antibody production. On the other hand, the results of IgG4 production in EoE and also during oral immunotherapy with peanut or milk provide evidence favoring local production of IgG4. The role of eosinophils in supporting PC survival [20•] may be relevant in this condition.
Mouse Versus Man: Different Types of TH2 Responses to Protein Antigens
The protocols used for the investigation of the IgE response in mice produce results that differ in important ways from the prototypic atopic IgE immune response. The close to 100 % response rate in the mouse models contrasts with the less than 25 % response rate (for a single allergen) in humans. Another relevant difference is the IgG/IgE ratio, which is usually much higher in the mouse model than in atopic patients. Also, the IgE antibody response in the mouse is typically transient, whereas the atopic IgE response persists for many years. The TH2 response in the mouse models fits much better with the TH2 response observed initially in non-allergic cat owners that were found to have a high IgG4 response without detectable IgE (the “modified TH2 response” [10]) than with the atopic IgE response.
Three Levels of Epitope Spreading
A further limitation of the commonly used experimental protocols in mice is the use of a single antigen. In the atopic condition, multiple IgE responses are the rule. The initial set of antibodies is reactive with different epitopes on the same allergen. Interestingly, the IgE antibodies that are present in the plasma of an individual are by no means all directed to the same allergen but tend to be preferentially directed to several distinct allergens from the same source material and, to a lesser extent, to allergens from different source materials. Epitope spreading occurs at three levels: (1) at the level of the molecular allergen; (2) at the level of the allergen source (physically related allergens); (3) at the level of physically unrelated allergens. This “molecular atopic march” has been suggested to occur in a fixed sequence with an initiator allergen as trigger. This process occurs in parallel with the pathology-related atopic march (eczema, rhinitis, asthma) [33].
The Mucosal IgE Immune Response
Mechanisms involved in the sensitization following the initiating event have been proposed to depend on IgE-mediated reactions at mucosal sites that generate a milieu that supports class switching and B cell differentiation without the involvement of secondary lymphoid organs. For a review of these mucosal responses, see [8•].
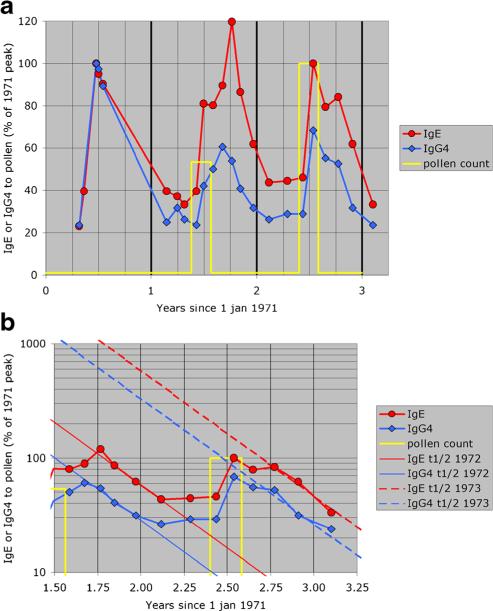
The post-seasonal increase in plasma IgE to pollen is an illustration of such a mucosal response. It is not a classical booster effect, because it presumably depends on prior allergen-induced mucosal inflammation and the development of (poorly organized) lymphoidal structures permitting class-switch recombination (CSR) and SHM. The post-seasonal decline of the antibodies in serum was very similar for IgE and for IgG4 (the half-life of the serum antibody levels is 100 days), despite the 10-fold longer half-life of IgG4 antibodies compared to IgE. This result suggests that the local IgE (and IgG4) plasma cells have a life span of 2–4 months (Fig. 1), possibly related to mucosal eosinophilia [20•].
