Abstract
Vitamin K is an enzyme cofactor required for the carboxylation of vitamin K dependent proteins, several of which have been implicated in diseases of aging. Inflammation is recognized as a crucial component of many chronic aging diseases and evidence suggests vitamin K has an anti-inflammatory action that is independent of its role as an enzyme co-factor. Vitamin K-dependent proteins and inflammation have been implicated in cardiovascular disease and osteoarthritis, which are leading causes of disability and mortality in older adults. The purpose of this review is to summarize observational studies and randomized trials focused on vitamin K status and inflammation, cardiovascular disease, and osteoarthritis. Although mechanistic evidence suggests a protective role for vitamin K in these age-related conditions, the benefit of vitamin K supplementation is controversial because observational data are equivocal and the number of randomized trials is few.
Keywords: Cardiovascular disease, inflammation, older adults, menaquinone, osteoarthritis, phylloquinone, vitamin K
Introduction
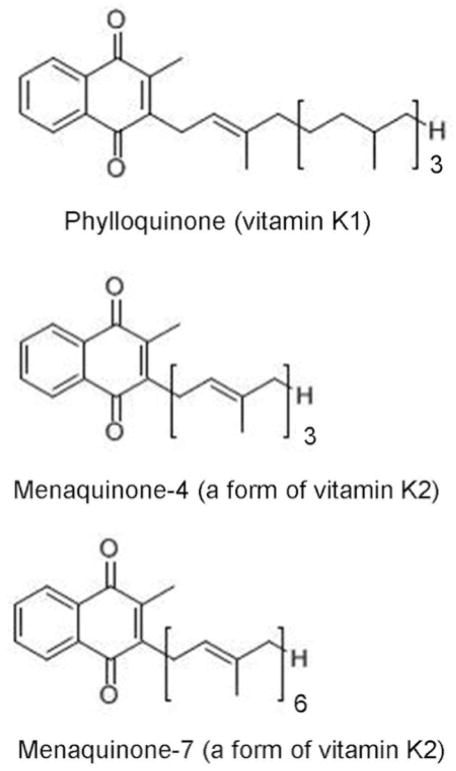
Vitamin K is a fat soluble vitamin found in two natural forms: phylloquinone (vitamin K1) and menaquinones (collectively known as vitamin K2), which differ from phylloquinone in length and saturation of the side chain [1*] (Figure 1). Phylloquinone, found predominantly in dark green leafy vegetables and vegetable oils is the primary dietary form of vitamin K in Western diets [2,3]. Menaquinones, which are found in animal based foods such as dairy and meats, as well as in fermented foods, are thought to contribute less than phylloquinone to overall vitamin K intakes in Western diets [4–6]. The current recommended Adequate Intake for vitamin K set by the United States’ Institute of Medicine is 120 micrograms and 90 micrograms per day for adult males and females respectively [3]. Vitamin K intake has shown great variation among age groups and geographic location [7]. Among those at greater risk for low vitamin K status are older adults [8,9].
Figure 1.
Forms of Vitamin K.
The only known function of vitamin K is as an enzymatic co-factor for the post-translational carboxylation of certain proteins (called vitamin K-dependent proteins). Carboxylation confers function to these proteins. While the most common vitamin K-dependent proteins are clotting proteins, vitamin K dependent proteins have been discovered in several extra-hepatic tissues and have important physiological functions, for example in soft-tissue calcification and bone metabolism [1*,10–12]. Additionally, vitamin K has been shown to have anti-inflammatory effects, through mechanisms that appear to be independent of its role as an enzymatic co-factor [13–15]. Vitamin K and vitamin K-dependent proteins have been linked to several age-related diseases in observational and intervention studies. The purpose of this review is to summarize key population-based studies and randomized trials focused on vitamin K nutritional status and age-related health outcomes in community-dwelling adults, namely inflammation, cardiovascular disease and osteoarthritis (Table 1). Vitamin K has been implicated in age-related bone loss and studied extensively in that regard. This body of literature was reviewed extensively in 2014 [16], so studies focused on bone loss and fractures are not included here. Additionally, kidney function declines with age and a unique role for vitamin K in kidney disease has been proposed, which has been thoroughly reviewed [17]. Therefore studies focused on kidney disease are not included here.
Table 1.
