Abstract
Objective
To report experience with negative pressure wound therapy (NPWT) in 45 consecutive dogs admitted with extensive cutaneous wounds and to determine if NPWT is feasible in veterinary hospital practice.
Study Design
Prospective descriptive study
Animals
Dogs (n = 45)
Methods
Collected data were organized into 6 categories: patient data, wound data, NPWT data, adjunctive treatments, complications, and final outcome
Results
Wounds (53 in 45 dogs) were largely traumatic in origin, and distributed fairly evenly to the trunk, proximal and distal aspects of the limbs. Most wounds (34 dogs, 76%) had no granulation tissue and were treated a mean of 4.2 days after wounding, whereas 11 dogs had granulating wounds that were initially treated a mean of 87 days after wounding. Median NPWT use was 3 days with a mean hospitalization of 7.8 days. Most wounds (33; 62%) were closed surgically after NPWT and were healed by 14 days. The other 18 wounds healed (mean, 21 days) by second intention after hospital discharge. Overall, 96% of the wounds healed; 2 dogs died before definitive closure could be attempted.
Conclusion
NPWT is applicable to a wide variety of canine wounds is well tolerated, allows for several days between dressing changes, and can used to optimize the wound bed for surgical closure or second intention healing.
INTRODUCTION
Extensive traumatic cutaneous wounds are challenging, often requiring repeated daily sedation or anesthesia for wound management and dressing changes until the wound is suitable for reconstruction or continued healing by second intention. Definitive wound closure should not be attempted until the wound is free of necrotic tissue, debris and infection, preferably supporting a healthy bed of granulation tissue.1,2 Clearly, any therapy or dressing that can facilitate conversion of a contaminated or dirty wound into a clean, vascularized wound bed would be beneficial.
Negative pressure wound therapy (NPWT), involves the application of sub-atmospheric pressure to a wound and is used extensively in human wound care. NWPT is gaining popularity in veterinary medicine as an adjunctive therapy before wound closure.3–8 Also termed “vacuum-assisted closure”, “topical negative pressure therapy”, and “sub-atmospheric pressure dressing”, NPWT involves placing a porous primary dressing (typically open-cell polyurethane ether foam) into the wound bed, sealing the wound with a non-permeable, adhesive drape, and applying an intermittent or continuous vacuum through tubing connected to a fenestrated pad on the foam. The open wound is converted into a closed environment where the entire wound surface is subject to a controlled negative pressure; wound fluid is evacuated through the tubing into a reservoir canister (Fig. 1).
Figure 1.
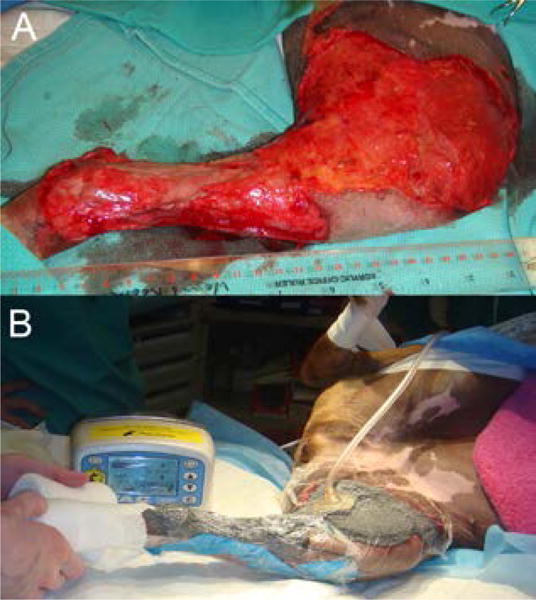
A) extensive open wound on medial aspect of left thigh and crus of a dog. B) after application of NPWT using open cell foam (KCI, San Antonio, TX) as the contact layer. The wound has been sealed with adhesive drapes and a vacuum applied through the pump unit. Negative pressure is achieved via tubing leading to a disc applied to a fenestration created in the drape. This converts the open wound into a controlled, closed environment.
Initially studied in pigs, NPWT increased blood flow to the wound and immediate periwound environment, removed exudate, and stimulated granulation tissue formation.9–12 Subsequent investigations have supported some of these findings (eg, early granulation tissue formation and increased wound perfusion), but other claims remain to be fully validated.13–16 Nevertheless, NPWT technology has increased in popularity and is used in all 5,000 primary care hospitals in the US.17 There is an extensive literature reporting use of NPWT in people where it is applied to both acute and chronic wounds, and in surgical applications (eg, free skin grafts, compromised flaps, incisional dehiscence, cytotoxic sloughs, open abdominal drainage, orthopedic trauma, and burns).5,18–33 NPWT is applied to over 90% of admitted extremity wounds in the military.34–41 Shorter hospitalization and lower treatment cost have been reported for NPWT.42–44
Information on NPWT use in veterinary medicine is limited. There are 7 case reports of various species (a dog, tiger, tortoise, horse, rhinoceros, and 2 cats), 1 retrospective case series in dogs, and more recently, 2 controlled, experimental studies in dogs.8,45–53 The variety of veterinary case reports indicate that NPWT is of interest in wound management. The 2 controlled, experimental veterinary studies concluded that NPWT was beneficial for management of open wounds and free skin grafts in dogs.52,53 Not documented is the feasibility of learning and using NPWT in a busy veterinary hospital setting. Information about the practical application of NWPT, wound types where NPWT is used, complications and pitfalls associated with NWPT use in wound care in a clinical setting would be helpful to those considering investing in NWPT. Thus, our purpose was to collate patient and wound data on 45 dogs treated with NPWT, detail the difficulties and complications associated with the NPWT use, and report wound outcome.