Fig. 1.
a, b Post-seasonal increase of IgE and IgG4 antibodies to grass pollen extract. Pollen counts were available only for the last two seasons. The yellow line indicates pollen season and the cumulative pollen count for that season relative to the 1973 season. The antibody results are expressed relative to the peak level in the first season (1971). In b, the antibody levels are indicated on a log scale to facilitate the visual comparison of the half-lives of IgE and IgG4 antibodies. The straight lines indicate the calculated half-life (100 days) for each of the two seasons that could be evaluated (data from [34])
Antigen-Independent Generation of IgE B Cells
While the maturation program of the pre-switch B cell is typically initiated by antigen exposure, B cells may alternatively develop along antigen-independent routes. In some conditions, such as bacterial colonization, or more extreme situations, such as CD4 deficiency, IgE can be produced in the absence of T cell help [35]. These B cells produce “natural” antibodies with a broad specificity and may be the precursors of isotype-switched B cells producing antibodies to glycans such as the antibodies to the galactose-alpha-1,3 galactose epitope [36, 37] and low-molecular compounds such as quaternary ammonium compounds related to muscle relaxants [38, 39] and drugs like pholcodine [40–43].
Unusual IgE+ Memory Cells in Mouse and Man
The spectrum of IgE+ B cells might be broader than expected. While conventional memory B cells are CD27+, CD27−IgE+ memory B cells have been described that had a limited replication history (3–4 cell divisions) and low SHM levels and were present in CD40L-deficient patients, supporting their origin from a T cell-independent pathway [44]. Patients with atopic dermatitis had increased numbers of CD27−IgE+ memory B cells with higher SHM rates compared with those seen in healthy control subject.
Recently, a “rogue B cell” was described, a rare GC B cell that does not depend on BCR signals to survive [45]. These GCrogue B cells have a striking propensity to become IgE-producing plasma cells, particularly if they succeed in avoiding Fas-mediated apoptosis. This deviant behavior may be the consequence of the high activity of activation-induced (cytidine) deaminase (AID) in GC B cells in view of a neglected role for AID in the GC environment: next to its roles in class switching and somatic hypermutation, it has been found to be also a major factor in modifying in a semi-random fashion the “methylome” of the GC B cell [46•].
How to Deal with Next-Generation Sequencing Information
An overwhelming amount of information on the transcriptional status of immunoglobulins in individual B cells can be obtained by a technology usually referred to as next-generation sequencing (NGS). A recent study demonstrates the feasibility to detect clonal relationships between IgE cells and other isotypes [47•]. Starting from sequences of single B cells specific for peanut allergens, four different clonal lineages could be identified by NSG. In all cases, the lineage contained IgE and IgG1 members, in only one case were also members of other isotypes were found, including IgG4. Interestingly, successive samples during oral immunotherapy (OIT) showed a gradual accumulation of mutations for IgG4, but not for IgE. This supports the notion that IgE is predominantly produced by pre-existing long-lived plasma cells, which are not influenced by OIT.
Another study similarly identifies B cell lineages starting from allergen-specific IgE clones to a number of allergens obtained during subcutaneous immunotherapy (SCIT) [48•]. For over 30 clones, corresponding reads were obtained using NGS on samples obtained at the same time point as the specific IgE clone, whereas in 13 cases, reads could also be identified at other time points. In a few cases, reads from isotypes other than IgE were identified. It is perhaps a little surprising that so few clon-ally related B cells of other isotypes could be identified in these subjects undergoing SIT. This is likely in part related to the low frequency of specific clone members and illustrates that even with the current technology, the sampling depth may be a limiting factor for identifying clonal lineages. Furthermore, it may indicate that most of the clonal expansion of other isotypes, notably IgG4, is not related to allergen-specific IgE clones.
Looney et al. explored clonal relationships and lineages regardless of antigenic specificity [49•]. This led to a much larger number of identified lineages containing IgE members. In allergic subjects, the average number of identified lineages was twice that of healthy subjects. Evidence for both direct switching as well as indirect switching from all possible precursor isotypes was observed, including IgG4, but with a predominant contribution of switch via IgG1 that is much larger than the fraction of directly switched IgE clones. Interestingly, the putative directly switched IgE clones display much lower mutational frequencies compared to the indirectly switched ones.