Observational studies of vitamin K, inflammation, cardiovascular disease, and osteoarthritis
| Participants | Study Design | Vitamin K status exposure | Outcome(s) | Overall finding | Reference |
|---|---|---|---|---|---|
| Inflammation | |||||
| PREDIMED N=510 Age: (yrs) 67.2±6 55% female |
Cross-sectional | Dietary phylloquineon intake (mcg/d) | Inflammatory biomarkers (12+ including TNF-a, IL-6) | Individuals who increased dietary phylloquinone intake had reduced IL-6. | [23] |
| Framingham Offspring Study N=1,381 Age: 59±8 52% female |
Cross-sectional | Plasma phylloquinone (nmol/L) Dietary phylloquinone intake (mcg/d) %ucOC |
Inflammation summary statistic Inflammatory biomarkers (13+) |
Plasma phylloquinone inversely correlated with grouped inflammatory markers, and CD40 ligand, IAM-1, IL-6, OPG, TNF-α individually. | [11] |
| MESA N=662 Age: 62 ±10 46% female |
Cross-sectional | Plasma phylloquinone (nmol/L) | Inflammatory biomarkers (6+ including IL-6, CRP) | Individuals with highest plasma phylloquinone had lower IL-6 levels. | [26] |
| N=388 Age: 68±6 60% female |
RCT | phylloquinone supplementation (500mcg/d) MGP (ng/mL) |
IL-6, CRP | No effect of supplementation on inflammatory markers. | [27] |
| N= 244 Post-menopausal women Age: 59.5±3.3 |
RCT | Menaquinone-7 supplementation (180mcg/d) Dp-ucMGP (pmol/L) |
IL-6, CRP, TNF-a | No effect of supplementation on inflammatory markers. | [28] |
| Arterial calcification, stiffness, and CVD | |||||
| MESA N=857 Age: 64±10 45% female |
Case-cohort | Plasma phylloquinone (nmol/L) | CAC Agatston score category: 0 AU 1–400 AU >400 AU |
Low phylloquinone status is associated with greater CAC progression in antihypertensive users. | [37] |
| N=508 post-menopausal women Age: 56±6 |
Longitudinal | Plasma phylloquinone (nmol/L) | Aortic valve, mitral valve, or aortic artery calcification | Detectable circulating phylloquinone was not associated with reduced vascular calcification. | [39] |
| N=388 Age: 68±6 60% female |
RCT | phylloquinone supplementation (500mcg/d) MGP (ng/mL) |
CAC Agatston score | Intent to treat analysis: no difference in supplemented group. In those who adherence >85% had slowed progression. |
[27] |
| N=564 Post-menopausal women Age: 67±5 |
Cross-sectional | Dietary menaquinone intake (mcg/d) | CAC Agatston score | High dietary menaquinone intake is associated with decreased coronary calcification. | [59] |
| Rotterdam Study N=4807 Age: 67±8 60% female |
Longitudinal | Dietary menaquinone intake (mcg/d) | Aortic calcification | Menaquinone intake in inversely associated with severe aortic calcification. | [60] |
| Prospect-EPIC study N=16,057 post menopausal women Age: 57±6 |
Longitudinal | Dietary phylloquinone intake (mcg/d) Dietary menaquinone intake (mcg/d) |
CVD | Higher menaquinone intake associated with lower incidence in CVD. | [61] |
| N=60 Age 60±3 60% female |
RCT | Menaquinone-7 supplementation: 180 μg/d, 360 μg/d MK-7 or placebo for 12 weeks |
Plasma dp-ucMGP, dp-cMGP, HDL, TGs | Menaquinone-7 decreased dp-ucMGP in a dose dependent manner. No effect on cardiovascular risk factors. | [64] |
| N=374 Age: 68±6 60% female |
RCT | phylloquinone supplementation (500mcg/d) | Plasma ucMGP (ng/mL) | Phylloquinone supplementation significantly reduced plasma ucMGP over 3 years, but 3 year changein ucMGP was not associated with CAC | [43] |
| N=200 Post menopausal women Age: 66.9±5.5 |
Cross-sectional | Plasma dp-ucMGP | CAC | Trend towards high ucMGP associated with decreased CAC. | [45] |
| EPIC-NL N= 518 Type 2 diabetics Age: 58.1±7.1 82% female |
Longitudinal | Plasma dp-ucMGP | CVD risk | Higher circulating dp-ucMGP was associated with significant increased risk of CVD in type 2 diabetics with peripheral artery disease (PAD) and heart failure. | [46] |
| EPIC-NL N=2985 Age: 49.5±11.8 75% female |
Case-cohort | Dp-ucMGP | CVD and stroke risk | No association between circulating dp-ucMGP and stroke risk or CVD risk. | [47] |
| LASA N=577 Age: > 55 |
Longitudinal | Plasma dp-ucMGP | CVD incidence | Increased risk of CVD in highest tertile of dp-ucMGP indicative of vitamin K insufficiency. | [48] |
| NHANES N=5296 Age: >50 |
Cross-sectional | Dietary phylloquinone intake (mcg/d) | Arterial stiffness by Pulse pressure | Inadequate dietary phylloquinone intake was a strong and significant predictor of higher arterial pulse pressure. | [55] |
| Czech MONICA study N=1087 Age: 54.8±13 53% female |
Cross-sectional | Dp-ucMGP | Arterial stiffness by aortic and distal pulse wave velocities | Individuals with highest circulating dp-ucMGP had highest risk of elevated aortic pulse wave velocity, indicating aortic stiffness. | [56] |
| N=1001 Age: 46.5±17.2 52% female |
Cross-sectional | Dp-ucMGP | Arterial stiffness by aortic pulse wave velocity | Circulating dp-ucMGP were positively associated with dp-ucMGP before and after adjusting for lifestyle and health factors | [57] |
| N= 244 Post-menopausal women Age: 59.5±3.3 |
RCT | Menaquinone-7 supplementation (180mcg/d) | Aterial stiffness by aortic and arm pulse wave velocity | Menaquinone-7 supplementation improved arterial stiffness in individuals with higher baseline stiffness index. | [28] |
| Osteoarthritis | |||||
| ROAD study N=719 Age: >60 60% female |
Cross-sectional | Dietary phylloquinone (mcg/d) | Radiographic knee OA | Dietary intake was inversely associated with presence of knee OA and JSN | [70] |
| Framingham Offspring Study N=672 Age: 65.6±8.5 53% female |
Cross-sectional | Plasma phylloquinone (nmol/L) | Osteoarthritis (OA) prevalence Osteophytes Joint space narrowing (JSN) |
Low plasma phylloquinone associated with increase prevalence of OA in the hand and knee | [71] |
| MOST study N=1180 Age: 62±8 62% female |
Longitudinal | Plasma phylloquinone (nmol/L) | Radiographic knee OA | Individuals with subclinical circulating phylloquinone were more likely to develop knee OA | [72] |
| Health ABC N=791 Age: 74±3 67% female |
Cross-sectional Longitudinal |
Plasma phylloquinone (nmol/L) Dp-ucMGP |
Knee OA structural features by MRI | Longitudinally, adults with low plasma phylloquinone more likely to have articular and meniscus cartilage damaged. Cross sectionally, higher plasma dp-ucMGP was associated with increased odds of OA features including osteophytes, and lesions. | [73] |
| N=376 Age: 71±5.5 60% female |
RCT | Serum MGP (ng/mL) | Radiographic hand OA | No association between circulating MGP and hand OA | [75] |
PREDIMED: PREvención con DIeta MEDiterránea study
MESA study: Multiethnic Study on Atherosclerosis
Prospect-EPIC study: The European Prospective Investigation into Cancer and Nutrition Prospect cohort
EPIC NL- The European Prospective Invesigation into Cancer Dutch cohort
LASA: Longitudinal Aging Study in Amsterdam
NHANES: National Health and Nutrition Examination Surveys
ROAD study: Research on Osteoarthritis Against Disability
MOST study: The Multicenter Osteoarthritis Study
Health ABC: Health, Aging, and Body Composition study
Vitamin K and inflammation
Aging is characterized by a chronic low-grade pro-inflammatory state [18]. Age related increases in C-reactive protein (CRP), interleukin- (IL-6), and tumor necrosis factor-α (TNF-α) resulting in the low-grade inflammation appear to contribute to the onset and progression of chronic aging diseases including cardiovascular disease, osteoarthritis and other chronic diseases [19–22].