MATERIALS AND METHODS
Inclusion Criteria
Inclusion criteria were the first 45 dogs with open wounds that had NPWT (July 2006 – June 2011). All dogs with open wounds were considered for NPWT, but the final decision on study enrollment was made after consultation between the senior author (BJS) and owner. Dogs were excluded if their wounds did not penetrate the full thickness of the skin, were closed immediately, if it was thought the NPWT system could not be secured (e.g., face) or if owner declined to participate.
Wounds were photographed on admission and at each dressing change using a high-resolution digital camera (DSC-T200, Sony USA, New York, NY). Data collected was categorized: patient data, wound data, NPWT data, adjunctive treatments, complications associated with NPWT, and final wound outcome (Table 1). Medical records of these 45 dogs were additionally reviewed (KAP) after data accrual.
TABLE 1.
Data collected from each of the cases enrolled in this study were organized into six major categories.
| Categories | Data collected |
|---|---|
| Patient Data | Medical record, name, owner |
| Species, Breed | |
| Gender status | |
| Age | |
| Weight, Body Condition Score* | |
| Date of admission; date of discharge | |
| Comorbidities | |
| Wound Data | Age of wound (days) |
| Granulation tissue present or absent | |
| Wound cause | |
| Wound description | |
| Location ID1=trunk, 2=head/neck, 3=proximal limb, 4=distal limb | |
| Location description | |
| Size ID at widest point: 1<5cm, 2=5–10cm, 3>10cm | |
| Size description | |
| NPWT Data | Primary dressing type – foam or gauze; pressure setting |
| Days from admission to NPWT application | |
| Days from wounding to NPWT application | |
| Total days on NPWT | |
| Number of dressing changes | |
| Intermittent or continuous setting | |
| Days between dressing changes | |
| Sedation protocol for dressing changes | |
| Definitive closure type | |
| Definitive closure day | |
| Days from end of NPWT to reconstruction | |
| Adjunctive Treatments | |
| Complications associated with NPWT | |
| Final outcome | |
The Ohio State University: Body Condition Scoring Chart. (http://vet.osu.edu/vmc/body-condition-scoring-chart).
Initial Wound Management Protocol
After any required cardiovascular or respiratory stabilization of the dog, wounds had a standardized management protocol before NPWT use. Under general anesthesia, the open wound area was covered with a sterile water-based lubricant jelly (MediChoice® Lubricating Jelly, Owens & Minor, Mechanicsville, VA). The periwound skin was liberally clipped for a minimum 10 cm around the wound, and cleansed with chlorhexidine scrub (Nolvasan Surgical Scrub®, Fort Dodge Animal Health, Fort Dodge, IA) and isopropyl alcohol (Sun Mark®, McKesson, San Francisco, CA). The open wound was then cleansed thoroughly with gauze swabs soaked in 0.05% chlorhexidine solution (Nolvasan Solution®, Fort Dodge Animal Health). The wound was then draped and gently explored using aseptic technique. The full extent of the wound was identified, hemorrhage controlled, and communications established between any pocketed areas. After surgical debridement, copious wound lavage (3L bag minimum) with a sterile, buffered solution (lactated Ringers) was performed with pulsatile irrigation system (Interpulse®, Stryker, Kalamazoo, MI).
NPWT Protocol
During the reporting period, both foam- and gauze-based NPWT commercial systems were used because of inconsistent availability of a single system. The foam-based system involved the application of a proprietary polyurethane ether foam, wound sealing kit and suction pad connected to the pump units (V.A.C. ® Granufoam®, T.R.A.C. Pad ®, V.A.C.® Freedom units and canisters, Kinetic Concepts Inc., San Antonio, TX). The 2 gauze-based systems (EZCare, Smith & Nephew, Largo, FL and Venturi™, Talley Medical, Hampshire, UK) used saline-moistened, wide-weave gauze into which a fenestrated tube was embedded. The proprietary wound sealing kits (containing primary dressing, tubing, hydrogels, adhesive drapes) were used for all dressing changes, and the dressings were applied according to manufacturer instructions.54–57 In accord with a change in manufacturers’ recommendations at the beginning of the study, most dogs (42/45) were managed using the continuous setting. When applying the adhesive drapes, care was taken to eliminate potential loss of seal by filling all depressions. On wounded areas close to joints, digits and other irregular areas, the use of ostomy pastes (Stomahesive®, ConvaTec USA, Skillman, NJ; Coloplast USA, Minneapolis, MN), to fill crevices or depressions facilitated the smooth application of the adhesive drape. The evacuation tubing was then connected to the canister and the pump activated to the appropriate pressure: −80mmHg for gauze based systems, −125mmHg for the foam-based system. In all cases, successful dressing placement was confirmed by shrinkage, hardening and wrinkling of the foam or gauze once the machine was turned on (Fig. 2). If a leak was suspected, the investigators would listen closely to the dressing for a low, whistling sound to determine its location, and reinforce the seal if necessary. Once an appropriate seal was obtained, the NPWT dressing was covered with a soft, padded bandage, where possible (eg, limb), to help maintain the integrity of the primary dressing. Tubing was arranged and bandaged in such a way that it would not impede movement of the dog. Each bandage change was performed in similar fashion.
Figure 2.