The NGS data from non-allergic subjects are important. In the prototypic atopic patient, specific IgE is >25 % of total IgE, which is high compared to other isotypes. Yet the relatively minor differences between allergic and non-allergic patients suggests a high contribution of “background” IgE-producing cells in the NGS studies, particularly those based on B cell obtained from peripheral blood. These may have been generated by one of the above-mentioned non-canonical developmental pathways and thus be non-informative about the canonical allergen-specific IgE B cells. Additionally, some biases are hard to avoid in the interpretation of NGS data. The use of RNA as starting material skews the results in favor of plasmablasts. This problem can be minimized by sorting specific B cell subsets, but most studies use total PBMCs. Obviously, the residence time in circulation influences the chance for a B cell to be sampled. Another bias is the extent of initial and/or autonomous (antigen and T cell independent) ongoing clonal expansion. A smaller expansion of IgM precursor cells renders them less likely to be picked up compared to a more expanded IgG precursor cell population.
Conclusion
It is now firmly established that in the mouse, IgE-switched B cells (1) are briefly present in GCs (mostly in the dark zone, but Yang et al.[5••] also find them in the light zone) and (2) expand a little in these GCs. Some develop into plasmablasts/plasma cells well before the IgG-switched B cells do, but most IgE-switched B cells (including plasma cells) are short-lived. IgE-switched memory cells are found in some of the mouse models, but their number is small. Indirect switching is an important source of IgE-producing cells, both in the primary and in the secondary response. The current mouse models are more similar to the modified TH2 response in man than to the atopic immune response. The latter is characterized by an early age of onset, a low IgG/IgE ratio, and persistent IgE antibody production due to long-lived plasma cells in the bone marrow niche.
Secreted IgE (i.e., as found in serum) might be of at least four types: (1) induced during sensitization in childhood; (2) adult-onset induced in a previously non-sensitized (non-atopic) subject; (3) clinically irrelevant specific IgE, for example, IgE to tetanus toxoid [50, 51]; and (4) “baseline” IgE with no known specificity. It would be interesting to know more about the kinetics of these four types of IgE (stable or fluctuating level, transient or persistent) and the effect of pharmacological manipulation (corticosteroids, rituximab, quilizumab (anti-MPED), or anti-CD38.
The two main postulates of our 2004 model of the atopic immune response [9] still seem to hold (but are still in need of substantiation): 1) IgE-switched B cells are not compatible with late GCs and 2) the short residence time of directly switched IgE B cells in GCs severely limits, but does not fully prevent, clonal expansion, somatic hypermutation, and the development of IgE B memory. How does this new information help to understand why allergy is not universal? We now more clearly understand the differences between atopic and non-atopic IgE responses. In both situations, the level and timing of IL-4 production is critical. However, the induction of atopic IgE antibodies in childhood is possible only within a narrow window of antigen stimulation: just strong enough to induce IgE but not enough to initiate a full-blown GC reaction. The IgG/IgE ratio is typically low. In contrast, the adult-onset induction of IgE antibodies is more dependent on indirect class-switching and thus has a higher IgG/IgE ratio.
Acknowledgments
Thomas A. Platts-Mills has received a grant from the National Institutes of Health (grant no. RO1 AI-20565-32).