In vitro and animal experiments have found vitamin K suppresses production of pro-inflammatory cytokines [13–15]. At this time, however, the relevance of vitamin K nutritional status to inflammation in humans remains unclear. In a cross-sectional analysis of the Framingham Offspring (n= 1,381; mean age = 59±8 years), higher vitamin K intake was associated with lower inflammation overall and with lower concentrations of several individual pro-inflammatory biomarkers [11]. In a secondary analysis of the PREDIMED trial, a Mediterranean diet intervention being conducted in Spain, (n= 510; mean age = 67.2±6 years) participants who increased their dietary phylloquinone intake the most (≥70 mcg/d) had the greatest reductions in IL-6, and TNF-α over 1 year. In this analysis, the intervention and control groups were combined, which is not consistent with the RCT design [23]. In both studies [11,23] vitamin K intake was estimated using self-report measures, which are inherently limited (as recently reviewed [24**]). Phylloquinone is found in generally healthy foods, so it cannot be discounted that the reported associations reflect a healthy diet rather than phylloquinone intake specifically.
Nutritional biomarkers are considered more objective measures of nutrient status and reflect nutrient intake, metabolism, and absorption [25]. There are multiple biomarkers indicative of vitamin K status, but no single biomarker is considered the ‘gold-standard’ [24**]. Circulating phylloquinone, a global indicator of vitamin K status, has been evaluated in relation to inflammation. In the Framingham Offspring higher plasma phylloquinone was also associated with lower inflammatory-burden cross-sectionally, consistent with the findings of vitamin K intake [11]. Higher serum phylloquinone was also associated with lower inflammation cross-sectionally in a multi-ethnic cohort of adults without clinically apparent CVD (the Multi-ethnic Study of Atherosclerosis, MESA, n= 662; mean age=62±10 years) [26].
Vitamin K status can also be estimated by measuring the uncarboxylated fractions of certain vitamin K dependent proteins in circulation, as recently reviewed [24**]. Osteocalcin (OC) is a vitamin K dependent protein synthesized in bone and higher circulating uncarboxylated OC (ucOC) reflects low vitamin K status [24**]. In the Framingham Offspring, ucOC, however, was overall not associated with inflammation [11]. That plasma phylloquinone, but not ucOC, was associated with an inflammatory burden, suggests vitamin Ks role in inflammation is independent of its role as an enzyme factor. Since this has not been well studied, it is premature to draw conclusions about the mechanism underlying the apparent anti-inflammatory effects of vitamin K. Additionally, these studies are cross-sectional [11,26], so causal relationship between vitamin K status and inflammation is uncertain.
Randomized trials can address causality and inflammatory measures have been evaluated as secondary outcomes in two trials conducted in older adults. However, neither phylloquinone [27] nor menaquinone-7 [28] supplementation reduced circulating inflammatory biomarkers in older men and women over 3 years. Both of these studies enrolled generally healthy older adults, who are less likely to have substantial increases in inflammation, so any ability to see an effect of vitamin K (or any nutrient) in reducing inflammation may have been blunted. It is plausible the anti-inflammatory effects of vitamin K may be more relevant to groups with a higher inflammatory burden.
Vitamin K, arterial calcification, and cardiovascular disease
Cardiovascular disease (CVD) is the leading cause of mortality in adults ≥ 65 years old [29]. Coronary artery calcification (CAC) is indicative of subclinical CVD and predicts clinical cardiovascular events and all-cause mortality [30–33]. A role for vitamin K in CVD has been proposed, based on the presence of vitamin K-dependent proteins, such as matrix Gla protein (MGP), in vascular tissue [34,35*]. When MGP is carboxylated, which requires vitamin K, it inhibits calcification in arterial and other soft tissues.
The association between vitamin K status, CAC, and CVD has been evaluated, with equivocal results, as reviewed in 2012 [36*]. Results of more recent studies are also inconclusive. In a case-cohort analysis of the MESA, low serum phylloquinone was associated with a 34% higher odds of CAC progression over 3 years, but statistical significance was not reached [OR(95%CI) 1.34(0.94–1.90)] [37]. In secondary analysis, low serum phylloquinone was associated with a 2-fold higher odds of CAC progression in persons treated for hypertension [OR (95% CI): 2.37 (1.38 – 4.09)] but was not associated with CAC progression in persons not treated for hypertension. Although this was not hypothesized a priori, it was replicated in a post hoc analysis of a phylloquinone supplementation trial [37]. The circulating concentration of phylloquinone considered sufficient is not yet defined clinically, so in this study low plasma phylloquinone was defined as < 1.0nmol/L, which is the concentration achieved when Adequate Intakes are met [8,38]. In a sub-study of the Dutch Prospect cohort, post-menopausal women (n=508 mean±SD age 57±5 years), with higher plasma phylloquinone, defined as >0.7 nmol/L, had a higher prevalence of CAC [39]. However in this study, CAC was defined as absent or present at a single time point measured 7 – 11 years after plasma phylloquinone was measured, so it is not known if CAC was actually prevalent at the time phylloquinone status was assessed. Furthermore, this analysis did not account for triglycerides, which may have confounded the findings. Phylloquinone is transported on triglyceride-rich lipoproteins [40], so adjustment for triglycerides is imperative in studies utilizing circulating phylloquinone as a biomarker, especially in relation to CVD since elevated triglycerides are a CVD risk factor [41].