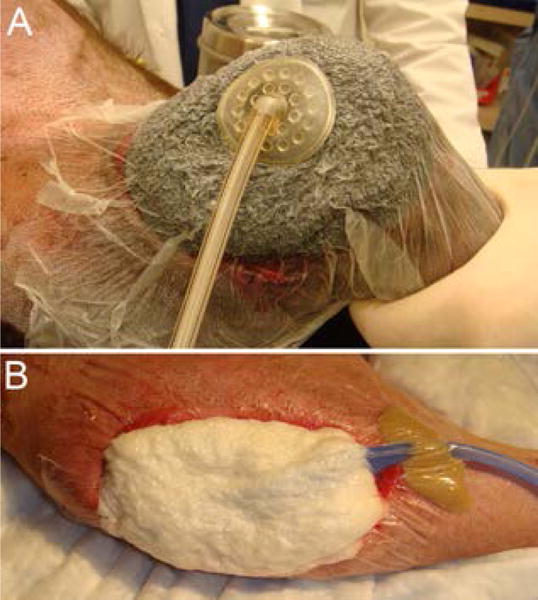
A) Successful placement of the NPWT dressing is confirmed as it takes on a shrunken, hard and wrinkled appearance when the vacuum is applied. A foam dressing (KCI, San Antonio, TX) placed on an open elbow hygroma in a dog. B) Successful placement of the NPWT dressing is confirmed as it takes on a shrunken, hard and wrinkled appearance when the vacuum is applied. A gauze dressing (EZCare, Smith & Nephew, Largo, FL) placed on a dehisced stifle incision in a dog.
RESULTS
Most dogs remained within the hospital, but 3 were managed with NPWT at home, and came in for dressing bandage changes.
Patient Data
Although 22 different dog breeds were treated, the most common dog type was mixed breed (n=11). There were 26 males (16 neutered) and 19 females (15 neutered), with a mean age on admission, 5.6 years (range, 1 – 12.4 years). Mean weight was 31.1kg (range, 3.1 – 62.3 kg) and mean body condition score (BCS) was 5.2/9 (range, 1.5 – 8). Mean hospitalization was 7.75 days (range, 1 – 21 days). Comorbidities were widely varied and depended on the individual dog´s health before injury, as well as the cause of the injury. The most common comorbidities thought to have an effect on wound healing were: orthopedic injuries (14 dogs), anemia (12), concurrent infection (8), endocrinopathies (4), respiratory disease (3), wound lymphedema (2), coagulopathy (2), renal disease (2), shock (2), neoplasia (2), neurologic (2), cardiovascular (1) and hepatic (1) disease. Less relevant comorbidities included conjunctivitis (3 dogs), lumbosacral disease (3), and arthritis (2).
Wound Data
In 45 dogs, 53 wounds had NPWT. Mean wound age was 7 days (range, 0 – 365 days). Thirty-four dogs (76%) had no granulation tissue in their wounds (mean wound age, 4.2 days; range, 0 – 21 days) whereas 11 dogs (24%) had granulation tissue in their wounds and were considered chronic (mean wound age, 87 days; range, 7 – 365 days). Three chronic wounds were >140 days old and had been classed before referral as chronic, non-healing wounds.
Wound cause varied; 28 (62%) dog had wounds of traumatic origin (vehicular trauma, bite wounds, gunshot, or unknown trauma) and 17 had wounds caused by incisional dehiscence (7%), hygroma/pressure wounds (5%), abscesses (13%), envenomation (4%) and chronic non-healing wounds of unknown origin (9%).
All 53 wounds had full thickness skin loss and were anatomic degloving or shear injuries (31%), punctures (20%), lacerations (16%), abscesses (13%), chronic non-healing open wounds (9%), dehiscence (7%), and physiologic degloving (4%). Wounds were distributed fairly evenly to the trunk (28%), proximal (32%) and distal (34%) aspects of the limbs. Only 3 wounds (6%) were located on the head/neck. Six (11%) wounds were <5 cm diameter, 22 (42%) were >5 cm but < 10 cm in largest diameter, and 25 (47%) were >10 cm in largest diameter.
NPWT Data
Twenty-four (53%) dogs were treated with foam-based NPWT (−125 mmHg), and 21 (47%) had gauze-based NPWT (−80 mmHg). Mean time from admission to NPWT use was 2 days (range, 0 – 14). Time from wounding to NPWT varied widely (range, 0–368 days; mean, 26 days) because of the chronicity of some wounds. Median time (right skewed data) of NPWT use was 3 days (range, 1 – 22 days) with 42 dogs (93%) treated < 8 days (Figs. 3, 4). Dressing changes were consistently performed every 2 or 3 days (mean, 2.4 days; range, 1 – 3.5 days). Forty-one (82%) dogs required heavy sedation or anesthesia for dressing changes. A consistent sedation protocol was not recorded in 9 dogs (ie, it differed each dressing change). The most commonly used drugs were acepromazine (51%), hydromorphone (49%), and propofol with isoflurane (40%). Additional drugs used were fentanyl boluses (9%), butorphanol (16%), ketamine and diazepam (20%), and medetomidine (4%) at dosages based on anesthesia service recommendations.
Figure 3.
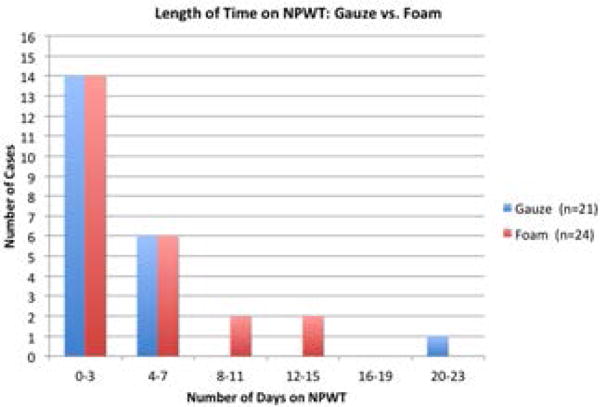
Graph depicting the number of dogs receiving NPWT in each stratified time period, and primary dressing (foam or gauze) that was used.
Figure 4.