Abbreviations
- ISAC
Immuno Solid-phase Allergen Chip (ISAC is a registered trade mark)
- mIgE
membrane-anchored IgE (=IgE BCR)
- MPED
membrane-proximal extracellular domain (of mIgE)
- sIgE
allergen-specific IgE
- sIgG
allergen-specific IgG
- AID
Activation-induced (cytidine) deaminase
- BCR
B cell receptor
- CSR
Class-switch recombination
- EoE
Eosinophilic esophagitis
- IgG4-RD
IgG4-related diseases
- GC
Germinal center
- LLPC
Long-lived plasma cell
- NGS
Next-generation sequencing
- OIT
Oral immunotherapy
- SCIT
Subcutaneous immunotherapy
- SHM
Somatic hypermutation
Footnotes
This article is part of the Topical Collection on Immunologic/Diagnostic Tests in Allergy
Compliance with Ethical Standards
Conflict of Interest Drs. Aalberse, Platts-Mills, and Rispens declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Davies JM, Platts-Mills TA, Aalberse RC. The enigma of IgE+ B-cell memory in human subjects. J Allergy Clin Immunol. 2013;131:972–6. doi: 10.1016/j.jaci.2012.12.1569. [DOI] [PubMed] [Google Scholar]
- 2.Talay O, Yan D, Brightbill HD, Straney EE, Zhou M, Ladi E, et al. IgE+ memory B cells and plasma cells generated through a germinal-center pathway. Nat Immunol. 2012;26:396–404. doi: 10.1038/ni.2256. [DOI] [PubMed] [Google Scholar]
- 3••.Talay O, Yan D, Brightbill HD, Straney EE, Zhou M, Ladi E, et al. Addendum: IgE+ memory B cells and plasma cells generated through a germinal-center pathway. Nat Immunol. 2013;14:1302–4. doi: 10.1038/ni.2770. [Important addendum on how to deal with the incomplete specificity of this IgE reporter mouse system.] [DOI] [PubMed] [Google Scholar]
- 4••.He JS, Meyer-Hermann M, Xiangying D, Zuan LY, Jones LA, Ramakrishna L, et al. The distinctive germinal center phase of IgE+ B lymphocytes limits their contribution to the classical memory response. J Exp Med. 2013;18:2755–71. doi: 10.1084/jem.20131539. [Important new information on the origin and fate of the IgE-switched B cell.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Yang Z, Sullivan BM, Allen CD. Fluorescent in vivo detection reveals that IgE+ B cells are restrained by an intrinsic cell fate predisposition. Immunity. 2012;36:857–72. doi: 10.1016/j.immuni.2012.02.009. [Important new information on the origin and fate of the IgE-switched B cell.] [DOI] [PubMed] [Google Scholar]
- 6•.Laffleur B, Duchez S, Tarte K, Denis-Lagache N, Péron S, Carrion C, et al. Self-restrained B cells arise following membrane IgE expression. Cell Rep. 2015 Feb 12;:S2211–1247(15)00048-0. doi: 10.1016/j.celrep.2015.01.023. [This study describes several striking effects of the mere expression of the membrane-form of IgE (effects that are independent of signals from the cell's environment).] [DOI] [PubMed] [Google Scholar]
- 7.Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ. A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity. 2016;44:116–30. doi: 10.1016/j.immuni.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Gould HJ, Ramadani F. IgE responses in mouse and man and the persistence of IgE memory. Trends Immunol. 2015;36:40–8. doi: 10.1016/j.it.2014.11.002. [A clear and comprehensive overview of the IgE response of mice and man.] [DOI] [PubMed] [Google Scholar]
- 9.Aalberse RC, Platts-Mills TA. How do we avoid developing allergy: modifications of the TH2 response from a B-cell perspective. J Allergy Clin Immunol. 2004;113:983–6. doi: 10.1016/j.jaci.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 10.Platts-Mills TA, Vaughan JW, Blumenthal K, Pollart Squillace S, Sporik RB. Serum IgG and IgG4 antibodies to Fel d 1 among children exposed to 20 microg Fel d 1 at home: relevance of a nonallergic modified Th2 response. Int Arch Allergy Immunol. 2001;124:126–9. doi: 10.1159/000053689. [DOI] [PubMed] [Google Scholar]