Assays that measure uncarboxylated MGP in plasma have been developed [42] and are now commercially available. The dephosphorylated uncarboxylated MGP ((dp)ucMGP) responds to changes in vitamin K intake [43,44] and is thought to be a functional indicator of vitamin K status in tissues that use MGP. Higher plasma (dp)ucMGP reflects lower vitamin K status. (Dp)ucMGP has been evaluated in relation to cardiovascular outcomes in clinical observational studies. (There are other circulating forms of MGP, some of which have been evaluated in relation to CVD, but do not reflect vitamin K status [42], so are not considered here.) In 195 post-menopausal women analyzed cross-sectionally, higher (dp)ucMGP was associated with more CAC, although statistical significance was borderline (p=0.065) [45]. In a secondary analysis of the randomized controlled trial that found phylloquinone supplementation reduced CAC progression in older men and women, (dp)ucMGP was reduced by phylloquinone supplementation, but the change in (dp)ucMGP did not correlate with change in CAC [43]. In type II diabetics in the Dutch European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, each standard deviation increase in (dp)ucMGP (reflecting lower vitamin K status) was associated with a 21% higher risk for CVD over 11 years of follow-up (HR(95%CI) 1.21 (1.06–1.38) [46]. However, a case-cohort analysis of the same cohort, did not find (dp)ucMGP to be associated with coronary heart disease or stroke [47]. In contrast, a longitudinal study conducted in 577 community dwelling older men and women in the Logitudinal Aging Study Amersterdam (LASA) who were free of CVD at baseline found that after a mean follow-up of 5.6+1.2 years, individuals with the highest circulating (dp)ucMGP had a 2-fold significantly higher risk of CVD [HR(95%CI) 2.69(1.09–6.62)] [48].
A growing body of evidence suggests arterial calcification is also implicated in arterial stiffness [49–51]. Arterial stiffness increases with age and is also an independent risk factor for CVD [52–54]. Examination of data from the National Health and Nutrition Examination Surveys 2007–2008, 2009–2010 of older adults (n=5296, age >50 yrs) showed that inadequate phylloquinone intake was a significant independent predictor of high arterial stiffness [55], although it is plausible this association is due, in part, to participants reporting low phylloquinone intake also consuming a less healthy diet. A cross sectional analysis of the Czech post-MONICA study found individuals in the highest dp-ucMGP quartile had a higher risk of elevated aortic stiffness [OR(95% CI): 1.73 (1.17–2.5)] [56]. Similar findings were obtained in a cross-sectional analysis of a family based study in Switzerland, in which (dp)ucMGP was positively associated with arterial stiffness after adjusting for various confounders [57]. Menaquinone-7 supplementation improved some parameters of arterial stiffness over 3 years in post-menopausal women (n=244; mean±age = 60±3) in a randomized trial designed test the effect of menaquinone supplementation on bone strength, but measured arterial stiffness as a secondary outcome [28]. It is not known if these findings are generalizable to men or other groups.
There are two systematic reviews examining the association between vitamin K status and CVD. The first one, published in 2010, suggested a beneficial effect of menaquinone intake, but not phylloquinone intake, in lowering CVD risk [58]. This is primarily due to three studies conducted in the Netherlands that all reported inverse associations between menaquinone intake and CVD [59–61]. Whether or not these findings generalize to other countries or nationalities is not known. Menaquinone intake was assessed using food frequency questionnaires, which carry inherent limitations [62]. At the time these studies were conducted food composition databases for menaquinones were limited. (They are currently being expanded) [24**]. It is therefore premature to draw conclusions regarding the relative importance of phylloquinone and menaquinones with respect to CVD based on the available studies. A systematic review of vitamin K and CVD published in 2015 sought to include only vitamin K supplementation trials of at least 3 months in duration and conducted in healthy adults or adults at high risk for CVD [63]. Ultimately only one trial that tested the effect of menaquinone-7 supplementation on blood pressure and lipid levels over 12 weeks 60 men and women 45–60 years old was included in the review [63,64]. Overall, the authors concluded there is insufficient evidence to conclude vitamin K affects CVD [63]. Of note, this review did not include the only randomized trial designed to test the effect of phylloquinone supplementation on CAC progression in older adults (n=388, mean±SD; age = 68±6 yrs) because the intervention and control groups both received calcium and vitamin D (to assure all participants were replete in those nutrients); hence the trial lacked a pure placebo group [27,63]. This trial found older adults who adhered to the 3-year phylloquinone supplementation intervention had significantly less CAC progression compared to adherent participants in the control group, suggesting a protective effect of phylloquinone against subclinical CVD. In the intent-to-treat analysis, however, phylloquinone supplementation did not significantly affect CAC progression [27]. In a subsequent post-hoc analysis of this trial it was found that phylloquinone supplementation reduced CAC progression in participants treated for hypertension but did not affect CAC progression in participants not treated for hypertension [37]. While this suggests vitamin K may be particularly beneficial to hypertension therapeutic regimens, these findings need to be confirmed in studies designed to study treated hypertensives.
Although some observational studies suggest improving vitamin K status may reduce subclinical and clinical CVD, data are conflicting [27,37,39,59,61]. Additional prospective studies are necessary to determine whether increasing vitamin K intake decreases risk for cardiovascular events and subclinical CVD and whether this is modulated by (dp)ucMGP.
Vitamin K and osteoarthritis
Osteoarthritis is the leading cause of lower-extremity disability in older adults, and there is currently no therapy known to reduce osteoarthritis progression. Osteoarthritis is characterized by pathological changes in all joint tissues, including cartilage and bone. Vitamin K has been implicated in osteoarthritis because vitamin K dependent proteins are found in cartilage and bone [65–67]. MGP is among the most studied vitamin K dependent protein expressed in human cartilage. Uncarboxylated MGP (the nonfunctional form) is elevated in human arthritic cartilage, while carboxylated (functional) MGP is more abundant in healthy cartilage [65]. Gla rich protein (GRP), which is another vitamin K dependent implicated in calcification, has recently identified in human articular cartilage [68]. Similar to MGP, GRP from arthritic cartilage is primarily uncarboxylated, whereas GRP from healthy cartilage is primarily carboxylated [69].