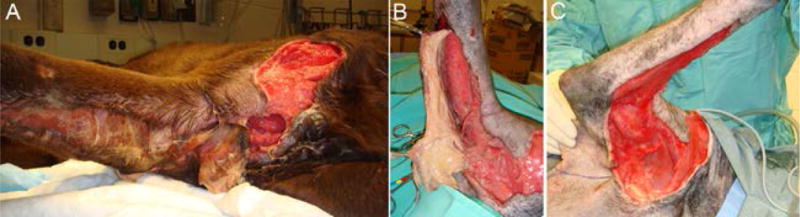
A) Necrotic wound on the right brachium and antebrachium caused by an untreated impalement in a 2 year male neutered Labrador retriever. B) during debridement, before placement of NPWT. C) wound appearance after 3 days of NPWT, immediately before reconstruction. Note the early appearance of granulation tissue in the wound.
Definitive closure method in 19 (36%) wounds was delayed primary closure (mean, 2.7 days of NWPWT; range, 1–5 days) defined as closure before appearance of an established granulation tissue bed but presence of some early granulation tissue. Fourteen wounds (26%) had secondary closure (closure over well-established granulation tissue) after 2 – 22 days of NPWT (mean, 6.9 days). Definitive closure procedures included direct apposition, skin flap, or free cutaneous graft. For wounds that had surgical closure, the NPWT device was removed either immediately before, or the day before reconstructive surgery. When free skin grafts were used, NPWT was re-applied at a continuous pressure of −80 mmHg, with a layer of petrolatum-impregnated knitted cellulose acetate (Adaptic®, Johnson & Johnson, Arlington, TX) over the graft. Eighteen wounds (34%) were managed until they healed by second intention; NPWT was discontinued once a smooth, vascular granulation tissue bed was evident (mean, 5.4 days; range, 1–15 days), after which, semi-occlusive, non-adherent wound dressings (Adaptic®, Johnson & Johnson, Arlington, TX or Telfa ™, Covidien, Mansfield, MA) were applied. In 2 dogs, no definitive wound closure occurred because the dogs died before reconstruction.
Adjunctive Treatment
Adjunctive treatments varied depending mainly on comorbidities and concurrent illnesses and the severity of injuries. All dogs were administered intravenous crystalloids and systemic antibiotics at some time during hospitalization. Common antibiotics administered were clavamox, cephalexin, and enrofloxacin. Adjunctive treatments included orthopedic repairs (7 dogs), blood component therapy (2), abdominal drains (1), hyaluronic acid (2), teeth extractions (2), topical oxygen emulsion (1), and nasogastric tube placement (1).
Complications Associated with NPWT
Complications were minor, especially as clinicians and staff gained experience applying the dressings and became familiar with the machines (e.g., intensity setting, tampering lock). The major issue reported with NPWT was the loss of vacuum because of inadequate periwound adhesion. This was most notable in the gauze-based systems, where the evacuation tubing had to be secured to the skin. It was also recorded for the digits, where crevices and movement caused drape detachment and leakage. Meticulous attention to clipping and cleansing the periwound skin, using a spray adhesive, and creating a dam across the uneven surfaces (such as interdigital areas) with stoma paste mitigated this problem. Chewing or kinking of the tubing caused device failure in 3 dogs and was resolved by appropriate attention to E-collar placement and cage confinement. In 1 young dog with a large abscessed elbow hygroma, granulation tissue was noted to adhere slightly to the foam dressing. Although not included in the analysis, we concurrently treated 3 cats and 2 horses and found that cats were intolerant of intermittent therapy – flinching and occasional vocalization was noted upon pump activation. One dog developed mild skin irritation with prolonged NPWT (after 7 – 10 days). This complication was reduced by allowing the original adhesive drape to remain on the periwound skin, and simply cutting off the dressing immediately over the wound bed, and any other non-adherent portion. With increasing experience using NPWT, far fewer management and technical issues occurred.
Outcome
One dog was lost to follow up after definitive closure, so outcome was assessed for 44 dogs (52 wounds); 50 wounds (96%; 23 dogs foam-based, 20 of 21 dogs gauze-based dressings) healed and no statistical difference in outcome was detected between dressing types (χ2 2-tailed, P =.98). All 32 surgically closed wounds were considered healed by the 10–14-day postoperative recheck. All 18 wounds healing by second intention were considered healed (fully epithelialized) between 14 and 32 days (mean, 21 days) after discharge. Nine of 32 wounds (28%) had minor complications after closure, including seroma (2) superficial flap tip or partial superficial graft necrosis (3), partial incisional dehiscence after delayed primary closure (1), and wound lymphedema (3); all complications resolved and the wounds healed. Two dogs (4%) died before wound healing was achieved. One had cardiac arrest before final closure could be attempted, and 1 was euthanatized because of progression of concurrent neurologic disease. Both these dogs had substantial and multiple comorbidities, including systemic infection, shock, coagulopathy, thoracic and neurologic trauma. Another dog had an extensive necrotizing wound of the right thoracic limb, axilla, and sternum caused by envenomation. Although the wound responded well to NPWT, the initial reconstructive effort (a thoracodorsal axial pattern flap) failed and this dog subsequently had successful forequarter amputation.
DISCUSSION
We found that NPWT is feasible for a wide variety of wound causes and types, and a useful mechanical adjunct to wound management in a well-staffed veterinary hospital setting. Although this study was not comparative, the short time to reconstruction appears to be consistent with previous reports of NPWT showing a rapid formation of a smooth granulation tissue bed.8,52,53
Although the dogs were fairly evenly distributed between early reconstruction, late reconstruction, and second intention healing, NPWT was generally used for less than a week, similar to the 15 dogs reported by Ben-Amotz.8 This suggests it is most useful in stimulating the development of a healthy wound bed suitable for a reconstructive effort, rather than a long-term management option. In these and other authors’ opinions, wounds undergoing NPWT were closed in a shorter time (first or second dressing change) than is typical for traumatic wounds.8,49,52 In our dogs, NPWT also appeared to accelerate or “kick-start” the chronic wound beds into the reparative phase, optimizing the wound environment for epithelialization and contraction.