- 11.Corcoran LM, Tarlinton DM. Regulation of germinal center responses, memory B cells and plasma cell formation-an update. Curr Opin Immunol. 2016;39:59–67. doi: 10.1016/j.coi.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular helper T cells. Annu Rev Immunol. 2016;34:335–68. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Garcia-Ibanez L, Toellner KM. Regulation of germinal center B-cell differentiation. Immunol Rev. 2016;270:8–19. doi: 10.1111/imr.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anand S, Batista FD, Tkach T, Efremov DG, Burrone OR. Multiple transcripts of the murine immunoglobulin epsilon membrane locus are generated by alternative splicing and differential usage of two polyadenylation sites. Mol Immunol. 1997;34:175–83. doi: 10.1016/s0161-5890(96)00110-1. [DOI] [PubMed] [Google Scholar]
- 15.Karnowski A, Achatz-Straussberger G, Klockenbusch C, Achatz G, Lamers MC. Inefficient processing of mRNA for the membrane form of IgE is a genetic mechanism to limit recruitment of IgE-secreting cells. Eur J Immunol. 2006;36:1917–25. doi: 10.1002/eji.200535495. [DOI] [PubMed] [Google Scholar]
- 16.Kelly KA, Butch AW. Antigen-specific immunoglobulin E+ B cells are preferentially localized within germinal centres. Immunology. 2007;120:345–53. doi: 10.1111/j.1365-2567.2006.02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erazo A, Kutchukhidze N, Leung M, Christ AP, Urban JF, Jr, Curotto de Lafaille MA, et al. Unique maturation program of the IgE response in vivo. Immunity. 2007;26:191–203. doi: 10.1016/j.immuni.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batista FD, Anand S, Presani G, Efremov DG, Burrone OR. The two membrane isoforms of human IgE assemble into functionally distinct B cell antigen receptors. J Exp Med. 1996;184:2197–205. doi: 10.1084/jem.184.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oberndorfer I, Schmid D, Geisberger R, Achatz-Straussberger G, Crameri R, Lamers M. HS1-associated protein X-1 interacts with membrane-bound IgE: impact on receptor-mediated internalization. J Immunol. 2006;177:1139–45. doi: 10.4049/jimmunol.177.2.1139. [DOI] [PubMed] [Google Scholar]
- 20•.Chu VT, Berek C. The establishment of the plasma cell survival niche in the bone marrow. Immunol Rev. 2013;251:177–88. doi: 10.1111/imr.12011. [Describes among others the unexpected positive contribution of the eosinophil to plasma cell survival.] [DOI] [PubMed] [Google Scholar]
- 21.Gauvreau GM, Harris JM, Boulet LP, Scheerens H, Fitzgerald JM, Putnam WS, et al. Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production. Sci Transl Med. 2014;6(243):243ra85. doi: 10.1126/scitranslmed.3008961. [DOI] [PubMed] [Google Scholar]
- 22.Mei HE, Wirries I, Frölich D, Brisslert M, Giesecke C, Grün JR, et al. A unique population of IgG-expressing plasma cells lacking CD19 is enriched in human bone marrow. Blood. 2015;125:1739–48. doi: 10.1182/blood-2014-02-555169. [DOI] [PubMed] [Google Scholar]
- 23.van der Zee JS, van Swieten P, Aalberse RC. Serologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J Immunol. 1986;137:3566–71. [PubMed] [Google Scholar]
- 24.Lighaam LC, Vermeulen E, Bleker T, Meijlink KJ, Aalberse RC, Barnes E, et al. Phenotypic differences between IgG4+ and IgG1+ B cells point to distinct regulation of the IgG4 response. J Allergy Clin Immunol. 2014;133:267–70. doi: 10.1016/j.jaci.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 25.Lighaam LC, Aalberse RC, Rispens T. IgG4-related fibrotic diseases from an immunological perspective: regulators out of control? Int J Rheumatol. 2012;2012:789164. doi: 10.1155/2012/789164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH. IgG4-related disease. Annu Rev Pathol. 2014;9:315–47. doi: 10.1146/annurev-pathol-012513-104708. [DOI] [PubMed] [Google Scholar]