In a cross-sectional analysis of older men and women from Japan, low vitamin K intake was associated with a higher prevalence of radiographic knee osteoarthritis [70]. In a cross-sectional analysis of the Framingham Offspring, low plasma phylloquinone, was associated with higher knee and hand osteoarthritis prevalence [71]. In the Multicenter Osteoarthritis (MOST) Study (mean±SD age = 62±8 yrs), participants with subclinical vitamin K deficiency (defined as plasma phylloquinone < 0.5 nmol/L) were 1.5 – 2 times more likely to develop radiographic knee osteoarthritis and cartilage damage over 30 months (risk ratio (95%CI) 1.56(1.08–2.25) and 2.39(1.05–5.40) respectively, compared to those without subclinical deficiency) [72]. In the Health Aging and Body Composition (Health ABC) Study (mean±SD age = 74±3 yrs), older community-dwelling adults with very low plasma phylloquinone (defined as below the assay limit of detection, <0.2 nmol/L) had a 1.7-and 2.6-fold higher odds of worsening cartilage damage and meniscal damage over 3 years. This analysis also suggested participants with very low phylloquinone were more likely to have bone attrition, subarticular cyst, and osteophyte progression, but statistical significance was not reached [OR (95% CI): 1.9(0.9–3.6); 1.5(0.8–2.7); 1.5(0.8–2.8) respectively] [73]. In this same study, individuals with elevated (dp)ucMGP (reflecting low vitamin K status) were more likely to have osteophytes, bone marrow lesions, subarticular cysts, and meniscus damage cross-sectionally, but this was not associated with progression of any structural abnormalities in the longitudinal analysis [73]. This may suggest vitamin Ks role in these pathologies is independent of its function as an enzymatic co-factor in the carboxylation of MGP. Neogi et al assessed the effect of phylloquinone supplementation on radiographic hand osteoarthritis using x-rays obtained at the end of study and did not find any effect of 3 years phylloquinone supplementation [74]. No baseline measures of hand osteoarthritis were available in this study. When participants with baseline plasma phylloquinone <1.0nM were analyzed separately, there was a trend towards less joint space narrowing (a measure of radiographic osteoarthritis) in the phylloquinone supplemented group, suggesting persons with low vitamin K status are more likely to benefit from vitamin K supplementation, but this finding needs to be confirmed in trials designed specifically to test the effect of vitamin K on osteoarthritis development and progression. In this same cohort, MGP genotype was found to be associated with hand OA, but circulating total MGP concentrations were not [75].
While collective data suggest a protective role for vitamin K in osteoarthritis, several questions remain. Low circulating phylloquinone was associated with more OA in three cohorts, but low circulating phylloquinone has not been consistently defined [72,73,75]. It is not clear what level is ‘sufficient’ in terms of joint health. Vitamin Ks role in osteoarthritis was proposed because vitamin K dependent proteins are present in cartilage and bone. However, alternate mechanisms may exist. Osteoarthritis has been characterized as having an inflammatory component with measureable cytokines and inflammatory mediators present in the synovium of the joint resulting in joint pain, swelling, and stiffness [22]. Vitamin K appears to have anti-inflammatory effects [13–15], suggesting an alternate pathway through which vitamin K may affect joint health – an area of research that merits attention. Clinical trials are needed to evaluate the efficacy of vitamin K supplementation in OA development and progression.
Conclusion
There is accumulating evidence to support a protective role for vitamin K in chronic aging conditions and diseases, such as inflammation, cardiovascular disease, and osteoarthritis, but there are also inconsistencies in the studies conducted to date. It is therefore premature to make recommendations regarding vitamin K’s efficacy in improving inflammation, and cardiovascular and joint health. Clinical trials designed to test the effect of vitamin K supplementation on these chronic age-related conditions are needed. As the aging population continues to grow, the prevalence of these diseases will rise dramatically. Identifying and understanding nutritional factors that impact the progression or treatment of these diseases is imperative to address the health and function of older adults worldwide.
Acknowledgments
All authors have read and approved the final manuscript. Supported by the National Institute of Arthritis Musculoskeletal and Skin Diseases (K01AR063167) and U.S. Department of Agriculture, Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture.
Footnotes
Conflict of Interest
Stephanie G. Harshman and M. Kyla Shea declare that they have no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Stephanie G. Harshman, Email: stephanie.harshman@tufts.edu, Jean Mayer Human Nutrition Research Center on Aging, Tufts University 711 Washington Street, Boston, MA 02111, Phone number: 617-556-3151, Fax number: 617 556 3149.
M. Kyla Shea, Email: kyla.shea@tufts.edu, Jean Mayer Human Nutrition Research Center on Aging, Tufts University 711 Washington Street, Boston, MA 02111, Phone number: 617-556-3073, fax number: 617 556 3344.