One interesting finding was the lack of dogs where NPWT was used for head and neck wounds, considering that we often see wounds (e.g., bite wounds) in these areas. In this study, we selected against head and neck wounds due to our lack of expertise in obtaining a seal and maintaining integrity of the dressings in this anatomical region. In people, application of NPWT in the nuchal area not only improves healing, but allows increased patient mobility (neck movement is socially and functionally very important).21,58 As we gain further experience with NPWT, innovative approaches should be considered for wounds around the head and neck. Such approaches may include more extensive clipping of the face, head, and neck, use of temporary tarsorrhaphies, additional protective padding with loop sutures over the NPWT dressings, and meticulous attention being paid to ‘building up’ the initial application of the dressing (e.g. with stoma paste).
A limitation of our study is the lack of data on wounds that presented to the VTH and did not receive NPWT. However, it appears that this modality was utilized much more frequently in larger wounds; almost 90% of the wounds in this study were > 5 cm diameter, and 50% were >10 cm diameter. Wounds in published reports have typically been large; however, in the series of 15 dogs with extremity wounds, dimensions were not stated.8,45–49,51 Mean hospitalization time in our study was 1 week; this appears to be a shorter time than typical for wounds of this size, and is slightly less than the 10 days in the reported case series.8 Smaller wounds (<5cm) were often not selected for NPWT because these wounds do not pose the same management challenges.
One major advantage of using NPWT is avoidance of daily anesthesia/sedation as required for traditional wet-to-dry dressings. As evident from the types of drugs used, NPWT dressing changes require heavy sedation (or anesthesia), but the 3 days between NPWT dressing changes allows patients to recover adequately to eat, drink, eliminate, ambulate, and interact with visiting owners. In comparison, the wet-to-dry dressing needs to be re-dressed every 12–24 hours, requiring some form of sedation or anesthesia each time, thus increasing patient morbidity and cost. Nutritional intake (providing calories) and ambulation (promoting lymphatic drainage) are both critical for optimizing wound healing. An additional advantage that was noted with NPWT was that strike-through is completely eliminated, as all exudate and wound fluids are collected into the canister.
Most dogs tolerated the NPWT well. Because of the intolerance to intermittent therapy in cats, presumably because of pain when the vacuum is activated, we now use continuous mode in cats. The negative outcomes in 2 dogs were considered to be unrelated to the use of NPWT.
Although there are around a dozen different proprietary NPWT systems in the human wound care market, the main difference is in the contact layer, consisting of either foam or gauze. The most commonly reported is a polyurethane ether foam-based system, marketed by Kinetic Concepts Incorporated (KCI; San Antonio, TX), which has >80% market share.60 Both primary dressings reportedly have similar mechanisms of action,61,62 and we did not find any difference in wound outcome between the 2 dressings.
Maintenance of therapy was quite simple once initial technical hurdles were overcome. The steepest learning curve was in securing of a good periwound seal in areas where the skin was uneven, e.g., between the digits, over the hock, the perineum. Techniques that were developed during our study included using various stoma pastes, and adding a liquid skin adhesive to ensure adequate periwound adhesion. The gauze-based systems were more difficult to secure compared with foam-based systems, because of the way the evacuation tubing had to be applied to the skin. Interestingly, placing the system over areas of mobility (e.g., axilla, inguinal area, over joints) did not appear to cause a loss of dressing integrity, possibly because of the disruptive shear forces were neutralized by “splinting” of the area. When possible, NPWT dressings were covered with a soft, padded bandage for protection. As dogs started to heal and became more mobile, they would sometimes start to circle before they lay down. This behavior should be noted, and any twisting of the tubing can be corrected at that time.
Although human patients are now frequently managed in the community with NPWT, in our study, only 3 dogs were treated at home with NPWT. These owners were extremely competent in managing their dog’s conditions. Although initially hesitant, we have now increased our home management of NPWT, in conjunction with training of owners on using the therapy, and the animals only come in for dressing changes or if a complication arises.
The machines we used were designed for human hospital use with many functions unnecessary for animals (eg, tampering lock). Veterinary-specific VAC® units are now available that are smaller, lighter, more robust and display fewer pump alerts than when we conducted this study. These smaller devices can be carried in a halter or harness, which would reduce any kinking complications.
One of the most controversial claims of NPWT is the ability to decrease the bacterial burden of a wound.14 Although nearly all wounds were cultured, NPWT placement was not influenced by this test. Culture results were generally not reported until NPWT had been in place for several days (or sometimes even removed). Because of lack of a control group in this study we are unable to provide any data to support or refute any claims of increased bacterial clearance with NPWT.
Cost comparison studies in human wound care have shown decreased overall cost of care when NPWT is used.44,65–68 We did not attempt to compare costs of using NPWT with standard-of-care. The costs of initial machine purchase and dressing materials need to be offset against the cost of decreased dressing changes, shortened time to reconstruction, and shorter hospitalization times.
Overall, our clinical experience with NPWT over the course of this study and since (over 150 cases) has been very positive, concurring with the outcome of the other case series.8 In our hospital, NPWT has replaced the wet-to-dry dressing as standard-of-care for acute traumatic wounds, skin grafts, and dehiscence. The encouraging findings of our study should be validated with randomized, controlled, clinical trials comparing NPWT with wet-to-dry and other dressings, specifically for time to reconstruction, and hospitalization time and cost, before widespread recommendations are made.