- 27.Kruszewski PG, Russo JM, Franciosi JP, Varni JW, Platts-Mills TA, Erwin EA. Prospective, comparative effectiveness trial of cow's milk elimination and swallowed fluticasone for pediatric eosinophilic esophagitis. Dis Esophagus. 2015 doi: 10.1111/dote.12339. doi:10.1111/dote.12339 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.van Rhijn BD, van Ree R, Versteeg SA, Vlieg-Boerstra BJ, Sprikkelman AB, Terreehorst I, et al. Birch pollen sensitization with cross-reactivity to food allergens predominates in adults with eosinophilic esophagitis. Allergy. 2013;68:1475–81. doi: 10.1111/all.12257. [DOI] [PubMed] [Google Scholar]
- 29.van Rhijn BD, Vlieg-Boerstra BJ, Versteeg SA, Akkerdaas JH, van Ree R, Terreehorst I, et al. Evaluation of allergen-microarray-guided dietary intervention as treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;136:1095–7. doi: 10.1016/j.jaci.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 30.Erwin EA, Tripathi A, Ogbogu PU, Commins SP, Slack MA, Cho CB, et al. IgE antibody detection and component analysis in patients with eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2015;3:896–904. doi: 10.1016/j.jaip.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erwin EA, Kruszewski PG, Russo JM, Schuyler AJ, Platts-Mills TA. IgE antibodies and response to cow's milk elimination diet in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2016:S0091–6749(16)30003-3. doi: 10.1016/j.jaci.2016.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147:602–9. doi: 10.1053/j.gastro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 33.Aalberse RC, Matricardi PM. Immunological aspects of the atopic march. In: Wahn U, Sampson HA, editors. Allergy, immunity and tolerance in early childhood. The first steps of the atopic march. Elsevier Academic Press London; UK: 2016. pp. 19–31. [Google Scholar]
- 34.van der Giessen M, Homan WL, van Kernbeek G, Aalberse RC, Dieges PH. Subclass typing of IgG antibodies formed by grass pollen-allergic patients during immunotherapy. Int Arch Allergy Appl Immunol. 1976;50:625–40. doi: 10.1159/000231566. [DOI] [PubMed] [Google Scholar]
- 35.Dullaers M, De Bruyne R, Ramadani F, Gould HJ, Gevaert P, Lambrecht BN. The who, where, and when of IgE in allergic airway disease. J Allergy Clin Immunol. 2012;129:635–45. doi: 10.1016/j.jaci.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 36.Cabezas-Cruz A, Mateos-Hernández L, Pérez-Cruz M, Valdés JJ, Mera IG, Villar M, et al. Regulation of the immune response to αGal and vector-borne diseases. Trends Parasitol. 2015;31:470–6. doi: 10.1016/j.pt.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Commins SP, Jerath MR, Cox K, Erickson LD, Platts-Mills T. Delayed anaphylaxis to alpha-gal, an oligosaccharide in mammalian meat. Allergol Int. 2016;65:16–20. doi: 10.1016/j.alit.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldo BA, Fisher MM, Pham NH. On the origin and specificity of antibodies to neuromuscular blocking (muscle relaxant) drugs: an immunochemical perspective. Clin Exp Allergy. 2009;39:325–44. doi: 10.1111/j.1365-2222.2008.03171.x. [DOI] [PubMed] [Google Scholar]
- 39.Aalberse RC, Kleine Budde I, Mulder M, Stapel SO, Paulij W, Leynadier F, et al. Differentiating the cellular and humoral components of neuromuscular blocking agent-induced anaphylactic reactions in patients undergoing anaesthesia. Br J Anaesth. 2011;106:665–74. doi: 10.1093/bja/aer028. [DOI] [PubMed] [Google Scholar]