References
(*) of importance
(**) of major importance
- 1*.Booth SL. Roles for vitamin K beyond coagulation. Annu Rev Nutr. 2009;29:89–110. doi: 10.1146/annurev-nutr-080508-141217. A review of emerging evidence suggesting physiological and biochemical functions of vitamin K beyond that as an enzyme cofactor for vitamin K dependent clotting proteins. [DOI] [PubMed] [Google Scholar]
- 2.Booth SL, Suttie JW. Dietary intake and adequacy of vitamin K. J Nutr. 1998;128:785–8. doi: 10.1093/jn/128.5.785. [DOI] [PubMed] [Google Scholar]
- 3.Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr. 2012;3:182–95. doi: 10.3945/an.111.001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 2000;30:298–307. doi: 10.1159/000054147. [DOI] [PubMed] [Google Scholar]
- 5.Elder SJ, Haytowitz DB, Howe J, Peterson JW, Booth SL. Vitamin k contents of meat, dairy, and fast food in the u. Diet J Agric Food Chem. 2006;54:463–7. doi: 10.1021/jf052400h. [DOI] [PubMed] [Google Scholar]
- 6.Booth SL. Vitamin K: food composition and dietary intakes. Food Nutr Res. 2012:56. doi: 10.3402/fnr.v56i0.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth SL, Al Rajabi A. Determinants of vitamin K status in humans. Vitam Horm. 2008;78:1–22. doi: 10.1016/S0083-6729(07)00001-5. [DOI] [PubMed] [Google Scholar]
- 8.Booth SL, Martini L, Peterson JW, Saltzman E, Dallal GE, Wood RJ. Dietary phylloquinone depletion and repletion in older women. J Nutr. 2003;133:2565–2569. doi: 10.1093/jn/133.8.2565. [DOI] [PubMed] [Google Scholar]
- 9.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 10.Reumann S. Biosynthesis of vitamin k1 (phylloquinone) by plant peroxisomes and its integration into signaling molecule synthesis pathways. Subcell Biochem. 2013;69:213–29. doi: 10.1007/978-94-007-6889-5_12. [DOI] [PubMed] [Google Scholar]
- 11.Shea MK, Booth SL, Massaro JM, Jacques PF, D’Agostino RB, Sr, Dawson-Hughes B, Ordovas JM, O’Donnell CJ, Kathiresan S, Keaney JF, Jr, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167:313–320. doi: 10.1093/aje/kwm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suttie JW, Booth SL. Vitamin K. Adv Nutr. 2011;2:440–1. doi: 10.3945/an.111.000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohsaki Y, Shirakawa H, Hiwatashi K, Furukawa Y, Mizutani T, Komai M. Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat. Biosci Biotechnol Biochem. 2006;70:926–932. doi: 10.1271/bbb.70.926. [DOI] [PubMed] [Google Scholar]
- 14.Ohsaki Y, Shirakawa H, Miura A, Giriwono PE, Sato S, Ohashi A, Iribe M, Goto T, Komai M. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor kappaB through the repression of IKKalpha/beta phosphorylation. J Nutr Biochem. 2010;21:1120–1126. doi: 10.1016/j.jnutbio.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Reddi K, Henderson B, Meghji S, Wilson M, Poole S, Hopper C, Harris M, Hodges SJ. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine. 1995;7:287–90. doi: 10.1006/cyto.1995.0034. [DOI] [PubMed] [Google Scholar]
- 16.Shea MK, Booth SL. Vitamin K’s Role in Age-Related Bone Loss: A Critical Review. In: Holick M, Nieves J, editors. Nutrition and Bone Health. Springer Sciences; 2014. pp. 471–486. [Google Scholar]
- 17.McCabe KM, Adams MA, Holden RM. Vitamin K status in chronic kidney disease. Nutrients. 2013;5:4390–8. doi: 10.3390/nu5114390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ershler WB. Interleukin-6: A Cytokine for Gerontolgists. J Am Geriatr Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 20.Strandberg TE, Tilvis RS. C-Reactive Protein, Cardiovascular Risk Factors, and Mortality in a Prospective Study in the Elderly. Arterioscler Thromb Vasc Biol. 2000;20:1057–1060. doi: 10.1161/01.atv.20.4.1057. [DOI] [PubMed] [Google Scholar]
- 21.Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK. A High Plasma Concentration of TNF- Is Associated With Dementia in Centenarians. Journals Gerontol Ser A Biol Sci Med Sci. 1999;54:M357–M364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- 22.Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1966–71. doi: 10.1016/j.joca.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juanola-Falgarona M, Salas-Salvadó J, Estruch R, Portillo MP, Casas R, Miranda J, Martínez-González MA, Bulló M, Salas-Salvado J, Martinez-Gonzalez MA, et al. Association between dietary phylloquinone intake and peripheral metabolic risk markers related to insulin resistance and diabetes in elderly subjects at high cardiovascular risk. Cardiovasc Diabetol. 2013;12:7. doi: 10.1186/1475-2840-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Shea MK, Booth SL. Concepts and Controversies in Evaluating Vitamin K Status in Population-Based Studies. Nutrients. 2016:8. doi: 10.3390/nu8010008. A recent review outlining the various methods to estimate vitamin K nutrition status in population and clinical studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potischman N. Biologic and methodologic issues for nutritional biomarkers. J Nutr. 2003;133(Suppl):875S–880S. doi: 10.1093/jn/133.3.875S. [DOI] [PubMed] [Google Scholar]
- 26.Shea MK, Cushman M, Booth SL, Burke GL, Chen H, Kritchevsky SB. Associations between vitamin K status and haemostatic and inflammatory biomarkers in community-dwelling adults. The Multi-Ethnic Study of Atherosclerosis. Thromb Haemost. 2014;112:438–44. doi: 10.1160/TH13-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shea MK, O’Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009;89:1799–807. doi: 10.3945/ajcn.2008.27338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knapen M, Braam L, Drummen N, Bekers O, Hoeks APG, Vermeer C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. Thromb Haemost. 2015;113:1135–1144. doi: 10.1160/TH14-08-0675. [DOI] [PubMed] [Google Scholar]
- 29.National Center for Health Statistics. Health, United States 2014: With Special Feature on Adults Aged 55–64. 2015. [PubMed] [Google Scholar]