Acknowledgments
We thank Chris Phipps, LVT for maintaining log of cases, and to all NCU technicians involved in the care of patients on NPWT.
Footnotes
DISCLOSURE
Following acceptance of this manuscript, Dr. BJ Stanley has acted as a consultant to Kinetic Concepts, Inc.
References
- 1.Swaim SF. Veterinary Clinics of North America. Vol. 36. Philadelphia: Saunders; 2006. Wound Management. [Google Scholar]
- 2.Pavletic MM. Atlas of Small Animal Wound Management and Reconstructive Surgery. 3rd. Ames, Iowa: Wiley-Blackwell; 2010. [Google Scholar]
- 3.Kirkby K, Wheeler J, Farese J, et al. Surgical views: vacuum-assisted wound closure: clinical applications. Compend Contin Educ Vet. 2010;32:E1–7. [PubMed] [Google Scholar]
- 4.Bovill E, Banwell PE, Teot L, et al. Topical negative pressure wound therapy: a review of its role and guidelines for its use in the management of acute wounds. Int Wound J. 2008;5:511–529. doi: 10.1111/j.1742-481X.2008.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanakaris NK, Thanasas C, Keramaris N, et al. The efficacy of negative pressure wound therapy in the management of lower extremity trauma: review of clinical evidence. Injury. 2007;38(Suppl 5):S9–18. doi: 10.1016/j.injury.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Hunter JE, Teot L, Horch R, et al. Evidence-based medicine: vacuum-assisted closure in wound care management. Int Wound J. 2007;4:256–269. doi: 10.1111/j.1742-481X.2007.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parrett BM, Matros E, Pribaz JJ, et al. Lower extremity trauma: trends in the management of soft-tissue reconstruction of open tibia-fibula fractures. Plast Reconstr Surg. 2006;117:1315–1322. doi: 10.1097/01.prs.0000204959.18136.36. discussion 1323–1314. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Amotz R, Lanz OI, Miller JM, et al. The use of vacuum-assisted closure therapy for the treatment of distal extremity wounds in 15 dogs. Vet Surg. 2007;36:684–690. doi: 10.1111/j.1532-950X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 9.Morykwas MJ, Argenta LC, Shelton-Brown EI, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553–562. doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Morykwas MJ, David LR, Schneider AM, et al. Use of subatmospheric pressure to prevent progression of partial-thickness burns in a swine model. J Burn Care Rehabil. 1999;20:15–21. doi: 10.1097/00004630-199901001-00003. [DOI] [PubMed] [Google Scholar]
- 11.Morykwas MJ, Kennedy A, Argenta JP, et al. Use of subatmospheric pressure to prevent doxorubicin extravasation ulcers in a swine model. J Surg Oncol. 1999;72:14–17. doi: 10.1002/(sici)1096-9098(199909)72:1<14::aid-jso4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Morykwas MJ, Faler BJ, Pearce DJ, et al. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg. 2001;47:547–551. doi: 10.1097/00000637-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Morykwas MJ, Simpson J, Punger K, et al. Vacuum-assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg. 2006;117:121S–126S. doi: 10.1097/01.prs.0000225450.12593.12. [DOI] [PubMed] [Google Scholar]
- 14.Xie X, McGregor M, Dendukuri N. The clinical effectiveness of negative pressure wound therapy: a systematic review. J Wound Care. 2010;19:490–495. doi: 10.12968/jowc.2010.19.11.79697. [DOI] [PubMed] [Google Scholar]
- 15.Peinemann F, Sauerland S. Negative-pressure wound therapy: systematic review of randomized controlled trials. Dtsch Arztebl Int. 2011;108:381–389. doi: 10.3238/arztebl.2011.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glass GE, Nanchahal J. The methodology of negative pressure wound therapy: Separating fact from fiction. J Plast Reconstr Aesthet Surg. 2012 doi: 10.1016/j.bjps.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Grellner D. Pers comm. Kinetic Concepts Inc; San Antonio, TX: 2012. [Google Scholar]
- 18.Genecov DG, Schneider AM, Morykwas MJ, et al. A controlled subatmospheric pressure dressing increases the rate of skin graft donor site reepithelialization. Ann Plast Surg. 1998;40:219–225. doi: 10.1097/00000637-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Moisidis E, Heath T, Boorer C, et al. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg. 2004;114:917–922. doi: 10.1097/01.prs.0000133168.57199.e1. [DOI] [PubMed] [Google Scholar]
- 20.Scherer LA, Shiver S, Chang M, et al. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg. 2002;137:930–933. doi: 10.1001/archsurg.137.8.930. discussion 933–934. [DOI] [PubMed] [Google Scholar]
- 21.Schneider AM, Morykwas MJ, Argenta LC. A new and reliable method of securing skin grafts to the difficult recipient bed. Plast Reconstr Surg. 1998;102:1195–1198. doi: 10.1097/00006534-199809040-00045. [DOI] [PubMed] [Google Scholar]