- 40.Florvaag E, Johansson SG, Oman H, Venemalm L, Degerbeck F, Dybendal T, et al. Prevalence of IgE antibodies to morphine. Relation to the high and low incidences of NMBA anaphylaxis in Norway and Sweden, respectively. Acta Anaesthesiol Scand. 2005;49:437–44. doi: 10.1111/j.1399-6576.2004.00591.x. [DOI] [PubMed] [Google Scholar]
- 41.Brusch AM, Clarke RC, Platt PR, Phillips EJ. Exploring the link between pholcodine exposure and neuromuscular blocking agent anaphylaxis. Br J Clin Pharmacol. 2014;78:14–23. doi: 10.1111/bcp.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brusch AM, Clarke RC, Platt PR, Phillips E. Response to letter regarding article ‘Exploring the link between pholcodine exposure and neuromuscular blocking agent anaphylaxis’. Br J Clin Pharmacol. 2014;78:931–2. doi: 10.1111/bcp.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uyttebroek A, Leysen J, Bridts C, Ebo D. Letter to the authors concerning the accepted manuscript: exploring the link between pholcodine and neuromuscular anaphylaxis by Brush et al. Br J Clin Pharmacol. 2014;78:929–30. doi: 10.1111/bcp.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berkowska MA, Heeringa JJ, Hajdarbegovic E, van der Burg M, Thio HB, van Hagen PM, et al. Human IgE+ B cells are derived from T cell-dependent and T cell-independent pathways. J Allergy Clin Immunol. 2014;134:688–697. doi: 10.1016/j.jaci.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 45.Butt D, Chan TD, Bourne K, Hermes JR, Nguyen A, Statham A, et al. FAS inactivation releases unconventional germinal center B cells that escape antigen control and drive IgE and autoantibody production. Immunity. 2015;42:890–902. doi: 10.1016/j.immuni.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 46•.Dominguez PM, Teater M, Chambwe N, Kormaksson M, Redmond D, Ishii J, et al. DNA methylation dynamics of germinal center B cells are mediated by AID. Cell Rep. 2015;12:2086–98. doi: 10.1016/j.celrep.2015.08.036. [Describes a potentially important effect of AID on the control of gene expression (in addition to its role in CSR and SHM).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Hoh RA, Joshi SA, Liu Y, Wang C, Roskin KM, Lee JY, et al. Single B-cell deconvolution of peanut-specific antibody responses in allergic patients. J Allergy Clin Immunol. 2016;137:157–67. doi: 10.1016/j.jaci.2015.05.029. [Proof of principle demonstration of the combination of 3 techniques: 1) Cloning of antibody genes from fluorescent- allergen-sorted single-cells, 2) expressed Fab fragments; and 3) NGS from the same donors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Levin M, King JJ, Glanville J, Jackson KJ, Looney TJ, Hoh RA, et al. Persistence and evolution of allergen-specific IgE repertoires during subcutaneous specific immunotherapy. J Allergy Clin Immunol. 2016;137:1535–44. doi: 10.1016/j.jaci.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Looney TJ, Lee JY, Roskin KM, Hoh RA, King J, Glanville J, et al. Human B-cell isotype switching origins of IgE. J Allergy Clin Immunol. 2016;137:579–86. doi: 10.1016/j.jaci.2015.07.014. [Impressive demonstration of the power of NGS for the investigation of IgE-producing cells. The major overlap between allergic and non-allergic subjects is a strong warning against overinterpreting NGS data into an allergic framework.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aalberse RC, van Ree R, Danneman A, Wahn U. IgE antibodies to tetanus toxoid in relation to atopy. Int Arch Allergy Immunol. 1995;107:169–71. doi: 10.1159/000236967. [DOI] [PubMed] [Google Scholar]
- 51.Grüber C, Lau S, Dannemann A, Sommerfeld C, Wahn U, Aalberse RC. Down-regulation of IgE and IgG4 antibodies to tetanus toxoid and diphtheria toxoid by covaccination with cellular Bordetella pertussis vaccine. J Immunol. 2001;167:2411–7. doi: 10.4049/jimmunol.167.4.2411. [DOI] [PubMed] [Google Scholar]