- 30.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 31.Thompson GR, Partridge J. Coronary calcification score: the coronary-risk impact factor. Lancet (London, England) 2004;363:557–9. doi: 10.1016/s0140-6736(04)15544-x. [DOI] [PubMed] [Google Scholar]
- 32.Vliegenthart R, Oudkerk M, Hofman A, Oei H-HS, van Dijck W, van Rooij FJA, Witteman JCM. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–7. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 33.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3:1229–36. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 35*.Berkner KL, Runge KW. The physiology of vitamin K nutriture and vitamin K-dependent protein function in atherosclerosis. J Thromb Haemost. 2004;2:2118–32. doi: 10.1111/j.1538-7836.2004.00968.x. A review of vitamin K-dependent protein function and vitamin K nutriture in atherosclerosis. [DOI] [PubMed] [Google Scholar]
- 36*.Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158–65. doi: 10.3945/an.111.001644. A review summarizing the current evidence of vitamin K and vascular calcification in communnity based studies and clinic based studies focusing on patients with kidney disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shea MK, Booth SL, Miller ME, Burke GL, Chen H, Cushman M, Tracy RP, Kritchevsky SB. Association between circulating vitamin K1 and coronary calcium progression in community-dwelling adults: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;98:197–208. doi: 10.3945/ajcn.112.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Institute of Medicine. Dietary references intakes for vitamin K, arsenic, chromium, copper, iodine, iron, managanses, molybeum, silicon, vanadium, and zinc. 2001. [Google Scholar]
- 39.Dalmeijer GW, van der Schouw YT, Booth SL, de Jong PA, Beulens JWJ. Phylloquinone concentrations and the risk of vascular calcification in healthy women. Arterioscler Thromb Vasc Biol. 2014;34:1587–90. doi: 10.1161/ATVBAHA.114.303853. [DOI] [PubMed] [Google Scholar]
- 40.Lamon-Fava S, Sadowski JA, Davidson KW, O’Brien ME, McNamara JR, Schaefer EJ. Plasma lipoproteins as carriers of phylloquinone (vitamin K1) in humans. Am J Clin Nutr. 1998;67:1226–31. doi: 10.1093/ajcn/67.6.1226. [DOI] [PubMed] [Google Scholar]
- 41.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet (London, England) 2014;384:626–35. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 42.Cranenburg ECM, VAN Spaendonck-Zwarts KY, Bonafe L, Mittaz Crettol L, Rödiger LA, Dikkers FG, VAN Essen AJ, Superti-Furga A, Alexandrakis E, Vermeer C, et al. Circulating matrix γ-carboxyglutamate protein (MGP) species are refractory to vitamin K treatment in a new case of Keutel syndrome. J Thromb Haemost. 2011;9:1225–35. doi: 10.1111/j.1538-7836.2011.04263.x. [DOI] [PubMed] [Google Scholar]
- 43.Shea MK, O’Donnell CJ, Vermeer C, Magdeleyns EJP, Crosier MD, Gundberg CM, Ordovas JM, Kritchevsky SB, Booth SL. Circulating uncarboxylated matrix gla protein is associated with vitamin K nutritional status, but not coronary artery calcium, in older adults. J Nutr. 2011;141:1529–34. doi: 10.3945/jn.111.139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theuwissen E, Cranenburg EC, Knapen MH, Magdeleyns EJ, Teunissen KJ, Schurgers LJ, Smit E, Vermeer C. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br J Nutr. 2012;108:1652–7. doi: 10.1017/S0007114511007185. [DOI] [PubMed] [Google Scholar]
- 45.Dalmeijer GW, van der Schouw YT, Vermeer C, Magdeleyns EJ, Schurgers LJ, Beulens JWJ. Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J Nutr Biochem. 2013;24:624–8. doi: 10.1016/j.jnutbio.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Dalmeijer GW, van der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WMM, Boer JMA, Beulens JWJ. Matrix Gla protein species and risk of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2013;36:3766–71. doi: 10.2337/dc13-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalmeijer GW, van der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WMM, Boer JMA, Beulens JWJ. Circulating desphospho-uncarboxylated matrix γ-carboxyglutamate protein and the risk of coronary heart disease and stroke. J Thromb Haemost. 2014;12:1028–1034. doi: 10.1111/jth.12609. [DOI] [PubMed] [Google Scholar]
- 48.van den Heuvel EGHM, van Schoor NM, Lips P, Magdeleyns EJP, Deeg DJH, Vermeer C, den Heijer M. Circulating uncarboxylated matrix Gla protein, a marker of vitamin K status, as a risk factor of cardiovascular disease. Maturitas. 2014;77:137–41. doi: 10.1016/j.maturitas.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Tsao CW, Pencina KM, Massaro JM, Benjamin EJ, Levy D, Vasan RS, Hoffmann U, O’Donnell CJ, Mitchell GF. Cross-sectional relations of arterial stiffness, pressure pulsatility, wave reflection, and arterial calcification. Arterioscler Thromb Vasc Biol. 2014;34:2495–500. doi: 10.1161/ATVBAHA.114.303916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blaha MJ, Budoff MJ, Rivera JJ, Katz R, O’Leary DH, Polak JF, Takasu J, Blumenthal RS, Nasir K. Relationship of Carotid Distensibility and Thoracic Aorta Calcification: Multi-Ethnic Study of Atherosclerosis (MESA) Hypertension. 2009;54:1408–1415. doi: 10.1161/HYPERTENSIONAHA.109.138396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sekikawa A, Shin C, Curb JD, Barinas-Mitchell E, Masaki K, El-Saed A, Seto TB, Mackey RH, Choo J, Fujiyoshi A, et al. Aortic stiffness and calcification in men in a population-based international study. Atherosclerosis. 2012;222:473–7. doi: 10.1016/j.atherosclerosis.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Assmann G, Cullen P, Evers T, Petzinna D, Schulte H. Importance of arterial pulse pressure as a predictor of coronary heart disease risk in PROCAM. Eur Heart J. 2005;26:2120–6. doi: 10.1093/eurheartj/ehi467. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–11. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–45. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 55.Vaccaro JA, Huffman FG. Phylloquinone (vitamin K1) intake and pulse pressure as a measure of arterial stiffness in older adults. J Nutr Gerontol Geriatr. 2013;32:244–57. doi: 10.1080/21551197.2013.809045. [DOI] [PubMed] [Google Scholar]