- 22.Paul JC. Vacuum assisted closure therapy: a must in plastic surgery. Plast Surg Nurs. 2005;25:61–65. doi: 10.1097/00006527-200504000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Uygur F, Duman H, Ulkur E, et al. The role of the vacuum-assisted closure therapy in the salvage of venous congestion of the free flap: case report. Int Wound J. 2008;5:50–53. doi: 10.1111/j.1742-481X.2007.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Argenta PA, Rahaman J, Gretz HF, 3rd, et al. Vacuum-assisted closure in the treatment of complex gynecologic wound failures. Obstet Gynecol. 2002;99:497–501. doi: 10.1016/s0029-7844(01)01752-5. [DOI] [PubMed] [Google Scholar]
- 25.de Vooght A, Feruzi G, Detry R, et al. Vacuum-assisted closure for abdominal wound dehiscence with prosthesis exposure in hernia surgery. Plast Reconstr Surg. 2003;112:1188–1189. doi: 10.1097/01.PRS.0000077233.64957.30. [DOI] [PubMed] [Google Scholar]
- 26.Fife CE, Otto G, Walker D, et al. Healing dehisced surgical wounds with negative pressure wound therapy. Ostomy Wound Manage. 2004;50:28–31. [PubMed] [Google Scholar]
- 27.DeFranzo AJ, Pitzer K, Molnar JA, et al. Vacuum-assisted closure for defects of the abdominal wall. Plast Reconstr Surg. 2008;121:832–839. doi: 10.1097/01.prs.0000299268.51008.47. [DOI] [PubMed] [Google Scholar]
- 28.Fackler ML. Vacuum-assisted closure of the abdomen. J Am Coll Surg. 2008;206:400. doi: 10.1016/j.jamcollsurg.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Stone PA, Hass SM, Flaherty SK, et al. Vacuum-assisted fascial closure for patients with abdominal trauma. J Trauma. 2004;57:1082–1086. doi: 10.1097/01.ta.0000149248.02598.9e. [DOI] [PubMed] [Google Scholar]
- 30.Schlatterer D, Hirshorn K. Negative pressure wound therapy with reticulated open cell foam-adjunctive treatment in the management of traumatic wounds of the leg: a review of the literature. J Orthop Trauma. 2008;22:S152–160. doi: 10.1097/BOT.0b013e318188e2d7. [DOI] [PubMed] [Google Scholar]
- 31.Pollak AN. Use of negative pressure wound therapy with reticulated open cell foam for lower extremity trauma. J Orthop Trauma. 2008;22:S142–145. doi: 10.1097/BOT.0b013e318188e2a9. [DOI] [PubMed] [Google Scholar]
- 32.Terrazas SG. Adjuvant dressing for negative pressure wound therapy in burns. Ostomy Wound Manage. 2006;52:16–18. [PubMed] [Google Scholar]
- 33.Nugent N, Lannon D, O’Donnell M. Vacuum-assisted closure - a management option for the burns patient with exposed bone. Burns. 2005;31:390–393. doi: 10.1016/j.burns.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Lalliss SJ, Branstetter JG, Murray CK, et al. Infection rates in U.S. military personnel using vacuum-assisted closure. Plast Reconstr Surg. 2007;120:574–575. doi: 10.1097/01.prs.0000267671.78598.b4. author reply 575–576. [DOI] [PubMed] [Google Scholar]
- 35.Leininger BE, Rasmussen TE, Smith DL, et al. Experience with wound VAC and delayed primary closure of contaminated soft tissue injuries in Iraq. J Trauma. 2006;61:1207–1211. doi: 10.1097/01.ta.0000241150.15342.da. [DOI] [PubMed] [Google Scholar]
- 36.Powell ETt. The role of negative pressure wound therapy with reticulated open cell foam in the treatment of war wounds. J Orthop Trauma. 2008;22:S138–141. doi: 10.1097/BOT.0b013e318188e27d. [DOI] [PubMed] [Google Scholar]
- 37.Geiger S, McCormick F, Chou R, et al. War wounds: lessons learned from Operation Iraqi Freedom. Plast Reconstr Surg. 2008;122:146–153. doi: 10.1097/PRS.0b013e3181773d19. [DOI] [PubMed] [Google Scholar]
- 38.Kumar AR, Grewal NS, Chung TL, et al. Lessons from operation Iraqi freedom: successful subacute reconstruction of complex lower extremity battle injuries. Plast Reconstr Surg. 2009;123:218–229. doi: 10.1097/PRS.0b013e3181904da9. [DOI] [PubMed] [Google Scholar]
- 39.Kumar AR, Grewal NS, Chung TL, et al. Lessons from the modern battlefield: successful upper extremity injury reconstruction in the subacute period. J Trauma. 2009;67:752–757. doi: 10.1097/TA.0b013e3181808115. [DOI] [PubMed] [Google Scholar]
- 40.Marsh DJ, Abu-Sitta G, Patel H. The role of vacuum-assisted wound closure in blast injury. Plast Reconstr Surg. 2007;119:1978–1979. doi: 10.1097/01.prs.0000259773.52889.68. [DOI] [PubMed] [Google Scholar]
- 41.Hinck D, Franke A, Gatzka F. Use of vacuum-assisted closure negative pressure wound therapy in combat-related injuries–literature review. Mil Med. 2010;175:173–181. doi: 10.7205/milmed-d-09-00075. [DOI] [PubMed] [Google Scholar]
- 42.Braakenburg A, Obdeijn MC, Feitz R, et al. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg. 2006;118:390–397. doi: 10.1097/01.prs.0000227675.63744.af. discussion 398–400. [DOI] [PubMed] [Google Scholar]
- 43.Apelqvist J, Armstrong DG, Lavery LA, et al. Resource utilization and economic costs of care based on a randomized trial of vacuum-assisted closure therapy in the treatment of diabetic foot wounds. Am J Surg. 2008 doi: 10.1016/j.amjsurg.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 44.Niezgoda JA. The economic value of negative pressure wound therapy. Ostomy Wound Manage. 2005;51:44S–47S. [PubMed] [Google Scholar]
- 45.Mullally C, Carey K, Seshadri R. Use of a nanocrystalline silver dressing and vacuum-assisted closure in a severely burned dog. J Vet Emerg Crit Care (San Antonio) 2010;20:456–463. doi: 10.1111/j.1476-4431.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- 46.Harrison TM, Stanley BJ, Sikarskie JG, et al. Surgical amputation of a digit and vacuum-assisted closure (V.A.C.) management in a case of osteomyelitis and wound care in an Eastern Black Rhinoceros (Diceros bicornis michaeli) Journal of Zoo and Wildlife Medicine. 2011;42:317–321. doi: 10.1638/2010-0149.1. [DOI] [PubMed] [Google Scholar]
- 47.Owen L, Hotston-Moore A, Holt P. Vacuum-assisted wound closure following urine-induced skin and thigh muscle necrosis in a cat. Vet Comp Orthop Traumatol. 2009;22:417–421. doi: 10.3415/VCOT-08-12-0123. [DOI] [PubMed] [Google Scholar]
- 48.Lafortune M, Fleming GJ, Wheeler JL, et al. Wound management in a juvenile tiger (Panthera tigris) with vacuum-assisted closure (V.A.C. Therapy) J Zoo Wildl Med. 2007;38:341–344. doi: 10.1638/1042-7260(2007)038[0341:WMIAJT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 49.Guille AE, Tseng LW, Orsher RJ. Use of vacuum-assisted closure for management of a large skin wound in a cat. J Am Vet Med Assoc. 2007;230:1669–1673. doi: 10.2460/javma.230.11.1669. [DOI] [PubMed] [Google Scholar]
- 50.Adkesson MJ, Travis EK, Weber MA, et al. Vacuum-assisted closure for treatment of a deep shell abscess and osteomyelitis in a tortoise. J Am Vet Med Assoc. 2007;231:1249–1254. doi: 10.2460/javma.231.8.1249. [DOI] [PubMed] [Google Scholar]
- 51.Gemeinhardt KD, Molnar JA. Vacuum-assisted closure for management of a traumatic neck wound in a horse. Equine Veterinary Education. 2005;17:27–33. [Google Scholar]
- 52.Demaria M, Stanley BJ, Hauptman JG, et al. Effects of negative pressure wound therapy on healing of open wounds in dogs. Vet Surg. 2011;40:658–669. doi: 10.1111/j.1532-950X.2011.00849.x. [DOI] [PubMed] [Google Scholar]
- 53.Stanley BJ, Pitt KA, Weder CD, et al. Effects of Negative Pressure Wound Therapy on Healing of Full-Thickness Skin Grafts in Dogs. Vet Surg Epub ahead of print. doi: 10.1111/j.1532-950X.2013.12005.x2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tally Medical. Venturi (TM) Negative Pressure Wound Therapy System: Clinical Guidelines Manual. 2009 [Google Scholar]
- 55.Kinetic Concepts. VAC Therapy Clinical Guidelines: A reference source for clinicians. 2007 [Google Scholar]
- 56.Kinetic Concepts. VAC Therapy Clinical Guidelines: A reference source for clinicians. 2005 [Google Scholar]
- 57.Smith, Nephew EZCare Negative Pressure Wound Therapy: User Guide. 2007 [Google Scholar]
- 58.Chang KP, Tsai CC, Lin TM, et al. An alternative dressing for skin graft immobilization: negative pressure dressing. Burns. 2001;27:839–842. doi: 10.1016/s0305-4179(01)00052-3. [DOI] [PubMed] [Google Scholar]
- 59.Malmsjo M, Gustafsson L, Lindstedt S, et al. The effects of variable, intermittent, and continuous negative pressure wound therapy, using foam or gauze, on wound contraction, granulation tissue formation, and ingrowth into the wound filler. Eplasty. 2012;12:e5. [PMC free article] [PubMed] [Google Scholar]
- 60.Clark T. In: MSU CVM Wounds. Stanley DB, editor. 2012. (E-mail communication), Vol. [Google Scholar]
- 61.Malmsjo M, Ingemansson R, Martin R, et al. Wound edge microvascular blood flow: effects of negative pressure wound therapy using gauze or polyurethane foam. Ann Plast Surg. 2009;63:676–681. doi: 10.1097/SAP.0b013e31819ae01b. [DOI] [PubMed] [Google Scholar]
- 62.Malmsjo M, Ingemansson R, Martin R, et al. Negative-pressure wound therapy using gauze or open-cell polyurethane foam: similar early effects on pressure transduction and tissue contraction in an experimental porcine wound model. Wound Repair Regen. 2009;17:200–205. doi: 10.1111/j.1524-475X.2009.00461.x. [DOI] [PubMed] [Google Scholar]
- 63.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563–576. discussion 577. [PubMed] [Google Scholar]
- 64.Argenta LC, Morykwas MJ, Marks MW, et al. Vacuum-assisted closure: state of clinic art. Plast Reconstr Surg. 2006;117:127S–142S. doi: 10.1097/01.prs.0000222551.10793.51. [DOI] [PubMed] [Google Scholar]
- 65.Apelqvist J, Armstrong DG, Lavery LA, et al. Resource utilization and economic costs of care based on a randomized trial of vacuum-assisted closure therapy in the treatment of diabetic foot wounds. Am J Surg. 2008;195:782–788. doi: 10.1016/j.amjsurg.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 66.Baharestani MM. Negative pressure wound therapy: an examination of cost-effectiveness. Ostomy Wound Manage. 2004;50:29S–33S. [PubMed] [Google Scholar]
- 67.Mokhtari A, Sjogren J, Nilsson J, et al. The cost of vacuum-assisted closure therapy in treatment of deep sternal wound infection. Scand Cardiovasc J. 2008;42:85–89. doi: 10.1080/14017430701744469. [DOI] [PubMed] [Google Scholar]
- 68.Philbeck TE, Jr, Whittington KT, Millsap MH, et al. The clinical and cost effectiveness of externally applied negative pressure wound therapy in the treatment of wounds in home healthcare Medicare patients. Ostomy Wound Manage. 1999;45:41–50. [PubMed] [Google Scholar]