- 56.Mayer O, Seidlerová J, Wohlfahrt P, Filipovský J, Vaněk J, Cífková R, Windrichová J, Topolčan O, Knapen MHJ, Drummen NEA, et al. Desphospho-uncarboxylated matrix Gla protein is associated with increased aortic stiffness in a general population. J Hum Hypertens. 2015 doi: 10.1038/jhh.2015.55. [DOI] [PubMed] [Google Scholar]
- 57.Pivin E, Ponte B, Pruijm M, Ackermann D, Guessous I, Ehret G, Liu Y-P, Drummen NEA, Knapen MHJ, Pechere-Bertschi A, et al. Inactive Matrix Gla-Protein Is Associated With Arterial Stiffness in an Adult Population-Based Study. Hypertension. 2015;66:85–92. doi: 10.1161/HYPERTENSIONAHA.115.05177. [DOI] [PubMed] [Google Scholar]
- 58.Rees K, Guraewal S, Wong YL, Majanbu DL, Mavrodaris A, Stranges S, Kandala N-B, Clarke A, Franco OH. Is vitamin K consumption associated with cardio-metabolic disorders? A systematic review. Maturitas. 2010;67:121–8. doi: 10.1016/j.maturitas.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Beulens JWJ, Bots ML, Atsma F, Bartelink M-LEL, Prokop M, Geleijnse JM, Witteman JCM, Grobbee DE, van der Schouw YT. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. 2009;203:489–93. doi: 10.1016/j.atherosclerosis.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 60.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MHJ, Van Der Meer IM, Hofman A, Witteman JCM. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The Rotterdam Study. J Nutr. 2004;134:3100–3105. doi: 10.1093/jn/134.11.3100. [DOI] [PubMed] [Google Scholar]
- 61.Gast GCM, de Roos NM, Sluijs I, Bots ML, Beulens JWJ, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PHM, van der Schouw YT. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis. 2009;19:504–10. doi: 10.1016/j.numecd.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Westerterp KR, Goris AHC. Validity of the assessment of dietary intake: problems of misreporting. Curr Opin Clin Nutr Metab Care. 2002;5:489–93. doi: 10.1097/00075197-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Hartley L, Clar C, Ghannam O, Flowers N, Stranges S, Rees K. Vitamin K for the primary prevention of cardiovascular disease. Cochrane database Syst Rev. 2015;9:CD011148. doi: 10.1002/14651858.CD011148.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dalmeijer GW, van der Schouw YT, Magdeleyns E, Ahmed N, Vermeer C, Beulens JWJ. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis. 2012;225:397–402. doi: 10.1016/j.atherosclerosis.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 65.Wallin R, Schurgers LJ, Loeser RF. Biosynthesis of the vitamin K-dependent matrix Gla protein (MGP) in chondrocytes: a fetuin-MGP protein complex is assembled in vesicles shed from normal but not from osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2010;18:1096–103. doi: 10.1016/j.joca.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 66.Loeser RF, Varnum BC, Carlson CS, Goldring MB, Liu ET, Sadiev S, Kute TE, Wallin R. Human chondrocyte expression of growth-arrest-specific gene 6 and the tyrosine kinase receptor axl: potential role in autocrine signaling in cartilage. Arthritis Rheum. 1997;40:1455–65. doi: 10.1002/art.1780400814. [DOI] [PubMed] [Google Scholar]
- 67.Ohno S, Tanaka N, Ueki M, Honda K, Tanimoto K, Yoneno K, Ohno-Nakahara M, Fujimoto K, Kato Y, Tanne K. Mechanical regulation of terminal chondrocyte differentiation via RGD-CAP/beta ig-h3 induced by TGF-beta. Connect Tissue Res. 2005;46:227–34. doi: 10.1080/03008200500346111. [DOI] [PubMed] [Google Scholar]
- 68.Viegas CSB, Cavaco S, Neves PL, Ferreira A, João A, Williamson MK, Price PA, Cancela ML, Simes DC. Gla-rich protein is a novel vitamin K-dependent protein present in serum that accumulates at sites of pathological calcifications. Am J Pathol. 2009;175:2288–98. doi: 10.2353/ajpath.2009.090474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rafael MS, Cavaco S, Viegas CSB, Santos S, Ramos A, Willems BAG, Herfs M, Theuwissen E, Vermeer C, Simes DC. Insights into the association of Gla-rich protein and osteoarthritis, novel splice variants and γ-carboxylation status. Mol Nutr Food Res. 2014 doi: 10.1002/mnfr.201300941. [DOI] [PubMed] [Google Scholar]
- 70.Oka H, Akune T, Muraki S, En-yo Y, Yoshida M, Saika A, Sasaki S, Nakamura K, Kawaguchi H, Yoshimura N. Association of low dietary vitamin K intake with radiographic knee osteoarthritis in the Japanese elderly population: dietary survey in a population-based cohort of the ROAD study. J Orthop Sci. 2009;14:687–92. doi: 10.1007/s00776-009-1395-y. [DOI] [PubMed] [Google Scholar]
- 71.Neogi T, Booth SL, Zhang YQ, Jacques PF, Terkeltaub R, Aliabadi P, Felson DT. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum. 2006;54:1255–61. doi: 10.1002/art.21735. [DOI] [PubMed] [Google Scholar]
- 72.Misra D, Booth SL, Tolstykh I, Felson DT, Nevitt MC, Lewis CE, Torner J, Neogi T. Vitamin K deficiency is associated with incident knee osteoarthritis. Am J Med. 2013;126:243–8. doi: 10.1016/j.amjmed.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shea MK, Kritchevsky SB, Hsu F-C, Nevitt M, Booth SL, Kwoh CK, McAlindon TE, Vermeer C, Drummen N, Harris TB, et al. The association between vitamin K status and knee osteoarthritis features in older adults: the Health, Aging and Body Composition Study. Osteoarthritis Cartilage. 2015;23:370–8. doi: 10.1016/j.joca.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neogi T, Felson DT, Sarno R, Booth SL. Vitamin K in hand osteoarthritis: results from a randomised clinical trial. Ann Rheum Dis. 2008;67:1570–1573. doi: 10.1136/ard.2008.094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Misra D, Booth SL, Crosier MD, Ordovas JM, Felson DT, Neogi T. Matrix Gla protein polymorphism, but not concentrations, is associated with radiographic hand osteoarthritis. J Rheumatol. 2011;38:1960–5. doi: 10.3899/jrheum.100985. [DOI] [PMC free article] [PubMed] [Google Scholar]